Abstract
Background
SoloSTAR® (SOL; sanofi-aventis, Paris, France) is a prefilled insulin pen device for the injection of insulin glargine and insulin glulisine. This is the first Australian survey to determine its usability, participant acceptance, and safety in clinical practice.
Methods
A 3-month, nonrandomized, noncomparative, observational survey in Australia was conducted in individuals with diabetes. Participants were given SOL pens containing glargine, the instruction leaflet, and a toll-free helpline number. Training was offered to all participants. Safety data, including product technical complaints (PTCs), were gathered from ongoing feedback given by the participant or health care professional (HCP) and by independent interviews conducted 6–10 weeks after study start.
Results
Some 2674 people consented to take part across 93 sites (150 HCPs), and 2029 participated in interviews. Of these, 52.6% had type 1 diabetes, 16.3% had manual dexterity problems, and 15.5% had poor eyesight not corrected by glasses. At the time of interview, 96.8% of participants were still using SOL. None of the eight PTCs reported were due to technical defects; most were related to handling errors. Some 62 participants reported 77 adverse events; none were related to a PTC. The vast majority of participants (95.4%) were “very satisfied” or “satisfied” with using SOL, and 89.7% of the participants had no questions or concerns using SOL on a daily basis. Similar positive findings were reported by participants with manual or dexterity impairments.
Conclusions
In this survey of everyday clinical practice, SOL had a good safety profile and was very well accepted by participants.
Keywords: clinical practice, diabetes, insulin glargine, safety, SoloSTAR
Introduction
Insulin pens have had a significant impact on the treatment of diabetes. Compared with a vial and syringe, they offer substantial advantages in terms of compliance, social acceptability, and flexibility for patients using insulin and have been shown to be preferred by both people with diabetes and the health care professionals (HCPs) who treat them.1–4 Since the first insulin pen was introduced in 1985, ongoing developments in technology have led to more advanced devices that offer larger maximum doses, smaller dose increments, and improved dose features, such as lower injection force and ease of identifying the insulin.5–7
SoloSTAR® (SOL) is a new prefilled insulin pen device developed for the administration of either insulin glargine (LANTUS®) or insulin glulisine (Apidra®; all sanofi-aventis, Paris, France). The SOL pen can be set in 1 U increments, similar to other devices, and is capable of delivering a maximum dose of 80 U, which is a larger dose volume compared with other commonly used disposable pen devices. SoloSTAR has a different pen body color for each insulin—gray for insulin glargine and blue for insulin glulisine. In addition, the insulin glulisine pen has a tactile differentiation of a raised ring on the dose button besides other differentiation features, including different colors in the labels and packaging.
The aim of this survey was to evaluate the safety, usability, and acceptance of SOL in a clinical setting and focuses on the administration of insulin glargine with SOL.
Participants and Methods
Objectives
The primary objective of this survey was to monitor SOL in actual everyday use in order to collect information on real use experience and detect any product technical complaints (PTCs), safety issues, or problems related to its use. This survey was designed to monitor the device and not the insulin. Secondary objectives included participant satisfaction with the use of the pen. This was a 3-month, prospective, observational survey based in Australia and was conducted between November 2006 and February 2007.
Participants
People with type 1 or type 2 diabetes with past or current use of injectable insulin or other prescribed antidiabetes agents or people considered by their health care provider to be candidates for initiation of injectable insulin therapy were invited to participate in the survey. Exclusion criteria included current addiction or current alcohol/drug abuse; diagnosis of dementia; severe visual or dexterity impairment; mental condition rendering the person unable to understand the nature, scope, and possible consequences of the survey; or any person deemed by the investigator as potentially uncooperative.
Potential participants who met the inclusion criteria were identified by their HCP, and the program was explained to them at either the next routine clinic visit or by telephone. Participants were informed that they would be required to report and keep records of any apparently broken or not properly functioning devices and participate in a 10 min telephone interview. All potential participants were clearly informed that participation was entirely voluntary and that they would continue to receive the best standard of care available, even if they chose not to participate. Those who were interested were then asked to sign an information sheet, which further described the program. The survey was conducted in accordance with the Declaration of Helsinki. Health care professionals who participated in the survey were reimbursed for costs associated with administration of the survey.
Survey Design
At the start of the observation period, participants were given SOL pens containing insulin glargine, the instruction leaflet, and a toll-free helpline number, which was operated by an independent agency. At this time, participants were also offered training by the HCPs on how to use SOL. Participants used SOL for 6–10 weeks and were asked to report any issues that they experienced during this time. At 6–10 weeks after initial use, participants were contacted to take part in a 10 min telephone survey (Appendix 1) to collect information on any problems experienced with SOL. Participants were also asked to rate their experience with SOL, including aspects of use. To maintain participant confidentiality, all telephone contact with participants was managed through an independent customer service group (International™ SOS) specializing in medical assistance.
Statistical Analysis
No comparisons were performed, and descriptive data are provided. Events are presented as number of participants and percentages with exact 95% confidence intervals (CIs; binomial distribution, as assessed by the Clopper–Pearson algorithm). All analyses were performed using SAS version 8.2 (SAS Inc., Cary, NC). An estimated sample size of 2000 participants was determined by taking into account potential device and handling problems, which were derived from already marketed insulin pen devices in terms of occurrence rate. In addition, the minimum sample size allowed the detection (with 95% confidence) of potential pen issues that occur at a rate of 0.005% or handling problems occurring at a rate of 0.0035%, based on an estimated 6 weeks of use, involving 10,000 pens and >105,000 injections.
