Abstract
Introduction
Four randomized controlled trials have compared mortality and morbidity of tight glycemic control versus conventional glucose for intensive care unit (ICU) patients. Two trials showed a positive outcome. However, one single-center trial and a large multicenter trial had negative results. The positive trials used accurate portable lab analyzers. The negative trial allowed the use of meters. The portable analyzer measures in filtered plasma, minimizing the interference effects.
OptiScan Biomedical Corporation is developing a continuous glucose monitor using centrifuged plasma and mid-infrared spectroscopy for use in ICU medicine. The OptiScanner draws approximately 0.1 ml of blood every 15 min and creates a centrifuged plasma sample. Internal quality control minimizes sample preparation error. Interference adjustment using this technique has been presented at the Society of Critical Care Medicine in separate studies since 2006.
Method
A good laboratory practice study was conducted on three Yorkshire pigs using a central venous catheter over 6 h while performing a glucose challenge. Matching Yellow Springs Instrument glucose readings were obtained.
Results
Some 95.7% of the predicted values were in the Clarke Error Grid A zone and 4.3% in the B zone. Of those in the B zone, all were within 3.3% of the A zone boundaries. The coefficient of determination (R2) was 0.993. The coefficient of variance was 5.02%. Animal necropsy and blood panels demonstrated safety.
Conclusion
The OptiScanner investigational device performed safely and accurately in an animal model. Human studies using the device will begin soon.
Keywords: accuracy, continuous monitor, critical care, glucose, interference, mid-infrared spectroscopy, tight glycemic control
Introduction
Clinical Study Background
Recent studies have focused attention on the implementation of tight glycemic control (TGC) in critically ill patients. This position is based on evidence that morbidity and mortality are improved not just in diabetes patients, but in all critically ill patients experiencing “stress hyperglycemia” in the intensive care unit (ICU) when compared to conventional glucose control.
In 2001, Van den Berghe and colleagues published the benefits of TGC and intensive insulin therapy in critically ill patients in the landmark Leuven I randomized controlled trial (RCT),1 showing a significant decrease in mortality and morbidity in a surgical ICU. Numerous TGC studies have since been conducted, including Leuven II and III2,3 and others at Stamford Hospital,4 R. Adams Cowley Shock Trauma Center,5,6 St. Vincent Medical Center,7 and Brooke Army Medical Center8 to name a few. Additionally, glycemic variability in the critically ill population has been shown to be independently associated with mortality and morbidity as well.9–11 Unfortunately, hypoglycemia has also been associated with death and has resulted in the termination of multicenter RCTs Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis study12 and GluControl.13
Later, the results of the multicenter Normoglycemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Reglation RCT14 were negative (albeit at a “tighter” or lowered implementation of conventional glucose control). There has been wide commentary that this and other negative trials could have been confounded with poor glucose measurement accuracy because of the use of meters, which have been shown to have limited accuracy.15 The trials that showed the positive benefits of TGC used more accurate portable lab analyzers, which filter the blood prior to measurements of glucose.
Controversy Concerning Meter Accuracy in Hospitals
Existing glucose meters suffer from a variety of error sources that can put intensively managed patients at risk for insulin overdosing or underdosing.16,17 Interferants from changes in a patient's pH,18 hematocrit,19,20 blood oxygen,21 and medications22 can all affect meter accuracy. The U.S. Food and Drug Administration has expressed concerns over the use of existing meters in hospitals, particularly in ICU medicine.23,24 Leading figures in clinical chemistry have questioned whether the accuracy of existing meters is sufficient for insulin administration in ICU medicine.25 In addition to insufficient accuracy, these meters require that ICU nurses invest considerable time in taking measurements, which puts an additional burden on patient care.26
The OptiScanner System
A consensus panel at the 2009 International Symposium on Intensive Care and Emergency Medicine meeting called for “continuous plasma accuracy” for the measurement of glucose in ICU medicine.27 OptiScan Biomedical Corporation is developing a continuous glucose monitoring technology to provide accurate blood glucose measurements over a wide range of glucose concentrations for ICU patients. The development goal of the OptiScanner is to create central laboratory quality plasma at the bedside, adjust for interference effects, and provide high accuracy for each glucose value reported. Enhanced quality assurance is accomplished by system checks as each sample is centrifuged and measured.
