Abstract
Intensive insulin therapy has an inherent risk of hypoglycemia that can lead to loss of consciousness, cardiac arrhythmia, seizure, and death (“dead-in-bed syndrome”). This risk of hypoglycemia is a major concern for patients, families, and physicians. The need for an automated system that can alert in the event of severe hypoglycemia is evident. In engineering systems, where there is a risk of malfunction of the primary control system, alert and safety mechanisms are implemented in layers of protection. This concept has been adopted in the proposed system that integrates a hypoglycemia prediction algorithm with a global position system (GPS) locator and short message service such that the current glucose value with the rate of change (ROC) and the location of the subject can be communicated to a predefined list. Furthermore, if the system is linked to the insulin pump, it can suspend the pump or decrease the basal insulin infusion rate to prevent the pending event. The system was evaluated on clinical datasets of glucose tracings from the DexCom Seven® system. Glucose tracings were analyzed for hypoglycemia events and then a text message was broadcast to a predefined list of people who were notified with the glucose value, ROC, GPS coordinates, and a Google map of the location. In addition to providing a safety layer to a future artificial pancreas, this system also can be easily implemented in current continuous glucose monitors to help provide information and alerts to people with diabetes.
Keywords: artificial pancreas, hypoglycemia, telemedicine, type 1 diabetes mellitus
Introduction
Intensive insulin therapy has an inherent risk of hypo-glycemia that can lead to loss of consciousness, cardiac arrhythmia, seizure, and death (“dead-in-bed syndrome”).1,2 This risk of hypoglycemia is a major concern for patients, families, and physicians. It is assumed that, in the future, fully automated insulin delivery systems, such as the artificial pancreatic β cell, will regulate the glucose concentrations yet minimize the risk of hypoglycemia. However, there is both a current and future need for an automated system that can provide remote monitoring as well as alert individuals, family members, and emergency services in the event of severe hypoglycemia.
Up-to-date information is the key to a successful operation. This is true for tactical military plans as well as civilian business decision making and medical triage. Telemedicine, the use of information and communication technology to support medical care and decision making, has been broadly used to enhance medical care and to provide up-to-date medical information.3 The use of telemedicine includes electronic medical records, telephone interviews and follow-up by health care providers, uploading of medical data to databases, remote analysis of x rays, remote medical consultation, and telesurgery.4–10
Telemedicine has been widely used as a means to improve glycemic control of people with type 1 diabetes mellitus (T1DM) and to reduce medical expenses.11 Chase and colleagues have demonstrated in a clinical study that the use of telemedicine in lieu of a clinical visit is as useful as the clinical visit and can reduce medical costs.12 Use of Internet tools, smart phones, and communication protocols such as short message service (SMS) and general packet radio service have been reported previously by several researchers such as Rami and associates,13 Gomez and coworkers,14 and Rigla and colleagues15 as tools that can be used to improve glycemic control. In those applications, the users have transmitted medical data such as glucose, insulin, and meal data to a Web site that is monitored by medical providers using smart phones and then received medical advice.13–15 All of these novel applications require some level of user interaction and aim to improve overall glycemic control and assist users in controlling their diabetes. Telemedicine technology can also be used to implement and automate systems that can monitor, alert, and locate people with T1DM who need medical assistance. Recently, the notion of the compatibility of the artificial pancreas (AP) with other devices and the use of a telemedicine system that will provide the needed safety layer was suggested by Dr. David C. Klonoff at the Food and Drug