Abstract
Bolus infusion of insulin along with a meal is a standard procedure with continuous subcutaneous insulin infusion. Modern insulin pumps allow applying this bolus in four different ways: infusion of the total dose at once or splitting the dose into two boluses, infusion of a part of the bolus in the usual manner plus infusion of the other part over a prolonged period of time (with a higher infusion rate than the basal rate), or infusion of the total dose in the form of an elevated basal rate. Depending on the composition of the given meal and its glycemic index, this is an attempt to match the circulating insulin levels to the rate of glucose absorption from the gut in order to minimize postprandial glycemic excursions. However, in the framework of evidence-based medicine, the benefits of this approach should be proven in appropriately designed clinical studies. Performance of meal-related studies requires careful attention to many aspects in order to allow meaningful evaluation of a given intervention (i.e., type of bolus). Critical evaluation of the clinical experimental studies and the one clinical study published about the impact of different types of boluses on postprandial metabolic control revealed fundamental shortcomings in study design and performance in these studies. Insufficient establishment of comparable preprandial glycemia and insulinemia on the different study days within and between the patients studied is one key aspect. Therefore, the recommendation made in most of these studies (i.e., use of dual-wave bolus) has to be accepted with care, until we have better evidence.
Keywords: insulin pumps, insulin therapy, prandial insulin
Introduction
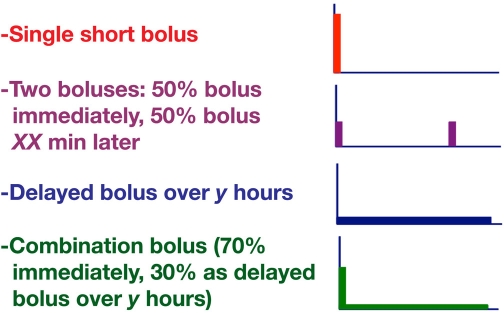
Optimization of postprandial metabolic control (i.e., to reduce excursions in glycemic levels as well as possible) by using different types of prandial insulin boluses is a feature offered by modern insulin pumps. By using “dual-wave” bolus and other types of insulin boluses, it should be possible to improve matching of insulin action to insulin requirements with different types of meals (Figure 1). It is still a matter of intensive scientific discussion whether an optimization is of relevance for the long-term outcome of patients with diabetes. The manufacturers of insulin pumps have selected different names used for the same type of bolus: single/quick/easy bolus, two boluses/split bolus, delayed/extended (Roche Diagnostics)/square wave (Medtronic), and combination/multiwave (Roche Diagnostics)/dual wave (Medtronic). The first two types of boluses are not pump-specific options; this is possible with subcutaneous (sc) injection as well. However, the latter ones are unique options of insulin pumps. In the end, the idea is always the same: distribute the meal-related insulin infusion in a manner that helps to optimize postprandial glycemic control. An interesting question is whether patients on continuous subcutaneous insulin infusion (CSII) use these different types of boluses in daily life. A small investigation in Europe indicates that less than half of the patients make use of, for example, the extended bolus.1
Figure 1.
Subcutaneous infusion of insulin with respect to a meal with different types of bolus. The time interval between the two boluses or the time during which the delayed bolus (i.e., a high basal rate for a selected period of time) is applied can be selected by the patients themselves (between 1 and 8 h). The proportion of the insulin dose applied as a bolus or as a square wave can also be varied (in 10% steps between 20%/80% and 50/50%).
At first glance, one might say there is no need for a combination bolus; patients eat something (e.g., fast food) and then they need a bolus of prandial insulin (like with a sc injection of prandial insulin) to cover the meal adequately. However, in many countries of the world, people sit together and eat for hours regularly. Even if this is not the case in most industrialized countries or only in the case of special events like business dinners, to cover such an extended meal appropriately with a single shot (or infusion) of insulin is an issue. Also, delayed carbohydrate absorption from the gut, be it due to certain diseases that come along with diabetes (gastroparesis) or by eating meals that require time-consuming digestion of complex carbohydrates (“muesli”) or that have a high fat content, are relevant aspects in this context. In a sense, one can also say these different types of boluses represent an option to convert the rapid metabolic action obtained with modern insulin into that of regular human insulin if appropriate for the type of meal consumed. This mini review aims to analyze the evidence for the use of different types of boluses from published literature and which factors are of relevance in this respect.
What Is the Target?
In a healthy subject, a rapid increase in endogenous insulin secretion occurs simultaneously as a meal is ingested. This secretion is also enhanced by a cephalic reaction. The rapid increase in circulating insulin levels not only enhance glucose storage in the musculature, it also induces a rapid suppression of hepatic glucose production to limit postprandial increase in glycemia. The amplitude and duration of the endogenous insulin secretion correlates with the amount of carbohydrates in the meal in a highly fine-tuned system. Aim of sc insulin therapy in patients with diabetes is to re-establish such physiological postprandial glycemic excursions by optimal coverage of the prandial insulin requirements (determined by the rate of glucose absorption in the gut) with sc injection or infusion of insulin. In view of the multitude of factors that have an impact on postprandial glycemic excursions (discussed later), it is fully understandable that a given pharmacodynamic effect induced by a sc injection of a given dose of regular human insulin or a rapid-acting insulin analogue—which, in a given sense, is a stereotype and not a fine-adjusted response—is far from being optimal in all cases. In contrast, CSII offers the unique opportunity to provide insulin with meals in different manners and thereby to fine-tune insulin administration.