The OptiScanner attaches to the patient's catheter (a peripheral IV, a peripherally inserted central catheter, or the proximal port of a central venous catheter) and handles all measurements and contact with blood without operator assistance. The system automatically rinses the flow cell and internal fluidics at every cycle. The system uses no reagents and requires no in-use calibration. No calibration solutions or individual patient calibrations are required for the OptiScanner. Only normal saline is used for flushing the patient line. The OptiScanner provides a glucose reading every 15 min, more frequently than can be accomplished in normal ICU nursing care with current glucose control methods.
Mid-Infrared Spectroscopy and Effect on Accuracy, Interference Correction, and Quality Assurance
Infrared (IR) spectroscopy is commonly used for analysis for substances where IR radiation interacts with molecular bonds. Infrared spectroscopy identifies covalent bonds through the detection of IR radiation absorption as the bonds vibrate, rotate, and bend. Both mid- and near-IR measurements of glucose have been studied. The mid- IR consists of distinct and stronger vibrational modes compared with the weaker overtone and combination band modes in the near-IR, allowing for more accurate identification and quantification of substances. The primary spectral peaks for glucose are in the mid-IR region.28,29
In 1990 and 1994, Heise and et al. published papers on the IR spectroscopic estimation of glucose concentration.30,31 In those papers, they reported that mid-IR was at least 70% more accurate than near-IR in measuring glucose and that plasma was approximately twice as accurate as whole blood. The coefficient of variance (CV) obtained was approximately 5% for the combination of the mid-IR plasma approach. The accuracy of this technique led the scientists at OptiScan to focus on plasma measurement using mid-IR for the OptiScan measurement platform. Plasma is used also in nonoptical systems to improve the accuracy of glucose measurement.32,33
OptiScan based their unique algorithm on a wide variety of sample measurements and drug spectra spanning hundreds of therapeutic drugs and blood chemistry combinations found in the critical care environment. Measured spectra are compared against the glucose “fingerprint” in the mid-IR region. When interferences are present, the OptiScanner utilizes an internally stored library of interfering substances to construct adaptive calibration vectors in real time and applies these to measured sample spectra. Should the OptiScanner be unable to adjust to interferences, it will not provide a reading. Quality assurance also ensures the blood sample is not significantly diluted from the nonheparinized saline flush. Quality checks during the measurement process ensure the integrity of OptiScan's glucose measurement.
OptiScan Biomedical Corporation has demonstrated the accuracy of glucose measurement using mid-IR spectrometry in previously approved studies involving a wide variety of ICU populations conducted from 2005 to early 2009. Much of this work has been presented at the Society of Critical Care Medicine meetings from 2006 to 2009.34–37 These studies were essential in developing and verifying the performance of OptiScan's interference correction algorithm.
Methods
Study Design
The purpose of this study, OptiScanner Performance in the pig model, was to evaluate the safe use of the OptiScanner for at least 6 h in three pigs under good laboratory practices performed at an independent preclinical facility by two physicians (doctors of veterinary medicine). Each pig was studied on a separate day. Device safety was assessed by consideration of changes in complete blood count (CBC), chemistry profile, plasma-free hemoglobin, haptoglobin, prothrombin time (PT), and activated partial thromboplastin time (APTT) tests collected at the beginning and end of the test period and by examination for thromboembolism during the gross necropsy on each animal. The ability of the OptiScanner to track glucose concentration in an animal model was determined by comparison with Yellow Springs Instrument (YSI) 2300 Stat Plus results. An OptiScanner was in operation for at least 6 h in each animal.