Results
Participant Characteristics and Disposition
A total of 2674 people agreed to participate in this observational survey of everyday clinical practice, which was conducted across 93 sites, involving 150 HCPs (Appendix 2). Health care professionals were a combination of primary care physicians, endocrinologists/diabetologists, and diabetes educators. Twenty participants withdrew consent prior to the survey; therefore, 2654 people used SOL. At 6–10 weeks after initial use of SOL, 2029 people provided feedback during solicited interviews. Participant characteristics and demographics are summarized in Table 1.
Table 1.
Characteristics and Demographics of the Participants, Collected from Spontaneous Interviews Conducted after 6–10 Weeks of SOL Use in the Overall Survey Population and in Specified Subgroups Of Participants, Including Diabetes Type, Prior Device Experience, Visual/Manual Impairments and Participant Age
| Overall population (n = 2029) | Diabetes type | Prior device experience | Self-reported impairments | Participant age | |||||
|---|---|---|---|---|---|---|---|---|---|
| Type 1 (n = 1067) | Type 2 (n = 926) | Naïve (n = 194) | Experienced (n = 1834) | Visual (n = 170) | Manual (n = 130) | <18 years (n = 21) | ≥70 years (n = 230) | ||
| Age (years) a | 50.5 ± 16.1 | 42.6 ± 15.4 | 59.3 ± 11.6 | 55.8 ± 16.4 | 50.0 ± 16.0 | 60.4 ± 13.2 | 60.8 ± 12.9 | 14.9 ± 3.0 | 75.0 ± 4.4 |
| Females/males (%) | 49/51 | 49/51 | 48/52 | 46/54 | 49/51 | 57/43 | 58/42 | 43/57 | 48/52 |
| Type 1/2 diabetes (%) | 54/46 | 100 | 100 | 29/71 | 56/44 | 42/58 | 34/66 | 100/0 | 22/78 |
| Never used an injection pen (%) | 10 | 5 | 15 | 100 | 0 | 8 | 8 | 24 | 16 |
| Satisfied with using SOL (%) | |||||||||
| Very satisfied | 74 | 74 | 75 | 77 | 74 | 72 | 78 | 76 | 77 |
| Satisfied | 21 | 22 | 20 | 21 | 21 | 19 | 18 | 19 | 19 |
| Neutral | 3 | 3 | 3 | 1 | 3 | 5 | 2 | 5 | 3 |
| Unsatisfied | <1 | <1 | <1 | 1 | <1 | <1 | <1 | 0 | <1 |
| Very unsatisfied | 1 | <1 | 1 | <1 | 1 | 2 | 2 | 0 | <1 |
| Participants without concerns/questions/issues (%) | 90 | 90 | 90 | 92 | 89 | 88 | 91 | 86 | 94 |
| Questions raised during interviews (%) | |||||||||
| Needle attachment | <1 | <1 | <1 | <1 | <1 | 2 | <1 | 5 | <1 |
| Dose dialing | 3 | 3 | 3 | 3 | 3 | 5 | 2 | 5 | 2 |
| Injecting | 4 | 4 | 5 | 3 | 4 | 4 | 5 | 0 | 2 |
| Reading anything on the pen | <1 | 1 | <1 | <1 | <1 | <1 | 1 | 0 | 0 |
| Removing bubbles | <1 | <1 | <1 | 0 | <1 | 0 | 0 | 5 | 0 |
| Continuing to use SOL after the end of survey period (%) | 97 | 97 | 97 | 96 | 97 | 98 | 95 | 100 | 97 |
Mean ± standard deviation.
Similar numbers of participants with type 1 and type 2 diabetes were included in the survey, the majority had used a pen device in the past, and 9.6% of the participants (n = 194/2029) reported no prior experience of using insulin devices. Training was offered to all participants, and 87% (n = 1770/2029) were trained. Training by one-to-one demonstration was carried out in 74% (n = 1501) of participants, 12.5% (n = 253) of participants were trained using a group demonstration, 36.1% (n = 732) received both demonstration and a user guide booklet, 32.1% (n = 651) were trained with the user guide booklet, and 12.6% (n = 256) received no training (multiple answers were allowed). The median age was 42 years for participants with type 1 diabetes and 60 years for participants with type 2 diabetes. Overall, 16.3% (n = 329/2024) of participants had manual dexterity problems, 15.5% (n = 314/2023) had poor eyesight not corrected by glasses, and 12.1% (n = 245/2022) had other disabilities considered unrelated to the ability to use SOL.
At the time of interview, 96.8% (n = 1962/2027) of participants were still using SOL. Of the participants who discontinued use of SOL, 17 (0.8%) ceased use SOL 1–6 days prior to interview, 19 (0.9%) 2–4 weeks previously, 16 (0.8%) 3–4 weeks previously, and 13 (0.6%) 5–6 weeks previously; reasons for discontinuation were not recorded. In total, 21.2% (n = 430/2028) were using insulin glargine as the only insulin, and 78.8% (n = 1598/2028) reported that they were using insulin glargine plus one or more other insulin product. The mean duration of SOL use prior to the interviews was 60.5 ± 15.7 days.
