Abstract
Background
The primary objective of this review was to determine the strength of evidence for the effectiveness of self-monitoring devices and technologies for individuals with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) based on specific health-related outcome measures. Self-monitoring devices included those that assist patients with managing diabetes and preventing cardiovascular complications (CVCs). A secondary objective was to explore issues of feasibility, usability, and compliance among patients and providers.
Methods
Study criteria included individuals ≥14 years and youth (7–14 years) with T1DM or T2DM, intervention with a self-monitoring device, assessment of clinical outcomes with the device, literature in English, and ≥10 participants. Relevant published literature was searched from 1985 to 2008. Randomized controlled trials and observational studies were included. Data were extracted for clinical outcomes, feasibility and compliance methods, and results. Selected studies were independently evaluated with a validated instrument for assessing methodological quality.
Results
Eighteen trials were selected. Predominant types of device interventions included self-monitoring of blood glucose, pedometers, and cell phone or wireless technologies. Feasibility and compliance were measured in the majority of studies.
Conclusions
Self-monitoring of blood glucose continues to be an effective tool for the management of diabetes. Wireless technologies can improve diabetes self-care, and pedometers are effective lifestyle modification tools. The results of this review indicate a need for additional controlled trial research on existing and novel technologies for diabetes self-monitoring, on health outcomes associated with diabetes and CVCs, and device feasibility and compliance.
Keywords: diabetes mellitus, health technologies, monitoring, systematic review
Introduction
Diabetes mellitus is a serious chronic disease that imposes substantial social and economic burdens around the world. The prevalence of diabetes for all age groups worldwide is estimated to be 2.8% and is expected to nearly double by 2030 (4.4%).1 In the United States, the prevalence of diabetes is estimated to be 23.6 million people, roughly 7.8% of the population.2 According to projected prevalence estimates, the number of people with diabetes in Canada will increase from approximately 1.4 million to 2.4 million, and estimated diabetes-related health care costs in Canada are projected to increase by 75% (for the years 2000–2016).3 It is likely, however, that the true prevalence of diabetes is dramatically underestimated.4 Diabetes is associated with a number of health-related complications, and the level of hyperglycemia and the duration of the disease are associated with an increased risk of developing macrovascular and microvascular complications such as neuropathy, nephropathy, retinopathy, myocardial infarction, and stroke.5,6 One way to prevent the progression of these complications is through improved blood glucose (hemoglobin A1c [HbA1c]) control, as was shown in the UK Prospective Diabetes Study.7,8 and the Diabetes Control and Complications Trial.9 The results from these trials also highlight the positive effects that increased contact with clinicians can have on improving patient glycemic control. However, health care provision deficits, physician shortages, and the inability of many patients to increase their clinic visits have prompted the clinical research community and individuals themselves to search for feasible solutions. This reality supports the need for effective self-management of the disease through self-monitoring of blood glucose (SMBG) and blood pressure, as well as increasing levels of exercise. Exercise programs have been shown to reduce blood pressure, improve glycemic control, and improve overall cardiovascular health.10,11 Current self-management interventions for patients with type 2 diabetes mellitus (T2DM) who are at risk for cardiovascular complications (CVCs) include the use of devices that monitor blood glucose, blood pressure, heart rate, and physical activity. Although recent surveys indicate that patients are willing to become more actively involved in managing their own care,.12,13 it is unclear how much patients know about these self-monitoring techniques or how accessible and feasible they are to implement into daily life. The main objective of this systematic review, therefore, was to determine the strength of evidence for the effectiveness of any type of established or emerging self-monitoring device for improving key health outcomes (HbA1c, blood pressure, low-density lipoproteins [LDLs]) in adults and youth with diabetes (type 1 or type 2) who are at risk of developing CVCs. Secondary objectives were to critically examine the factors that may affect patient and provider adherence to these established or emerging self-monitoring devices and to assess the feasibility or usability of these technologies.
Methods
Study Inclusion/Exclusion Criteria
The population of interest included individuals ≥14 years and youth 7–14 years with type 1 diabetes mellitus (T1DM) or T2DM. Studies were included if there were measures of clinical outcomes, such as HbA1c, blood pressure, body mass index (BMI), LDL, or other related outcomes important for understanding the progression of CVCs. All types of self-monitoring devices and technologies were included in our search: SMBG devices, blood pressure devices, heart rate monitors, pedometers or accelerometers, wireless data technologies such as mobile messaging (e.g., short message service [SMS] and pagers), devices that use Web-enabled technologies, and global information systems. Research literature was also considered where there was an inclusion of measured outcomes related to the usability and feasibility of these technologies, both from the patient's or the clinician's perspectives. Literature in English was primarily considered for this review. All types of experimental study designs were included (randomized controlled trials [RCTs] and nonrandomized, observational studies) for possible evaluation. We excluded studies with <10 participants, cross-sectional data, primary interventions with medications, studies assessing accuracy of devices, telemedicine applications, continuous glucose monitoring devices, or where the self-monitoring device was not part of the main intervention being assessed. Studies scoring lower than 20 points on the Downs and Black instrument for assessing study quality14 were excluded due to an inability to form strong conclusions with low-scoring studies (fair to poor quality levels). Downs and Black is a validated instrument for rating the methodological quality of both RCTs and non-RCTs15 and is sensitive to key qualities of research design (see Table 1).
Table 1.