Administration/National Institutes of Health/Juvenile Diabetes Research Foundation AP workshop with the idea of integrating continuous glucose monitoring (CGM) and a global positioning system (GPS) known as KlonStar (Knowledge of Loop Operations Necessary System to Assist Repairs).16 This automated system is very important due to an altered level of consciousness resulting from severe hypoglycemia that may prevent a person with T1DM from taking corrective action or giving a clear report to emergency responders. We propose an intelligent telemedicine system that can detect adverse events in real time, suggest a solution, and then alert and broadcast the location of the event. For example, a health care provider or 911 operator can receive notification of a medical event with an electronic record of glucose level and its rate of change (ROC), analyze the situation, and assign an optimal solution. Such a system can be a lifesaving solution, enhance medical treatment, and optimize the workload on the medical personnel.17
Alerts and Alarms
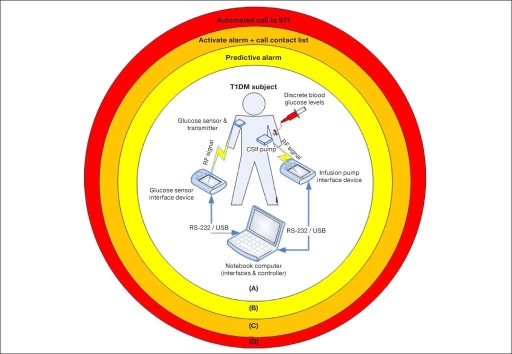
One way to address the need for automated response to life-threatening situations can be adopted from the process industry (chemical and nuclear), where a risk of malfunction of the primary control system causes implementation of alert and safety mechanisms in layers of protection. In this case, each chemical plant or unit that has inherent risk is protected by several layers of passive and active alarms and fully automated actuators; therefore, the overall risk associated will be minimal, and the likelihood for a catastrophic event is remote.18,19 As can be seen from Figure 1, the layers in the context of diabetes are as follows: (a) AP system that combines CGM, continuous subcutaneous insulin infusion (CSII), and a control algorithm that should be able to regulate the glucose level (this is not a safety layer but rather a primary system that aims to regulate glycemia); (b) predictive alarm that shall alert the patient and suggest a correction; (c) active alarm in case of severe hypoglycemia that will make an audible sound and/or call an emergency contact; and (d) automated dialer to emergency medical services. All the different algorithms are based on glucose information from continuous glucose sensors and have been developed and evaluated using computer simulation of T1DM subjects using historical CGM data.
Figure 1.
Layers of protection diagram divided into four layers: (A) AP system (sensor, pump, and controller), the primary system that aims to regulate glycemia; (B) predictive alarm that should alert the patient and suggest mitigation; (C) active alarm when the patient reaches the severe hypoglycemic range; and (D) call emergency medical service since the patient is not responsive to any other alarm.20 RF, radio frequency.
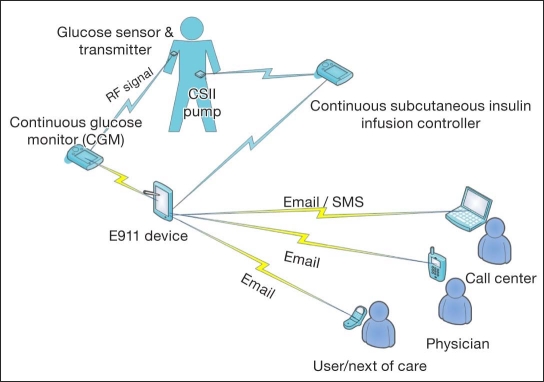
Figure 2 illustrates the monitoring layers in which CGM data are analyzed by an alert algorithm. If predefined conditions such as hypoglycemia are flagged by the algorithm, appropriate actions are performed, including the following: (a) alert the patient to take corrective action, (b) issue an emergency call, and (c) suspend insulin delivery. The system integrates a hypoglycemia prediction algorithm with a GPS locator, SMS, and email, such that the current glucose value with the ROC and the location of the subject at risk for a hypoglycemic event can be communicated to the user, a caregiver, a physician, and a call center. Furthermore, if the system is linked to the insulin pump, it can suspend the pump or decrease the basal insulin infusion rate to prevent the hypoglycemic event.
Figure 2.
Schematic illustration of the prototype telemedicine system in the context of diabetes, where the E911 device can broadcast an alert to the user, caregiver, physician, and call center by either email or text (SMS) message. RF, radio frequency.
This alert system has been realized using a communication module from the artificial pancreas software (APS), which was approved by the Food and Drug Administration to be used in clinical studies,21 along with a GPS receiver and wireless Internet connection to a personal computer. The E911 module, where safety algorithms analyze the data and suggest action, was monitoring real-time CGM data.