Product Technical Complaints
A total of eight problems were considered to be PTCs, of which seven were reported during the solicited interviews. In three instances, the pens jammed; in two instances, the pens leaked; in two instances, the pens were hard to push; and in one instance, the pen or plunger was reported as “faulty.” Investigations were performed according to standard methods, and results were logged into the PTC database. None of the PTCs were due to a technical defect, and five PTCs were considered to be related to handling errors by the participants. The eight participants who reported a PTC rated their satisfaction with SOL to be either “very satisfied” (n = 6) or “satisfied” (n = 2).
Safety
A total of 77 adverse events (AEs) were reported by 62 people, none of which were related to a PTC. The most commonly reported AEs were injection-site reactions, hypoglycemia, dizziness, and hyperglycemia (Table 2). The reported rate of occurrence for injection-site reactions was 1.7% based on the number of enrolled participants. Injection site reactions are expected and are a listed event for insulin glargine. No AE was considered to be related to the SOL pen. There were four cases of serious AEs, none of which were related to a PTC. The majority of these events were most likely related to the participants' underlying diabetes or other confounding factors.
Table 2.
Adverse Events Reported
| AE | Nonserious | Serious | Total |
|---|---|---|---|
| Injection-site reaction | 33 | 1 | 34 |
| Hypoglycemia | 7 | 1 | 8 |
| Dizziness | 3 | – | 3 |
| Swelling | 2 | 1 | 3 |
| Abdominal pain | 2 | – | 2 |
| Pain | 2 | – | 2 |
| Headache | 2 | – | 2 |
| Hyperglycemia | 3 | – | 3 |
| Nausea | 2 | – | 2 |
| Rhinorrhea | 2 | – | 2 |
| Drug exposure during pregnancy | 2 | – | 2 |
| Back pain | 1 | – | 1 |
| Cystitis | – | 1 | 1 |
| Deatha | – | 1 | 1 |
| Diarrhea | 1 | – | 1 |
| Drug ineffective | 1 | – | 1 |
| Emotional disorder | 1 | – | 1 |
| Hunger | 1 | – | 1 |
| Hypotension | – | 1 | 1 |
| Kidney infection | – | 1 | 1 |
| Loss of consciousness | – | 1 | 1 |
| Edema peripheral | – | 1 | 1 |
| Renal dysfunction | – | 1 | 1 |
| Respiratory disorder | 1 | – | 1 |
| Visual acuity reduced | 1 | – | 1 |
| Total | 67 | 10 | 77 |
This participant had long-standing medical history of renal and heart failure. Death was considered due to these conditions and not related to the use of SOL.
Acceptance
Overall, the large majority (n = 1934/2028; 95.4%) of participants reported that they were either “satisfied” or “very satisfied” with using SOL (Figure 1). When the participants were asked to report on the occurrence of any issue or question during the 6–10 weeks period of SOL use, the majority of participants (n = 1820/2029; 89.7%; 95% CI: 88.3, 91.0%) reported that they experienced none with using SOL on a daily basis. This was consistent between those participants who were device naïve (n = 179/194; 92.3%; 95% CI: 87.6, 95.6%) and those who had previously used a device (n = 1641/1834; 89.5%; 95% CI: 88.0, 90.8%) and between people with type 1 diabetes (n = 957/1067; 89.7%; 95% CI: 87.7, 91.5%) and type 2 diabetes (n = 830/926; 89.6%; 95% CI: 87.5, 91.5%; Table 1). Similar findings were also reported by participants with manual or dexterity impairments and by young and elderly participants (Table 2). A small proportion of participants suggested aspects that could be improved, including injection (n = 86/2029; 4.2%; 95% CI: 3.4, 5.2%), dialing a dose (n = 65/2029; 3.2%; 95% CI: 2.5, 4.1%), reading anything on the pen (n = 16/2029; 0.8%; 95% CI: 0.5, 1.3%), attaching a needle (n = 13/2029; 0.6%; 95% CI: 0.4, 1.1%), removing air bubbles (n = 7/2029; 0.3%; 95% CI: 0.1, 0.7%), or something else (n = 21/2029; 1.0%).
Figure 1.
Participant satisfaction with SOL (percent ± 95% confidence bounds).
Of the 32 participants who reported that they were “unsatisfied” or “very unsatisfied,” only seven subsequently provided further comments, of which four were related to injecting and one each of dialing a dose, attaching a needle, and reading anything on the pen.
Discussion
In this survey of everyday clinical practice, SOL had a good safety profile and was very well accepted by participants with a low incidence of participant-reported questions or concerns during use, confirming its convenience in everyday practice. The results from this observational survey support the findings of Haak and colleagues that SOL demonstrates high patient usability and high patient preference in people with diabetes.8
Although patients with type 1 diabetes must accept the need for insulin from diagnosis, patients with type 2 diabetes are often resistant to the addition of insulin to their regimen of oral antidiabetes agents.9,10 Delaying insulin therapy in type 2 diabetes may lead to deleterious effects on glycemic control and, as a result, increase the risk of diabetes-specific complications, such as retinopathy, nephropathy, and neuropathy.11 Insulin pens have the potential to help patients overcome barriers to the initiation of insulin, such as fear of needles, social acceptability, and the inconvenience of a vial and syringe.4
The number of participants with type 1 versus type 2 diabetes who reported no concerns with using SOL on a daily basis and were either “satisfied” or “very satisfied” with the device were similar in this survey; a very small proportion of participants reported SOL as “acceptable,” “poor,” or “very poor.” These positive experiences with SOL suggest that it may be a very convenient and useful tool in overcoming some of the barriers associated with the initiation of insulin in patients with type 2 diabetes and encouraging earlier use.