Factors Influencing Postprandial Glycemic Excursions
Variation of insulin secretion by the beta cells in healthy subjects, not only with respect to the amount of carbohydrates ingested but also to the timing of glucose absorption in the gut—determined mainly by the properties of the meal ingested—resulted in the “magic” conclusion that the postprandial glycemic excursions in healthy subjects vary only relatively moderately despite huge differences in the amount and type of carbohydrates ingested.2 In patients with diabetes treated with insulin, many factors have an impact on postprandial glycemic excursions:
preprandial glycemia
circulating amount of insulin (due to basal insulin infusion/injection or the last prandial insulin application in the case of CSII)
insulin formulation/dose/site/time of day
time interval between start of prandial insulin infusion/injection and start of meal
type of meal (low/high glycemic index [GI])
amount of carbohydrates
preparation of the meal (e.g., fried or grilled)
rapidity/order of consumption (with/without a drink)
time elapsed since previous meal (second-meal phenomenon)
time of the day (breakfast versus dinner)
variability of glucose absorption in the gut3
Most probably, this list is not complete. By means of a continuous glucose monitoring system (CGMS), we now have the option to monitor postprandial glycemic excursions much more closely than was possible in the past. This will help us in understanding the impact of all meal-related factors on postprandial glycemic swings and allow us to learn more about the factors that influence these excursions and which therapeutic maneuvers allow us to reduce them. The variability of glycemic excursions in daily practice related to meals was more or less unknown until this new diagnostic option become available, as, in most cases, patients measure only preprandial glycemia by means of conventional blood glucose self-measurement to adjust their insulin dose accordingly.
Is the Metabolic Effect of Infused Insulin Identical to That of Injected Insulin?
Most of the clinical experimental studies investigating different factors influencing postprandial glycemic excursions were performed with sc injection of insulin. However, there is a question whether the pharmaco-dynamic effects observed after, for example, sc injection of 10 IU regular human insulin were identical to those observed after sc infusion of the same bolus dose with an insulin pump? The bolus is infused over a period of some minutes (most often 3 min), whereas the injection is applied at once. Another question is whether the insulin was injected into the same layers of the sc tissue, which is known to have an impact on insulin absorption rates. Additionally, with insulin pump therapy, the bolus is applied into a small insulin depot already existing around the tip of the catheter. Is the absorption into the bloodstream from a volume of sc tissue in which a catheter has been inserted for a period of time (inducing local trauma) different from that of insulin applied into a portion of tissue that is “naïve” to insulin? It appears that this has not been investigated thoroughly, at least not in past years with modern pumps, insulin, and research methods. It is not clear if differences exist between insulin pumps from different manufacturers, based on the individual pump technique used, that are relevant to the questions just raised. It would be of interest to run head-to-head studies to investigate multiple scenarios (which would also take differences in catheter sets into account). Nevertheless, despite our lack of knowledge (i.e., appropriate studies), one can assume that the differences in the metabolic action induced by the two routes of insulin administration are not fundamental.
Requirements for Studying Postprandial Glycemic Excursions
As with scientific experiments in general, when performing clinical experimental studies that investigate factors influencing postprandial glycemic excursions, all other factors should be kept as constant as possible with the exception of the intervention studied. In other words, all conditions that have an impact on postprandial glycemic excursions should be kept as constant/identical as possible on the different study days; preprandial glycemia and insulinemia are key factors in this respect. Without very careful standardization of the preprandial experimental conditions, no meaningful results will come out of such studies. Unfortunately, in most studies, not much attention was paid to these fundamental requirements (discussed later).
Selection of patients is also a critical factor/topic to ensure that reliable results are obtained. Patients with type 1 diabetes who are C-peptide negative (i.e., no remaining endogenous insulin secretion) are the ideal model for such studies. In patients with type 2 diabetes who have measurable serum C-peptide levels, the post-prandial glycemic response to a given glucose load will be influenced by the physiological activity of the remaining beta cells, i.e., the endogenously secreted insulin. This can also be an issue in young patients with type 1 diabetes and/or with a short duration of the disease. The subjects should also be in good to moderate metabolic control to avoid “chronic” changes in glucose handling (glucose toxicity) after a meal. The preprandial glycemia should be at a euglycemic starting level of 120 mg/dl and not at a lower one. No additional antidiabetic medication or medication that interferes with absorption of glucose from the gut should be taken by the patients. The patients should have no (prominent) symptoms of diabetes-related complications that influence glucose absorption (e.g., diabetic gastroparesis). The patients should also avoid alcohol/caffeine/smoking in the last 12 h before the study days, as this can have an impact on glucose metabolism. The same holds true for strenuous physical exercise. To establish reproducible conditions on the different study days, patients should stay in-house the night before the experiments to
reduce the risk of hypoglycemic events (release of counter-regulatory hormones has an impact on insulin sensitivity),
ensure a low level of physical activity in the last 12 h (to avoid an increase in insulin sensitivity), and
make sure identical blood glucose and circulating insulin levels are prevailing for some period of time prior to the test meal.
Ideally, the patients are in such a “stable” preprandial metabolic situation that no or only a low intravenous (IV) glucose infusion is required to balance the metabolic effect induced by a low-dose constant IV insulin infusion. This insulin IV infusion of regular insulin should just suppress hepatic glucose production and not induce a blood glucose lowering effect in the periphery, e.g., in the musculature. With such a low-dose insulin infusion, the metabolic effect of sc injected or infused basal insulin administration shall be mimicked, and identical insulin levels shall be established on all study days. Therefore, patients with a CSII should stop their pump during the study days to several hours prior to the intervention. It is also advisable to remove the pumps altogether to avoid any misunderstanding. If patients were studied who make use of sc injections of basal insulin formulations, patients should not have injected basal insulin preparation for at least 12 h (with neutral protamine Hagedorn insulin) or 24 h (with long-acting insulin analogues). Clearly, the time interval between the insulin application and the start of the meal has a massive impact on postprandial glycemic excursions; in case of CSII, this is the bolus meal interval instead of the injection meal interval with sc insulin injection.