Pilot Evaluation
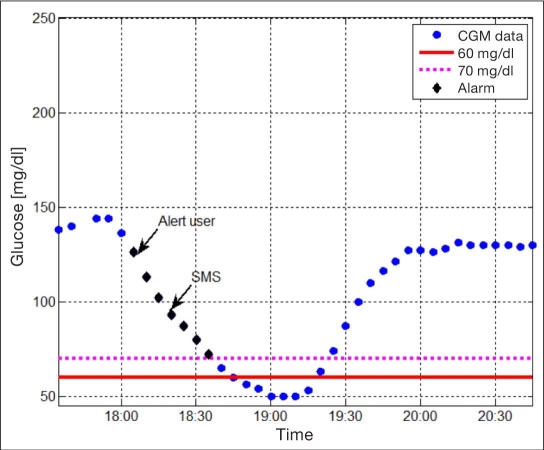
The prototype system was evaluated on clinical data records of glucose tracings from the DexCom Seven® system (DexCom, San Diego, CA) using the hardware-in-the-loop evaluation platform.22 Historical glucose readings were introduced to the DexCom transmitter and then obtained from the DexCom receiver using the APS. These glucose readings were analyzed for hypoglycemia events, and if the alert condition was met, a text message was broadcast to a predefined list with the glucose value, ROC, GPS coordinates, and a Google map of the location. As demonstrated in Figures 3 and 4, the E911 module flagged a pending hypoglycemia event where glucose was 90 mg/dl and the ROC was -1 mg/dl. An SMS message was then sent to the health care provider with the information and the location of the subject, including a Google map.
Figure 3.
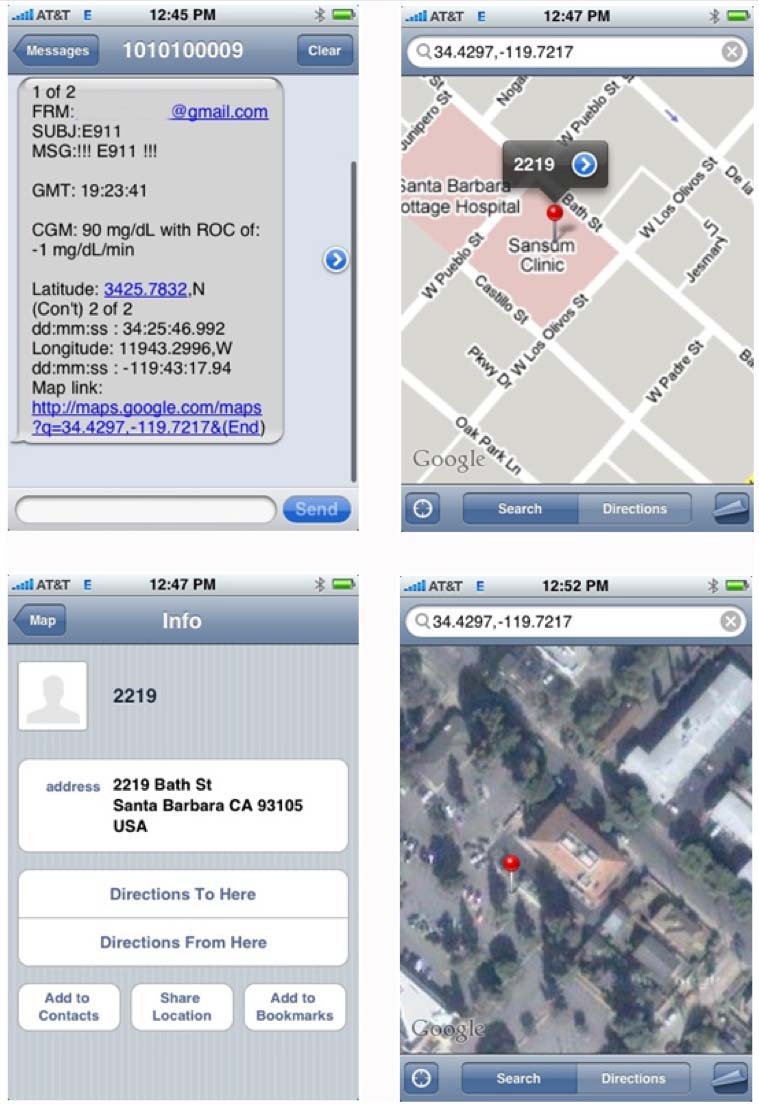
An example of the message received by the physician to his mobile device, where (a) the message including time, glucose, ROC, and GPS coordinates with a hyperlink to Google Maps are presented in the upper left panel; (b) the Google map pinpointing the subject location is presented in the upper right panel; (c) detailed address information is presented in the lower left panel; and (d) a satellite view of the location is presented in the lower right panel.