In the population of people included in this survey, only a small percentage were device naïve. Further studies in this group, and in insulin-naïve subjects, would help to strengthen this hypothesis. Nevertheless, results from this population of people, the majority of whom had type 2 diabetes, confirms the results given by Haak and colleagues8 that SOL is rated positively by people with no experience of using insulin pen devices.
In addition, results of participants who were device naïve were similar to those in people who were experienced with devices. Of particular interest, the majority of people who had no experience of insulin devices had no problems using SOL and were either “satisfied” or “very satisfied” with the device. This ease of initiation with SOL could be expected to translate to benefits in everyday clinical practice for both people with diabetes and their HCPs. These findings were also consistent among the participants with manual or visual dexterity impairments and among the young and elderly participants.
The results of this survey, which suggest that SOL was easy to use since the majority of device-naïve participants were “satisfied” or “very satisfied,” are consistent with a laboratory-based study where the injection force of SOL was compared with FlexPen (FP) and Next Generation FlexPen (NGFP). A realistic dispense rate (6 U/s, constant volume flow rate) was used. The mean ± standard deviation plateau dispense force for each pen to dispense 60 U at a rate of 6 U/s was 7.15 ± 0.69 N for SOL versus 12.51 ± 0.96 N for FP and 9.72 ± 0.72 N for NGFP. This finding mirrors the study by Clarke and Spollett, who also reported lower injection force dynamics for SOL versus FP.6 Although it is unknown whether this lower injection force of SOL compared with other available pens on the market is clinically significant, it may potentially be of benefit for those who have limited levels of manual dexterity.
Information regarding the likelihood of participants continuing using SOL as part of their daily management of diabetes was not captured in this survey. Nevertheless, a high proportion of patients (over 95%) were still using SOL at the time of the interview. Unfortunately, reasons for discontinuation were not recorded; however, it seems probable that participants may have stopped using SOL owing to therapy changes, for example, a change in insulin regimen or a switch to oral therapy, or the participant preferred other pen devices. It would be of further interest to investigate the continuation rates of SOL use after the survey was conducted, as this would provide valuable information on the longer-term impact of SOL.
One of the main limitations of surveys is that participants may not feel comfortable in reporting negative feedback, especially as the participants were invited to participate in the survey by their HCP, which may have introduced some bias to the results; re-interviewing participants could help to overcome this problem. We endeavored to minimize this effect by ensuring all telephone contact and data collection was mediated through an independent agency providing anonymity from the HCPs and sponsor. Nevertheless, the results of this survey of people with type 1 or type 2 diabetes were consistent with the previously reported findings for 65 of the 150 HCPs involved in managing the participants. Indeed, 85% of the HCPs reported that SOL had made it easier to train people to use the device, while 98% reported that it was quicker to train participants.12 Such findings from people with diabetes who use the device described in the present report and those findings reported for the prescribing HCPs demonstrate the ease of use and indicate the potential saving of resources used in training people how to use the device.
Finally, it would be of interest to repeat this observation in a larger population of people with no prior experience of using insulin and insulin devices, given the increasing prevalence of type 2 diabetes13 and the known benefit of adding insulin to existing therapy versus intensification of oral therapy.14 As this was an observational study, further randomized trials with a crossover design that compare SOL with other available devices in a clinical setting could be performed; however, participants may compare the type of insulin given, which may confound the results.
Conclusions
In this noninterventional, observational survey of everyday clinical practice, SOL was well accepted by participants with both type 1 and type 2 diabetes, with the majority of participants reporting that they were either very satisfied or satisfied with the device. SoloSTAR also had a good safety profile. Most of the PTCs reported were likely due to participant handling errors and not to technical deficiencies. This is the first survey to demonstrate the high usability, high participant acceptance, and safety of SOL in everyday clinical practice. Further comparative studies and follow-up surveys conducted in the clinical practice setting would be of interest to confirm the findings presented here.
Acknowledgements
Editorial support for this article was provided through the global publications group of sanofi-aventis. We thank all the investigators for their commitment to this survey.
