Abstract
Activation of medial olivocochlear efferents through contralateral acoustic stimulation (CAS) has been shown to modulate distortion product otoacoustic emission (DPOAE) level in various ways (enhancement, reduction, or no change). The goal of this study was to investigate the effect of a range of CAS levels on DPOAE fine structure. The 2f1-f2 DPOAE was recorded (f2∕f1=1.22, L1=55 dB, and L2=40 dB) from eight normal-hearing subjects, using both a frequency-sweep paradigm and a fixed frequency paradigm. Contamination due to the middle ear muscle reflex was avoided by monitoring the magnitude and phase of a probe in the test ear and by monitoring DPOAE stimulus levels throughout testing. Results show modulations in both level and frequency of DPOAE fine structure patterns. Frequency shifts observed at DPOAE level minima could explain reports of enhancement in DPOAE level due to efferent activation. CAS affected the magnitude and phase of the DPOAE component from the characteristic frequency region to a greater extent than the component from the overlap region between the stimulus tones. This differential effect explains the occasional enhancement observed in DPOAE level as well as the frequency shift in fine structure patterns.
INTRODUCTION
The peripheral auditory system is primarily a receiver of acoustic signals and the gateway to higher processing centers. However, activity at this first way station is not a result of simple passive characteristics of the structures involved; an active “cochlear amplifier” modifies acoustic signals in specific and complex ways (Dallos, 2008). Otoacoustic emissions (OAEs), low-level signals produced in the cochlea, are an essential byproduct of the cochlear amplifier (Kemp, 1978).
Distortion product (DP) OAEs are evoked by simultaneous stimulation with two pure tones (f1 and f2, f1<f2), and are measured at frequencies that are arithmetic combinations of the stimulus frequencies. Although DPOAEs at several combination frequencies can be recorded from the normally functioning human ear, the DPOAE at 2f1-f2 is the most extensively studied and used for clinical purposes (Gorga et al., 1997).
DPOAEs are commonly measured at a few frequencies per octave, resulting in a general picture of overall cochlear function in that region. When recorded with higher frequency resolution, however, a pseudo-periodic pattern of peaks (maxima) and dips (minima) in DPOAE level as a function of frequency, known as fine structure, is routinely observed (e.g., Heitmann et al., 1998; Talmadge et al., 1999; Dhar et al., 2002; Dhar and Shaffer, 2004). DPOAE fine structure is a consequence of constructive and destructive interferences between two components: one from the region where the activity patterns of the stimulus tones overlap on the basilar membrane, and the other from the characteristic frequency (CF) region of the DPOAE (Talmadge et al., 1999). This detailed response allows further investigation of the cochlea within one region.
In this study, we are interested in the modulation of DPOAEs, and specifically DPOAE fine structure, by the medial olivocochlear (MOC) efferent system stimulated acoustically via the contralateral ear. The MOC fibers are thick, myelinated fibers that originate in the medial part of the superior olivary complex. The fibers project through the vestibular nerve and innervate the outer hair cells (OHCs) directly. Mediated by the neurotransmitter acetylcholine, MOC neurons change the conductance and the stiffness of the OHC, leading to a general attenuation of cochlear amplifier gain (see Guinan, 2006 for a recent review). The effects of efferent-induced attenuation of the cochlear amplifier is evident in reduced basilar membrane vibrations (Murugasu and Russell, 1996) as well as altered auditory nerve fiber responses (Kawase et al., 1993) and tuning (Guinan and Gifford, 1988).
With more invasive measurement techniques being impractical, OAEs have become an important tool for studying the effects of the MOC efferents in live humans. Mountain (1980) was among the first to demonstrate a reduction in OAE level following efferent stimulation. These results were later corroborated and extended by Siegel and Kim (1982), who also reported reduction, enhancement, or no change in OAE levels upon efferent activation. In many ways, we are still unraveling the underlying physiology linked to these diverse changes in OAE level due to efferent stimulation.
The early promise of gleaning clinically useful information from the modulation of OAEs by the efferent system has led to a search for the most robust and stable measure. In the case of DPOAEs in humans, the vast majority of reports have demonstrated a small reduction in DPOAE level (1–3 dB) due to the activation of the MOC efferent system (Bassim et al., 2003; Abdala et al., 2009). Enhancement of DPOAE level upon stimulation of the efferent system, much akin to the results of Siegel and Kim (1982), has been reported as well (Maison and Liberman, 2000; Müller et al., 2005). While reduction in DPOAE level is easily explained as a consequence of attenuation of cochlear gain by the MOC efferents, a differential alteration of the two DPOAE components has been proposed as the cause behind the observed enhancement at certain frequencies. The observation of enhancement almost exclusively at minima in DPOAE level supports this theory (Zhang et al., 2007). Minima are a result of cancellation due to phase opposition between the two DPOAE components (from the overlap and CF regions). Selective reduction in the CF component at such a frequency would “release” the overlap component from cancellation thereby causing an enhancement of the DPOAE level in the ear canal.
The bipolar effect of efferent stimulation on DPOAE level can be observed in the time domain as well. When the MOC efferents are stimulated while monitoring DPOAE level at a specific frequency, a reduction in DPOAE level is observed upon activation of the MOC efferents when the monitored frequency is a known level maximum. In contrast, an enhancement is observed when the monitored frequency is a known level minimum (Sun, 2008b).