Figure 4.
Illustration of the performance of a simple alert algorithm on historical CGM tracing denoted as blue circles, where the algorithm alerts are marked as black diamonds. As can be seen, a warning is issued to the user when glucose is ∼120 mg/dl, and an SMS is sent when crossing the 90 mg/dl threshold. The red and magenta dotted lines denote the 60 mg/dl (clinical) and 70 mg/dl (algorithm) hypoglycemia threshold, respectively.
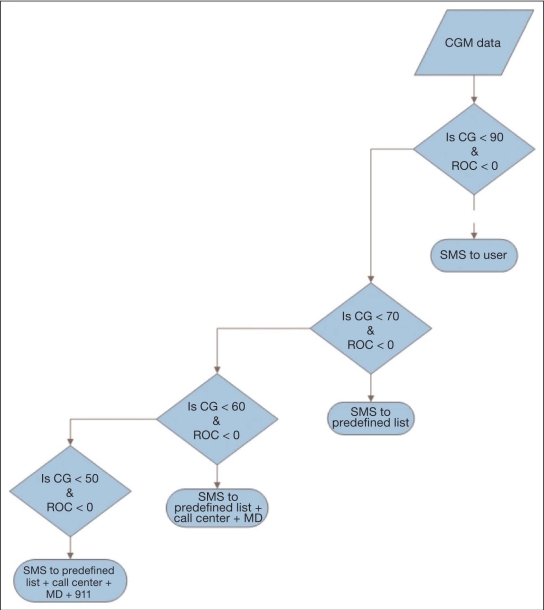
In the context of diabetes management, this alert and mitigation system not only provides a safety layer to a future AP using predictive alarms, but can also be easily implemented in current CGMs using a series of threshold alarms with predefined actions, as illustrated in Figure 5. The E911 module analyzes glucose data (continuous glucose) and sends different alerts as glucose levels continue to fall and no user interaction is detected.
Figure 5.
A simplified version of a decision support system to be embedded in CGM devices, where CGM data are analyzed and, as predefined thresholds are crossed as defined by both continuous glucose and its ROC, predefined actions are preformed. Alerts are sent in the form of a text messages (SMS) to the user, physician, and emergency medical service, depending on the severity of the hypoglycemic event. CG, continuous glucose; MD, physician.
It should be disclosed that the conceptual design needs to be further evaluated with extensive field studies. Both communication interference as well as the use of cellular networks in lieu of wireless communication need to be evaluated. The use of cellular triangulation in cases that the GPS signal is inefficient or unavailable should be examined as well in a final prototype.
Conclusions
The proposed E911 alert system can be used in the general population and/or can be directly transferred to medical military applications, such as the Warfighter Physiological Status Monitoring23,24 and the Electronic Information Carrier for use on the battlefield to transmit data immediately to a central command post. Use can be extended to more comprehensive applications in surgical units to optimally address different medical conditions that need to be detected, analyzed, and treated with limited resources.