Abbreviations
- AE
adverse event
- CI
confidence interval
- FP
FlexPen
- HCP
health care professional
- NGFP
Next Generation FlexPen
- PTC
product technical complaint
- SOL
SoloSTAR
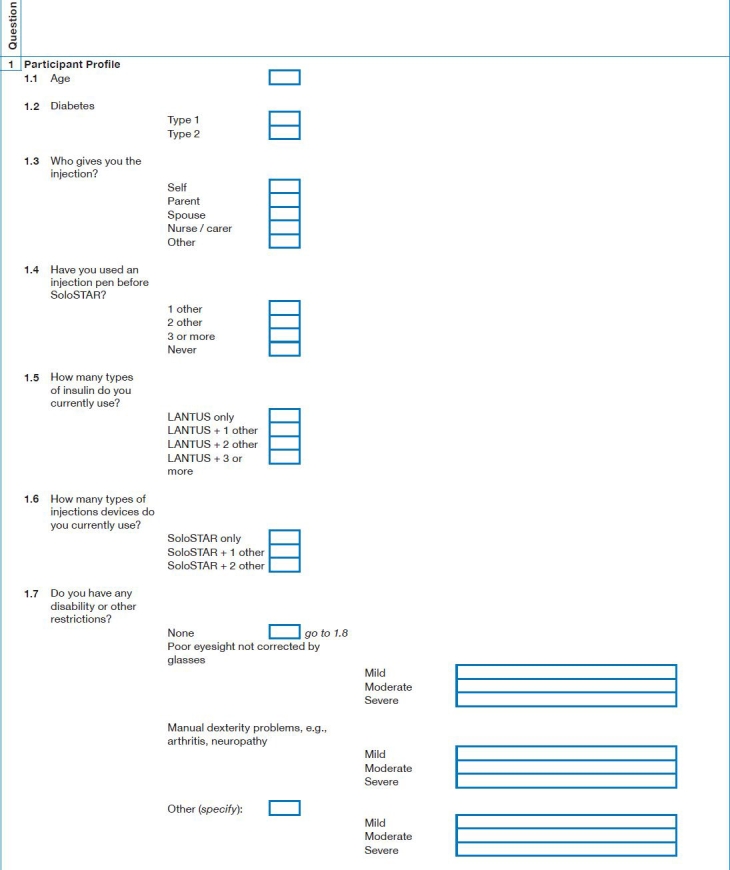
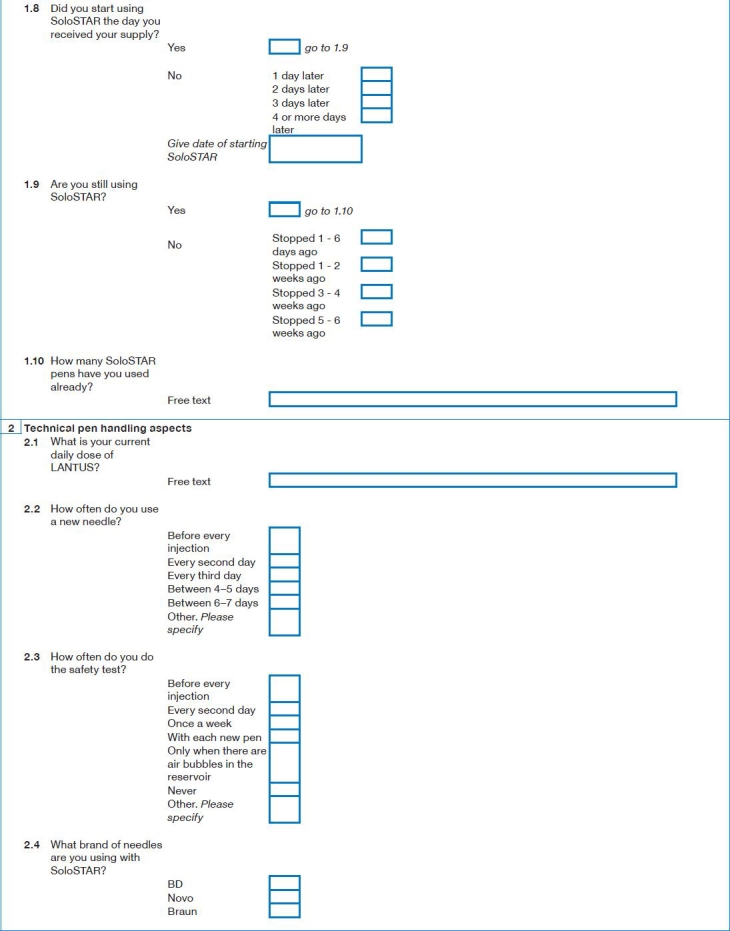
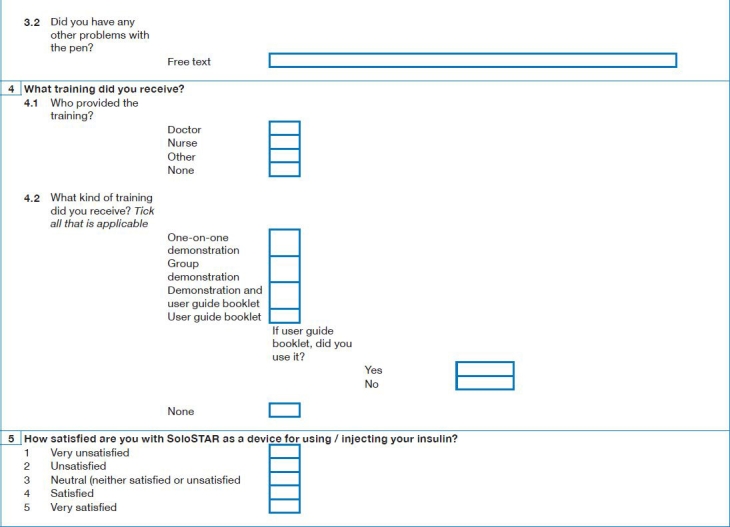
Appendix 1: Survey Questions

Appendix 2: Clinical Sites and Investigators
| Investigator | Address | Primary care or speciality clinic |
|---|---|---|
| Professor John Carter | Consulting Suites 39 Palmerston Road Hornsby NSW 2077 | Speciality |
| Dr. J. Yamba | Mt. Gambier Hospital Pharmacy Dept. 276 - 300 Wehl Street Mt. Gambier, SA 5290 | Speciality |
| Dr. Gregory Fulcher | Royal North Shore Hospital Dept. of Endocrinology 209 Pacific Highway St. Leonards, 2065 | Speciality |
| Dr. Martyn Sulway | 40 Shirley Road Wollstonecraft NSW 2065 | Speciality |
| A/P Patricia Crock | John Hunter Children's Hospital Paediatric Endocrinology and Diabetes University of Newcastle Newcastle, 2310 | Speciality |
| Dr. Stephen Thornley | Consulting Suites Suite 17 468 - 472 Kingsway Miranda, 2228 | Speciality |
| Dr. Gabrielle Howard | Consulting Suites 27 Belgrave Street Manly, 2095 | Speciality |
| Dr. Harry Grunstein | Consulting Suites Suite 707 Waverley Street Bondi Junction, 2022 | Speciality |
| Dr. Soji Swaraj | Suite 6, No 4 Browne St. Campbelltown 2560 | Speciality |
| Dr. Soji Swaraj | Sydney Southwest Pvte. Hospital Consulting Suite 40 Bigge Street Liverpool, 2170 | Speciality |
| Dr. Soji Swaraj | Suite 5A 28 Burwood Rd. Burwood 2134 | Speciality |
| Dr. Robert Schmidli | Consulting Suites John James Medical Centre 175 Strickland Crescent Deakin NSW, 2600 | Speciality |
| Dr. Adam Morton | Mater Adults Hospital Diabetes Centre 53 Raymond Terrace South Brisbane, 4101 | Speciality |
| Dr. David Roberts | Logan Hospital Dept. of General Medicine 8-48 Armstrong Road Meadowbrook, 4131 | Speciality |
| Dr. Winnifred Lee | Consulting Suites Alexandra House 5/201 Wickham Terrace Brisbane, 4000 | Speciality |
| Dr. Margaret Layton | 54 William Street, Gosford NSW 2250 | Speciality |
| Professor Geoff Nicholson | Geelong Hospital Douglas Hocking Research Institute Bellerine Street Geelong VIC 3220 | Speciality |
| Dr. Alison Nankervis | Suite 9, 3rd Floor Private Medical Centre Melbourne Private Hospital Royal Parade Parkville 3050 | Speciality |
| Professor Kong Wah Ng | St. Vincents Public Hospital Dept. of Endocrinology 4th Floor, Daly Wing 35 Victoria Parade Fitzroy, 3065 | Speciality |
| Dr. Duncan Topliss | Alfred Hospital Dept. of Endocrinolgy and Diabetes Commercial Road Melbourne 3004 | Speciality |
| Dr. Richard Arnott | Consulting Suites Diabetes and Specialist Centre Suite 3, 2nd floor 517 St. Kilda Road St. Kilda Road Melbourne, 3004 | Speciality |
| Dr. Serge Tang-Fui | Consulting Suites Diabetes and Specialist Centre 517 St Kilda Road St. Kilda Melbourne VIC 3004 | Speciality |
| Dr. Serge Tang-Fui | Consulting Suites 21 Kars Street Frankston VIC 3199 | Speciality |
| Dr. Christopher Gilfillan | Consulting Suite 11 Hastings Road Frankston, 3199 | Speciality |
| Dr. Murray Gerstman | Ringwood Specialist Centre 325 Maroondah Highway Ringwood, 3199 | Speciality |
