Abstract
Significant fluctuations in serum glucose levels accompany the stress response of surgery or acute injury and may be associated with vascular or neurologic morbidity. Maintenance of euglycemia with intensive insulin therapy (IIT) continues to be investigated as a therapeutic intervention to decrease morbidity associated with derangements in glucose metabolism. Hypoglycemia is a common side effect of IIT with potential for significant morbidity, especially in the neurologically injured patient. Differences in cerebral versus systemic glucose metabolism, the time course of cerebral response to injury, and heterogeneity of pathophysiology in neurosurgical patient populations are important to consider in evaluating the risks and benefits of IIT. While extremes of glucose levels are to be avoided, there are little data to support specific use of IIT for maintenance of euglycemia in the perioperative management of neurosurgical patients. Existing data are summarized and reviewed in this context.
Keywords: cerebral glucose metabolism, hyperglycemic brain injury, intensive insulin therapy, perioperative glucose control
Introduction
Hyperglycemia contemporaneous with cerebral ischemia is associated with deleterious clinical sequelae in some neurosurgical patient populations.1–8 These include a longer intensive care unit (ICU) stay, poorer recovery of neurologic function, symptomatic cerebral vasospasm, and increased infarct size.9,10 Still debated is whether plasma glucose level is primarily a surrogate marker of disease severity and its associated metabolic derangements, the response to stress, or a causative agent of damage exacerbation.
Therapeutic interventions to lower glucose levels to a tight range (80–120 mg/dl) with intensive insulin therapy (IIT) have been studied in a variety of critical care settings with mixed results. Clinical data regarding glucose control in the general surgical population and during neurocritical care have been reviewed.11,12 Clear benefit has only been demonstrated in cardiac and vascular surgery populations.13,14 However, a near universal finding in these studies is an increased incidence of iatrogenic hypoglycemia in patients receiving IIT. The clinical consequences of iatrogenic hypoglycemia are just beginning to be investigated. For example, the Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial reported both increased hypoglycemia and increased risk of death in a tight control group of ICU patients on IIT.15
Generalization of clinical studies of IIT to neurosurgical patients must be done with caution and in the context of an understanding of brain glucose metabolism. Brain glucose metabolism is altered by neurologic disease. The mixed data on tight glucose control presents neurointensivists and anesthesiologists with the clinical conundrum that persistent hyperglycemia creates numerous untoward consequences while iatrogenic hypoglycemia may initiate brain metabolic crises that are even worse. Moreover, the upper and lower thresholds of plasma glucose for these adverse effects are not clearly defined, and peripheral glucose measurements do not consistently correlate with brain glucose levels.
Hypoglycemia in the neurosurgical patient, both peripheral and cerebral, is of particular concern. The negative physiologic effects of hypoglycemia in the setting of neuropathology may be exaggerated when compared to healthy patients; and clinical signs are frequently masked by the underlying condition. Several new studies investigating cerebral glucose metabolism in the critical care setting have advanced our understanding and reinforced these concerns regarding tight glucose control.
This review focuses on the physiology of brain glucose metabolism and available clinical data on glucose control and IIT in neurosurgical patients that inform the highlighted controversies.
Brain Glucose Metabolism
The brain is critically dependent on a continuous supply of both oxygen and glucose for normal metabolic function. This includes synaptic transmission, maintenance of transmembrane ionic gradients and cellular integrity, and biochemical synthesis. The brain contains multiple cell types (e.g., neurons, glial cells such as astrocytes, oligodendrocytes, microglia, and, finally, the cells that constitute the brain's vascular system). Each of these cell types appears to have different metabolic profiles and, presumably, vulnerabilities. The neurologic effects of transient or sustained alterations in both blood and brain glucose must be interpreted in this context. Here we highlight several critical aspects of brain glucose metabolism. Readers are referred to a comprehensive review of brain metabolism by Fitch16 and a recent in-depth discussion of brain biochemistry in Gibson and Dienel.17
Glucose delivered to the brain is taken up into cells (10% of delivered; 25% of total body glucose utilization) and undergoes glycolytic breakdown to adenosine tri-phosphate (ATP) and pyruvate under aerobic conditions (i.e., oxygen available and normal mitochondrial function). Pyruvate is converted to acetyl-CoA via the tricarboxylic acid (TCA) cycle to generate ATP and reducing equivalents. When oxidative phosphorylation is impaired (lack of oxygen, mitochondrial dysfunction, brain injury), pyruvate is instead converted to lactate. Most of these biosynthetic reactions are under tight feedback control. Delicate equilibriums are modulated by many factors including substrate availability/concentration, acid–base status, and redox status.
Normal brain glucose metabolism is altered under conditions of hyperglycemia, hypoglycemia, and pharma-cologic therapy. Animal and human studies indicate a significantly altered normal glucose metabolism in the setting of traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and cerebral ischemia.18 The metabolic derangements associated with neuro-pathology are heterogeneous and continue to change with the evolution of the underlying pathologic process.
Anesthetic drugs such as propofol or barbiturates used during surgery or ICU sedation decrease the cerebral metabolic rate of oxygen (CMRO2) and, to some extent, cerebral glucose metabolism. Dexamethasone, commonly used in the neurosurgical population, decreases cerebral glucose consumption in patients with intracerebral tumors; and patients with Cushing's disease may have decreased brain glucose utilization.19 Traumatic brain injury and SAH are associated, at least in early phases, with acute hyperglycolysis (increased anaerobic glycolysis despite dysfunctional mitochondrial oxidation).20–22 The pyruvate generated, in the absence of functioning mitochondria, is predominantly converted to lactate. It is not clear if the increased glycolysis reflects a temporary equilibrium shift to lactate as a major energy source, simple dysregulation of glycolytic feedback, or a local metabolic crisis in the injured tissue. Episodes of cerebral hyperglycolysis may be linked to low extracellular brain glucose despite peripheral blood hyperglycemia. In this clinical situation, further lowering of blood glucose values could theoretically decrease brain glucose levels below critical values.
Lactate is worth special mention. It is produced in the brain by astrocytes during synaptic activation even under normal conditions. Lactate dehydrogenase interconverts lactate and pyruvate. The anaerobic conversion of pyruvate to lactate produces less ATP per glucose molecule than occurs in the TCA cycle. In addition, the conversion of pyruvate to lactate causes an intracellular lactic acidosis. A reversal of the normal equilibrium direction (pyruvate → lactate) allows lactate to be used as an energy source. Glenn and colleagues studied 49 patients with severe TBI and 31 healthy volunteers.23 They compared cerebral metabolic rates for glucose, oxygen, and lactate using cerebral blood flow (CBF) and arterial and jugular venous bulb measurement. A large percentage of TBI patients demonstrated brain lactate uptake that was suggestive of a metabolic role.24,25Others have found that post-injury lactate infusion improves cognitive outcome in an animal model of TBI.26 The astrocyte-neuron lactate shuttle hypothesis is a controversial theory that holds that, under conditions of high-energy demand (i.e., neuronal activation) or impaired oxygen/glucose supply (ischemia), neurons use available lactate as a principal energy source. If this theory proves correct, then temporarily increased cerebral lactate in the setting of SAH, TBI, or ischemia may not be deleterious,27–29 and decreasing the blood glucose in attempt to avoid excess lactate production could potentiate injury. In addition, the excitatory neurotransmitter glutamate, typically released in excess as a consequence of brain injury, may also play a role in modulating glycolytic function.30 The biochemistry involved in these processes and applicability to the in vivo situation remains under active investigation.