Great care also has to be taken with the selection and preparation of the meal provided to the subjects. Something that might sound simple at first glance is tricky when it comes to the challenge of producing “standardized” meals in all respects on different study days. The composition of the meal should represent a “typical” meal for patients with diabetes (this must not mean that it is according to guidelines; the meals used in the studies are discussed later). Also, precise recommendations should be given for how the meal should be consumed, i.e., in which order the meal should be eaten and within which timeframe. It simply makes a difference with respect to postprandial glycemic changes if a glass of orange juice is consumed before or after the breakfast was eaten. As stated before, this also holds true for the time point when a drink (e.g., Coke) is allowed to be consumed. Also, the time interval since the last meal was eaten (second-meal phenomenon) and the time of day it was eaten have an impact on the postprandial glycemic excursions.3 Gastric emptying and glucose absorption are more rapid with low blood glucose values, whereas gastric emptying is slower with high blood glucose values (>15 mmol/liter).
Now the critical question is in regards to how much attention was paid to all these aspects in the relatively small number of clinical experimental studies performed to address the evidence of using different types of boluses on postprandial glycemic excursions. One has to acknowledge that the performance of such studies taking all these aspects into account is a demanding and challenging task. When rapid-acting insulin analogues were developed in the 1990s, most of the meal-related studies performed with sc insulin injection were of mediocre quality. In later years, attempts were made to take all factors mentioned into account in meal-related studies with novel rapid-acting insulin analogues.4
Variability of Insulin and Glucose Absorption
Even if great care is taken to reduce the interstudy day variability as much as possible by taking all aspects mentioned into account, one has to acknowledge that glucose absorption from the gut into the bloodstream and insulin absorption from the insulin depot in the sc tissue is highly variable, i.e., it is practically impossible to avoid differences in postprandial glycemic excursions. However, the number of studies focusing on this reproducibility is small. Usually, in such studies, certain factors (like the type of bolus) are varied. With sc insulin injection, the metabolic effect depends on the injection site, the depth of the injection, and the local skin temperature. Clearly, the type and dose of prandial insulin (regular human insulin versus rapid-acting insulin analogues) applied also has an impact on the induced effect. The variability of the metabolic effect induced can be assumed to be in the range of 20–30% (expressed as coefficient of variation for key pharmaco-dynamic parameters) with sc insulin injection. Absorption of insulin is known to be slower in subjects with a higher body mass index (i.e., thicker layers of sc fat). It is not clear if this has an impact on the variability of absorption as well.
Now, the interesting question is related to what we know about the variability of insulin absorption when the prandial insulin was infused rather than injected and whether this variability is identical or not. Was the insulin infused in the same layer of the sc tissue as the injected insulin, especially when using short pen needles? Again, one has to acknowledge that this question was not studied in the past decades. It is worth mentioning that no studies were performed to measure variability in the metabolic effects induced by different types of insulin boluses with CSII.
Another source of variability of insulin absorption with unknown significance are histological changes to the sc tissue. If the insulin is applied repeatedly with an inserted catheter into the same region of skin, for example, the abdomen, there is a risk of lipodystrophic changes in the sc tissue. Unfortunately, we have only clinical experience that such changes at the injection/infusion site have a profound influence on insulin absorption in an unpredictable manner. No systematic investigation has been performed thus far regarding this practical and relevant issue (independent from the route of insulin administration).
Under daily life conditions, most probably the variability of insulin absorption/action and glucose uptake in the gut is even higher than in highly controlled clinical experimental conditions. Besides the variability in insulin sensitivity, which depends on several factors like physical activity, many of the factors mentioned interact with each other in a manner that is difficult to predict, but most probably will increase the variability. With CSII, an additional question is related to the impact of the duration that the catheter is inserted into the sc tissue on insulin absorption rates, i.e., whether they are identical on day 1 and day 3. Older data suggest that this has no impact; however, subsequent data with more up-to-date methods have shown that, with a longer duration of catheter use, the pharmacodynamic properties of an applied bolus are different from that of a bolus applied in a newer infusion site.5,6
It is also important to determine the exact carbohydrate content of a given meal and its blood glucose increasing effect, even if this might appear to be trivial at first glance. Different types of carbohydrates differ from each other considerably with respect to their rapidity of absorption from the gut and their potential to increase glycemia. For many foods, the GI is not clear and may vary to a given extent if the preparation conditions are not truly identical.
Under experimental conditions, it is not the aim to achieve optimal postprandial metabolic control but to study if different interventions induce differences in the relevant outcome parameters of glycemic excursions. Therefore, the selection of the insulin dose should be realistic but more on the low side in order to allow a certain increase in postprandial glycemic excursions. With optimal selection of insulin doses, it is possible not to induce postprandial glycemia at all. Also, the duration of the evaluation of the postprandial glycemic excursions has to be sufficient; with a measurement period of 3 or 4 h, the risk of postprandial hypoglycemic excursions cannot be evaluated. In the studies reviewed later, the observation period varied between 3–16 hours. Unfortunately, authors sometimes feel pressured by editors/reviewers to focus on the time immediately after the meal (such as in the case of the pizza-Coke-tiramisu study) and to ignore the full data set (4 h were reported, but 8 h were measured).7
All these factors mean that the sample size to be studied in a given study must be high enough to have sufficient power to detect a certain difference in postprandial glycemia. It is noteworthy that, in the studies presented later, no formal sample size calculation was performed; at least, none was mentioned in the manuscripts.