Summary of Downs and Black Instrument
| A 27-item checklist was used to assess the methodological quality of both randomized and nonrandomized studies of health care interventions. Answers are scored 0 or 1, except for one item in the reporting subscale, which is scored 0 to 2. The power item responses were collapsed from the original 0 to 5, to either 0 or 1. The total maximum score is 28. |
| Reporting (10 items) |
| Assesses whether the information provided in the paper was sufficient to allow the reader to make an unbiased assessment of the findings of the study. |
| External Validity (3 items) |
| Addresses the degree to which the findings from the study can be generalized to the population from which the participants were derived. |
| Internal Validity-Bias (7 items) |
| Addresses biases in the measurement of the intervention and the outcome. |
| Internal Validity-Confounding (6 items) |
| Addresses bias in the selection of study participants. |
| Power (1 item) |
| Addresses whether the negative findings from a study could be due to chance. |
Search Strategy
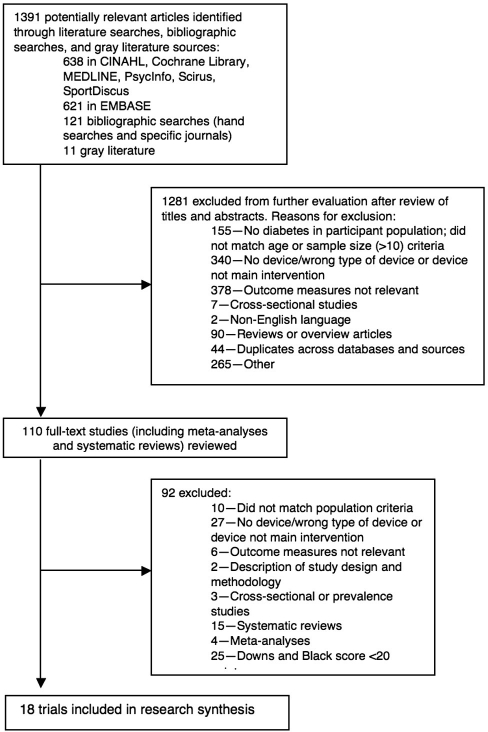
Topic-related online databases were searched from 1985 to May 2008 using a detailed search strategy: CINAHL, Cochrane Library, EMBASE, MEDLINE, PsycInfo, SportDiscus, and Scirus. Relevant journals, health-related evidence-based practice centre Web sites, clinical guidelines, and unpublished literature sources were also searched. Two research assistants, Elizabeth Russell-Minda and Kaitlin Bradley, conducted the literature searches and organized the material in reference management software. Abstracts were reviewed by authors Elizabeth Russell-Minda, Jeffrey Jutai, Kaitlin Bradley, and Robert Petrella. See Figure 1 for study flow and selection.
Figure 1.
Study flow diagram.
Study Selection, Review, and Assessment
Three reviewers (Elizabeth Russell-Minda, Kaitlin Bradley, and Anna Chudyk) evaluated the selected studies using the Downs and Black instrument.14 The highest possible score is 28 for RCTs and 25 for non-RCTs. Studies were assigned the following levels where applicable: RCT (I), cohort (II), case control (III), case series (IV), and expert opinion (V).16 Downs and Black score ranges were given corresponding quality levels: excellent (26–28), good (20–25), fair (15–19), and poor (≤14). Only RCTs could be assigned a quality level of excellent. These quality levels were then mapped to strength of evidence levels and used to formulate results. In order to assess inter-rater reliability using Downs and Black, Pearson R correlations were conducted among three reviewers (Elizabeth Russell-Minda, Anna Chudyk, and Kaitlin Bradley). The results showed adequate inter-rater reliability among reviewers (range 0.71–0.90, statistically significant at the alpha level of p < .05). After a study was scored with the Downs and Black checklist, it was assigned an evidence level. This grading system is based on a hierarchical scale developed by the Centre for Evidence-Based Medicine (Oxford, UK),17 where evidence levels provide grades of recommendation and assist with formulating evidence-based conclusions. In order to determine conclusions for the research evidence, Downs and Black score ranges were given corresponding quality levels based on a methodology used in other projects affiliated with the research team.18,19 In conjunction with determining accepted scoring ranges, strength of evidence levels were then used to formulate conclusions. The following strength of evidence levels were based in part on methods used in other health care systematic reviews:.20,21 level 1a (very strong), the findings were supported by the results of two or more studies of at least excellent quality; level 1b (strong), the findings were supported by at least one study of excellent quality; level 2a (moderate), the findings were supported by two or more studies of at least good quality; level 2b (limited), the findings were supported by at least one study of good quality; level 2c (weak), the findings were supported by at least one study of fair or poor quality; level 3 (consensus), in the absence of evidence, agreement by a group of experts on the appropriate treatment course—consensus opinion is regarded as the lowest form of evidence; and level 4 (conflicting), disagreement between the findings of at least two RCTs. Where there are more than four RCTs and the results of only one is conflicting, the conclusion is based on the results of the majority of the studies, unless the study with conflicting results was of higher quality.
Results
Eighteen trials were selected and grouped according to the following major device categories: (1) SMBG, (2) pedometers, and (3) cell phone/wireless devices. Studies were from the following countries: United States (seven), France (three), South Korea (four), UK/Scotland (two), Canada (one), and Norway (one). Four meta-analyses, one Cochrane review, five systematic reviews, one health technology assessment, and one criteria-based review were reviewed but not evaluated with Downs and Black. The details of the selected studies and health outcomes results are listed in corresponding tables. Where studies included measures of usability, feasibility, compliance, and patient satisfaction, these are noted in the tables. Comprehensive evidence tables may be requested from the authors.