Astrocytes and neurons may metabolize lactate to pyruvate within the mitochondria. Moreover, in the presence of hyperglycemia, astrocytes, but not neurons, show increased rates of glycolysis. It remains to be seen if these metabolic effects are generalizable across patients with SAH, ischemic stroke, or tumor pathology. The effects of glucose levels on injury patterns after ischemia, SAH, or TBI are likely to vary based on the mechanism of neurologic injury at the cellular level and the time course of associated cellular dysfunction in various cell subtypes.31
Studies of glucose in neurosurgical patients increasingly report the changes in microdialysis measurements of intracerebral metabolites such as lactate, glutamate, and glucose in a small region of the brain.32 Using such data, Payne and associates discuss the “glucose paradox of cerebral ischemia.”33 The paradox is an apparent need for increased glucose levels to support cellular function during ischemia but that glucose is also associated with deleterious effects by its role in promoting anaerobic glycolysis to generate lactate and acidosis. This associated cellular acidosis may damage neurons or inhibit critical homeostatic processes.
Glucose is not only metabolized through glycolysis and the TCA cycle [via pyruvate dehydrogenase (PDH)] for energy, but it is also metabolized [via pyruvate carboxylase, (PC)] toward the synthesis of key TCA intermediates. Bartnick and coworkers used carbon-13 labeling of pyruvate in animal models to show that TBI influences these relative pathways, producing a significant increase in the PDH/PC ratio in injured animals in the presence of adequate glucose substrate.34 This is consistent with a shift away from substrate maintenance and toward critical energy production during metabolic stress. Auer and colleagues studied substrate flux in a rat model of hypoglycemia-induced metabolic coma.35 As glucose levels decline, flux through the TCA cycle declines and chemical intermediates such as oxaloacetate accumulate. Oxaloacetate is then available, in higher concentrations, to participate in side reactions or shunt pathways, such as aspartate synthesis. Elevated concentrations of excitatory amino acids in the setting of ischemia may promote brain injury via calcium entry, substrate depletion, and cell death.36
Hyperglycemia and Brain Function
Neurological insults of all varieties stimulate a sympathetically mediated stress response that results in increased metabolic rate, activation of the hypothalamic-pituitary-adrenal axis, release of inflammatory mediators, and, ultimately, consumption of glucose reserves to generate ATP via enhanced glycolysis and glycogenolysis.37 In view of the absolute dependence of neurons on blood glucose delivery, plasma hyperglycemia in TBI and SAH patients was once thought to be physiologically necessary for metabolic support of injured tissue; however, hyperglycemia has increasingly been associated with worsened outcome.38
The physiologic effects of hyperglycemia are multifold. When significantly elevated, glucose, a functional osmole, stimulates diuresis. Diuresis sufficient to produce hypovolemia can produce hypotension and decreased cerebral perfusion [especially in the presence of elevated intracranial pressure (ICP)]. Associated hyperosmolarity, or electrolyte imbalances, can alter mental status or produce seizures. Similarly, hyperglycemia may result in an autoregulatory decrease in CBF that could worsen brain ischemia.39
Chronic hyperglycemia, as occurs in diabetes mellitus, is the stimulus for a cascade of biological events with profound consequences for the cardiovascular and neurologic systems in particular. The deleterious impact on neurologic function of chronic hyperglycemia in diabetes patients is well established.40 The effects of chronic hyperglycemia on neurologic function, including cognitive dysfunction, were reviewed.41
Hyperglycemia is associated with disruption of the blood brain barrier (BBB) in rodents. Evidence suggests that the disruption, and associated cerebral edema, may be mediated by activation of enzymatic pathways involved in the generation of reactive oxygen species.42 Blood brain barrier disruption may promote cerebral edema and diffusion of excess calcium, lactate, and glutamate. Alternatively, it may promote diffusion of substrates, such as TCA cycle intermediates, that might serve as a critical energy source. In nonpathologic states, insulin crosses the BBB by active transport to enter the brain, where it acts mainly as a counter-regulatory hormone.43 The disruption of the BBB in neurologic injury may alter this process as may the use of corticosteroids.
Some of the pro-inflammatory effects of hyperglycemia are likely mediated by advanced glycation end products. These are glycosylated proteins that bind to naturally occurring receptors for advanced glycation end products (RAGEs). Receptor activation initiates cellular signaling cascades with pro-inflammatory sequelae such as secretion of tumor necrosis factor-α and interleukins.44 Receptors for advanced glycation end products are found in the central and peripheral nervous system, and activation is likely involved in the development of peripheral neuropathy and microvascular pathology. Receptors for advanced glycation end products are upregulated in the brain tissue of diabetes patients and may be associated with cognitive impairment and stroke risk.45,46The time course of RAGE formation involves a period of days to weeks such that chronic glucose control in an extended ICU setting may be relevant to this mechanism of injury in the neurosurgical patient at risk; but acute, perioperative management of blood glucose levels should not be a factor.
Current insight suggests that peripheral blood hyper-glycemia is both a marker of injury severity and the potential for poor outcome. Studies supporting the association of hyperglycemia with poor outcome during ischemic stroke and SAH/intracranial hemorrhage (ICH) are numerous and summarized in Table 1. While early studies looked solely at peripheral glucose values, and often only at admission values, later investigations utilize multimodal neuromonitoring with intracerebral microdialysis catheters, brain oxygen monitors, and frequent measurement of peripheral and cerebral blood glucose.
Table 1.
Studies of Hyperglycemia and Outcome (Excluding Traumatic Brain Injury)a
| Author(s) | Design | Patients | Glucose cutoff (mg/dl) | Results and comments |
|---|---|---|---|---|
| SAH/ICH | ||||
| Kimura and colleagues9 | PO | 100 | >150 | ↑ Risk poor outcome/death |
| Frontera and associates4 | P | 281 | >105 | Measured “mean glucose burden” in ICU stay (i.e., time > 105) Mean glucose burden correlated with ↑ risk poor outcome/death |
| Badjatia and coworkers47 | R | 352 | >140 | ↑ Risk symptomatic vasospasm (also mean blood glucose) |
| Kerner and colleagues48 | P | 170 | >120 | ↑ Risk poor outcome, ↑ L/P but not ↑ cerebral glucose |
| Schlenk and associates49 | P | 28 | >140 | ↑ Risk symptomatic vasospasm and ↓ ECGlc Blood glucose does not correlate with ECGlc |
| Thiele and coworkers50 | R | 834 | >180 | ↑ In-patient blood glucose correlates with ↑ mortality, ↑ vasospasm |
| Oertel and colleagues21 | PO | 21 | Cont. | ↑ AVDGlc, ↓ CMRO2, ↑ SAH and assoc. with poor outcome |
| Claassen and associates2 | PO | 413 | >180 | ↑ Risk poor outcome/death |
| Godoy and coworkers51 | P | 295 | >164 | ↑ Risk poor outcome/death |
| Schlenk and colleagues49 | – | 187 | >140 | ↑ Risk poor outcome/death Blood Glucose >140 correlated with ↑ ECGlc only at days 4-5 after SAH |
| Ischemic Stroke | ||||
| Pulsinelli and associates52 | P | 31 | >120 | ↑ Admission blood glucose associated with poor outcome in nondiabetes subjects |
| Parsons and coworkers53 | P | 63 | none | ↑ Blood glucose associated with ↑ infarct size and poor outcome |
| Baird and colleagues1 | P | 20 | >126 | ↑ Post-admission blood glucose associated with ↑ infarct size and poor outcome; No correlation with admission hemoglobin A1c |
| Stead and associates54 | P | 447 | >130 | ↑ Stroke severity and poor outcome only in nondiabetes subjects |
| Fuentes and coworkers55 | P | 476 | >155 | “GLIAS” trial, ↑ blood glucose < 48 h poststroke ↑ risk poor outcome |
| Poppe and colleagues56 | P | 1098 | >144 | ↑ Admission blood glucose associated with poor outcome after TPA |
| Mass/Other | ||||
| McGirt and associates8 | R | 367 | 180 | Astrocytoma/GBM patients status post-resection Persistent outpatient hyperglycemia associated with mortality risk Most patients were receiving corticosteroids |
| Woodworth and coworkers57 | R | 78 | 170 | Intramedullary spinal cord tumors status post-resection Preoperative ↑ blood glucose associated ↓ post-op functional status |
P, prospective; R, retrospective; PO, prospective observational; L/P, lactate/pyruvate; ECGlc, extracellular glucose; GBM, glioblastoma multiforma.