Publications on the Effect of Different Types of Boluses on Postprandial Glycemic Excursions
Only four full publications and two abstracts could be identified by a literature search about the impact of different types of boluses on postprandial glycemic excursions.8–12 It appears as if mainly clinical experimental studies have been performed thus far and only one clinical study.13 Clearly, only a clinical study can prove if the use of different boluses is advantageous when it comes to harder endpoints. Therefore, it is of note that no results of such a study are available.
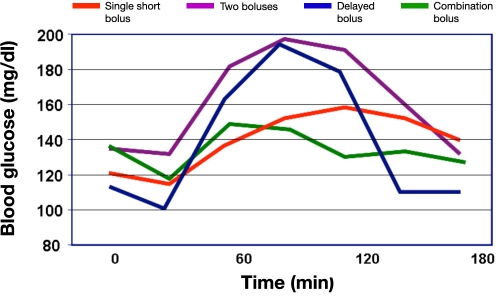
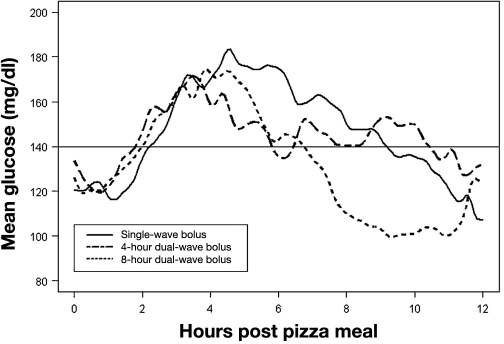
In the first publication about this topic (which, therefore, is the most cited one), nine young (age 14–28 years) C-peptide-negative patients with type 1 diabetes were studied.8 Four different types of insulin boluses were studied (Figure 2; mean insulin dose was 9.6 U). Boluses were started 10 min prior to the meal. Insulin lispro was infused with an individualized but constant dose for each subject. The meal consisted of pizza, tiramisu, and regular Coke (11% protein, 53% carbohydrate, 36% fat, 820 cal). The fasting glycemia upon arrival in the study center was in the range of 3.2–11.1 mmol/liter. Blood glucose measurements were performed with a nonlaboratory system (HemoCue blood glucose meter) in 30 min intervals.
Figure 2.
Postprandial glycemic excursions with four different types of prandial insulin boluses in nine patients with type 1 diabetes on CSII.
In this study, after the test meal, postprandial glycemia was lowest at 90 min with the dual-wave bolus. Glycemia tends to become higher in the following order: single bolus, double bolus, and square-wave. However, the glucose profiles were not significantly different. Glycemia after 240 min was lower with dual- and square-wave than with single and double bolus. The incidence of hypoglycemia (blood glucose < 2.8 mmol/liter) was similar with all four types of insulin boluses. It appears as if postprandial glycemia is controlled best with the dual-wave bolus (70%/30% ratio, over 2 h).
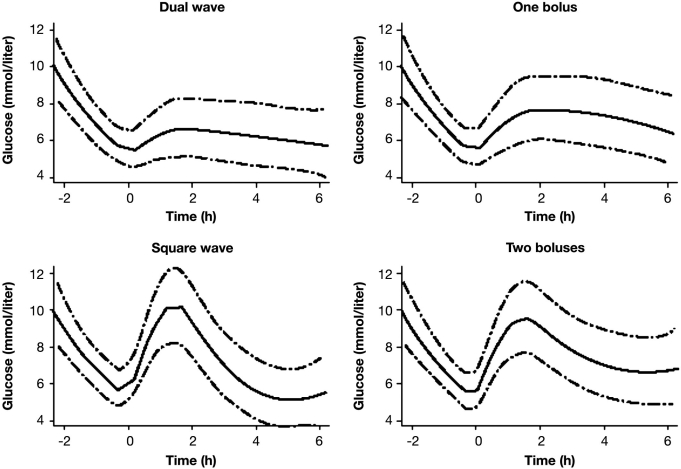
This study has some shortcomings that questioned the conclusion drawn. It appears as if the subjects had high preprandial glycemia (10 mmol/liter) in the 2 h prior to the meal (Figure 3). Also, the preprandial glycemia varied significantly between the study days (no statistical comparison is reported). Blood glucose levels were measured rather infrequently. No data about preprandial and postprandial insulinemia were reported. That this was not reported for this study (and all other studies) might also be due to space limitation of publications that prohibit reporting all details/data of a given study. The final recommendation made may be appropriate for meals that are high in fat and carbohydrates and are, however, inappropriate for meals with another composition (discussed later). It is also questionable if patients apply the insulin bolus 10 min prior to the start of the meal in daily life.14,15
Figure 3.
Best-fitting models for the behavior of blood glucose levels using the four methods of bolus administration. Please acknowledge that, in these figures, the preprandial glycemia is also presented.