Several measures of efferent-induced alteration of OAEs, the so-called MOC reflex, have been proposed in recent literature. Maison and Liberman (2000) measured a change in DPOAE level at a fixed frequency over time (∼300 ms) following onset of the stimulus. Both reduction and enhancement were observed in response to varying stimulus level combinations. The authors dealt with this by designating the absolute value of the biggest change in an animal (guinea pig) as the MOC reflex strength. Absolute reflex values in this group of animals were distributed over a range of approximately 20 dB. Following a similar paradigm in a group of human subjects, using contralateral acoustic stimulation (CAS) of the efferent system (Müller et al., 2005) recommended using the difference between the largest enhancement and the largest reduction as the MOC reflex metric. Since the largest differences between enhancement and reduction have been typically observed at frequencies of DPOAE level minima, recommendations for measuring the MOC reflex at frequencies of fine structure minima have also been made in the literature (Müller et al., 2005; Wagner et al., 2007).
A counterpoint to the recommendation of measuring the MOC reflex only at DPOAE level minima has been set forward in a group of very recent publications (Zhang et al., 2007; Purcell et al., 2008; Abdala et al., 2009). While results from this set of experiments align with the previously reported robustness of the MOC reflex at DPOAE level minima, they also show extreme variability at such frequencies. These publications recommend measuring the MOC reflex strength at DPOAE level maxima if magnitude can be sacrificed for the sake of stability.
Using stimuli swept continuously in frequency yields a more comprehensive picture of the effects of MOC activation on DPOAE level (Purcell et al., 2008; Abdala et al., 2009). Purcell et al. (2008) reported the effect of the MOC efferents (stimulated acoustically via the contralateral ear) on DPOAE recordings with a fixed f2 frequency while varying f1 around f2∕f1 ratios of either 1.10 or 1.22. Since this recording technique allows the comparison of a continuous DPOAE level versus frequency function with and without CAS, several metrics of the MOC reflex can be extracted, such as the maximum change at any given frequency, average change at all frequencies, or change in the area under the fine structure curve. These authors did not advocate for any particular metric but simply demonstrated the feasibility of using stimuli swept in frequency to evaluate multiple measures of the effect of MOC activation on DPOAEs. These data, dense in their frequency distribution, expose a consistent shift in DPOAE level toward higher frequencies upon activation of the MOC efferents.
A similar shift in DPOAE fine structure toward higher frequencies is also reported by other groups (Purcell et al., 2008; Sun, 2008b; Abdala et al., 2009). Abdala et al. (2009), in particular, focused on the effects of MOC activation on DPOAE fine structure and demonstrated that the level of the DPOAE CF component is affected more than that of the overlap component. Departing from the previous recommendation of measuring MOC effects at DPOAE level minima (Müller et al., 2005; Wagner et al., 2007), Abdala et al. (2009) explicitly recommended using the smaller but more stable reduction in DPOAE level observed at frequencies of DPOAE level maxima.
Acoustic stimuli used to activate the MOC efferents can also activate the middle ear muscle reflex (MEMR). The possibility of the MEMR co-occurring with the MOC reflex casts a constant shadow of doubt in the interpretation of data such as those reported here. A simple approach would be to stimulate the MOC efferents at levels lower than the MEMR threshold. However, the detected MEMR threshold is critically dependent on the limits of the measurement system and technique. Laboratory techniques (Guinan et al., 2003; Goodman and Keefe, 2006) often yield thresholds significantly lower than those detected using commercially available impedance audiometers.
The relative contribution of the MEMR and the MOC reflex to changes in OAEs (or other measures of cochlear output, such as the compound action potential) appears to be species dependent. For example, the effect appears to be dominated by the MEMR in the rat, as sectioning the middle ear muscle eradicates the response almost entirely (Relkin et al., 2005). In contrast, the effect appears to be dominated by the MOC efferents in the cat (Liberman et al., 1996; Puria et al., 1996) and humans (Giraud et al., 1995). Nonetheless, the MEMR is an issue to contend with and we made a concerted effort to isolate the MOC reflex from the MEMR in the data reported here.
The quest to understand the effects of MOC efferents on OAEs in general, DPOAEs and DPOAE fine structure in particular, and to find a reliable and robust measure applicable for clinical use, is ongoing. Here we extend previous work by presenting results from eight normal-hearing healthy young adult humans, documenting the effects of multiple levels of CAS on DPOAEs. The subjects chosen for these experiments had unusually high MEMR but behavioral hearing thresholds within the normal range, and robust DPOAEs.
METHODS
Data from the right ears of eight normal-hearing female human subjects (mean age=20 years, st. dev.=1 year) are reported. These eight subjects, selected from a database of approximately 90 subjects, displayed pronounced DPOAE fine structure, modulation of DPOAEs due to contralateral stimulation (see top panel of Fig. 3 for example), and, most importantly, high MEMR thresholds. All tests were conducted in a sound-treated audiometric booth with the subject comfortably seated in a recliner. Subjects were paid for their participation and all measurements were conducted in accordance with the guidelines of the Institutional Review Board at Northwestern University.