Measures of brain glucose utilization [e.g., arteriovenous glucose difference (AVDGlc,)]; and related values (cerebral glucose lactate/pyruvate ratio, PC/PDH flux) in the penumbra of pathologic or ischemic tissue may be relevant markers of metabolic flux. Many of the studies in Table 1 have focused on TBI, SAH/ICH, and ischemic stroke patients outside of the acute operating room setting. Many of these studies use limited glucose data points such as admission blood glucose while some are retrospective or small-scale, but there is a relatively consistent pattern between higher glucose levels and greater brain damage. Several editorials have discussed these data and their implications.3,5,58
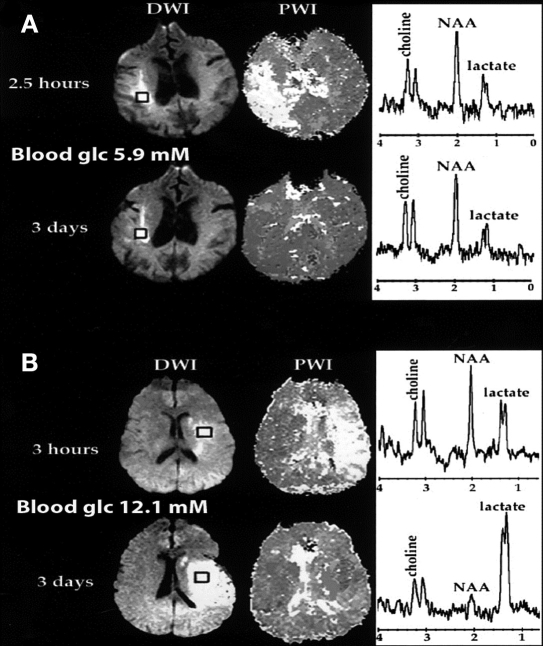
In an elegant study, Parsons and associates studied 63 acute stroke patients with magnetic resonance imaging (MRI, perfusion/diffusion weighted mismatch) and magnetic resonance spectroscopy (MRS, lactate). They found that hyperglycemia (>144 mg/dl) within an hour of the MRI was predictive of both decreased penumbral salvage and increased brain lactate levels in the ischemic region.53 In a dramatic set of images (Figure 1), Parsons and associates showed a patient with normal blood glucose and a patient with high blood glucose at the time of initial imaging. Three days later, the patient with the higher blood glucose had a large increase in brain lactate as detected by MRS, and much of the at-risk tissue had progressed to infarction. In contrast, the patient with normal blood glucose had no increase in brain lactate and major salvage of the area around the stroke core (penumbra).
Figure 1.
Perfusion-weighted imaging, diffusion-weighted imaging, and MRI spectra in patient A imaged at 2.5 h and 3 days and in patient B imaged at 3 h and 3 days. Patients A and B both had large areas of acute perfusion-weighted-imaging–diffusion-weighted-imaging mismatch or at-risk tissue. Acute blood glucose was 5.9 mmol/liter for patient A and 12.1 mmol/liter for patient B. At 3 days, patient A had no increase in lactate level and major penumbral salvage. In contrast, patient B had a large increase in lactate level, and much of the at-risk tissue progressed to infarction. Copyright 1996; reprinted with permission of John Wiley & Sons, Inc.53
In a prospective, cerebral microdialysis study of 170 post-clipping, aneurysmal/SAH patients with no specific glucose control protocol, Sakowitz and coworkers found admission and postoperative ICU plasma hyperglycemia (>120 mg/dl) both to be associated with poor outcome. The peripheral hyperglycemia, however, did not correlate with increased extracellular brain glucose in the dialysate.59
Schlenk and colleagues measured peripheral glucose and cerebral metabolites via microdialysis catheters in 31 patients with SAH of varying grade and severity.49 Extracellular cerebral glucose did not consistently correlate with peripheral blood glucose and sometimes correlated inversely. Peripheral hyperglycemia (>140 mg/dl) was sometimes coincident with low levels of cerebral glucose, which occurred in patients regardless of insulin infusion. Notably, symptomatic SAH patients (with worse outcome) tended to have both increased episodes of peripheral hyperglycemia and decreased cerebral glucose. These periods of low cerebral glucose (averaging 8 lactate, lactate/pyruvate ratio, glutamate, and glycerol (a membrane breakdown product) that are consistent with metabolic distress. Notably, some peripheral hypo-glycemia episodes occurred spontaneously.
Prado and associates studied the effects of acute hyperglycemia on infarct size in rats subject to global ischemia (bilateral carotid occlusion) versus focal ischemia [unilateral middle cerebral artery (MCA) occlusion]. Infarct size was worsened in the presence of hyper-glycemia, but only in the focal ischemia model.60 Global ischemia (cardiac arrest, bilateral carotid occlusion) is pathophysiologically distinct from the regional ischemia that is common to most neurosurgical patients. Hamilton and coworkers made similar findings in insulin-treated rats subject to global and focal ischemia.61 Global ischemia induces a metabolic crisis (severely increased lactate, glycolysis, decreased phosphorylated energy substrates, and free glucose levels), whereas the effects of regional ischemia may be less pronounced. Focal ischemia produces an ischemic core and a peri-infarct penumbra that can vary in size depending on the local physiologic milieu at the time of injury. The metabolic profiles of the two regions, particularly with respect to glucose metabolism, are strikingly different in rats.31 The infarct core demonstrates a dramatic drop in glucose metabolism, whereas the penumbra maintains glucose consumption near control values.
In animal models, timing of the hyperglycemic episode (early versus immediate pre-ischemia) and an associated rise in plasma corticosterone levels were both related to the extent of ischemia-mediated cellular damage in the hippocampus. Relative to the hyperglycemia episode early (120–240 min prior) but not immediate pre-ischemic hyperglycemia was actually protective. Similarly, administration of the steroid antagonist metapyrone prior to the ischemic episode was protective, even in the presence of acute hyperglycemia.33
Studies have tried to tease out the relevance of diabetes to the role of glucose in neurologic injury. No firm conclusions can be drawn, but some studies suggest that nondiabetes patients are more likely to demonstrate hyperglycemia and poor outcome.54
Hypoglycemia and Brain Function
Studies have demonstrated that, with currently available technology, IIT to achieve tight glucose control in critically ill patients is frequently associated with an increased incidence of peripheral blood hypoglycemia.62 Spontaneous hypoglycemia can also occur in the critically ill population. A newly published study investigated morbidity associated with iatrogenic hypoglycemic episodes in the ICU. This retrospective analysis of acute myocardial infarction patients enrolled in a tight glucose control/IIT study showed that episodes of hypoglycemia occurred both iatrogenically (IIT group) and spontaneously (control group). The researchers found spontaneous but not iatrogenic induced hypoglycemia to be associated with significant morbidity.63 However, patients admitted with neurologic injury have not been specifically studied.