Self-Monitoring of Blood Glucose Devices
Five trials using SMBG devices were selected for this systematic review (Table 2). The use of SMBG devices has been suggested as an effective way of maintaining healthy blood glucose levels in patients with T2DM. According to previous meta-analyses and systematic reviews on SMBG, there is some disagreement regarding the level of effectiveness of SMBG for patients with both insulin- and noninsulin-dependent forms of the disease.6,22,23 In addition, there is a lack of consensus as to the recommended frequency of SMBG testing. Welschen and colleagues,24 Coster and associates,23 and Faas and coworkers22 concluded that results showing positive effects of SMBG on glycemic control for patients with T2DM were inconclusive due to study heterogeneity and poor study designs.
Table 2.
Health-Related Outcomes for Self-Monitoring of Blood Glucose Devices
| Study detailsa | Intervention | Outcome measures | Health outcomes resultsb | Downs and Black scorec |
|---|---|---|---|---|
| Farmer et al.25 England I RCT | N = 453; SMBG (alone or with instruction) versus usual care (noninsulin-dependent T2DM) |
|
|
26 |
| Davidson et al.26 USA I RCT | N = 89; effectiveness of SMBG improving HbA1c responses in noninsulin-dependent T2DM patients |
|
|
24 |
| Fontbonne et al.27 France I RCT | N = 208; SMBG versus usual care in noninsulin-dependent T2DM; duration of trial, 6 months |
|
|
24 |
| Guerci et al.28 France I RCT | N = 689; SMBG (in addition to conventional laboratory work-up) with education on weight loss and physical activity versus conventional laboratory work-up based solely on lab measurement of HbA1c every 12 weeks; education on weight loss and physical activity |
|
|
21 |
| Allen et al.29 USA I RCT | N = 54; compared the effectiveness of SMBG to routine urine testing as part of standardized treatment program for patients with noninsulin-treated T2DM |
|
|
21 |
Study details are listed according to level of evidence and in order of quality assessment score (Downs and Black). Study levels: I = RCT; II = cohort; III = case control; IV = case series.
↔ indicates no difference in health outcomes; + indicates improvements in health outcomes; and - indicates decline in health outcomes.
Downs and Black score ranges were given corresponding quality levels: excellent (26–28) and good (20–25). Studies that scored either in the fair (15–19) or poor (≤14) ranges were excluded, except where it was the only available evidence.
Level 1b (strong) evidence based on results of one excellent (based on Downs and Black score) RCT25 indicates that, when compared with usual care, the effectiveness of a less versus more intensive SMBG intervention for improving glycemic control is inconclusive. This trial included a sample size of 453 patients with noninsulin-treated T2DM and a primary outcome measure of HbA1c measured at 12 months. The “diabetes glycemic education and monitoring” intervention was conducted as a 4-year, open, randomized, three-arm, parallel group study. The primary goal was to determine if HbA1c levels at 12 months were different between patients with T2DM receiving one of the three allocations: (1) standardized usual care with measurements of HbA1c levels every 3 months (control group); (2) use of a blood glucose meter, with advice for patients to contact their provider to interpret the results (less intensive monitoring); and (3) use of a blood glucose meter, with training in self-interpretation and application of results to physical activity, diet, and drug adherence (more intensive monitoring). At 12 months, no difference was found in HbA1c levels between the groups (after adjusting baseline HbA1c levels) (p = .12). The mean difference in change in HbA1c levels from baseline to 12 months between the controls and the less intensive intervention group was -0.14% (95% confidence interval -0.35% to 0.07%) and between the control group and more intensive intervention group was -0.17% (-0.37% to 0.03%). For secondary outcome measures, there was a significant difference found in the change in total cholesterol levels between the groups (p = .010). Although Farmer and colleagues25 scored relatively high on the Downs and Black instrument, there are several limitations of this study: the inclusion of patients with good metabolic control may have skewed their treatment plans, the intensified treatment was not detailed, and there was an overall inadequacy of adherence to self-monitoring.
Level 2a (moderately strong) evidence based on the combined results of four good quality RCTs26–29 suggests that SMBG may be effective in improving glycemic control in patients with noninsulin-treated T2DM, but due to the heterogeneity of interventions and outcomes across the selected studies, it is difficult to compare study results adequately. Fontbonne and associates27 and Davidson and coworkers26 showed no statistically significant differences in the decrease of HbA1c between SMBG and control groups. In the Davidson et al.26 trial, HbA1c decreased 0.8% in the SMBG group and 0.6% in the control group. For Fontbonne and associates,27 HbA1c improved 0.4% in the SMBG group, compared to 0.1% and 0.5% in the self-monitoring of urine glucose and control groups. Guerci and colleagues28 reported a statistically significant difference of 0.4% in HbA1c levels at the close of the study between SMBG and control groups. The results from Allen and associates29 found that both groups (SMBG and self-monitoring of urine glucose) had lower HbA1c at the end of the study. No difference was detected between groups (both had a decrease of 2.0%). In summary, there is moderately strong evidence that SMBG is an effective tool for maintaining stable metabolic control for both T1DM and T2DM, thus reducing the risk of developing additional microvascular and macrovascular complications. Self-monitoring of blood glucose remains an integral part of diabetes self-care. There may still be debate about the frequency of testing as well as the accuracy of the devices themselves.