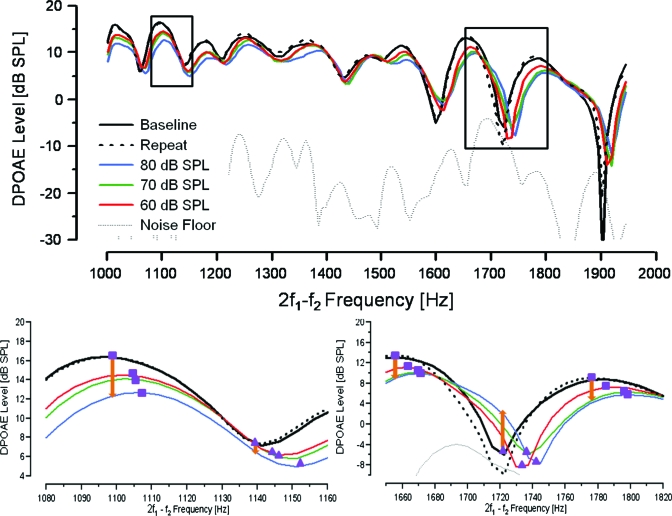
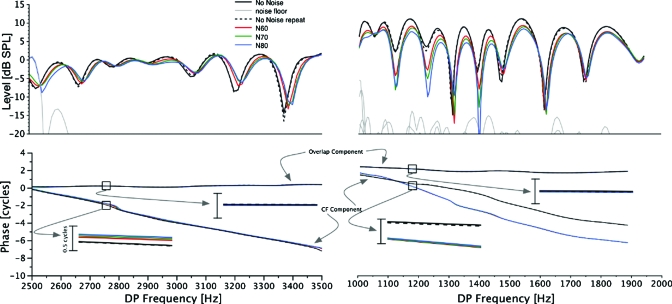
Figure 3.
Top panel: example of changes in DPOAE fine structure in response to several levels of CAS in an individual subject. Black boxes surround areas magnified in lower panels. Bottom panels: magnified view of fine structure extrema under different noise conditions. Purple squares and triangles represent maxima and minima, respectively. Orange arrows indicate the trend that traditional fixed frequency DPOAE analysis would have shown. Note that the minimum in the bottom left panel shows reduction (arrow pointing downwards) while the minimum in the bottom right panel shows enhancement (arrow pointing upwards).
General methods
Signal generation and recording for OAE measurements were done using custom software running on an Apple Macintosh computer. A MOTU 828 MkII input∕output FireWire device was used for analog-to-digital (44 100 Hz, 24 bits) and digital-to-analog conversions. Generated signals were passed through a Behringer Pro XL headphone amplifier to MB Quart 13.01HX drivers. The drivers were coupled to the subjects’ ear canal via an Etymotic Research ER10A probe assembly. The broadband noise (BBN) used for some experimental conditions was delivered through an independent channel of the MOTU and Behringer using an additional MB Quart driver. The signal from this driver was delivered to the ear canal using a plastic tube and foam tip assembly similar to one used in clinical audiometry. Signals from the test ear were recorded using the ER10A microphone and preamplifier combination, digitized using the MOTU and stored on disk for analyses.
Stimuli used for DPOAE measurements were calibrated using a coupler calibration procedure that allowed us to approximately compensate for the depth of insertion in the ear canal [Siegel (personal communication)]. The frequency responses of the transducers were measured in a long lossy tube (50 ft long, 0.375 in. outside diameter, copper plumbing tubing) using a slow chirp between 200 and 20 000 Hz. The absence of standing waves in this long tube with its diameter matched to that of the probe allows the recording of the combined frequency response of the sound source and the microphone. These frequency responses were also measured in an IEC 711 coupler (Bruel and Kjaer 4157) for various depths of insertion. The response was recorded using the microphone of the OAE probe as well as a Bruel and Kjaer 0.5-in. microphone (BK4134) attached at the distal end of the coupler. The recording obtained using the OAE probe microphone was normalized with that obtained in the long lossy tube at each insertion depth. This normalization resulted in the isolation of the frequency response of the cavity with a half-wave resonance that was related to the depth of insertion. The recording from the BK4134 was used to generate a correction filter for each insertion depth to yield a uniform stimulus level at the distal end of the coupler. During experiments, the frequency response for a specific insertion in each subject was measured in the ear canal using a slow chirp between 200 and 20 000 Hz. This response was normalized to that obtained in the long lossy tube and used to detect a half-wave resonance frequency. The compensation function for that particular half-wave frequency was then used to alter the stimuli before delivery to the ear canal.
Preliminary screening
The screening process consisted of otoscopy, pure-tone audiometry, tympanometry, measurement of contralateral acoustic reflex thresholds, and an OAE protocol described below. Audiometry was performed using standard clinical procedures (ASHA, 2005) bilaterally at 0.25, 0.5, 1, 2, 4, and 8 kHz using a Maico MI 26 clinical audiometer and TDH headphones. Tympanometry and contralateral reflex thresholds, using a 1000-Hz pure tone and a BBN as the activator in the non-test ear, were measured in the right ear (the test ear for all subjects) using an Interacoustic Audio Traveler AA220.
Spontaneous (S) OAEs and DPOAEs were measured during the screening procedure as well. A 3-min recording from the ear canal without any stimulation was analyzed using a 44,100-point fast Fourier transform (FFT) to detect SOAEs. The presence of SOAEs and their frequency location were noted and used in interpretation of results. Frequency regions with prominent SOAEs were not included in the DPOAE measurements.
DPOAEs were recorded by sweeping two stimulus tones between 500 and 6000 Hz [L1=55 dB sound pressure level (SPL), L2=40 dB SPL, and f2∕f1=1.22] over 32 s (Long et al., 2008). At least six sweeps were averaged before using a least-squares-fit (LSF) procedure (Long and Talmadge, 1997; Talmadge et al., 1999; Dhar et al., 2002, 2005) to estimate the level and phase of the DPOAE at the frequency 2f1-f2. DPOAEs were also recorded over a narrower frequency range between 1000 and 2000 Hz (L1=55 dB SPL, L2=40 dB SPL, f2∕f1=1.22, and stimuli swept over 8 s) with a BBN (0.1–10 kHz) presented in the contralateral ear at three levels (60, 70, and 80 dB SPL). The noise conditions were interleaved for level, with a 2-s silent interval between conditions, and were always preceded and followed by a recording without the contralateral noise. This entire sequence of five conditions was repeated eight times, allowing sufficient averaging to obtain an acceptable signal-to-noise ratio (SNR). The BBN was presented in the contralateral ear 1 s prior to the stimulus tones for all noise conditions to allow for the activation of the efferent response prior to initiation of DPOAE generation and recording. While this paradigm assures the activation of both fast and slow contralateral efferent effects, the stimulus tones themselves are likely to evoke ipsilateral efferent effects. Since our stimulus tones were swept in frequency always starting at the low frequencies, gradual activation of the slow ipsilateral efferent effects could affect the high frequencies more than the low frequencies. However, no frequency effects were found in our data leading us to collapse the results across frequencies in this report.