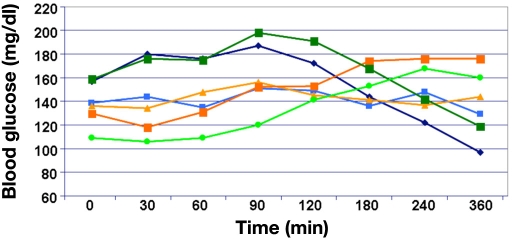
Lee and colleagues studied 10 patients with type 1 diabetes (mean age, 48 years; duration of type 1 diabetes, 18 years) on CSII.9 From the standard deviation (SD) provided for age (13 years) and duration of the disease (12 years), this was a heterogeneous group of patients (no information about serum C-peptide levels [i.e., remaining endogenous insulin secretion capacity] were provided). Three combi-nations of meals (evening meal) and bolus types were investigated in this nonrandomized study:
control meal with normal bolus delivery
high-fat meal with normal bolus delivery
high-fat meal with dual-wave insulin bolus delivery
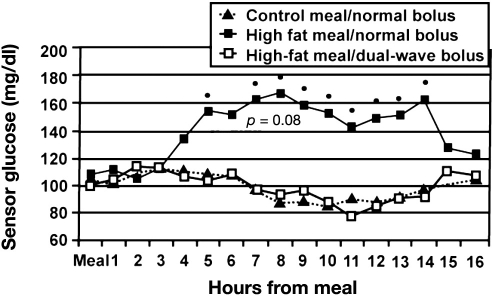
The control meal consisted of two Rosarita brand frozen bean burritos (14% protein, 62% carbohydrate, 24% fat). The high-fat meal consisted of one-third of a medium Red Baron brand four-cheese pizza with two-thirds cup additional cheese (20% protein, 26% carbohydrate, 54% fat). The individualized insulin doses applied on the three study days were different (bolus part 8.1, 9.2, and 8.7 U, mean values; the SD was > 3 U, not significant). On the study day with the dual-wave bolus (70%/30% ratio over 5 h), the total insulin dose was 13.4 U. The boluses were started immediately prior to the meals. It appears as if regular insulin was used in this study. Postprandial glucose profiles were monitored by means of a CGMS (Medtronic), but blood glucose was not measured with a laboratory system with a high measurement reliability. No comment was made about how fasting glycemia was controlled on the different study days. Mean preprandial glycemia were comparable with 106, 99, and 108 mg/dl on the three study days, but the high SD values of >20 mg/dl indicated massive differences between the study days with respect to preprandial glycemia. A significant increase in glycemia from baseline was observed only with the high-fat meal/normal bolus; on the other two study days, glycemia remained constant or even decreased (Figure 4). The increase in glycemia with the high-fat meal was slow, most probably due to the meal composition. The massive difference between the mean glucose profiles observed with the high-fat meal with the single bolus and that with the dual-wave bolus is puzzling in view of the relatively small difference of 4.7 U insulin, which was additionally applied on the study days with the square-wave bolus. Unfortunately, no information about the number of low blood glucose values is provided. The final recommendation of this study was to make use of the dual-wave bolus. Again, this was a small study, preprandial glycemia on the study differed widely intra-individually and interindividually, and no data about the preprandial and postprandial insulinemia were provided. It also appears as if the insulin doses were too high in general. The meals studied are a bit unusual; however, it might be a typical meal for many Hispanic patients. In summary, again, the number of critical questions challenges the validity of the recommendation given.
Figure 4.
Mean average hourly glucose sensor values compared for 16 consecutive hours following each of the three combinations of meal and bolus.
Twenty-four patients with type 1 diabetes (mean ± SD, age 40 ± 10 years; duration of the disease 21 ± 10 years) on CSII (no information about serum C-peptide levels, but minimal duration of the disease >3 years) participated in the study of Jones and associates.16 On three consecutive evenings, these patients consumed the same plain cheese pizza from Pizza Hut and water. The size of the meal was determined by each of the patients individually on the amount he/she would typically consume (2–3 slices, constant on the three days). One slice of pizza contained 14 g fat, 30 g carbohydrates, 2 g of fiber, 2 g of sugar, and 15 g protein. The patients applied on day 1 a standard insulin bolus immediately prior to meal; on day 2, a dual-wave bolus (50%/50%) over a 4 h period; and on day 3, a dual-wave bolus over an 8 h period. Six different summary measures were used to characterize the postprandial glucose profiles registered by means of a CGMS (Figure 5).
Figure 5.
Postprandial glycemic excursions with a standard bolus or dual-wave bolus in 24 patients with type 1 diabetes.
Unfortunately, no data about the level of preprandial glycemia were provided, but the statement was made that “mean glucose levels do not differ markedly at time 0.” Also, no data were provided indicating the variability of the measurement results obtained. However, the steep decline in the glucose levels at t = 0 min indicates higher preprandial values, at least on the two study days with dual-wave bolus. Glycemic profiles were not different until several hours after the meal. Divergence between glucose profiles occurred at 4–12 h after the meal and was greatest 8–12 h postmeal. During this period, significant differences were observed. The publication also stated that “blood glucose values for individual patients (data not shown) contained more variability,” indicating that many of the individual profiles differed from the mean curves substantially. The authors also commented about the delayed increase in glycemia observed (“pizza is a complex food”). However, they recommended a dual-wave bolus (50%/50% ratio, over 8 h, with 8 h better than 4 h). Again, no data about the preprandial and postprandial insulinemia or the incidence of hypoglycemic events were provided. One can discuss whether differences in glycemia 8–12 h after the meal are of practical relevance or not. In other words, one can say that a dual bolus over 8 h is not a bolus anymore and that this is an increased basal rate over one third of the day.