The three Yorkshire pigs were in good health and weighed, on the study day, between 50.0 and 52.6 kg. Each pig was anesthetized for the study procedure using Telazol and Xylazine intramuscularly and Isoflurane via a mask and then intubated. Several other drugs were given to the animals during the course of the study to maintain anesthesia. Heparin was not administered during the entire study procedure.
A central venous multilumen catheter was inserted into the jugular vein and was connected to the OptiScanner using the proximal lumen. Another lumen of the central venous catheter was used for simultaneous YSI sample collection. A 16-gauge catheter was inserted into the femoral vein and was used for the administration of drugs and fluids during the study procedure. All catheters were flushed with saline. Each anesthetized pig was subjected to controlled excursions of blood glucose through the administration of dextrose or insulin. The target blood glucose range was 40 to 300 mg/dl.
The total anesthesia time for the pigs were similar: pig 290 (study day 1), 8 h 19 min; pig 291 (study day 2), 8 h 31 min; and pig 294 (study day 3), 8 h 45 min. The esophageal temperature, blood pressure, and respiratory parameters were maintained within acceptable ranges for each animal.
At the end of the study procedure, heparin was administered (300 IU/kg) and was allowed to circulate for at least 5 min prior to euthanasia, and the gross necropsy was performed by a veterinarian.
Animal welfare for this study was in compliance with the U.S. Department of Agriculture Animal Welfare Act implementing regulations (9 CFR parts 1, 2, and 3). The Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Academy Press, Washington DC, 1996, as amended by the Animal Welfare Act of 1970 (P.L. 91-579) and the 1976 amendments to the Animal Welfare Act (P.L. 94-279) were followed. In order to ensure compliance, this protocol was reviewed and approved by the Institutional Animal Care and Use Committee before the initiation of treatment.
Sample Collection and Processing
The OptiScanner collected a blood sample automatically every 15 min. Paired YSI samples were collected simultaneously from an additional lumen of the same catheter from which the OptiScanner was drawing samples. The measured value was displayed on the OptiScanner monitor, and YSI samples were centrifuged and the plasma removed for testing. The displayed glucose measurements on the OptiScanner screen and YSI determined blood glucose measurements were recorded.
Data Analysis
Safety of the OptiScanner 5000 in the pig was assessed by consideration of changes in clinical laboratory tests of samples collected at the beginning and end of the test period (CBC and chemistry profile, plasma-free hemoglobin, haptoglobin, PT, and APTT) and the gross necropsy assessment of organs for the presence of thromboembolism.
Blood samples collected from the pig at the beginning of the anesthetic period prior to beginning the OptiScanner data collection were compared to blood samples collected at the completion of the performance data collection prior to the administration of heparin immediately before the animal was euthanatized.
The focus of the gross necropsy performed at the end of each study day on each animal was thromboembolism. The vascular course of the triple lumen catheter was assessed for any signs of clotting. The location of the end of the catheter was photographed in situ.
The lungs were carefully examined for any gross evidence of thromboembolism. Lung, heart, liver, and kidney sections were collected for histological evaluation. The heart was evaluated grossly, with particular attention to the valves and chambers for any adhesion of fibrin or thrombus formation. The liver and kidneys were also evaluated for any gross abnormalities, and two sections from each were collected for storage. Any other lesions or abnormalities identified were also documented and collected for possible histological analysis. The organs specified and any other abnormal findings were photographed.
The glucose measurements for both the OptiScanner and the YSI were tracked during the study. Post-study, the glucose values for all three animals were combined for analysis on the Clarke Error Grid (CEG) and by linear regression.
Results
Clinical Pathology
The CBC and serum chemistry values were compared with a reference range established from blood collected from clinically normal pigs anesthetized with similar drugs and with the tests performed by the same subcontractors as used in this study. A partial list of the blood test results is shown in Table 1. Most results from these pigs were within the reference range. The red blood cell count, hemoglobin, hematocrit, albumin, and total protein were slightly below the low reference value, but not to clinically relevant levels. The lower values may be due to a dilutional effect associated with anesthesia and fluid administration. The glucose at time point 2 was elevated because the animals were receiving an infusion of 50% dextrose prior to the end of the study. No reference values are available for either PT or APTT for Yorkshire pigs. A clinically relevant change was not present in the time point 1 compared with time point 2 values for PT and APTT, so the values are considered to be within normal range. Plasma-free hemoglobin and haptoglobin were within the reference range for both time points.