MEMR
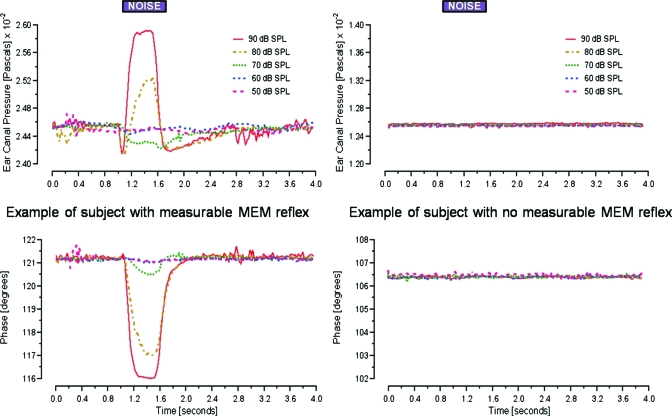
To identify activation of the MEMR, the magnitude and phase of a 602-Hz probe-tone at 60 dB SPL were monitored in the test ear. A BBN (described above) was presented contralaterally in 10-dB steps between 50 and 90 dB SPLs. The noise was played for a period of 500 ms between 1 and 1.5 s, while the tone was monitored over a period of 4 s. Between 8 and 12 recordings were made at each noise level and averaged before extracting the magnitude and phase of the 602-Hz tone using a LSF analysis. See Fig. 1 (left panel) for an example of a subject where systematic changes in magnitude and phase of the 602-Hz tone induced by the contralateral noise were observed; we considered the increase in the magnitude of the 602-Hz tone, time-locked to the contralateral noise, as an indicator of activation of the MEMR. The subject in the left panel of Fig. 1 was not included in the current subject pool because her MEMR threshold was below our criterion (described below). In contrast, no significant and systematic changes in the magnitude and pressure of the 602-Hz tone were observed at any noise level for the subject in the right panel of Fig. 1. Results such as these were interpreted to indicate no significant middle ear involvement in response to contralateral noise stimulation up to 90 dB SPL.
Figure 1.
Illustration of suspected MEMR recorded using laboratory technique. For all subjects a 602-Hz probe-tone was presented for 4 s in the test ear. A BBN activator was presented at five levels (50, 60, 70, 80, and 90 dB SPLs) in the contralateral ear between 1 and 1.5 s. Left panel: example of a subject showing measurable change in probe magnitude and phase starting at 70 dB SPL. Right: example of a subject with no measurable change in probe magnitude or phase at 90 dB SPL.
All subjects included in these experiments had an MEMR threshold greater than 90 dB hearing loss (HL) for both a 1-kHz tone and wide-band noise measured using the Interacoustics AA220. Three of our subjects showed observable changes in the magnitude and phase of the 602-Hz tone for a contralateral noise level of 90 dB SPL, while no changes in the magnitude and phase of the 602-Hz tone were observed at any noise level for the remaining five subjects. Other techniques for the same purpose have been reported in the literature (Guinan et al., 2003; Goodman and Keefe, 2006) and such laboratory methods have typically been found to be more sensitive in detecting MEMRs than clinical methods. Our method of monitoring middle ear muscle activity was at least 20 dB more sensitive for these subjects than the thresholds obtained using the Interacoustics AA220.
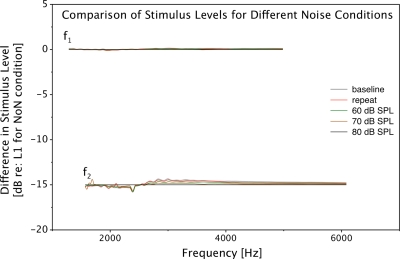
A final control to ensure that the changes observed in DPOAEs due to contralateral stimulation were not dominated by the MEMR was implemented by comparing the levels of the stimulus tones across all test conditions. We hypothesized that if the contralateral noise induced a MEMR, the level of the stimulus tones in the ear canal would be higher as compared to the recording without contralateral stimulation. An example of such a comparison is presented in Fig. 2. As demonstrated by the virtual overlap of all traces in the figure, no significant or systematic differences in the levels of the stimulus tones were induced by the contralateral noise. Close examination of Fig. 2 reveals some variations in the level of f2. However, these variations were not systematically related to the level of the contralateral noise. The level of f2 is reduced below approximately 2100 Hz and increased beyond that. Further, the greatest deviation is observed for the no-noise (NoN) conditions. Given these idiosyncrasies, we attributed these deviations to errors in calibration and not to the MEMR. Results from all other subjects were indistinguishable from the example displayed in Fig. 2.
Figure 2.
Comparison of stimulus levels for different test conditions plotted as the difference from the level of the low-frequency stimulus tone (f1) for the NoN condition. The traces overlap signifying no systematic difference in the stimulus levels for the various noise conditions. Some separation between the f2 levels can be observed. These changes were not found to be systematically related to the contralateral noise level.
These results guided the establishment of an upper limit of 80 dB SPL for the contralateral noise presentation during the DPOAE recordings and allowed us to be reasonably certain that the changes observed in the DPOAE recordings were dominated by the efferent system.