| Dr. Richard MacIsaac | Consulting Suites Thomas Town Consulting Rooms 113 High Street Thomastown, 3074 | Speciality |
| Dr. Mario De Luise | Ivanhoe Specialist Centre 61 Livingstone Street Ivanhoe, 3079 | Speciality |
| Dr. Sunghamitra Guha | Royal Adelaide Hospital Dept. of Endocrinology North Terrace Adelaide, 5000 | Speciality |
| Dr. Patrick Phillips | TQEH Endocrinology and Diabetes Services 8 Woodville Road Woodville Woodville South, 5011 | Speciality |
| Dr. Anthony Roberts | 8A Hampton Road Keswick SA 5035 | Speciality |
| Dr. Kim Stanton | Dept. of Endocrinology and Diabetes Royal Perth Hospital Goderich St. Clinic 196 Goderich St. Perth WA 6000 | Speciality |
| Professor Timothy Davis | Fremantle Hospital School of Medicine Block T, Level 7, Education Centre 2 Alma Street Fremantle, 6160 | Speciality |
| Dr. Tim Jones | Princess Margaret Childrens Dept. of Diabetes Roberts Road Subiaco, 6008 | Speciality |
| Professor Timothy Welborn | Consulting Suites Suite 2 55 Hampden Road Nedlands, 6009 | Speciality |
| Dr. Andrew Lowy | Suite 6 / 50-52 Urunga Parade Miranda NSW 2228 | Speciality |
| Dr. Andrew Lowy | St. Vincent's Clinic Suite 505 438 Victoria Street Darlinghurst NSW 2010 | Speciality |
| Dr. Chris Michealides | Mater Hill Family Medical Centre 7-40 Annerley Road, Woolloongabba Qld. 4102 | Primary |
| Dr. Yong Mong Tan | 36 Fulham Road Pimlico Qld. 4812 | Speciality |
| Dr. Yong Mong Tan | Townsville Hospital Diabetes Clinic Dept. of Endocrinology and Diabetes | Speciality |
| Dr. Kunwar Jit Singh Sangla | Townsville Hospital 100 Angus Smith Drive Douglas Qld. 4814 | Speciality |
| Dr. Birrell | St. Andrews Hospital 280 North Street Toowoomba Qld. 4350 | Speciality |
| Dr. Ashim Sinha | Cairns Diabetes Centre 249 Lake Street Cairns Qld. 4870 | Speciality |
| Dr. Ashim Sinha | Primary Health Care Douglas Street Thursday Island Qld. 4875 | Primary |
| Dr. Estella | Consulting Rooms 11 Roderick St. Ipswich Qld. 4305 | Speciality |
| Dr. Jonathan Beilin | Royal Perth Hospital Level 3, Diabetes Clinic Wellington Street Perth, 6000 | Speciality |
| Dr. P. Bastian | Unit 1 / 66 Great Northern Highway Midland WA 6056 | Speciality |
| Dr. D. Hurley | Kirkman House 10 Murray Street Perth 6000 | Speciality |
| Dr. J. Kaye | Dept. of Endocrinology and Diabetes SCGH Nedlands, WA 6009 | Speciality |
| Dr. Perry-Keene | Level 4, Alexandra 201 Wickham Terrace Brisbane 4000 | Speciality |
| Dr. H. Eaton | Repatriation Hospital Dept. of Endocrinology Daws Park Road Daws Park SA 5041 | Speciality |
| Dr. Joanne Shaw | Prince Charles Hospital 4th Floor Private Practice Rode Road Chermside Qld. 4032 | Speciality |
| Dr. Julia Lowe | Hunter Diabetes Service 7th Floor, Nixon Wing Royal Newcastle Centre Pacific Street Newcastle 2300 | Speciality |
| Dr. Julia Lowe | Salamander Diabetes Centre Sundew Rooms Tomaree Library & Community Centre Town Centre Circuit Salamander Bay 2317 | Speciality |
| Dr. Sultan Linjawi | Professional Centre Suite 1 No 9 Park Ave. Coffs Harbour NSW 2450 | Speciality |
| Dr. Robert Coles | 70 Derby Street Kingswood NSW 2747 | Speciality |
| Dr. P. Harding | Diabetes Centre Burnside Hospital 2 Kensington Parade Rose Park SA 5067 | Speciality |