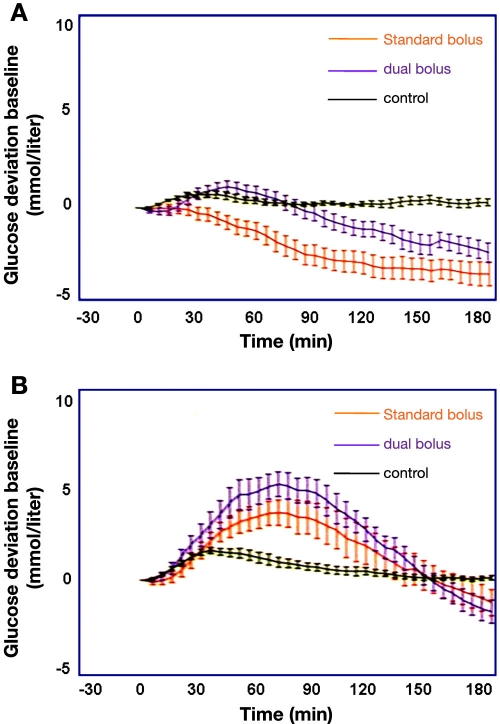
In view of the meals studied in the three studies presented thus far, it is clearly of interest to see the results of a study in which the appropriateness of two different types of insulin boluses (in this case, standard bolus versus dual-wave bolus [50%/50% over 2 h]) in combination with meals of low and high GI on four study days were studied in randomized order.11 The meals had equal macronutrient, energy, and fiber content but opposing GI (34 versus 76, low GI and high GI meals, respectively). Again, a CGMS was used to monitor postprandial glucose profiles (for 3 h only). Twenty children and adolescents with type 1 diabetes [mean (range): age 12 (9–17) years, duration of diabetes 5 (2–9) years] on CSII and 10 healthy subjects as a control group participated in this study. Fasting subjects arrived in the research unit at 8:00 AM and ate a standardized breakfast to negate any confounding second-meal effect at the time of the subsequent test meal eaten at lunchtime. Bolus application of insulin aspart was started immediately prior to the meal.
A dual-wave bolus before low GI meals decreased the area under the curve (AUC) in the postprandial glucose profile by up to 47% (p = .004) when the data from those with treated hypoglycemia (11 with low GI meals [7 with standard bolus, 4 with dual-wave bolus]; 2 hypoglycemic episodes with high GI and standard bolus) were excluded (Figure 6A). This type of insulin bolus also lowered the risk of hypoglycemic events for the same premeal glucose (p = .005) compared with the standard bolus. In contrast, premeal bolus type had no effect on postprandial AUC following the high GI meals (Figure 6B). In this case, a significant upward postprandial glycemic excursion was observed with greater AUC (p = .45) than in the controls, regardless of the bolus type applied.
Figure 6.
Relative mean ± SD of postprandial glycemic changes with a standard bolus or dual-wave bolus in 20 young patients with type 1 diabetes versus 10 healthy subjects (control) after consuming a meal with (A) low GI or (B) high GI.
A regression analysis performed to analyze whether the preprandial glycemic values had an effect on the subsequent postprandial glucose profiles revealed no significant effect. However, this can also be interpreted as an indication of widely varying preprandial glycemic values of the patients. Also, the fact that only relative changes were shown but no absolute values can be interpreted in this way, i.e., standardization of the preprandial conditions, was most probably not sufficient in this study. Again, no information about the preprandial and postprandial insulinemia or about the insulin dose applied is provided. One can also argue that, in this study, the insulin dose applied with the low GI meal was too high and too low with the high GI meal, as judged by the moderate increase in glycemia with the low GI meal and the difference in the glycemia between the two study days. In summary, again, the basis for the recommendation of a dual-wave bolus for low GI meals appeared to be not well funded.
Twenty-six kids and adolescents with type 1 diabetes on CSII [age 15 (range 4–22) years; duration of diabetes 8 (1–19) years] participated in the fifth study about this topic, which was reported as an abstract only thus far.12 The aim was to identify the optimal bolus with respect to postprandial glycemic excursions after eating different pizzas with identical carbohydrate content on consecutive days:
pizza margherita: dual bolus (30%/70% over 6 h) with a bolus–meal interval of 15 min or 0 min
pizza margherita: regular bolus (100% insulin) with a bolus–meal interval of 15 min or 0 min
pizza vegetable: dual bolus (30%/70% over 6 h) with a bolus–meal interval of 15 min or 0 min
Blood glucose levels were measured every 30 min for 6 h, and insulin aspart was applied for insulin treatment.
In combination with meals with a low GI, usage of dual-wave bolus over 6 h induced lower glycemic excursions as the standard bolus (Figure 7). However, regular bolus is better with a pizza with vegetables. Again, young patients were studied, and it is not clear if all were serum C-peptide negative. Apparently, the preprandial glycemia was quite different on the different study days. Postprandial glycemic monitoring by means of a CGMS would have been advantageous. It would also have been of interest to study dual-wave boluses with shorter bolus duration.
Figure 7.
Mean postprandial glycemic changes with a dual bolus over 6 h, with a 15 min bolus–meal interval prior to consumption of a pizza margherita (dark blue line) or without a bolus–meal interval (light blue line); a dual-bolus over 6 h, with a 15 min bolus–meal interval prior to consumption of a pizza vegetable (dark green line) or without a bolus–meal interval (light green line); and regular bolus with a 15 min bolus–meal interval and a pizza margherita (light brown line) or without a bolus–meal interval (light red line) in 26 young patients with type 1 diabetes.