Pedometers
Regular physical activity and exercise can have positive effects on glycemic control, weight management, and insulin resistance in patients with T2DM.30–33 Recommendations for exercise in adults from the Centers for Disease Control and the American College of Sports Medicine include at least 30 min of moderate-intensity exercise during the week.34 This can also be achieved by walking at least 10,000 steps per day, also in agreement with clinical recommendations.35,36 A total of four trials with pedometer-based interventions were selected for this review (see Table 3). Two trials included some component or measurement of compliance with the pedometer-based intervention.37,38 None of the selected studies on pedometer-based interventions assessed issues of feasibility or usability. One trial using an accelerometer-based exercise intervention was reviewed39 but did not fit the inclusion criteria.
Table 3.
Health-Related Outcomes in Studies Using Pedometers
| Study detailsa | Intervention | Outcome measures | Health outcomes resultsb | Downs and Black scorec |
|---|---|---|---|---|
| Bjørgaas et al.40 Norway I RCT | N = 70; subjects with T2DM randomized pedometer or no-pedometer group to determine if pedometer increases walking or increases beneficial health-related effects; 6-month intervention |
|
|
22 |
| Tudor-Locke et al.37 Canada I RCT | N = 47; effectiveness of 16-week physical activity intervention (24-week follow-up): the First Step Program for adults with T2DM; examined if increased physical activity was related to improvements in cardiovascular health, glycemic control, and lipid profiles |
|
|
22 |
| Richardson et al.38 USA I RCT (pilot) | N = 30 (35 randomized); T2DM; 6-week study comparing two goal-setting strategies: (1) lifestyle goals targeting total daily accumulated steps and (2) structured goals targeting bout steps defined as walking that lasts for 10 min or longer at a pace of 60 steps/min, to determine which strategy was most effective in increasing bout steps |
|
|
21 |
| Araiza et al.41 USA I RCT | N = 30; effectiveness of accumulation of daily steps (10,000/day) for improving metabolic outcomes in patients with T2DM; 6-week intervention; the active group (N = 15) instructed to walk at least 10,000 steps/day, 5 or more days/week for 6 weeks |
|
|
19 |
Study details are listed according to level of evidence and in order of quality assessment score (Downs and Black). Study levels: I = RCT; II = cohort; III = case control; IV = case series.
↔ indicates no difference in health outcomes; + indicates improvements in health outcomes; and – indicates decline in health outcomes.
Downs and Black score ranges were given corresponding quality levels: excellent (26–28) and good (20–25). Studies that scored either in the fair (15–19) or poor (≤14) ranges were excluded, except where it was the only available evidence.
MDA, malondialdehyde; REE, resting energy expenditure
Level 2b (limited) evidence based on the results of one good quality RCT40 indicates that pedometers do not increase walking (number of steps) or improve metabolic outcomes, based on results from a 6-month intervention. Level 2b (limited) evidence based on the results of one good quality RCT37 suggests that the First Step Programincreases daily physical activity (>3000 steps/day, p < .0001) and improves long-term health outcomes (no statistically significant difference between groups on cardiovascular fitness, glycemia, or lipid status). Relapses in activity indicate that reminder sessions are important for continued success. This is based on results from a16-week intervention with a 24-week follow-up. Level 2b (limited) evidence from one good quality RCT38 suggests that using a pedometer may increase the number of daily accumulated steps, whether the person is involved in a structured or unstructured goal-setting program. A structured lifestyle-goal program may improve participant satisfaction with pedometer-based programs, based on the results from a 6-week intervention. There were no clinical outcomes measured in this study. Level 2c (weak) evidence from one fair quality RCT41 indicates that use of a pedometer can increase step counts in patients with T2DM, based on the results of a 6-week intervention instructing participants to walk 10,000 steps/day on five or more days of the week. There is an absence of evidence on the effectiveness of using accelerometers for improving diabetes self-management and improving clinical outcomes.
Cell Phones and Wireless Devices
Existing and emerging technologies such as wireless devices (cell phones) with email and text messaging (SMS) functionality, pagers, and the Internet can help facilitate patient self-management of diabetes. These types of devices are practical and cost-effective methods for monitoring clinical outcomes and increasing patient adherence to treatments.42,43 Wireless technologies can be used as intermediary tools to facilitate the information between patient and provider and treatment advice between clinic visits. Results from studies incorporating the use of remote patient monitoring devices (cell phones and other wireless tools) have indicated significant decreases in HbA1c levels and improved health-related outcomes in diabetes.43,44 The use of these devices may encourage patients to adhere to their monitoring regimens by acting as reminders to self-manage their disease. Nine trials using cell phones with SMS interventions for diabetes management were selected for this review (see Table 4) and are discussed below.
Table 4.