Table 1.
Comparison of Blood Test Results
| Partial list of results for CBC test c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test Animal ID | Average Measurements | |||||||||||
| Units | Reference Range | 290 | 291 | 294 | Mean | SD | Mean | SD | ||||
| 1a | 2b | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |||
| WBC c | ×103/μl | 8.8–20.4 | 15.0 | 11.6 | 15.2 | 11.5 | 13.9 | 12.1 | 14.7 | 0.7 | 11.7 | 0.3 |
| RBCc | ×103/μl | 5.13–7.20 | 4.94 | 4.68 | 5.28 | 4.74 | 5.54 | 5.22 | 5.25 | 0.30 | 4.88 | 0.30 |
| HBGc | g/dl | 9.0–12.9 | 8.1 | 7.7 | 9.1 | 8.2 | 9.7 | 9.1 | 9.0 | 0.8 | 8.3 | 0.7 |
| HCTc | % | 26.2–36.1 | 24.7 | 23.7 | 27.6 | 24.6 | 28.5 | 26.6 | 26.9 | 2.0 | 25.0 | 1.5 |
| PLTc | ×103/μl | 148–556 | 377 | 360 | 320 | 256 | 280 | 255 | 326 | 49 | 290 | 60 |
| Partial list of results for serum chemistry test | ||||||||||||
| Test Animal ID | Average Measurements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | Reference Range | 290 | 291 | 294 | Mean | SD | Mean | SD | ||||
| 1a | 2b | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |||
| g/dl | 2.8–4.2 | 3.2 | 2.8 | 3.2 | 2.5 | 2.7 | 2.4 | 3.0 | 0.3 | 2.6 | 0.2 | |
| Total | Proteing/dl | 5.6–6.7 | 6.3 | 5.5 | 6.0 | 5.0 | 6.5 | 5.8 | 6.3 | 0.3 | 5.4 | 0.4 |
| ALT | U/liter | 19–43 | 25 | 22 | 30 | 28 | 23 | 20 | 26 | 4 | 23 | 4 |
| BUNc | mg/dl | 4–11 | 7 | 10 | 9 | 9 | 6 | 8 | 7 | 2 | 9 | 1 |
| Creatinine | mg/dl | 0.9–1.9 | 1.2 | 1.3 | 1.6 | 1.6 | 1.4 | 1.5 | 1.4 | 0.2 | 1.5 | 0.2 |
| Glucose | mg/dl | 48–185 | 74 | 177 | 89 | 269 | 132 | 167 | 98 | 30 | 204 | 56 |
| CO2 | mEq/liter | 25–30 | 32 | 32 | 32 | 33 | 30 | 32 | 31 | 1 | 32 | 1 |
| PT | s | none | 11.4 | 11.4 | 11.5 | 11.5 | 11.9 | 11.4 | 11.6 | 0.3 | 11.4 | 0.1 |
| APTT | s | none | 10.9 | 10.6 | 13.0 | 13.4 | 12.4 | 10.5 | 12.1 | 1.1 | 11.5 | 1.6 |
| Haptoglobin and plasma-free hemoglobin results | ||||||||||||
| Test Animal ID | Average Measurements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | Reference Range | 290 | 291 | 294 | Mean | SD | Mean | SD | ||||
| 1a | 2b | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |||
| Plasma haptoglobin | mg/ml | 0–1.96 | 0.81 | 0.98 | 0.17 | 0.24 | 0.32 | 0.51 | 0.43 | 0.33 | 0.58 | 0.37 |
| Plasma-free hemoglobin | mg/dl | 0.2–1.9 | 0.4 | 1.7 | 1.4 | 1.1 | 1.1 | 1.4 | 1.0 | 0.5 | 1.4 | 0.3 |
Lab value at the start of the study before attachment to the OptiScanner.