In the critically ill neurosurgical population, there exists the additional concern that cerebral hypoglycemia may occur without associated peripheral blood hypoglycemia. Accumulated data support the hypothesis that cerebral hypoglycemia occurs and has physiologically significant effects. In an experimental cat model of focal ischemia, Strong and colleagues demonstrated that, after 3 h of MCA occlusion, brain lactate increased and brain glucose progressively decreased. The magnitude and timing of the decrease in brain glucose correlated with the frequency of peri-infarct depolarizations.64 Peri-infarct depolarizations are mechanistically associated with infarct expansion and, probably, outcome in models of ischemic stroke and TBI.65–67
Auer and associates undertook seminal histopathologic studies of hypoglycemia and brain damage and provided an excellent review on the subject.68–70 The fundamental pathology in hypoglycemia is necrosis; this differs from the injury that occurs in ischemic infarction. Neuronal injury from hypoglycemia only occurs at glucose levels that produce coma. A spectrum of changes in the electroencephalogram (EEG) coincides with decreases in plasma glucose levels. In humans, when starting with the conscious state, profound hypoglycemia induces a coma that, if maintained for >30 min, is associated with irreversible brain damage. Cerebral energy failure occurs at extremely low peripheral blood glucose concentrations (<1.4 mM, <25 mg/dl) and is associated with an isoelectric EEG. It is important to note that Auer and associates studied only changes in blood glucose; they did not measure extracellular cerebral glucose. In the clinical setting, it seems unlikely that peripheral blood glucose would drop and persist at the extremely low, <1.4 mM, glucose concentration associated with isoelectric EEG and neuronal cell death. Clinical studies of IIT have induced temporary hypoglycemia in the range of 55–60 mg/dl. Neuronal susceptibility to hypoglycemic injury is tissue specific.71 The hippocampus and cerebral cortex are highly susceptible to hypoglycemia-mediated damage, whereas the cerebellum, in particular, is relatively resilient. Finally, in some contexts, a physiologic response to hypoglycemia in the brain is increased CBF. The resultant increase in brain volume could contribute to elevated ICP in the setting of TBI, stroke, or other clinical situations in which intracranial compliance is already decreased.
Tight Glucose Control in Neurosurgical Patients
Clinical consensus regarding perioperative glucose control in critically ill patients is lacking, especially in the neurosurgical population. No clinical study in neurosurgical patients has demonstrated significantly improved outcome with IIT, and much of the data is retrospective or small scale. Moreover, the heterogeneity of pathology in neurologic surgery calls for caution in the generalization of other study results, such as those in cardiac surgical patient, to this patient population. Furthermore, in some studies, peripheral and cerebral glucose values do not correlate or correlate inversely. Importantly, the “normal” level of cerebral glucose is poorly defined, and optimal levels in the presence of anesthesia or brain pathology are unknown. In addition, while some studies have investigated possible clinical differences between diabetes and nondiabetes patients with regard to the association of hyperglycemia and outcome, no data exists to guide any type of differential management with IIT or fluid therapy.
The human clinical studies at the core of this debate, in the context of the biochemical issue presented earlier, are discussed later. The data on clinical trials of IIT in neurosurgical patients are summarized in Table 2.
Table 2.
Intensive Insulin Therapy and Outcome in Neurosurgical Patients (Excluding Traumatic Brain Injury)a
| Author(s) | Study Type | # Patients | Glucose target (mg/dl) | Findings/comments |
|---|---|---|---|---|
| SAH/ICH | ||||
| Ho and colleagues72 | R | 12 | 74-136 | ↓ ICP, ↑ PtiO2, ↓ L/P; small study |
| Schlenk and associates49 | P | 31 | <140 | ↓ ECGlc; ↔ L/P |
| Theile and coworkers50 | R | 834 | 90-120 | No Δ mortality with IIT, small ↑ risk of death with hypoglycemia |
| Godoy and colleagues51 | P | 295 | 60-150 | ↓ Mortality with IIT only after the first 12 h |
| Ischemic Stroke | ||||
| Bruno and associates73 | PRn | 46 | <130 or <200 | “THIS” trial, no Δ 3 months outcomes, No adverse effects of IIT 35% IITs experienced hypoglycemia (<60 mg/dl) |
| Gray and coworkers74 | PRn | 933 | 72-126 | “GIST-UK” Trial; stopped early due to slow enrollment Used glucose-potassium-insulin infusion No Δ mortality or secondary outcomes at 90 days |
| Johnston and colleagues75 | PRn | 73 | 70-110 or 110-200 or 70-300 | “GRASP” Trial No significant Δ outcomes between the three groups |
| Mixed Neurosurgical Populations | ||||
| Van den Berghe and associates76 | PRn | 63b | 80-110 | ↓ ICP, ↓ vasopressors, ↑ seizures, ↑ neuro outcome 12 months |
| Azevedo and coworkers77 | PRn | 47b | 80-120 | No Δ outcome (14 months); low power |
P, prospective; PRn, prospective-randomized; R, retrospective; L/P, lactate/pyruvate; ECGlc, extracellular glucose.
Subgroup of analysis of larger study.
Traumatic Brain Injury
A substantial body of literature exists regarding glucose management in the patient with TBI. It is clear that TBI represents a continuum of injury with heterogeneous changes in regional brain function and glucose metabolism. Under certain circumstances, cerebral hypoglycemia may be a significant concern that complicates management. This subset of patients is discussed in the section discussing trauma and was recently reviewed by Gentile and Siren.78
LeRoux and coworkers at the University of Pennsylvania are enrolling patients in a Phase IIa randomized trial of effects of tight glycemic control on cerebral glucose metabolism after severe TBI. Lactate/pyruvate ratio, brain glucose, systemic glucose, short-term outcome, and CBF (via Xenon-CT) will be measured in an attempt to resolve the many issues discussed earlier in this review.