Fixed frequency experiment
A second set of recordings was obtained at four fixed DPOAE frequencies in each subject. These data points were selected to represent DPOAE fine structure extrema (one maximum and one minimum from the same fine structure period in the NoN condition, two periods per subject). The stimuli were identical to those used in the DPOAE sweep measurements (i.e., L1=55 dB SPL, L2=40 dB SPL, f2∕f1=1.22, and DPOAE at 2f1-f2) but with stimulus frequencies fixed to generate a DPOAE at the frequency of interest. Also, the same contralateral BBN conditions were used during these recordings (i.e., NoN; 60, 70, and 80 dB SPL; NoN for repeatability). Stimulus tones were played for 2 s, with the contralateral noise coming on at 0.5 s and turning off at 1 s with 5-ms on and off ramps. Thirty-two repetitions for each fixed frequency and each BBN condition were averaged.
Separation of DPOAE components
Components contributing to the DPOAE signal in the ear canal were separated using an inverse fast Fourier transform (FFT), time-windowing, and conversion to the frequency domain using an FFT (see Shaffer and Dhar, 2006 for methodological details). Briefly, subjecting the amplitude and phase of the DPOAE signal in the ear canal to an inverse FFT yields a pseudo-time domain representation of the DPOAE signal where the components from the overlap and DPOAE CF regions are distinguishable due to their differences in phase behavior as a function of frequency. Time domain filters were constructed for 400-Hz slices of data after visual inspection of the results of the inverse FFT. The two DPOAE components were then separated using these filters, and magnitude and phase of each component were individually reconstructed for the entire frequency range. Similar techniques have been used by several research groups in the literature (e.g., Stover et al., 1996; Kalluri and Shera, 2001; Dhar et al., 2005; Shaffer and Dhar, 2006).
Quantifying the effect of contralateral noise
The effect of varying levels of contralateral noise on DPOAE level and phase, as well as the level and phase of DPOAE components, was quantified using multiple measures. As has been the tradition, the effect of contralateral noise was computed as the difference in DPOAE level at a fixed frequency between the NoN condition and all noise conditions. This computation was done on three selected DPOAE level maxima and minima in each subject. These data points were selected such that at least a 3-dB SNR was maintained even for the highest level of contralateral noise. The DPOAE levels reported at an SNR of 3 dB could be influenced by external noise. However, choosing a higher SNR would not have allowed the analysis of DPOAE level minima. In parallel, the change in DPOAE level at these maxima and minima was also computed by tracking the maxima or minima as they shifted to different frequencies due to the contralateral noise. Finally, the shift in frequency of these maxima and minima was computed.
The change in level and phase of DPOAE components due to the contralateral noise was computed from the values for the NoN condition. These differences across frequency were then condensed to averages over third octave bands between 1 and 2 kHz. Statistical comparisons were made using SPSS version 17.0 (SAS, 2008).
RESULTS
General effects of contralateral noise
A general reduction in DPOAE level, an attenuation of fine structure depth, and a shift of fine structure patterns toward higher frequencies were observed for all three levels (60, 70, 80 dB SPLs) of contralateral BBN. These effects were more pronounced as the level of the contralateral noise increased. DPOAE level as a function of frequency from one subject (MOC038) for the initial and repeated NoN conditions (black traces), as well as for the three different noise conditions (color traces), is plotted in the top panel of Fig. 3 for illustrative purposes. Trends observed in Fig. 3 were consistent across subjects. The bottom two panels of Fig. 3 provide magnified views of two frequency regions enclosed in black boxes in the top panel. The fine structure extrema (maxima and minima) are tracked using purple squares and triangles, respectively. This tracking illustrates the shift of fine structure patterns toward higher frequencies.
The effects of contralateral BBN on DPOAE level were quantified in two ways. First, consistent with traditional analyses, the change in level at a fixed frequency was computed for each maximum or minimum. The orange arrows in the bottom panels of Fig. 3 schematically represent such a computation. Second, the change in DPOAE level for a given maximum or minimum was computed by tracking the level of that particular maximum or minimum, marked by the purple squares and triangles in the bottom panels of Fig. 3. Comparing the bottom panels of Fig. 3 clearly demonstrates the divergent results obtained when using the two different methods. The level of the minimum in the bottom right panel of Fig. 3 shows no systematic change with increasing contralateral BBN levels if the level at the exact minimum is tracked. However, an enhancement would be reported using the more traditional, fixed frequency method of comparison (orange arrow). In contrast, both methods would yield a reduction in DPOAE level in the case of the minimum in the bottom left panel of Fig. 3. These divergent results in two frequency regions within the same subject are due, primarily, to the shift in fine structure frequency. For a similar shift in frequency, the sharper minimum around 1720 Hz shows an apparent enhancement at the minimum frequency as compared to the reduction observed at the minimum around 1095 Hz.
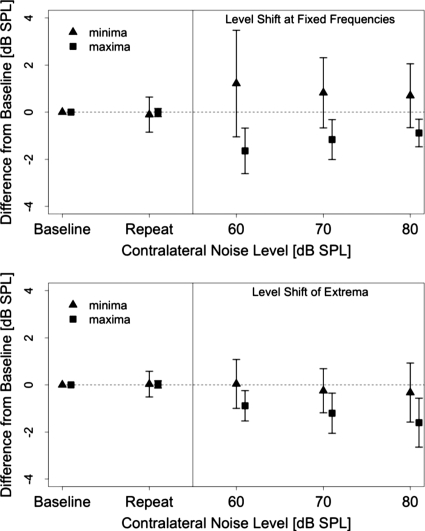
Three DPOAE level maxima and three minima were sampled from the eight subjects (total of 24 data points) where an SNR of at least 3 dB was maintained for all noise conditions. Maxima were chosen from the same fine structure period after an appropriate minimum was identified. Average changes at DPOAE level maxima (squares) and minima (triangles) computed using the traditional, fixed frequency method (top panel) and the tracking method (bottom panel) are presented in Fig. 4. The error bars represent one standard deviation. The averages for the baseline or NoN condition fall on the zero line by construction. Averages for the repeated NoN condition are also presented and show no differences from the baseline measures. DPOAE level at maxima shows a general reduction for the noise conditions using either method of calculation. The magnitude of reduction systematically increases with increasing noise level when the maximum frequency is tracked. The results are not as consistent for the fixed frequency method.