Lab value at the end of the study after 6 h of attachment to the OptiScanner.
WBC, white blood cells; RBC red blood cells; HBG hemoglobin; HCT, hematocrit; PLT, platelet; BUN, blood urea nitrogen.
Gross Necropsy
The gross necropsy findings were similar in all pigs. Necropsy photographs were taken and the photographs of the catheter in each pig were taken in situ. The tip of the triple lumen catheter was present in the caudal vena cava proximal to the diaphragm. The position of the proximal port, which was the port from which the test article was collecting blood, was positioned caudal to the heart in the caudal vena cava. No luminal damage to any part of the vessel in contact with the catheter was observed. All catheter ports were patent. No evidence of distal thromboembolism in the target organs was found. The target organs were normal except for the presence of hypostatic congestion in the caudal and dorsal lung fields. Hypostatic congestion in the dorsal and caudal lung fields is an expected finding immediately following prolonged anesthesia in animals positioned in dorsal recumbency and was not related to the use of the test article.
Glucose Tracking
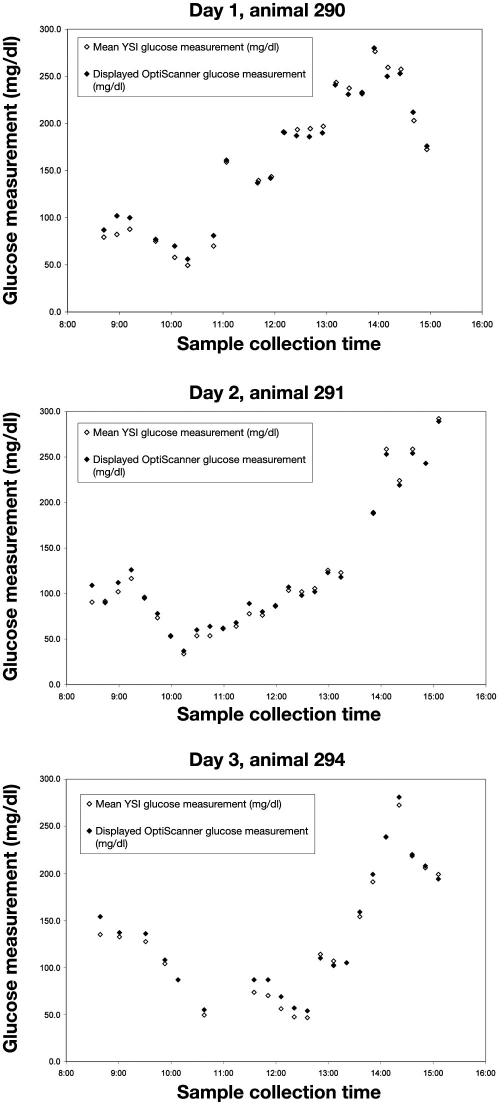
The glucose values from the YSI were compared to the OptiScanner glucose value for each paired sample. The glucose trend graph for each study day in Figure 1 shows the YSI glucose measurement followed the OptiScanner glucose measurement through the glucose challenges for each animal, spanning a glucose concentration range from 30 to 295 mg/dl. Some 75 paired measurements were collected. Six of the paired measurements did not contain an OptiScanner value, which were identified by the quality assurance module of the OptiScanner system in sample processing.
Figure 1.
Paired glucose measurement results.