Ischemic Stroke and Interventional Neuroradiology (Tissue Plasminogen Activator and Vasospasm)
Two pilot-trials of IIT in acute stroke patients have reported data. The Glucose Regulation in Acute Stroke Patients (GRASP) trial was designed to detect the incidence of hypoglycemia with outcome difference as a secondary end point, though it had insufficient power for the secondary end point with only 74 patients. Their data has only been reported in abstract form. This pilot study randomized hyperglycemic stroke patients (>109 mg/dl) within 24 h to tight glucose control (70–109 mg/dl), loose glucose control (70–200 mg/dl), or routine care. Only 43.5% of the tight-control group achieved the target glucose level and no statistical significance was demonstrated among the groups.75
The Treatment of Hyperglycemia in Ischemic Stroke trial randomized acute stroke patients (within 12 h) to usual care or tight control (70–130 mg/dl), but it was also a small trial (46 patients). A similar incidence of hypoglycemia was noted, and no neurologic outcome difference was measured at 3 months.73
The Glucose Insulin in Stroke Trial-United Kingdom trial enrolled a relatively large number of patients (933) and compared tight control (potassium glucose insulin, 72–126 mg/dl) to routine care and found no outcome difference at 90 days, but the trial was terminated early due to slow enrollment.74
With increasing frequency, patients present to the interventional radiology suite for aneurysm coiling, injection of intra-arterial tissue plasminogen activator (TPA) after acute stroke, and intra-arterial vasodilatory agents to treat symptomatic vasospasm. These are clinical circumstances in which a very acute ischemic episode has occurred and the clinical intervention may be accompanied by sudden reperfusion. In one study, nondiabetes patients with acute MCA ischemia who received intravenous TPA had larger cerebral stroke volume and worse 28-day outcome if hyperglycemia (>180 mg/dl) was present.79 However, glucose was measured on admission and immediately treated with insulin if elevated. One intriguing finding was that two patients in the hyperglycemic group who where were treated with insulin prior to emergency department arrival had outcomes similar to the normoglycemia group. In a very recent analysis of 1083 stroke patients, Poppe and colleagues reported that admission hyper-glycemia (>144 mg/dl) was associated with greater risk of ICH, mortality, and poor 90-day outcome after intravenous TPA.56 These data are retrospective and rest on a single glucose measurement in a largely diabetic population, so clear conclusions cannot be drawn.
However, it seems prudent to obtain peripheral blood glucose measurements in every patient who presents for intra-arterial thrombolysis or treatment of symptomatic vasospasm and to treat values greater than 145 mg/dl with insulin during the immediate periprocedure period.
Spine Surgery
There are no specific studies of perioperative glucose management in patients undergoing spinal surgery for tumor or correction of scoliosis. In 1989, Drummond and Moore reported that, in rabbits, glucose administration prior to temporary spinal cord ischemia dramatically increased the likelihood of paraplegia.80 Woodworth and associates reported, in a retrospective study, that a single preoperative episode of hyperglycemia in patients undergoing intramedullary spinal tumor resection correlated with a likelihood of poor postoperative ambulatory function.57
If the mechanism of anticipated spine injury is assumed to be focal ischemia, it seems reasonable to extend the models of acute focal ischemia to the spine and conclude that pronounced hyperglycemia immediately prior to hardware manipulation has the potential to worsen the extent of injury.60 Judicious use of insulin to maintain blood glucose less than 150 mg/dl before and during periods of potential ischemia is prudent and safe.
Neurosurgery for Tumor
There are no specific studies of perioperative management of glucose in patients with intracranial mass. Glucose may promote astrocytoma growth.81 Most patients presenting for tumor resection receive perioperative corticosteroids. This therapy is associated with increased plasma glucose and also with decreased cerebral glucose utilization. McGirt and coworkers retrospectively associated persistent postoperative hyperglycemia with mortality in patients undergoing tumor resection; but no causality can be concluded.8
Patients undergoing resection of a pituitary mass represent a special population. These patients frequently develop central diabetes insipidus during the late intra-operative, or more commonly, postoperative period. Diabetes insipidus is associated with hypernatremia, hyperosmolality, diuresis of dilute, high-volume urine, and a free-water deficit. Hypovolemia and cerebral cell shrinkage with neurologic changes will result if diabetes insipidus is not appropriately treated. Treatment includes DDAVP and consideration for free-water replacement, depending on the clinical circumstances. Traditionally, free-water replacement has been accomplished with glucose-containing solutions.82 Sieber and Smith have published an in-depth review of intraoperative transfusion of glucose-containing fluids,83 and intraoperative fluid management is nicely summarized by Zornow and Scheller.84 In asmall study, Sieber and Smith found that infusion of glucose-containing solutions during craniotomy for supratentorial tumor excision did in fact elevate blood glucose levels to those associated with injury in ischemia models.81 In view of the potential for harm with glucose infusions, particularly if regional ischemia occurs intraoperatively, the likelihood of elevated glucose with ongoing corticosteroid therapy and the safety of 0.45% saline solutions, most anesthesiologists and neurointensivists tend to avoid glucose-containing solutions in the perioperative period. At the Hospital of the University of Pennsylvania, we use 0.45% saline judiciously for free-water therapy in conjunction with intravenous DDAVP, as clinically indicated, with careful ongoing monitoring of serum sodium levels, plasma osmolarity, urine output, and vascular volume status.
Neurosurgery for Aneurysm and Hemorrhage
Hematoma evacuation and aneurysm clipping are common reasons for presentation to the operating room. Many patients who present for clipping have already experienced some degree of SAH/ICH, and the data on brain glucose metabolism, presented earlier, include many patients with ICH/SAH. After ICH/SAH regional abnormalities in CBF, posthemorrhage edema, vasospasm, and increased ICP all predispose the brain tissue to ischemia. Despite the apparent link between hyperglycemia and symptomatic vasospasm, infarct size, and outcome, the few studies of IIT in these populations have failed to demonstrate a significant difference in outcome with tight glucose control.48,50,51,72 All but one of these studies were retrospective, and target ranges were generally under 140 mg/dl.51 The Godoy and colleague study did detect a potential small mortality benefit during the first 12 h of therapy. Peripheral hypoglycemia and decreased intracerebral glucose were detected in some patients, albeit with unclear clinical significance.
Though no conclusive recommendations can be made, it seems reasonable to treat peripheral glucose values greater than 150 mg/dl with insulin. The data suggest that a benefit from tighter glucose control during acute episodes of ischemia (such as with temporary clip application or aneurismal rupture) would be mechanistically plausible. It seems appropriate to consider stricter control when acute, focal ischemia is occurring or anticipated, but continuation of tight control into the postoperative is not supported by the literature.
Conclusions and Future Directions
The available clinical data when interpreted in the context of a deeper understanding of the molecular biochemistry of glucose in the brain after neurologic injury do not support tight glucose control with IIT in this critically ill subpopulation. Two meta-analyses of all patient types treated with IIT arrived at similar conclusions.85,86Ongoing and future research promises to clarify the presently muddled picture. Examples include stratification of neurologic-injury-based protein biomarkers identified in high-throughput proteomic assays87–91 that will individualize disease management.
Further study of the multimodal effects of insulin via modulation of signaling pathways,92 including inflammation, cell adhesion, and activity of glucose transporters and pyruvate metabolism enzymes, will be important. Investigation of agents other than insulin for glucose-lowering effects may demonstrate a reduced rate of hypoglycemia or other beneficial metabolic effects.93–96 Elahi and associates at Johns Hopkins University are studying the efficacy of GLP-1 (glucagon-like peptide) for glycemic control in critically ill surgical patients (clinicaltrials.gov).
Microdialysis studies in patients undergoing IIT therapy continue to provide important insight into regional alterations of glucose metabolism in injured brain tissue. This has been referred to as multimodal neuroprotective monitoring. Patient populations are heterogeneous, and data interpretation is complicated. We need a better understanding of the microdisease processes before adoption of IIT.
Finally, work continues to determine the optimal technology for accurate, reliable, rapid glucose measurement and for developing closed-loop continuous glucose control systems. New technology may facilitate the avoidance of hypoglycemia under IIT regimen and the development of IIT protocols that can be individualized to the specific metabolic state of the patient under treatment.
Here are some issues to consider:
Hyperglycemia (>150 mg/dl) is associated with poorer outcome, but causality has not been definitively demonstrated.
Unknown clinical significance of peripheral hypo glycemia with IIT.
Unknown incidence and clinical significance of cerebral hypoglycemia with IIT.
Inconsistent correlation between blood glucose and cerebral extracellular glucose.