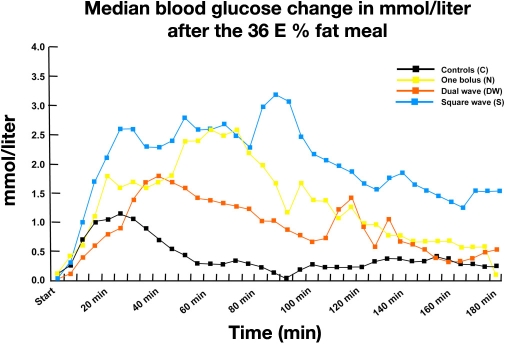
In another study that was reported as an abstract only, 13 adolescent girls (13–19 years) participated in a test with a meal that contained 36% fat given on three study days and were compared to a control group.17 On one study day, a single bolus was applied prior to the meal; on the second day, an extended bolus was started at 0 min and infused for 1 h; and on the third day, a multiwave bolus (60%/40%) was started at 0 min and infused for 1 h. The change in glycemia observed (Figure 8) indicates that the best postprandial metabolic control was achieved with the dual-wave bolus. As no details about study performance are available, it is difficult to comment on the quality/validity of this study.
Figure 8.
Median change in blood glucose in 13 adolescent girls after a meal with 36% fat and three different types of boluses in comparison to a control group.
In the only cross-sectional “clinical” study, the impact of dual-wave (DW) or square-wave (SW) bolus on metabolic control was evaluated.13 Also, the compliance of the pediatric patients studied with implementation of this system in daily practice was studied. Unfortunately, this was not a real clinical study but an uncontrolled evaluation of data collected in the outpatient clinic during routine visits. All patients were educated by the Warsaw School Program for dosing mealtime insulin in pump therapy. This also includes use of different types of boluses depending on food properties.
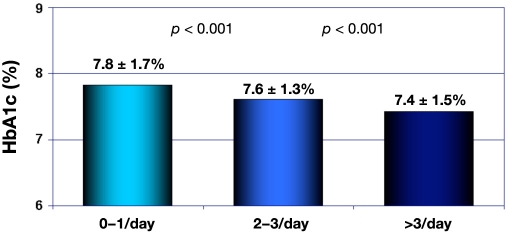
This study included 499 records of patients aged 0–18 years (246 girls/253 boys; age 11 ± 4 years; duration of diabetes 4 ± 3 years; hemoglobin A1c (HbA1c) 7.4 ± 1.5%; total daily insulin requirements 0.74 ± 0.2 IU; proportion of basal insulin on that 32 ± 14%). Only data from one type of pump (Medtronic MiniMed 508 or Paradigm 712, 722 pumps with rapid-acting insulin aspart or lispro) were considered. Data from the insulin pump memory provided information on the number of DW/SW boluses during a two-week period, the insulin requirement (U/kg/d), and the percentage of basal insulin. Mealtime dose of insulin in DW or SW bolus was calculated based on the amount of carbohydrate and fat/protein products. The number of applied DW/SW boluses was 16.6 ± 0.8/14 days (range 0–95) (or 1.2 of such boluses per day), while 18.8% of patients did not program DW/SW boluses. The lowest HbA1c value was observed in the group using two or more DW/SW boluses per day (p = .001) compared with the group administrating less than one DW/SW bolus per day (Figure 9). Patients with HbA1c level < 7.5% had a statistically higher relevant number of DW/SW boluses, 19.6 (95% confidence interval: 17.4–21.7) versus 12.4 (10.2–14.6) (p < .001). Therefore, the authors conclude that patients using at least one DW/SW bolus per day achieved a recommended level of HbA1c. Pediatric patients with type 1 diabetes mellitus were found to be able to apply DW/SW boluses in daily self-treatment process based on food counting. One has to acknowledge that these data were not collected during the conditions of a clinical study but during daily clinical work. Therefore, these data are of very limited value when it comes to the evidence level they do provide.
Figure 9.
Hemoglobin A1c in relation to the number of extended/dual-wave boluses.
Bolus Calculators and “Insulin on Board”
Modern insulin pumps offer support to the user in finding the right insulin dose for the meal; interestingly, they do not provide a suggestion for the type of bolus to be applied.18 Probably in the future, with more enhanced computer capabilities, more information about the different meals can be stored in the pump, and appropriate suggestions for the type of bolus will be made. To optimize postprandial glycemic control with a given meal is one topic; however, in daily practice, patients have more than one main meal per day, and probably also some snacks between these. If a given insulin dose was infused to cover a given meal and, additionally, some units of insulin are applied to cover the snacks, the question is in regards to how much of this insulin is still circulating in the blood stream and exhibiting a certain metabolic effect some hours later when the next meal is eaten and how much should be covered again with the next prandial insulin bolus. If the metabolic effect induced by this insulin infusion adds up to the effect of the previous infusion, the summation of the effects of the total amount of insulin might be a considerably stronger effect than anticipated; this in turn induces an increased risk of hypoglycemic events. The manufacturers of insulin pumps try to counteract this risk by something they call “insulin on board.” This is a rudimentary attempt to take care of this issue by assuming that, in relation to the insulin dose applied, the metabolic effect declines more or less linearly in the hours thereafter. It is interesting to see how differently, or similarly, the different pump manufacturers will handle this aspect in their pumps.
Summary and Outlook
In summary, this critical reappraisal of the studies published thus far about the advantages of different types of prandial insulin boluses with modern insulin pump therapy showed that the evidence for the use of different types of boluses is small, mainly due to shortcomings in the experimental procedures employed. One tends to believe that different types of prandial insulin bolus pumps enable better coverage of prandial insulin requirements in view of huge variability in which meals can be served. Unfortunately, the studies performed to date do not allow making firm statements, as all of them have severe shortcomings. Most of the studies had a too small sample size; most often, only young patients with type 1 diabetes were studied, preprandial glycemia was not adequately controlled on the different study days, no sufficient information/details about the insulin therapy/insulin levels were provided, only relative changes in glycemia were shown, and the measurement period was not of a sufficient duration. One has to acknowledge that performing good studies about this topic is a complex task. A large number of factors have to be controlled carefully to allow meaningful evaluation of the “intervention” applied. However, this is possible, and we clearly need better studies on the impact of different bolus types on postprandial glycemic excursions to enable us to make reliable recommendations to patients on CSII. Real-world studies making use of a CGMS might provide additional insight into the advantages offered by different types of boluses, if any.