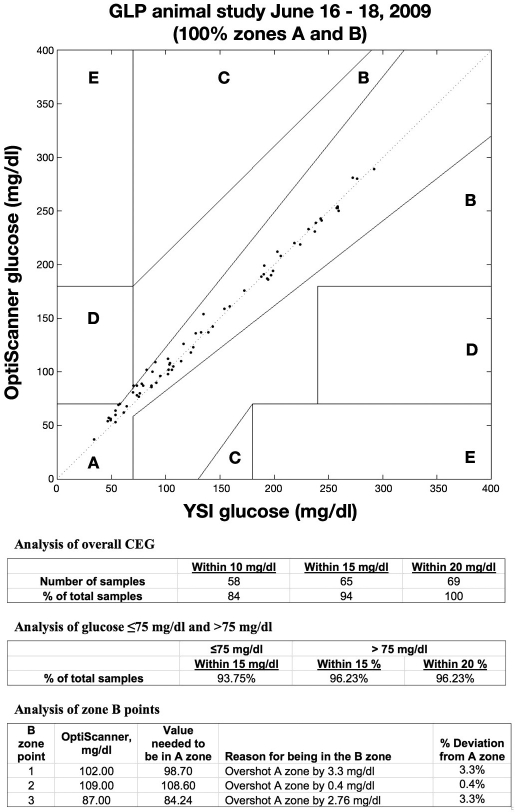
These data are shown on a CEG in Figure 2, where 100% of the points are in regions A and B (95.7% or 66 points are in region A, and 4.3% or 3 points are in region B). The overall analysis of the CEG shows that 94% of the measurements were within 15 mg/dl of the reference glucose value, and 84% of the measurements were within 10 mg/dl. B-zone values were within a maximum of 3.3% of the A-zone boundaries.
Figure 2.
Clarke Error Grid. GLP, good laboratory practice.
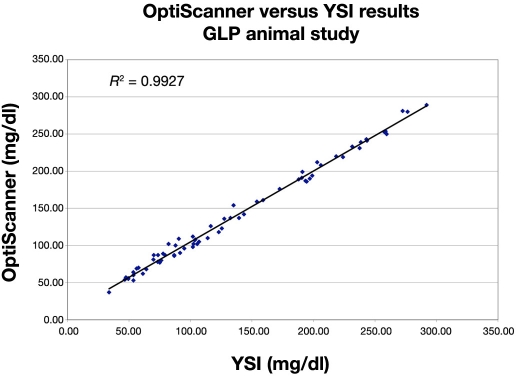
The differences between the OptiScanner and the YSI have a mean of 3.04 mg/dl and a standard deviation (SD) of 6.90 mg/dl. The root mean square error (the square root of the sum of the squares of the mean and the SD) is 7.53 mg/dl. The population CV is 5.02%. These results are consistent with the results of Heise and coworkers. To assess the linearity between YSI and OptiScanner, a linear model was used to regress the OptiScanner results on the YSI. Since the YSI is a reference method, it was treated as the independent variable. The coefficient of determination, R2, is 0.993 (r = 0.996). The graph showing this linear relationship is shown in Figure 3.
Figure 3.
OptiScanner and YSI linear relationship. GLP, good laboratory practice.
Data analysis was performed in the MATLAB (version 7, Natick, MA) environment. Each glucose estimation error is calculated as the result of the OptiScanner minus the result of the YSI reference method. The population CV is the SD of the glucose estimation errors divided by the mean YSI reading. This ratio is multiplied by 100.
The coefficient of determination (R2) is obtained by regressing the OptiScanner results on the YSI results using unweighted least squares and treating the YSI results as the independent variable as described earlier. R2 is used because it is a more stringent parameter than the correlation coefficient, r.
Discussion
The normal clinical pathology and coagulation values collected at time points 1 and 2 indicate that the use of the OptiScanner for 6 h did not cause abnormalities in these pigs. The normal plasma-free hemoglobin and serum haptoglobin for both time points indicate that there was no ongoing hemolysis.
The drugs administered during the performance data collection period were similar for the pigs. The drugs administered did not appear to significantly influence the clinical pathology results.
No thrombus was found that was associated with the OptiScanner. The absence of gross evidence of thromboembolism in the organs evaluated, the normal clinical pathology, and no evidence of ongoing hemolysis indicate that the OptiScanner 5000 system was safely used in these pigs.