Variation of brain glucose metabolism with the time course of the disease process.
Lack of large-scale, prospective trials demonstrating benefit from IIT.
Nutritional protocol of subject patients (total enteral nutrition; total parental nutrition; per os, by mouth) is heterogeneous.
Glucose target ranges, time, and duration of glucose measurement and IIT protocols vary.
Abbreviations
- ATP
adenosine triphosphate
- BBB
blood brain barrier
- CBF
cerebral blood flow
- CMRO2
cerebral metabolic rate of oxygen
- EEG
electroencephalogram
- ICH
intracranial hemorrhage
- ICP
intracranial pressure
- ICU
intensive care unit
- IIT
intensive insulin therapy
- GRASP
Glucose Regulation in Acute Stroke Patients
- MCA
middle cerebral artery
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- PC
pyruvate carboxylase
- PDH
pyruvate dehydrogenase
- RAGE
receptor for advanced glycation end product
- SAH
subarachnoid hemorrhage
- TBI
traumatic brain injury
- TCA
tricarboxylic acid
- TPA
tissue plasminogen activator
References
- 1.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34(9):2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 2.Claassen J, Vu A, Kreiter KT, Kowalski RG, Du EY, Ostapkovich N, Fitzsimmons BF, Connolly ES, Mayer SA. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32(3):832–838. doi: 10.1097/01.ccm.0000114830.48833.8a. [DOI] [PubMed] [Google Scholar]
- 3.Donnan GA, Levi C. Glucose and the ischaemic bratoo much of a good thing? Lancet Neurol. 2007;6(5):380–381. doi: 10.1016/S1474-4422(07)70086-8. [DOI] [PubMed] [Google Scholar]
- 4.Frontera JA, Fernandez A, Claassen J, Schmidt M, Schumacher HC, Wartenberg K, Temes R, Parra A, Ostapkovich ND, Mayer SA. Hyperglycemia after SAH: predictors, associated complications, and impact on outcome. Stroke. 2006;37(1):199–203. doi: 10.1161/01.STR.0000194960.73883.0f. [DOI] [PubMed] [Google Scholar]
- 5.Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37(1):267–273. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- 6.Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005;58(1):47–50. doi: 10.1097/01.ta.0000135158.42242.b1. [DOI] [PubMed] [Google Scholar]
- 7.Sakowitz OW, Wolfrum S, Sarrafzadeh AS, Stover JF, Dreier JP, Dendorfer A, Benndorf G, Lanksch WR, Unterberg AW. Relation of cerebral energy metabolism and extracellular nitrite and nitrate concentrations in patients after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2001;21(9):1067–1076. doi: 10.1097/00004647-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 8.McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, Olivi A, Quiñones-Hinojosa A. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63(2):286–291. doi: 10.1227/01.NEU.0000315282.61035.48. discussion 291. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Iguchi Y, Inoue T, Shibazaki K, Matsumoto N, Kobayashi K, Yamashita S. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J Neurol Sci. 2007;255(1–2):90–94. doi: 10.1016/j.jns.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Bilotta F, Spinelli A, Giovannini F, Doronzio A, Delfini R, Rosa G. The effect of intensive insulin therapy on infection rate, vasospasm, neurologic outcome, and mortality in neurointensive care unit after intracranial aneurysm clipping in patients with acute subarachnoid hemorrhage: a randomized prospective pilot trial. J Neurosurg Anesthesiol. 2007;19(3):156–160. doi: 10.1097/ANA.0b013e3180338e69. [DOI] [PubMed] [Google Scholar]
- 11.Lipshutz AK, Gropper MA. Perioperative glycemic control: an evidence-based review. Anesthesiology. 2009;110(2):408–421. doi: 10.1097/ALN.0b013e3181948a80. [DOI] [PubMed] [Google Scholar]
- 12.Bilotta F, Giovannini F, Caramia R, Rosa G. Glycemia management in neurocritical care patients: a review. J Neurosurg Anesthesiol. 2009;21(1):2–9. doi: 10.1097/ANA.0b013e31818f8a5c. [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam B, Panzica PJ, Novack V, Mahmood F, Matyal R, Mitchell JD, Sundar E, Bose R, Pomposelli F, Kersten JR, Talmor DS. Continuous perioperative insulin infusion decreases major cardiovascular events in patients undergoing vascular surgery: a prospective, randomized trial. Anesthesiology. 2009;110(5):970–977. doi: 10.1097/ALN.0b013e3181a1005b. [DOI] [PubMed] [Google Scholar]
- 14.Vanhorebeek I, Ingels C, Van den Berghe G. Intensive insulin therapy in high-risk cardiac surgery patients: evidence from the Leuven randomized study. Semin Thorac Cardiovasc Surg. 2006;18(4):309–316. doi: 10.1053/j.semtcvs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 16.Fitch W. Brain metabolism. In: Cottrell JE, Smith DS, editors. Anesthesia and neurosurgery. 4th. St. Louis: Mosby; 2001. pp. 1–17. [Google Scholar]
- 17.Gibson GE, Dienel GA. Integration of molecular and cellular processes. 3rd. New York: Springer; 2007. Brain energetics. [Google Scholar]
- 18.Lupi A, Bertagnoni G, Salgarello M, Orsolon P, Malfatti V, Zanco P. Cerebellar vermis relative hypermetabolism: an almost constant PET finding in an injured brain. Clin Nucl Med. 2007;32(6):445–451. doi: 10.1097/RLU.0b013e3180537621. [DOI] [PubMed] [Google Scholar]
- 19.Fulham MJ, Brunetti A, Aloj L, Raman R, Dwyer AJ, Di Chiro G. Decreased cerebral glucose metabolism in patients with brain tumors: an effect of corticosteroids. J Neurosurg. 1995;83(4):657–664. doi: 10.3171/jns.1995.83.4.0657. [DOI] [PubMed] [Google Scholar]
- 20.Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;86(2):241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 21.Oertel MF, Schwedler M, Stein M, Wachter D, Scharbrodt W, Schmidinger A, Böker DK. Cerebral energy failure after subarachnoid hemorrhage: the role of relative hyperglycolysis. J Clin Neurosci. 2007;14(10):948–954. doi: 10.1016/j.jocn.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Statler KD, Janesko KL, Melick JA, Clark RS, Jenkins LW, Kochanek PM. Hyperglycolysis is exacerbated after traumatic brain injury with fentanyl vs. isoflurane anesthesia in rats. Brain Res. 2003;994(1):37–43. doi: 10.1016/j.brainres.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, Hovda DA, Bergsneider M, Hillered L, Martin NA. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab. 2003;23(10):1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- 24.Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101(1):9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
- 25.Lemire J, Mailloux RJ, Appanna VD. Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (CCF-STTG1) PLoS ONE. 2008;3(2):e1550. doi: 10.1371/journal.pone.0001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice AC, Zsoldos R, Chen T, Wilson MS, Alessandri B, Hamm RJ, Bullock MR. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 2002;928(1–2):156–159. doi: 10.1016/s0006-8993(01)03299-1. [DOI] [PubMed] [Google Scholar]