Health-Related Outcomes in Studies Using Cell Phones and Wireless Devices
| Study detailsa | Intervention | Outcome measures | Health outcomes resultsb | Downs and Black scorec |
|---|---|---|---|---|
| Franklin et al.49 Scotland I RCT | N = 92; assessment of SweetTalk, a text-messaging support system to improve self-efficacy and adherence to intensive insulin therapy for youth (10–15 years) with T1DM |
|
|
25 |
| Benhamou et al.50 France I RCT | N = 30; randomized crossover trial of telecare for adults with T1DM under continuous subcutaneous insulin infusion, cell phone for transmission of retrospective data, and SMS for immediate feedback |
|
|
23 |
| Leu et al.51 USA I RCT | N = 42; T1DM and T2DM; effectiveness of a two-way pager for management of diabetes through medication, blood glucose testing reminders, and exercise reinforcement |
|
|
23 |
| Kim and Jeong45 South Korea I RCT | N = 51; diabetes management via nurse SMS by cellular phone and Internet for T2DM patients |
|
|
22 |
| Kim and Kim46 South Korea I RCT | N = 34; T2DM; decrease body weight and improve fasting plasma glucose levels through researcher recommendations via cell phone/SMS and Internet (Web site) |
|
|
22 |
| Yoon and Kim47 South Korea I RCT | N = 51; educational intervention using cellular phone with SMS and Internet for glycemic control (HbA1c < 7%) in patients with T2DM |
|
|
22 |
| Kim48 South Korea I RCT | N = 51; T2DM; weekly blood glucose based optimal recommendations via SMS |
|
|
21 |
| Kumar et al.52USA I RCT | N = 40; wireless, portable diabetes management system for youth with T1DM and T2DM; feasibility of system assessed in addition to clinical outcomes |
|
|
21 |
| Quinn et al.53 USA I RCT (pilot) | N = 30; T2DM; impact on HbA1c levels via cell-phone-based diabetes management software system (Web-based data analytics and therapy optimization tools); examine health care provider adherence to prescribing guidelines and assessed health care provider's adoption of the technology |
|
|
21 |
Study details are listed according to level of evidence and in order of quality assessment score (Downs and Black). Study levels: I = RCT; II = cohort; III = case control; IV = case series.
↔ indicates no difference in health outcomes; + indicates improvements in health outcomes; and - indicates decline in health outcomes.
Downs and Black score ranges were given corresponding quality levels: excellent (26–28) and good (20–25). Studies that scored either in the fair (15–19) or poor (≤14) ranges were excluded, except where it was the only available evidence.
HPG - 2-hour plasma glucose, HPMG - 2-hour post-meal glucose, HPPT - 2-hour post-prandial test
Level 2a (moderately strong) evidence from four good quality RCTs45–49 suggests that the use of a cell phone with SMS and Internet (some with nurse-directed educational component) may help to lower HbA1c levels and improve 2 h postmeal glucose values in patients with T2DM. With this intervention, patients initially set up their data on a Web site including blood glucose values, drug information, kinds and dosages of insulin, and other information important for diabetes control. After reviewing the patient information on the Web site, the diabetes educator or researcher sent recommendations and reminders via SMS to the patient's cell phone on a weekly basis (e.g., “please decrease the long-acting insulin by two units” or “lack of exercise may be the cause of aggravated glucose level”). Among the studies, the intervention periods ranged from 3 to 12 months; however, there were no measures of adherence, quality of life, patient satisfaction, or feasibility of the technology. Level 2b (limited) evidence from one good quality RCT49 suggests a text messaging support system (SweetTalk) may improve self-efficacy and adherence but does not improve glycemic control in children and adolescents (8–18 years) with T1DM. Level 2b (limited) evidence from one good quality RCT50 suggests that long-term telemedicine-based follow-up of insulin-pump-treated patients using a cell phone, SMS, and Web-based platforms is safe and feasible, and may improve metabolic control. There was a nonsignificant reduction in HbA1c (-0.25 ± 0.94%, p < .10) and mean glucose values (-9.2 ± 25 mg/dl, p = .06) for the 6-month SMS period. Level 2b (limited) evidence from one good quality RCT51 indicates that a self-managed wireless two-way pager able to send and receive text message reminders may improve metabolic control (average HbA1c decrease of 0.1%–0.3%). The primary outcome of this study was HbA1c, and secondary outcomes were blood pressure, patient perceptions of their disease and health care team, and if the pager system would improve their sense of well-being and adherence to the treatment plan. Level 2b (limited) evidence from one good quality RCT52 suggests that the use of a wireless personal digital assistant (PDA) with diabetes management software and an integrated motivational game (DiaBetNet) may assist youth 8–18 years with diabetes (type 1 and type 2) in managing their blood glucose levels. The use of the motivational game may also increase the frequency of monitoring and improve diabetes knowledge. Level 2b (limited) evidence based on the results of one good quality RCT53 suggests that a cell-phone-based diabetes management system, in conjunction with Web-based analytics and therapy optimization tools (WellDoc system), may significantly improve HbA1c in patients with T2DM. The average decrease in HbA1c for the intervention group was 2.03%, compared to 0.68% (p< .02) for control patients. Both provider and patient satisfaction with this system was found to be clinically and statistically significant. Patients in the intervention group used a Bluetooth-enabled OneTouch Ultra blood glucose meter and a cell phone with WellDoc diabetes management software. Patients were given a satisfaction survey at the end of the study and asked to give feedback on the usability of the system. For all major survey questions, 91% of the WellDoc users reported being satisfied with the system. Patients using the WellDoc system reported having better control over their diabetes based on their knowledge of food choices (91% versus 50%), confidence (100% versus 75%), and physician receiving regular blood sugars (100% versus 36%).