The paired YSI and OptiScanner measurements documented directional tracking of the consecutive blood glucose by the OptiScanner. Quality assurance was demonstrated by not reporting results for samples that could not meet the internal quality checks of the OptiScanner system. The results shown in the CEG demonstrate the clinical accuracy of testing a laboratory quality plasma sample and the ability of the mid-IR measurement technique.
Conclusions
The OptiScanner 5000 was safely used for 6 h in these pigs as demonstrated in this study. The paired glucose measurements show the accuracy of the OptiScanner measurements compared to the YSI reference instrument in an animal model as shown by the CEG and the linear regression analysis. The errors commonly observed and reported in current glucose measurement methods, due to measurement in whole blood samples with standard meter technology, were not observed in this study.
The OptiScanner is the first plasma-based continuous glucose monitor designed for correction of measurement interferences expected in ICU medicine. It has performed safely and accurately in an animal model. Human studies with the device are planned in the near future.
Abbreviations
- APTT
activated partial thromboplastin time
- CBC
complete blood count
- CEG
Clarke Error Grid
- CV
coefficient of variance
- ICU
intensive care unit
- IR
infrared
- PT
prothrombin time
- RCT
randomized controlled trial
- SD
standard deviation
- TGC
tight glycemic control
- YSI
Yellow Springs Instrument
References
- 1.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 3.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, Van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomized controlled study. Lancet. 2009;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 4.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 5.Bochicchio GV, Joshi M, Bochicchio KM, Pyle A, Johnson SB, Meyer W, Lumpkins K, Scalea TM. Early hyperglycemic control is important in critically injured trauma patients. J Trauma. 2007;63(6):1353–1358. doi: 10.1097/TA.0b013e31815b83c4. [DOI] [PubMed] [Google Scholar]
- 6.Scalea TM, Bochicchio GV, Bochicchio KM, Johnson SB, Joshi M, Pyle A. Tight glycemic control in critically injured trauma patients. Ann Surg. 2007;246(4):605–610. doi: 10.1097/SLA.0b013e318155a789. [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 8.Mann EA, Pidcoke HF, Salinas J, Holcomb JB, Wolf SE, Wade CE. The impact of intensive insulin protocols and restrictive blood transfusion strategies on glucose measurement in American Burn Association (ABA) verified burn centers. J Burn Care Res. 2008;29(5):718–723. doi: 10.1097/BCR.0b013e3181848c74. [DOI] [PubMed] [Google Scholar]
- 9.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105(2):244–252. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Ali N, O'Brien JM, Jr., Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Jr., Preiser JC. Glucose variability is independently associated with mortality in patients with sepsis. Crit Care Med. 2007;35:A924. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krinsley JS. Glycemic variability: a strong, independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36(11):3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 12.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 13.Preiser JC. Intensive glycemic control in medico-surgical patients: the European GluControl Trial. SCCM Congress; February 19, 2007; Orlando, FL. [Google Scholar]
- 14.The NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;369(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 15.Inzucchi SE, Siegel MD. Glucose control in the ICU—how tight is too tight? N Engl J Med. 2009;360(13):1346–1349. doi: 10.1056/NEJMe0901507. [DOI] [PubMed] [Google Scholar]
- 16.Krinsley JS, Preiser JC. Moving beyond tight glucose control to safe effective glucose control. Crit Care. 2008;12(3):149. doi: 10.1186/cc6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med. 2005;33(12):2778–2785. doi: 10.1097/01.ccm.0000189939.10881.60. [DOI] [PubMed] [Google Scholar]