Figure 4.
Average level shifts at DPOAE fine structure extrema as a function of contralateral noise level at fixed frequencies (top panel) or for tracked extrema (bottom panel). Triangles represent fine structure minima and squares represent fine structure maxima. The DPOAE level recorded in the NoN condition established the baseline from which the shifts were calculated. A second NoN condition was included for test∕retest reliability. Error bars represent one standard deviation. Three maxima and three minima from each of eight subjects (24 total points) were averaged.
On average, DPOAE level minima show enhancement when the effect of contralateral noise is computed using the fixed frequency method (top panel of Fig. 4). In contrast, small reductions are observed in the average level of minima in the bottom panel of Fig. 4. Greater variability is observed in the minima for either method of computation, as compared to the maxima. However, the variability in minima is observably higher for the fixed frequency calculation.
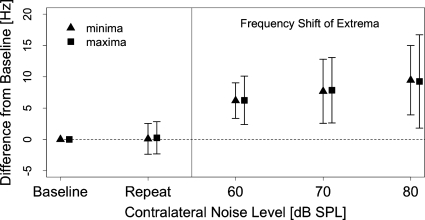
Average shifts in frequency for minima and maxima are presented in Fig. 5. The format of the figure is similar to the panels in Fig. 4. On average, the frequencies of maxima and minima appear to be consistent across the two NoN conditions. Both minima and maxima show a consistent shift toward the higher frequencies with increasing contralateral noise levels. The variability for both maxima and minima appears to increase with increasing contralateral noise levels.
Figure 5.
Average frequency shifts at DPOAE fine structure extrema as a function of contralateral noise level. Triangles and squares represent minima and maxima, respectively. The frequency of a given extrema in the NoN condition established the baseline from which the frequency shifts were calculated. A second NoN condition was included for test∕retest reliability. Error bars represent one standard deviation. Three maxima and three minima from each of eight subjects (24 total points) were averaged.
Effects on DPOAE components
The ear canal DPOAE was decomposed into the constituent components from the overlap and CF regions. Figure 6 displays DPOAE level as a function of frequency (top row) and phase (bottom row) of each DPOAE component from subjects MOC013 (left column) and MOC089 (right column). The range of the ordinate and the relatively small differences in DPOAE component phase for different noise conditions make it difficult to distinguish different traces in the bottom panels. The insets in the bottom panels highlight the phase behavior of each component over a 10-Hz frequency range. The bars next to the insets span a half cycle range. The phase of the overlap component is relatively invariant with frequency in both subjects. In contrast, the phase of the CF component accumulates approximately 6–8 cycles in the frequency span displayed.
Figure 6.
Ear canal DPOAE level (top) and DPOAE component phases (bottom) as a function of frequency in two subjects. See text for method used to separate DPOAE components. The insets in the bottom panel offer a detailed view of the phases of each component over a 10-Hz range with different levels of contralateral noise as the parameter. The typical response of a larger change in the phase of the CF component is observed in the bottom left panel. The bottom right panel depicts an atypical response, in only one of eight subjects, where the slope of the phase of the CF component was altered in the presence of contralateral noise.
Changes in the phase of the overlap component due to contralateral noise are difficult to detect in either subject. The phase of the CF component shows a visually detectable lead without a change in slope in the inset for subject MOC013 (bottom left) for the noise conditions. This was the pattern observed in seven of the eight subjects. The only exception to this pattern was seen in subject MOC089 (bottom right). In this case the slope of the phase of the CF component was steeper in the presence of contralateral noise between 1100 and 1300 Hz. While a consistent shift of fine structure toward higher frequencies is observed in subject MOC013 (left column), such a shift, especially between 1100 and 1300 Hz, is not observed in subject MOC089 (right column). We emphasize that the pattern seen in the bottom right panel was unique to subject MOC089; data from all other subjects showed a pattern consistent with that observed for subject MOC013 in the left column.
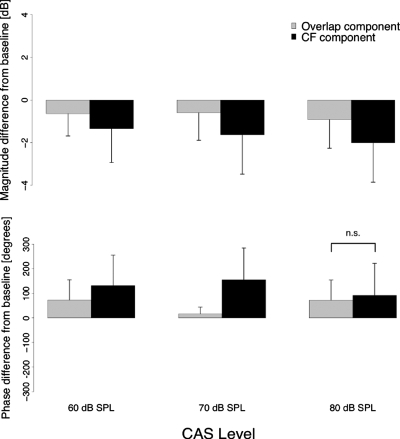
Changes in DPOAE component level (top) and phase (bottom) averaged across the entire frequency range and then averaged across subjects are displayed in Fig. 7 as a function of BBN level. The error bars represent one standard deviation from the mean. Greater reduction in the magnitude of the CF component and greater increase in the phase of the CF component were observed for all noise conditions. Paired t-tests indicated significant differences in both magnitude and phase between the two DPOAE components (p<0.008, compensated for multiple comparisons) for all but one paired contrast: The change in phase of the two components was not statistically significantly different for the contralateral noise level of 80 dB SPL [t(373)=2.601,p=0.01]. We draw the reader’s attention to the greater reduction in the level and greater increase in the phase of the CF component. Much of our interpretation of the data will hinge on this.