- 27.Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23(11):1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- 28.Schurr A. Lactate, glucose and energy metabolism in the ischemic brain (Review) Int J Mol Med. 2002;10(2):131–136. [PubMed] [Google Scholar]
- 29.Schurr A. Bench-to-bedside review: a possible resolution of the glucose paradox of cerebral ischemia. Crit Care. 2002;6(4):330–334. doi: 10.1186/cc1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellerin L, Magistretti PH. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. PNAS. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hertz L. Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology. 2008;55(3):289–309. doi: 10.1016/j.neuropharm.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Hutchinson PJ, O'Connell MT, Al-Rawi PG, Maskell LB, Kett-White R, Gupta AK, Richards HK, Hutchinson DB, Kirkpatrick PJ, Pickard JD. Clinical cerebral microdialysis: a methodological study. J Neurosurg. 2000;93(1):37–43. doi: 10.3171/jns.2000.93.1.0037. [DOI] [PubMed] [Google Scholar]
- 33.Payne RS, Tseng MT, Schurr A. The glucose paradox of cerebral ischemia: evidence for corticosterone involvement. Brain Res. 2003;971(1):9–17. doi: 10.1016/s0006-8993(03)02276-5. [DOI] [PubMed] [Google Scholar]
- 34.Bartnik BL, Hovda DA, Lee PW. Glucose metabolism after traumatic brain injury: estimation of pyruvate carboxylase and pyruvate dehydrogenase flux by mass isotopomer analysis. J Neurotrauma. 2007;24(1):181–194. doi: 10.1089/neu.2006.0038. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland GR, Tyson RL, Auer RN. Truncation of the krebs cycle during hypoglycemic coma. Med Chem. 2008;4(4):379–385. doi: 10.2174/157340608784872235. [DOI] [PubMed] [Google Scholar]
- 36.Martínez-Sánchez M, Striggow F, Schröder UH, Kahlert S, Reymann KG, Reiser G. Na(+) and Ca(2+) homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience. 2004;128(4):729–740. doi: 10.1016/j.neuroscience.2004.06.074. [DOI] [PubMed] [Google Scholar]
- 37.Falciglia M. Causes and consequences of hyperglycemia in critical illness. Curr Opin Clin Nutr Metab Care. 2007;10(4):498–503. doi: 10.1097/MCO.0b013e3281a3bf0a. [DOI] [PubMed] [Google Scholar]
- 38.Prakash A, Matta BF. Hyperglycaemia and neurological injury. Curr Opin Anaesthesiol. 2008;21(5):565–569. doi: 10.1097/ACO.0b013e32830f44e4. [DOI] [PubMed] [Google Scholar]
- 39.Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during hyperglycemia. Ann Neurol. 1985;17(3):267–272. doi: 10.1002/ana.410170308. [DOI] [PubMed] [Google Scholar]
- 40.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29(4):494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cukierman-Yaffee T. The relationship between dysglycemia and cognitive dysfunction. Curr Opin Investig Drugs. 2009;10(1):70–74. [PubMed] [Google Scholar]
- 42.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38(3):1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490(1–3):5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 44.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 45.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 46.Toth C, Martinez J, Zochodne DW. RAGE, diabetes, and the nervous system. Curr Mol Med. 2007;7(8):766–776. doi: 10.2174/156652407783220705. [DOI] [PubMed] [Google Scholar]
- 47.Badjatia N, Topcuoglu MA, Buonanno FS, Smith EE, Nogueira RG, Rordorf GA, Carter BS, Ogilvy CS, Singhal AB. Relationship between hyperglycemia and symptomatic vasospasm after subarachnoid hemorrhage. Crit Care Med. 2005;33(7):1603–1609. doi: 10.1097/01.ccm.0000168054.60538.2b. [DOI] [PubMed] [Google Scholar]
- 48.Kerner A, Schlenk F, Sakowitz O, Haux D, Sarrafzadeh A. Impact of hyperglycemia on neurological deficits and extracellular glucose levels in aneurysmal subarachnoid hemorrhage patients. Neurol Res. 2007;29(7):647–653. doi: 10.1179/016164107X248983. [DOI] [PubMed] [Google Scholar]
- 49.Schlenk F, Graetz D, Nagel A, Schmidt M, Sarrafzadeh AS. Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit Care. 2008;12(1):R9. doi: 10.1186/cc6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thiele RH, Pouratian N, Zuo Z, Scalzo DC, Dobbs HA, Dumont AS, Kassell NF, Nemergut EC. Strict glucose control does not affect mortality after aneurysmal subarachnoid hemorrhage. Anesthesiology. 2009;110(3):603–610. doi: 10.1097/ALN.0b013e318198006a. [DOI] [PubMed] [Google Scholar]
- 51.Godoy DA, Piñero GR, Svampa S, Papa F, Di Napoli M. Hyperglycemia and short-term outcome in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. 2008;9(2):217–229. doi: 10.1007/s12028-008-9063-1. [DOI] [PubMed] [Google Scholar]
- 52.Pulsinelli WA, Levy DE, Sigsbee B, Scherer P, Plum F. Increased damage after ischemic stroke in patients with hyper-glycemia with or without established diabetes mellitus. Am J Med. 1983;74(4):540–554. doi: 10.1016/0002-9343(83)91007-0. [DOI] [PubMed] [Google Scholar]
- 53.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52(1):20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 54.Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, Decker WW, Brown RD., Jr. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care. 2009;10(2):181–186. doi: 10.1007/s12028-008-9080-0. [DOI] [PubMed] [Google Scholar]
- 55.Fuentes B, Castillo J, San José B, Leira R, Serena J, Vivancos J, Dávalos A, Nuñez AG, Egido J, Díez-Tejedor E Stroke Project of the Cerebrovascular Diseases Study Group. Spanish Society of Neurology. The prognostic value of capillary glucose levels in acute stroke: the GLycemia in Acute Stroke (GLIAS) study Stroke. 2009;40(2):562–568. doi: 10.1161/STROKEAHA.108.519926. [DOI] [PubMed] [Google Scholar]
- 56.Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD Canadian Alteplase for Stroke Effectiveness Study Investigators. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32(4):617–622. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodworth GF, Chaichana KL, McGirt MJ, Sciubba DM, Jallo GI, Gokaslan Z, Wolinsky JP, Witham TF. Predictors of ambulatory function after surgical resection of intramedullary spinal cord tumors. Neurosurgery. 2007;61(1):105–106. doi: 10.1227/01.neu.0000279729.36392.42. 99,105; discussion. [DOI] [PubMed] [Google Scholar]
- 58.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of the critically ill? Diabetologia. 2006;49(8):1722–1725. doi: 10.1007/s00125-006-0306-4. [DOI] [PubMed] [Google Scholar]
- 59.Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR, Unterberg AW. On-line microdialysis following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2001;77:141–144. doi: 10.1007/978-3-7091-6232-3_30. [DOI] [PubMed] [Google Scholar]
- 60.Prado R, Ginsberg MD, Dietrich WD, Watson BD, Busto R. Hyperglycemia increases infarct size in collaterally perfused but not end-arterial vascular territories. J Cereb Blood Flow Metab. 1988;8(2):186–192. doi: 10.1038/jcbfm.1988.48. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton MG, Tranmer BI, Auer RN. Insulin reduction of cerebral infarction due to transient focal ischemia. J Neurosurg. 1995;82(2):262–268. doi: 10.3171/jns.1995.82.2.0262. [DOI] [PubMed] [Google Scholar]