The long-term advantages of using the E911 module may be that treatment and compliance will be improved with CGM,25 and thus long-term complications will be minimized. E911 could also be used to monitor pregnant women with diabetes, since tight glycemic control using insulin during pregnancy increases the risk of hypoglycemia. Thus it would be beneficial to monitor pregnant women with diabetes and increase the chances for a healthy, normal-weight baby.26
This monitoring, alert, and mitigation system can not only provide a safety layer to a future AP, but also be easily implemented in current CGMs to help provide information and alert to people with diabetes (T1DM, type 2 diabetes mellitus, and pregnant women with diabetes) as well as a monitoring and supervision system to health care providers. The E911 module is especially useful to people with T1DM, such as in cases of hypo-glycemia unawareness and prevention of seizure and death (dead-in-bed syndrome) and as a means for parents to monitor children with T1DM attending school or at play away from home.
Abbreviations
- AP
artificial pancreas
- APS
artificial pancreas software
- CGM
continuous glucose monitoring
- CSII
continuous subcutaneous insulin infusion
- GPS
global positioning system
- ROC
rate of change
- SMS
short message service
- T1DM
type 1 diabetes mellitus
References
- 1.Hanas R. Dead-in-bed syndrome in diabetes mellitus and hypo-glycaemic unawareness. Lancet. 1997;350(9076):492–493. doi: 10.1016/S0140-6736(05)63081-4. [DOI] [PubMed] [Google Scholar]
- 2.Weston PJ, Gill GV. Dead-in-bed syndrome in diabetes mellitus. Lancet. 1997;350(9083):1032–1033. doi: 10.1016/s0140-6736(05)64083-4. [DOI] [PubMed] [Google Scholar]
- 3.Bellazzi R. Telemedicine and diabetes management: current challenges and future research directions. J Diabetes Sci Technol. 2008;2(1):98–104. doi: 10.1177/193229680800200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleh M, Schoenlaub S, Desprez P, Bourcier T, Gaucher D, Astruc D, Speeg-Schatz C. Use of digital camera imaging of eye fundus for telemedicine in children suspected of abusive head injury. Br J Ophthalmol. 2009;93(4):424–428. doi: 10.1136/bjo.2008.147561. [DOI] [PubMed] [Google Scholar]
- 5.Bryson EO, Frost EA. Anesthesia in remote locations: radiology and beyond, international anesthesiology clinics: CT and MRI. Int Anesthesiol Clin. 2009;47(2):11–19. doi: 10.1097/AIA.0b013e3181939b0b. [DOI] [PubMed] [Google Scholar]
- 6.Gibelli G, Gibelli B, Nani F. Thyroid cancer: possible role of telemedicine. Acta Otorhinolaryngol Ital. 2008;28(6):281–286. [PMC free article] [PubMed] [Google Scholar]
- 7.Lienemann B, Hodler J, Luetolf M, Pfirrmann CW. Swiss teleradiology survey: present situation and future trends. Eur Radiol. 2005;15(10):2157–2162. doi: 10.1007/s00330-005-2764-3. [DOI] [PubMed] [Google Scholar]
- 8.Vuletić S. Teleradiology in Croatia. J Telemed Telecare. 2001;7(Suppl 1):73–75. doi: 10.1177/1357633X010070S131. [DOI] [PubMed] [Google Scholar]
- 9.Patterson V, Hoque F, Vassallo D, Farquharson Roberts M, Swinfen P, Swinfen R. Store-and-forward teleneurology in developing countries. J Telemed Telecare. 2001;7(Suppl 1):52–53. doi: 10.1177/1357633X010070S121. [DOI] [PubMed] [Google Scholar]
- 10.Klapan I, Vranjes Z, Risavi R, Simicić L, Prgomet D, Glusac B. Computer-assisted surgery and computer-assisted telesurgery in otorhinolaryngology. Ear Nose Throat J. 2006;85(5):318–321. [PubMed] [Google Scholar]