Abbreviations
- CGMS
continuous glucose monitoring system
- CSII
continuous subcutaneous insulin infusion
- DW
dual wave
- GI
glycemic index
- IV
intravenous
- sc
subcutaneous
- SD
standard deviation
- SW
square wave
References
- 1.Jankovec Z, Kusova H, Cechurova D, Krcma M, Lacigova S, Visek M, Zourek M, Rusavy Z. Prague: 2008. Frequency of available insulin pump functions use by patients with diabetes mellitus. 1st ATTD Congress (Advanced Technologies and Treatments for Diabetes) [Google Scholar]
- 2.Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, Haug C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007;1(5):695–703. doi: 10.1177/193229680700100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13:S16–S22. (9 Suppl 5) [PubMed] [Google Scholar]
- 4.Rave K, Klein O, Frick AD, Becker RH. Advantage of premeal-injected insulin glulisine compared with regular human insulin in subjects with type 1 diabetes. Diabetes Care. 2006;29(8):1812–1817. doi: 10.2337/dc06-0383. [DOI] [PubMed] [Google Scholar]
- 5.Hanas SR, Carlsson S, Frid A, Ludvigsson J. Unchanged insulin absorption after 4 days' use of subcutaneous indwelling catheters for insulin injections. Diabetes Care. 1997;20(4):487–490. doi: 10.2337/diacare.20.4.487. [DOI] [PubMed] [Google Scholar]
- 6.Swan KL, Dziura JD, Steil GM, Voskanyan GR, Sikes KA, Steffen AT, Martin ML, Tamborlane WV, Weinzimer SA. Effect of age of infusion site and type of rapid-acting analog on pharmaco-dynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care. 2009;32(2):240–244. doi: 10.2337/dc08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinemann L, Heise T, Wahl LC, Trautmann ME, Ampudia J, Starke AA, Berger M. Prandial glycaemia after a carbohydrate-rich meal in type I diabetic patients: using the rapid acting insulin analogue [Lys(B28), Pro(B29)] human insulin. Diabet Med. 1996;13(7):625–629. doi: 10.1002/(SICI)1096-9136(199607)13:7<625::AID-DIA134>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Chase HP, Saib SZ, MacKenzie T, Hansen MM, Garg SK. Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med. 2002;19(4):317–321. doi: 10.1046/j.1464-5491.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Cao M, Sajid S, Hayes M, Choi L, Rother C, de León R. The dual-wave bolus feature in continuous subcutaneous insulin infusion pumps controls prolonged post-prandial hyperglycaemia better than standard bolus in type 1 diabetes. Diabetes Nutr Metab. 2004;17(4):211–216. [PubMed] [Google Scholar]
- 10.Jones SM, Quarry JL, Caldwell-McMillan M, Mauger DT, Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther. 2005;7(2):233–240. doi: 10.1089/dia.2005.7.233. [DOI] [PubMed] [Google Scholar]
- 11.O'Connell MA, Gilbertson HR, Donath SM, Cameron FJ. Optimizing postprandial glycemia in pediatric patients with type 1 diabetes using insulin pump therapy: impact of glycemic index and prandial bolus type. Diabetes Care. 2008;31(8):1491–1495. doi: 10.2337/DC08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaramuzza AE, Iafusco D, Giani E, Spiri D, De Palma A, Bosetti A, Santoro L, Mameli C, Gazzarri A, Macedoni M, Riboni S, Zuccotti GV. The optimal type of bolus following a pizza meal in children and adolescents with type 1 diabetes. Diabetologia. 2008;51:S381. (Suppl 1) [Google Scholar]
- 13.Pańkowska E, Szypowska A, Lipka M, Szpotańska M, Blazik M, Groele L. Application of novel dual wave meal bolus and its impact on glycated hemoglobin A1c level in children with type 1 diabetes. Pediatr Diabetes. 2009;10(5):298–303. doi: 10.1111/j.1399-5448.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 14.Heinemann L, Overmann H, Mühlhauser I. How do patients with diabetes mellitus type I handle the injection-meal interval in daily life? Diabetes. 1997;46(Suppl 1):340A. [Google Scholar]
- 15.Overmann H, Heinemann L. Injection–meal interval: recom-mendations of diabetologists and how patients handle it. Diabetes Res Clin Pract. 1999;43(2):137–142. doi: 10.1016/s0168-8227(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 16.Jones SM, Quarry JL, Caldwell-McMillan M, Mauger DT, Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther. 2005;7(2):233–240. doi: 10.1089/dia.2005.7.233. [DOI] [PubMed] [Google Scholar]
- 17.Lindholm-Olinder A, Runefors J, Smide B, Kernell A. Post-prandial glucose levels following three methods of insulin bolusing: A study in adolescent girls and in comparison with girls without diabetes. Pract Diabetes Int. 2009;26(3):110–115. [Google Scholar]
- 18.Zisser H, Robinson L, Bevier W, Dassau E, Ellingsen C, Doyle FJ, Jovanovic L. Bolus calculator: a review of four “smart” insulin pumps. Diabetes Technol Ther. 2008;10(6):441–444. doi: 10.1089/dia.2007.0284. [DOI] [PubMed] [Google Scholar]