Discussion
According to the Canadian Diabetes Association Clinical Practice Guidelines for 2008,4 the targets for glycemic control for both T1DM and T2DM include maintaining HbA1c levels of ≤7.0%, a fasting plasma glucose or preprandial plasma glucose level of 4.0–7.0 mmol/liter, and a 2 h postprandial plasma glucose level of 5.0– 10.0 mmol/liter (5.0–8.0 if HbA1c targets are not being met). These levels are recommended in order to reduce the potential for developing microvascular and macro-vascular complications. The Canadian Diabetes Association Clinical Practice Guidelines also state that glycated hemoglobin is a valuable indicator of treatment effectiveness and should be measured every 3 months when glycemic targets are not being met and when diabetes therapy is being adjusted.4 Current Canadian Diabetes Association Clinical Practice Guidelines for diabetes and physical activity suggest that moderate to high levels of physical activity and cardiorespiratory fitness are associated with substantial reductions in morbidity and mortality in both men and women and in both T1DM and T2DM. Since many people with diabetes will develop hypertension, which can lead to CVCs, the recommended blood pressure targets are <130/80 mm Hg. The results from major trials indicate that both blood glucose as well as blood pressure should be adequately controlled to prevent further complications for individuals with diabetes.8,54,55 The results of this systematic review found an absence of trials devoted to the assessment of home blood pressure monitoring as a way of managing or developing the complications found in diabetes.
Based on the results of the studies discussed in this review, there is moderately strong evidence that SMBG is an effective way to improve glycemic control in patients with noninsulin-treated T2DM. The recommended frequency of SMBG testing for effective blood glucose control has yet to be determined. Furthermore, SMBG alone has not yet been proven to directly affect the ability to maintain stable metabolic levels—it is evident that behavior and lifestyle changes must accompany the self-monitoring activity to adequately self-manage this disease.56 The findings from this review compare positively with current clinical practice guidelines in terms of recommendations for SMBG, achieving targeted glycemic ranges, and increasing levels of physical activity. In addition to SMBG, regular monitoring of blood pressure and making lifestyle modifications such as increased exercise offer effective ways for patients to manage diabetes.
Although our research synthesis found limited levels of evidence supporting the effectiveness of pedometer-based interventions for improving overall fitness levels and metabolic control (based on our quality assessment methods), pedometers can still be an effective method of motivation for patients with diabetes to make these necessary lifestyle changes and increase their daily steps. Our review also uncovered an absence of trials involving the integration of diabetes self-management devices with blood pressure devices, heart rate monitors, and other emerging technologies. These are certainly areas where additional research is needed. Remote patient monitoring through the use of cell phones, smart phones, and other wireless technologies (Internet-based applications) are proving to be accessible, affordable methods for self-managing diabetes and adhering to exercise and diet regimens. These tools can be used in a home setting or while traveling at a minimal cost to the patient and the provider. Simple reminder schedules for self-monitoring can be established, and health care providers can oversee the progress via patient monitoring databases. Additional trials should be conducted that assess the effectiveness of remote patient monitoring on key health outcomes and lifestyle change (increased exercise or improved diet) for patients with diabetes.
Interventions and outcome measures related to feasibility, adherence, and satisfaction with diabetes self-management devices are frequently evaluated in the research; however, it is challenging to form solid conclusions as to the degree of feasibility or compliance with a particular device, as many of the trials used qualitative surveys or relied solely on the number of times a device was uploaded to a server to determine compliance with the device or intervention. Aside from calculating percentages of self-monitoring device or database usage, evaluating patient or provider compliance with statistically rigorous methods can pose challenges (difficult to assess usability or compliance in the patient's home, for example). In many cases, patients and their providers are generally satisfied with self-monitoring technologies, and adherence to self-management interventions is improved after using the device, perhaps due to increased motivation after learning how to use the technologies and receiving regular feedback. The impact of usability on device adherence is especially important in certain populations, such as younger patients with T1DM or T2DM who may need additional encouragement and support to use their devices and regulate their metabolic functions.
Systematic reviews aim to inform evidence-based practice and minimize bias by grouping and analyzing key research studies within an organized and rigorous framework. An important result of conducting systematic reviews is to close or locate gaps within healthcare research and highlight the need for additional studies to be conducted in the areas where they would be most beneficial. One limitation of this systematic review may be that our inclusion criteria for the population, the technologies, and the health-related outcomes were too broad. A consideration for a future review on diabetes monitoring technologies might be to conduct meta-analyses of the data on key health outcomes such as reduction in HbA1c, blood pressure, and cholesterol levels; lifestyle change (improved or increased exercise); compliance; and usability—and to assess these outcomes using a single type of self-monitoring technology. This review found an absence of trials incorporating outcome measures for assessing the usability and feasibility of self-monitoring devices for diabetes, which indicates a need for these types of measures to be included in future studies. If the methods for measuring device feasibility and usability outcomes were further developed, this could produce valuable findings for both patients and providers. Evaluations of adherence, compliance, and persistence with self-monitoring devices should also continue to be measured in future trials. Current clinical guidelines and health-related economic policies or programs could be greatly enhanced with the inclusion of strong evidence-based findings for device usability, feasibility, and costs. If a self-monitoring device is useful, and if patients are motivated to use the technology, then the degree of impediment to managing diabetes with the technology may potentially be lessened. Health care providers may in turn discover that they have more reliable clinical information and improved communication with their patients through the usability feedback they might obtain as a result of patient self-monitoring technologies.