Figure 7.
Average change in level (top) and phase (bottom) in DPOAE components due to different levels of contralateral noise. The reported differences were calculated by first averaging the component level or (unwrapped) phase over the entire recorded frequency range and then subtracting the average values of the NoN condition from the average values for each noise condition. Error bars represent one standard deviation. Top panel: change in each component level for three different noise levels. Bottom panel: change in each component phase for three different noise levels. All contrasts between the two components were statistically significant (p<0.05) except the change in phase of the two components for the contralateral noise level of 80 dB SPL.
Results at fixed frequencies
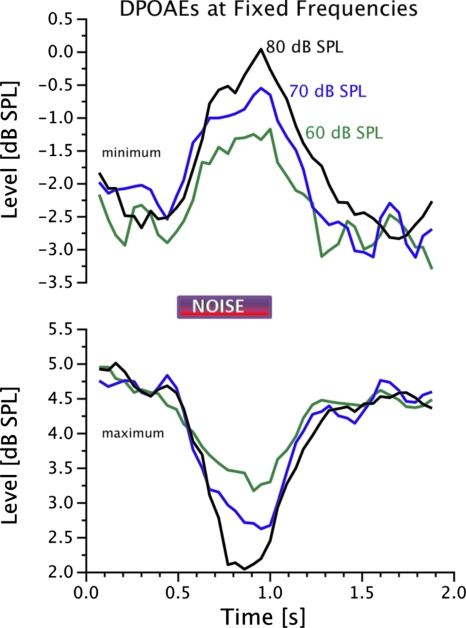
To confirm the results observed in the data obtained with swept stimulus frequencies, DPOAE levels were monitored over time at fixed frequencies corresponding to DPOAE level maxima or minima while presenting different levels of contralateral noise. Results of this experiment from one subject at one minimum (top) and one maximum (bottom) are presented in Fig. 8. The time window in which the contralateral noise was presented is marked in the figure. In the case of this minimum, DPOAE level increased due to the contralateral noise, and this increase was more pronounced for greater levels of contralateral noise. In the case of this maximum, the level of the DPOAE showed a consistent reduction due to the contralateral noise, and the reduction was more pronounced with increasing noise levels. While all 24 DPOAE level maxima monitored in this experiment showed consistent reduction, the effects of contralateral noise were more varied for the minima.
Figure 8.
Change in DPOAE level at a fixed frequency over time with different levels of contralateral noise presented for 500 ms between 0.5 and 1 s. Recordings were made for a fine structure minimum (top panel) or maximum (bottom panel) as determined from the NoN condition.
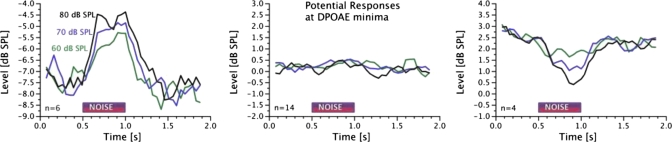
Examples of the three types of effects observed at minima are displayed in Fig. 9. An example of contralateral noise-induced enhancement is displayed in the left panel. This effect was observed in 6 of the 24 minima. The most common observation, in 14 of the 24 minima, was of no consistent change in DPOAE level, as shown in the middle panel. Finally, reduction in DPOAE level upon presentation of contralateral noise as shown in the right panel was observed in 4 of the 24 minima. When either enhancement or reduction was observed, the effect grew with increasing noise level. The time course of change in DPOAE level displayed in Figs. 89 should not be interpreted to be physiologically relevant as they were influenced by the averaging employed to enhance SNR.
Figure 9.
Examples of three types of responses observed at fine structure minima in the fixed frequency experiment. Upon the presentation of the contralateral noise, DPOAE level increased in 6 cases, did not show systematic change in 14 cases, and decreased in 4 cases of the 24 minima examined.
DISCUSSION
The results presented here continue the examination of the modulation of OAEs by the olivocochlear efferents that was started almost 3 decades ago (Mountain, 1980; Siegel and Kim, 1982). Most recently, an in-depth examination of efferent alteration of DPOAE fine structure has become one of the foci of this area of work (Zhang et al., 2007; Purcell et al., 2008; Sun, 2008a, 2008b; Abdala et al., 2009). We begin by highlighting the consistency of these results with previous work and then discussing the issues of the frequency shift observed in fine structure patterns and the most appropriate clinical measure of the efferent control of DPOAEs.
Reduction and enhancement of DPOAE level
The change in DPOAE level at fixed frequencies is the appropriate comparison with data from literature. Consistent with previous work (e.g., Lisowska et al., 2002; Zhang et al., 2007; Abdala et al., 2009), an average reduction of 1.64±0.96 dB at maxima and average enhancement of 1.21±2.83 dB at minima were observed for the contralateral noise condition of 60 dB SPL. For the 70 dB SPL condition, the average level shifts were 1.16±0.85 dB (reduction) at maxima and 0.82±1.40 dB (enhancement) at minima, also consistent with previous work (Zhang et al., 2007). The magnitude of the effects continued to decline at both maxima and minima (Fig. 4) for the 80 dB SPL condition. The consistency of these results with previous work survives the methodological differences between these reports. For example, Abdala et al. (2009) used stimulus levels of 65 and 55 dB SPLs and reported efferent-induced reduction in level at maxima averaged across frequency bands spanning 2 mm of the basilar membrane.