- 11.Klonoff DC. Using telemedicine to improve outcomes in diabetes—an emerging technology. J Diabetes Sci Technol. 2009;3(4):624–628. doi: 10.1177/193229680900300401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chase HP, Pearson JA, Wightman C, Roberts MD, Oderberg AD, Garg SK. Modem transmission of glucose values reduces the costs and need for clinic visits. Diabetes Care. 2003;26(5):1475–1479. doi: 10.2337/diacare.26.5.1475. [DOI] [PubMed] [Google Scholar]
- 13.Rami B, Popow C, Horn W, Waldhoer T, Schober E. Telemedical support to improve glycemic control in adolescents with type 1 diabetes mellitus. Eur J Pediatr. 2006;165(10):701–705. doi: 10.1007/s00431-006-0156-6. [DOI] [PubMed] [Google Scholar]
- 14.Gómez EJ, Hernando Pérez ME, Vering T, Rigla Cros M, Bott O, García-Sáez G, Pretschner P, Brugués E, Schnell O, Patte C, Bergmann J, Dudde R, de Leiva A. The INCA system: a further step towards a telemedical artificial pancreas. IEEE Trans Inf Technol Biomed. 2008;12(4):470–479. doi: 10.1109/TITB.2007.902162. [DOI] [PubMed] [Google Scholar]
- 15.Rigla M, Hernando ME, Gómez EJ, Brugués E, García-Sáez G, Torralba V, Prados A, Erdozain L, Vilaverde J, de Leiva A. A telemedicine system that includes a personal assistant improves glycemic control in pump-treated patients with type 1 diabetes. J Diabetes Sci Technol. 2007;1(4):505–510. doi: 10.1177/193229680700100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klonoff DC. Compatibility of an artificial pancreas with other devices towards an artificial pancreas: an FDA-NIH-JDRF workshop. Bethesda, MD. 2008 [Google Scholar]
- 17.Dassau E, Zisser H, Jovanovič L, Doyle III FJ. Enhanced 911/GPS Wizard for the prevention of severe hypoglycemia—monitor, alert, and locate. 69th American Diabetes Association Meeting, New Orleans, LA. Diabetes. 2009;59(Suppl 1):A506. doi: 10.1177/193229680900300632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bollinger RE, Clark DG, Dowell RM, III, Ewbank RM, Hendershot DC, Lutz WK, Meszaros SI, Park DE, Wixom ED, Crowl DA, Kletz T. Inherently safer chemical processes: a life cycle approach. Somerset: John Wiley & Sons; 1997. [Google Scholar]
- 19.Kletz T. Hazop and Hazan. 4th ed. Philadelphia: Taylor & Francis; 1999. [Google Scholar]
- 20.Dassau E, Cameron FM, Lee H, Bequette BW, Doyle III FJ, Niemeyer G, Chase P, Buckingham B. Real-time hypoglycemia prediction using continuous glucose monitoring (CGM), a safety net to the artificial pancreas. 68th American Diabetes Association Meeting, San Francisco, CA. Diabetes. 2008;57(Suppl 1):A13. [Google Scholar]
- 21.Dassau E, Zisser H, Palerm CC, Buckingham BA, Jovanovič L, Doyle III FJ. Modular artificial β-cell system: a prototype for clinical research. J Diabetes Sci Technol. 2008;2(5):863–872. doi: 10.1177/193229680800200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dassau E, Palerm CC, Zisser H, Buckingham BA, Jovanovič L, Doyle III FJ. In silico evaluation platform for artificial pancreatic β-cell development—a dynamic simulator for closed-loop control with hardware-in-the-loop. Diabetes Technol Ther. 2009;11(3):187–194. doi: 10.1089/dia.2008.0055. [DOI] [PubMed] [Google Scholar]
- 23.Friedl KE. Military diabetes and advanced technologies research. Diabetes Technol Ther. 2003;5(4):703–704. doi: 10.1089/152091503322250721. [DOI] [PubMed] [Google Scholar]
- 24.Friedl KE. Analysis: signs of illness: when will technology provide greater advantage than the practiced eye of the clinician (or the military commander)? Diabetes Technol Ther. 2003;5(5):857–859. doi: 10.1089/152091503322527067. [DOI] [PubMed] [Google Scholar]
- 25.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovič L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 26.Jovanovic L. Role of diet and insulin treatment of diabetes in pregnancy. Clin Obstet Gynecol. 2000;43(1):46–55. doi: 10.1097/00003081-200003000-00005. [DOI] [PubMed] [Google Scholar]