Abbreviations
- CVC
cardiovascular complication
- BMI
body mass index
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- PDA
personal digital assistant
- RCT
randomized controlled trial
- SMBG
self-monitoring of blood glucose
- SMS
short message service
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Bethesda MD National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. U.S. Department of Health and Human Services, National Institutes of Health. 2008. See: http://diabetes.niddk.nih.gov/DM/PUBS/statistics/. Accessed October 28, 2009.
- 3.Ohinmaa A, Jacobs P, Simpson S, Johnson JA. The projection of prevalence and cost of diabetes in Canada: 2000 to 2016. Can J Diabetes. 2004;28(2):1–5. [Google Scholar]
- 4.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(suppl 1):S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S33–S50. doi: 10.2337/diacare.26.2007.s33. [DOI] [PubMed] [Google Scholar]
- 6.Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R. Monitoring blood glucose control in diabetes mellitus: a systematic review. Health Technol Assess. 2000;4(12):1–93. i–iv. [PubMed] [Google Scholar]
- 7.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 9.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 10.Wing RR. Weight loss in the management of type 2 diabetes. In: Gerstein HC, Haynes RB, editors. Evidence-based diabetes care. BC Decker: Hamilton; 2001. pp. 252–276. [Google Scholar]
- 11.Kavookjian J, Elswick BM, Whetsel T. Interventions for being active among individuals with diabetes: a systematic review of the literature. Diabetes Educ. 2007;33(6):962–988. doi: 10.1177/0145721707308411. [DOI] [PubMed] [Google Scholar]
- 12.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program in patients with chronic disease. Eff Clin Pract. 2001;4(6):256–262. [PubMed] [Google Scholar]
- 13.Clark NM. Management of chronic disease by patients. Annu Rev Public Health. 2003;24:289–313. doi: 10.1146/annurev.publhealth.24.100901.141021. [DOI] [PubMed] [Google Scholar]
- 14.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Comm Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. http://www.cochrane.org/resources/handbook/. Accessed September 11, 2009.
- 16.Canadian Task Force on the Periodic Health Examination. The periodic health examination. Can Med Assoc J. 1979;121(9):1193–1254. [PMC free article] [PubMed] [Google Scholar]
- 17.Centre for Evidence Based Medicine. http://www.cebm.net/?o=1023.
- 18.Jutai J, Strong JG, Russell-Minda E. Effectiveness of assistive technologies for low vision rehabilitation: A systematic review. Journal of Visual Impairment and Blindness. 2009;103(4):210–222. [Google Scholar]
- 19.Teasell R, Foley N, Salter K, Bhogal SK, Jutai J, Speechley MR. London, Ontario Canada: Evidence-based review of stroke rehabilitation. See: http://www.ebrsr.com/. Accessed October 28, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10(1):1–7. [PubMed] [Google Scholar]
- 21.Gresham GE, Duncan PW, Statson WB. Poststroke Rehabilitation Guideline Panel. Clinical practice guideline no. 16. Rockville, MD. U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; 1995. AHCPR Publication No. 95–0662. [PubMed]
- 22.Faas A, Schellevis FG, Van Eijk JT. The efficacy of self-monitoring of blood glucose in NIDDM subjects. A criteria-based literature review. Diabetes Care. 1997;20(9):1482–1486. doi: 10.2337/diacare.20.9.1482. [DOI] [PubMed] [Google Scholar]
- 23.Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R. Self-monitoring in type 2 diabetes mellitus: a meta-analysis. Diabet Med. 2000;17(11):755–761. doi: 10.1046/j.1464-5491.2000.00390.x. [DOI] [PubMed] [Google Scholar]
- 24.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD005060.pub2. CD005060. [DOI] [PubMed] [Google Scholar]
- 25.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insula blinded, randomized trial. Am J Med. 2005;118(4):422–425. doi: 10.1016/j.amjmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Fontbonne A, Billault B, Acosta M, Percheron C, Varenne P, Besse A, Eschwege E, Monnier L, Slama G, Passa P. Is glucose self-monitoring beneficial in non-insulin-treated diabetic patients? Results of a randomized comparative trial. Diabetes Metab. 1989;15(5):255–260. [PubMed] [Google Scholar]
- 28.Guerci B, Drouin P, Grangé V, Bougnères P, Fontaine P, Kerlan V, Passa P, Thivolet Ch, Vialettes B, Charbonnel B. ASIA Group Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab. 2003;29(6):587–594. doi: 10.1016/s1262-3636(07)70073-3. [DOI] [PubMed] [Google Scholar]
- 29.Allen BT, DeLong ER, Feussner JR. Impact of glucose self-monitoring on non-insulin-treated patients with type II diabetes mellitus. Randomized controlled trial comparing blood and urine testing. Diabetes Care. 1990;13(10):1044–1050. doi: 10.2337/diacare.13.10.1044. [DOI] [PubMed] [Google Scholar]
- 30.Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 31.Schneider SH, Amorosa LF, Khachadurian AK, Ruderman NB. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1984;26(5):355–360. doi: 10.1007/BF00266036. [DOI] [PubMed] [Google Scholar]
- 32.Yamanouchi K, Shinozaki T, Chikada K, Nishikawa T, Ito K, Shimizu S, Ozawa N, Suzuki Y, Maeno H, Kato K. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1995;18(6):775–778. doi: 10.2337/diacare.18.6.775. [DOI] [PubMed] [Google Scholar]