- 62.Vriesendorp TM, DeVries JH, Hoekstra JB. Hypoglycemia and strict glycemic control in critically ill patients. Curr Opin Crit Care. 2008;14(4):397–402. doi: 10.1097/MCC.0b013e328306c7b1. [DOI] [PubMed] [Google Scholar]
- 63.Kosiborod M, Inzucchi SE, Goyal A, Krumholz HM, Masoudi FA, Xiao L, Spertus JA. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA. 2009;301(15):1556–1564. doi: 10.1001/jama.2009.496. [DOI] [PubMed] [Google Scholar]
- 64.Strong AJ, Smith SE, Whittington DJ, Meldrum BS, Parsons AA, Krupinski J, Hunter AJ, Patel S, Robertson C. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia. Stroke. 2000;31(1):214–222. doi: 10.1161/01.str.31.1.214. [DOI] [PubMed] [Google Scholar]
- 65.Hartings JA, Gugliotta M, Gilman C, Strong AJ, Tortella FC, Bullock MR. Repetitive cortical spreading depolarizations in a case of severe brain trauma. Neurol Res. 2008;30(8):876–882. doi: 10.1179/174313208X309739. [DOI] [PubMed] [Google Scholar]
- 66.Strong AJ. The management of plasma glucose in acute cerebral ischaemia and traumatic brain injury: more research needed. Intensive Care Med. 2008;34(7):1169–1172. doi: 10.1007/s00134-008-1045-4. [DOI] [PubMed] [Google Scholar]
- 67.Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129(Pt 12):3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 68.Auer RN, Hugh J, Cosgrove E, Curry B. Neuropathologic findings in three cases of profound hypoglycemia. Clin Neuropathol. 1989;8(2):63–68. [PubMed] [Google Scholar]
- 69.Auer RN, Siesjö BK. Hypoglycaemia: brain neurochemistry and neuropathology. Baillieres Clin Endocrinol Metab. 1993;7(3):611–625. doi: 10.1016/s0950-351x(05)80210-1. [DOI] [PubMed] [Google Scholar]
- 70.Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004;19(3-4):169–175. doi: 10.1023/b:mebr.0000043967.78763.5b. [DOI] [PubMed] [Google Scholar]
- 71.Auer RN, Wieloch T, Olsson Y, Siesjö BK. The distribution of hypoglycemic brain damage. Acta Neuropathol. 1984;64(3):177–191. doi: 10.1007/BF00688108. [DOI] [PubMed] [Google Scholar]
- 72.Ho CL, Ang CB, Lee KK, Ng IH. Effects of glycaemic control on cerebral neurochemistry in primary intracerebral haemorrhage. J Clin Neurosci. 2008;15(4):428–433. doi: 10.1016/j.jocn.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39(2):384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 74.Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG GIST Trialists Collaboration. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6(5):397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 75.Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR, van Wingerden J. The Glucose Regulation in Acute Stroke Patients (GRASP) trial outcome results. Abstracts from the 2009 International Stroke Conference. 2009;40(4):e106. [Google Scholar]
- 76.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 77.Azevedo JR, Lima ER, Cossetti RJ, Azevedo RP. Intensive insulin therapy versus conventional glycemic control in patients with acute neurological injury: a prospective controlled trial. Arq Neuropsiquiatr. 2007;65(3B):733–738. doi: 10.1590/s0004-282x2007000500001. [DOI] [PubMed] [Google Scholar]
- 78.Gentile NT, Siren K. Glycemic control and the injured brain. Emerg Med Clin North Am. 2009;27(1):151–169. doi: 10.1016/j.emc.2008.08.010. x. [DOI] [PubMed] [Google Scholar]
- 79.Els T, Klisch J, Orszagh M, Hetzel A, Schulte-Mönting J, Schumacher M, Lücking CH. Hyperglycemia in patients with focal cerebral ischemia after intravenous thrombolysis: influence on clinical outcome and infarct size. Cerebrovasc Dis. 2002;13(2):89–94. doi: 10.1159/000047756. [DOI] [PubMed] [Google Scholar]
- 80.Drummond JC, Moore SS. The influence of dextrose administration on neurologic outcome after temporary spinal cord ischemia in the rabbit. Anesthesiology. 1989;70(1):64–70. doi: 10.1097/00000542-198901000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Sieber F, Smith DS, Kupferberg J, Crosby L, Uzzell B, Buzby G, March K, Nann L. Effects of intraoperative glucose on protein catabolism and plasma glucose levels in patients with supratentorial tumors. Anesthesiology. 1986;64(4):453–459. doi: 10.1097/00000542-198604000-00007. [DOI] [PubMed] [Google Scholar]
- 82.Loh JA, Verbalis JG. Diabetes insipidus as a complication after pituitary surgery. Nat Clin Pract Endocrinol Metab. 2007;3(6):489–494. doi: 10.1038/ncpendmet0513. [DOI] [PubMed] [Google Scholar]
- 83.Sieber FE, Smith DS, Traystman RJ, Wollman H. Glucose: a reevaluation of its intraoperative use. Anesthesiology. 1987;67(1):72–81. [PubMed] [Google Scholar]
- 84.Zornow MH, Scheller MS. Cottrell JE, Smith DS, eds. Anesthesia and neurosurgery. 4th. St. Louis: Mosby; 2001. Intraoperative fluid management during craniotomy; pp. 237–249. [Google Scholar]
- 85.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Guo T, Wang H, He W, Mei H, Hong M, Yu J, Hu Y, Song S. Potential biomarkers of acute cerebral infarction detected by SELDI-TOF-MS. Am J Clin Pathol. 2008;130(2):299–304. doi: 10.1309/CA242R5VY14HUGE8. [DOI] [PubMed] [Google Scholar]
- 88.Schuhmann MU, Heine G, Skardelly M, Jaeger M, Selle H. Brain injury and proteomics/peptidomics: is it relevant? an overview. Acta Neurochir Suppl. 2005;95:465–470. doi: 10.1007/3-211-32318-x_95. [DOI] [PubMed] [Google Scholar]
- 89.Ottens AK, Kobeissy FH, Fuller BF, Liu MC, Oli MW, Hayes RL, Wang KK. Novel neuroproteomic approaches to studying traumatic brain injury. Prog Brain Res. 2007;161:401–418. doi: 10.1016/S0079-6123(06)61029-7. [DOI] [PubMed] [Google Scholar]
- 90.Nogoy N. Neuroproteomics: the hunt for biomarkers of neurotrauma. Andrew Ottens talks to Nicole Nogoy. Expert Rev Proteomics. 2007;4(3):343–345. doi: 10.1586/14789450.4.3.343. [DOI] [PubMed] [Google Scholar]
- 91.Prieto DA, Ye X, Veenstra TD. Proteomic analysis of traumatic brain injury: the search for biomarkers. Expert Rev Proteomics. 2008;5(2):283–291. doi: 10.1586/14789450.5.2.283. [DOI] [PubMed] [Google Scholar]
- 92.Krüger M, Kratchmarova I, Blagoev B, Tseng YH, Kahn CR, Mann M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci U S A. 2008;105(7):2451–2456. doi: 10.1073/pnas.0711713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenbloom AL. Mecasermin (recombinant human insulin-like growth factor I) Adv Ther. 2009;26(1):40–54. doi: 10.1007/s12325-008-0136-5. [DOI] [PubMed] [Google Scholar]
- 94.Kalfon P, Preiser JC. Tight glucose control: should we move from intensive insulin therapy alone to modulation of insulin and nutritional inputs? Crit Care. 2008;12(3):156. doi: 10.1186/cc6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18(1–2):7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 96.Funnell MM. The therapeutic role of incretin mimetics and DPP-4 inhibitors. Diabetes Educ. 2009;35(Suppl 1):12S–127S. doi: 10.1177/0145721709331521. [DOI] [PubMed] [Google Scholar]