| Dr. H. Teede | Consulting Suites Monash Medical Centre 246 Clayton Road Clayton, VIC 3168 | Speciality |
| Dr. H. Teede | Pharmacy Dept. Dandenong Hospital David Street Dandenong VIC 3178 | Speciality |
| Dr. A. Zimmermann | Lyell McEwin Hospital Dept of Endocrinology Haydown Road Elizabethvale SA 5112 | Speciality |
| Dr. Vern Hazelwood | Caboolture Hospital Department of Medicine McKean St. Caboolture QLD 4510 | Speciality |
| Dr. M. D'Emden | Royal Brisbane Hospital Dept of Endocrinology Level 1 East Block Butterfield St. Herston Qld. 4006 | Speciality |
| Dr. S. Hamwood | Nambour General Hospital Sunshine Coast Diabetes Centre Waterfall Clinic 1 Waterfall Road Nambour Qld. 4560 | Speciality |
| Dr. Dan Harmelin | 38 Junction Street Nowra NSW 2541 | Speciality |
| Dr. Marteen Kamp | Gold Coast Hospital Diabetes Centre 108 Nerang St. Southport Qld. 4215 | Speciality |
| Dr. Steve Morris | Consulting Suite 11 Hastings Road Frankston, 3199 | Speciality |
| Dr. Gary Kilov | Clarinda General Clinic 67 Bourke Rd. Clarinda VIC 3169 | Primary |
| Dr. K. Matheson | Consulting Suite 11 Hastings Road Frankston, 3199 | Speciality |
| Dr. Stephen Leow | Munno Para Medical Centre Munno Para Shopping City Munno Para SA 5115 | Primary |
| Dr. Stephen Hinton | 8 Minga Crt. Bunbury WA 6230 | Primary |
| Dr. Maggie Mackay | 28 Bamford La. Kirwan Townsville 4817 | Primary |
| Dr. Kym Daniel | 28 Bamford La. Kirwan Townsville 4817 | Primary |
| Dr. C. Strakosch | Suite 16 Greenslopes Specialist Centre Newdegate St. Greenslopes QLD 4120 | Speciality |
| Dr. D. Howard | Darwin Pvte. Hospital Specialist Centre Rocklands Drv. Tiwi 0810 | Speciality |
| Dr. Louise Maple-Brown | Darwin Pvte. Hospital Specialist Centre Rocklands Drv. Tiwi 0810 | Speciality |
| Dr. Tim Greenaway | Royal Hobart Hospital Diabetes and Endocrinolgy Dept. 3rd Floor, B Block 48 Liverpool Street Hobart TAS 7000 | Speciality |
| A/P Shane Hamblin | Consulting Suites Suite 24 Level 2 141 Grey Street East Melbourne, 3002 | Speciality |
| Dr. Jack Fowler | Suite 12 31 Watt Street Newcastle NSW, 2300 | Speciality |
| Dr. D. O'Neal | 77-79 Morris Road Hoppers Crossing, Vic. 3029 03 9748 6022 | Speciality |
| Dr. D. O'Neal | Werribee Mercy Hospital 300-310 Princess Hwy. Werribee, Vic. 3030 03 9216 8839 | Speciality |
| Dr. D. O'Neal | St. Vincents Public Hospital Dept. of General Medicine 41 Victoria Parade Fitzroy, Vic. 3065 | Speciality |
| Dr. Roger Chen | 11 Kempsey Street Blacktown NSW 2148 | Speciality |
| Dr. Roger Chen | Concord Hospital Level 6 Medical Centre Concord NSW | Speciality |
| Dr. Andrew Kryszton | Springwood Family Medical Centre 1 De Chair Ave. Springwood 2777 NSW | Primary |
| Dr. Tony Morrow | 506/20 Bungan St. Mona Vale NSW 2103 | Speciality |
| Dr. Parkin | Endocrinologist Private Consulting Suites Suite 201, Level 2 100 Victoria Parade East Melbourne, Vic. 3002 | Speciality |
| Dr. T. Hajicosta | Kent Road Clinic 124 Kent Road Pascoe Vale Vic. 3044 | Primary |
| Dr. T. Donnelly | 68 Belford Street Broadmeadow NSW 2292 | Speciality |
| Dr. Tucker | 13 / 9 Scott St P.O. Box 607 Toowoomba 4350 | Speciality |