- 33.Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 34.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 35.Tudor-Locke C, Bassett DR Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 36.Le Masurier GC, Tudor-Locke C. Comparison of pedometer and accelerometer accuracy under controlled conditions. Med Sci Sports Exerc. 2003;35(5):867–871. doi: 10.1249/01.MSS.0000064996.63632.10. [DOI] [PubMed] [Google Scholar]
- 37.Tudor-Locke C, Bell RC, Myers AM, Harris SB, Ecclestone NA, Lauzon N, Rodger NW. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes Relat Metab Disord. 2004;28(1):113–119. doi: 10.1038/sj.ijo.0802485. [DOI] [PubMed] [Google Scholar]
- 38.Richardson CR, Mehari KS, McIntyre LG, Janney AW, Fortlage LA, Sen A, Strecher VJ, Piette JD. A randomized trial comparing structured and lifestyle goals in an internet-mediated walking program for people with type 2 diabetes. Int J Behav Nutr Phys Act. 2007;4:59. doi: 10.1186/1479-5868-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paschali AA, Goodrick GK, Kalantzi-Azizi A, Papadatou D, Balasubramanyam A. Accelerometer feedback to promote physical activity in adults with type 2 diabetes: a pilot study. Percept Mot Skills. 2005;100(1):61–68. doi: 10.2466/pms.100.1.61-68. [DOI] [PubMed] [Google Scholar]
- 40.Bjørgaas MR, Vik JT, Stølen T, Lydersen S, Grill V. Regular use of pedometer does not enhance beneficial outcomes in a physical activity intervention study in type 2 diabetes mellitus. Metabolism. 2008;57(5):605–611. doi: 10.1016/j.metabol.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Araiza P, Hewes H, Gashetewa C, Vella CA, Burge MR. Efficacy of a pedometer-based physical activity program on parameters of diabetes control in type 2 diabetes mellitus. Metabolism. 2006;55(10):1382–1387. doi: 10.1016/j.metabol.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Albright CL, Pruitt L, Castro C, Gonzalez A, Woo S, King AC. Modifying physical activity in a multiethnic sample of low-income women: one-year results from the IMPACT (Increasing Motivation for Physical ACTivity) project. Ann Behav Med. 2005;30(3):191–200. doi: 10.1207/s15324796abm3003_3. [DOI] [PubMed] [Google Scholar]
- 43.Krishna S, Boren SA. Diabetes self-management care via cell phone: a systematic review. J Diabetes Sci Technol. 2008;2(3):509–517. doi: 10.1177/193229680800200324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faridi Z, Liberti L, Shuval K, Northrup V, Ali A, Katz DL. Evaluating the impact of mobile telephone technology on type 2 diabetic patients' self-management: the NICHE pilot study. J Eval Clin Pract. 2008;14(3):465–469. doi: 10.1111/j.1365-2753.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 45.Kim HS, Jeong HS. A nurse short message service by cellular phone in type-2 diabetic patients for six months. J Clin Nurs. 2007;16(6):1082–1087. doi: 10.1111/j.1365-2702.2007.01698.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim SI, Kim HS. Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform. 2008;77(6):399–404. doi: 10.1016/j.ijmedinf.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Yoon KH, Kim HS. A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Res Clin Pract. 2008;79(2):256–261. doi: 10.1016/j.diabres.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Hee-Sung K. Impact of Web-based nurse's education on glycosylated haemoglobin in type 2 diabetic patients. J Clin Nurs. 2007;16(7):1361–1366. doi: 10.1111/j.1365-2702.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 49.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med. 2006;23(12):1332–1338. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 50.Benhamou PY, Melki V, Boizel R, Perreal F, Quesada JL, Bessieres-Lacombe S, Bosson JL, Halimi S, Hanaire H. One-year efficacy and safety of Web-based follow-up using cellular phone in type 1 diabetic patients under insulin pump therapy: the PumpNet study. Diabetes Metab. 2007;33(3):220–226. doi: 10.1016/j.diabet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Leu MG, Norris TE, Hummel J, Isaac M, Brogan MW. A randomized, controlled trial of an automated wireless messaging system for diabetes. Diabetes Technol Ther. 2005;7(5):710–718. doi: 10.1089/dia.2005.7.710. [DOI] [PubMed] [Google Scholar]
- 52.Kumar VS, Wentzell KJ, Mikkelsen T, Pentland A, Laffel LM. The DAILY (Daily Automated Intensive Log for Youth) trial: a wireless, portable system to improve adherence and glycemic control in youth with diabetes. Diabetes Technol Ther. 2004;6(4):445–453. doi: 10.1089/1520915041705893. [DOI] [PubMed] [Google Scholar]
- 53.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther. 2008;10(3):160–168. doi: 10.1089/dia.2008.0283. [DOI] [PubMed] [Google Scholar]
- 54.Huang ES, Meigs JB, Singer DE. The effect of interventions to prevent cardiovascular disease in patients with type 2 diabetes mellitus. Am J Med. 2001;111(8):633–642. doi: 10.1016/s0002-9343(01)00978-0. [DOI] [PubMed] [Google Scholar]
- 55.Nazimek-Siewniak B, Moczulski D, Grzeszczak W. Risk of macrovascular and microvascular complications in type 2 diabetes: results of longitudinal study design. J Diabetes Complications. 2002;16(4):271–276. doi: 10.1016/s1056-8727(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 56.Institute of Health Economics Consensus Statements. Consensus statement on self-monitoring in diabetes. 2006;1(1):1–18. [Google Scholar]