- 18.Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124(4):577–582. doi: 10.5858/2000-124-0577-EOPOGM. [DOI] [PubMed] [Google Scholar]
- 19.Mann EA, Salinas J, Pidcoke HF, Wolf SE, Holcomb JB, Wade CE. Error rates resulting from anemia can be corrected in multiple commonly used point-of-care glucometers. J Trauma. 2008;64(1):15–20. doi: 10.1097/TA.0b013e318160b9e4. [DOI] [PubMed] [Google Scholar]
- 20.Tang Z, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurements with handheld meters for point- of-care testing. Arch Pathol Lab Med. 2000;124(8):1135–1140. doi: 10.5858/2000-124-1135-EODHLO. [DOI] [PubMed] [Google Scholar]
- 21.Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2(3):349–362. doi: 10.1089/15209150050194215. [DOI] [PubMed] [Google Scholar]
- 22.Tang ZP, Du XG, Louie RF, Kost GJ. Drug interference on glucose measurements with six handheld glucose meters and a portable analyzer for point-of-care testing in critical care. 51st Annual AACC Conference; July 25–29, 1999; New Orleans, LA. Abstract 97. [Google Scholar]
- 23.Harris G. FDA pushes for accurate glucose monitors. 2009 New York Times. July 19. [Google Scholar]
- 24.Hamburg M. FDA's Center for Devices and Radiological Health's Response. 2009 Jun 24; Department of Health and Human Services letter from the Commission of Food and Drugs, FDA. [Google Scholar]
- 25.Scott MG, Bruns DE, Boyd JC, Sacks DB. Tight glycemic control in the intensive care unit: are glucose meters up to the task? Clin Chem. 2009;55(1):18–20. doi: 10.1373/clinchem.2008.117291. [DOI] [PubMed] [Google Scholar]
- 26.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15(4):370–377. [PubMed] [Google Scholar]
- 27.Wernerman J. International Symposium on Intensive Care and Emergency Medicine Conference; March 25, 2009. [Google Scholar]
- 28.Kim YJ, Yoon G. Prediction of glucose in whole blood by near-infrared spectroscopy: influence of wavelength region, preprocessing, and hemoglobin concentration. J Biomed Opt. 2006;11(4):041128. doi: 10.1117/1.2342076. [DOI] [PubMed] [Google Scholar]
- 29.Druy MA. Applications for mid-IR spectroscopy in the pharmaceutical process environment. Spectroscopy. 2004;19(2):60–64. [Google Scholar]
- 30.Heise HM, Marbach R, Koschinsky T, Gries FA. Multicomponent assay for blood substrates in human plasma by mid-infrared spectroscopy and its evaluation for clinical analysis. Appl Spectrosc. 1994;48(1):85–95. [Google Scholar]
- 31.Kruse-Jarres JD, Janatsch G, Gless U, Marbach R, Heise HM. Glucose and other constituents of blood determined by ATR-FTIR- spectroscopy. Clin Chem. 1990;36(2):401–402. [PubMed] [Google Scholar]
- 32.Finkielman JD, Oyen LJ, Afessa B. Agreement between bedside blood and plasma glucose measurement in the ICU setting. Chest. 2005;127(5):1749–1751. doi: 10.1378/chest.127.5.1749. [DOI] [PubMed] [Google Scholar]
- 33.Brunkhorst FM, Wahl HG. Blood glucose measurements in the critically ill: more than just a blood draw. Crit Care. 2006;10(6):178. doi: 10.1186/cc5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krinsley J, Zheng P, Hall D, Magarian P. ICU validation of the OptiScanner, a continuous glucose monitoring device. Crit Care Med. 2006;34(12):A67. [Google Scholar]
- 35.Krinsley J, Hall D, Zheng P, Magarian P. Validation of the OptiScanner, a continuous glucose and lactate point of care monitor. SCCM 36th Critical Congress; February 2007; Poster 255. [Google Scholar]
- 36.Bochicchio G, Bochicchio K, Lettich K, Lambert P, Herrera A, Lumpkins K, Magarian P, Scalea T. Cutting edge technology in tight glycemic control. SCCM 37th Critical Care Congress; February 2008; Poster 519. [Google Scholar]
- 37.Bochicchio G, Bochicchio K, Lettich K, Lambert P, Herrera A, Lumpkins K, Magarian P, Scalea T. Mid infrared spectroscopy (MIS) is highly accurate in measuring glucose in ICU patients. SCCM 38th Critical Care Congress; February 2009; Poster 262. [Google Scholar]