| Dr. A. Moodley | Edgar Street Medical Center 7 Edgar Street Port Hedland WA 6721 | Primary |
| Dr. L. Woollard | Balo Street Medical Centre Moree NSW 2400 | Primary |
| Dr. G. Moore | Wesley Medical Centre Sandford Jackson Building Lvl 5 30 Chasely St.Auchenflower Qld. 4066 | Primary |
| Dr. C. Perera | Suite 7/256 Anson Street Orange NSW 2800 | Speciality |
| Professor T. Welborn | Consulting Suites Suite 2 55 Hampden Road Nedlands, 6009 | Speciality |
References
- 1.Bohannon NJ. Insulin delivery using pen devices. Simple-to-use tools may help young and old alike. Postgrad Med. 1999;106(5):57–58. doi: 10.3810/pgm.1999.10.15.751. 61–4, 68. [DOI] [PubMed] [Google Scholar]
- 2.Graff MR, McClanahan MA. Assessment by patients with diabetes mellitus of two insulin pen delivery systems versus a vial and syringe. Clin Ther. 1998;20(3):486–496. doi: 10.1016/s0149-2918(98)80058-1. [DOI] [PubMed] [Google Scholar]
- 3.Klonoff DC. The pen is mightier than the needle (and syringe) Diabetes Technol Ther. 2001;3(4):631–633. doi: 10.1089/15209150152811261. [DOI] [PubMed] [Google Scholar]
- 4.Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Clarke A, Dain MP. Dose accuracy of a reusable insulin pen using a cartridge system with an integrated plunger mechanism. Expert Opin Drug Deliv. 2006;3(5):677–683. doi: 10.1517/17425247.3.5.677. [DOI] [PubMed] [Google Scholar]
- 6.Clarke A, Spollett G. Dose accuracy and injection force dynamics of a novel disposable insulin pen. Expert Opin Drug Deliv. 2007;4(2):165–174. doi: 10.1517/17425247.4.2.165. [DOI] [PubMed] [Google Scholar]
- 7.Robertson KE, Glazer NB, Campbell RK. The latest developments in insulin injection devices. Diabetes Educ. 2000;26(1):135–138. doi: 10.1177/014572170002600114. 141–6, 149–52. [DOI] [PubMed] [Google Scholar]
- 8.Haak T, Edelman S, Walter C, Lecointre B, Spollett G. Comparison of usability and patient preference for the new disposable insulin device Solostar versus Flexpen, lilly disposable pen, and a prototype pen: an open-label study. Clin Ther. 2007;29(4):650–660. doi: 10.1016/j.clinthera.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Peyrot M, Rubin RR, Lauritzen T, Skovlund SE, Snoek FJ, Matthews DR, Landgraf R, Kleinebreil L. International DAWN Advisory Panel. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 10.Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28(10):2543–2545. doi: 10.2337/diacare.28.10.2543. [DOI] [PubMed] [Google Scholar]
- 11.Golden SH, Selvin E, Cunningham KE. Glycaemic status and cardiovascular disease in type 2 diabetes mellitus: re-visiting glycated haemoglobin targets for cardiovascular disease prevention. Diabetes Obes Metab. 2007;9(6):792–798. doi: 10.1111/j.1463-1326.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 12.Carter J, Roberts A. Usability of a pre-filled insulin injection device in a 3-month observational survey of everyday clinical practice in Australia. Curr Med Res Opin. 2008;24(10):2741–2749. doi: 10.1185/03007990802367579. [DOI] [PubMed] [Google Scholar]
- 13.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 14.Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med. 2006;23(7):736–742. doi: 10.1111/j.1464-5491.2006.01881.x. [DOI] [PubMed] [Google Scholar]