Both minima and maxima showed a more consistent change with increasing noise levels when the effects of the contralateral noise were quantified by tracking individual extrema (Fig. 4, bottom panel). Changes were smaller in magnitude at the minima using this method, but the average change was a reduction rather than an enhancement. The magnitude of the effect exhibited growth for both maxima and minima with increasing contralateral noise level. The effect was smaller for the minima as compared to the maxima, arguably due to the limitation imposed by the noise floor in the measurement ear.
Also consistent with literature, the effect of the contralateral noise was variable at minima, as evident in the larger variance in Fig. 4 and the variety of effects seen in the time domain in Fig. 9. Such variability has been reported by others (Zhang et al., 2007) with the greatest effect often observed at these minima, especially when the absolute value of the change (irrespective of the direction) is considered (Müller et al., 2005; Wagner et al., 2007).
An important question that many authors have addressed is that of the most appropriate clinical measure of the effects of the efferent system on DPOAEs. Some have argued for using the measure that yields the largest response, often seen at minima, especially when the range of change from the greatest reduction to the greatest enhancement is taken into account (Müller et al., 2005; Wagner et al., 2007). Others have supported the use of a more consistent (but smaller) effect by measuring the change only at maxima (e.g., Abdala et al., 2009). Yet others have offered an array of quantification schemes available from swept-frequency measurements such as those employed here (Purcell et al., 2008). In a similar vein, we have presented two alternate methods of quantifying the effects of efferent activation on DPOAEs, first by measuring the effect at a fixed frequency, and second by tracking individual maxima and∕or minima as they shift in frequency with the introduction of contralateral noise. In either case, data at maxima appear to yield more consistent results.
Given the capacity of current clinical equipment, it is unrealistic to expect a viable protocol where the clinician first searches for a DPOAE level maximum in a given frequency range and then measures the effects of contralateral stimulation at that target frequency. A more panoramic measure using stimulus tones swept continuously in frequency as employed here and elsewhere (Purcell et al., 2008; Abdala et al., 2009) is not available to the clinician either. An alternate approach could be to sample each fine structure period by making measurements at a few adjacent target frequencies and then using the largest reduction as the nominal measure of efferent alteration of DPOAE. Spontaneous, transient evoked, and stimulus frequency OAEs show a consistent spacing pattern that can be characterized to the value f∕Δf=16, where f is the center frequency and Δf is the frequency distance between adjacent SOAEs or maxima∕minima of transient or stimulus frequency OAEs (e.g., Zwicker, 1990; Shera, 2003). Although shown not to coincide exactly with other types of OAEs (Lutman and Deeks, 1999), approximate correspondence of DPOAE fine structure spacing to these other measures has been demonstrated (Reuter and Hammershoi, 2006; Dhar and Abdala, 2007). Based on this estimate of fine structure spacing, measurements made at f, f±(f∕4), and f±(f∕8), where f is the frequency of interest, should allow the sampling of a data point at or near the maximum of that particular fine structure period. Thus, measuring the effect of the MOC on five frequency points would give a realistic chance of sampling one frequency near or at a DPOAE level maximum. This data point could be identified as the one showing a small but consistent effect on multiple measures and then be used as the metric of MOC strength in that frequency range for that subject.
The second dimension: A shift in frequency
A key observation of this study is that contralateral noise causes a frequency shift in DPOAE fine structure. Such a shift in frequency has been observed (Purcell et al., 2008; Sun, 2008b) and quantified (Abdala et al., 2009) in recent reports. The magnitude and direction of frequency shifts observed here are consistent with previous reports. Both maxima and minima show consistent and parallel shifts in frequency (Fig. 5). Shifts in spontaneous emission frequency due to CAS have been reported before (Mott et al., 1989; Harrison and Burns, 1993) and are arguably mediated by similar mechanisms that cause shifts in DPOAE fine structure.
Although the shift in DPOAE fine structure has been demonstrated before, the cause behind this shift has not been explored in detail. Abdala et al. (2009) (p. 1593) commented the following: “Future studies are warranted to further elucidate the mechanism and biological significance of this frequency shift.” We are able to demonstrate at least the first layer of cochlear macromechanics responsible for this frequency shift.
It has long been speculated (Siegel and Kim, 1982; Maison and Liberman, 2000; Kim et al., 2001; Zhang et al., 2007; Purcell et al., 2008; Sun, 2008a, 2008b) and more recently documented (Abdala et al., 2009) that the activation of the efferents has a differential effect on the two DPOAE components, with the component from the CF region being affected more than the component from the overlap region. However, this greater effect has been demonstrated for component levels only. That is, the level of the CF component is reduced more than the level of the overlap component upon efferent stimulation (Abdala et al., 2009). Such a model can fully explain the enhancement of the DPOAE level observed in the ear canal at DPOAE level minima. The two DPOAE components are at phase opposition at minima and selective or greater reduction in the CF component would in effect release the overlap component from cancellation and cause an enhancement in the overall signal in the ear canal. A frequency shift in fine structure patterns is not as easily explained by this differential change in DPOAE component level. However, these and previous results (Zhang et al., 2007; Purcell et al., 2008; Sun, 2008a, 2008b; Abdala et al., 2009) definitively indicate that frequency shifts in fine structure patterns are at least as responsible as the release of the overlap component from cancellation for the enhancements observed at DPOAE minima due to MOC activation.
However, the frequency shift can be explained if the phases of the two DPOAE components are affected differently by efferent activation. One possibility is that the slope of the phase of the CF component is changed by the contralateral noise, while that of the overlap component is either unchanged or changed to a relatively lesser degree. In order to result in a shift toward the higher frequencies, the slope of the CF component would have to become shallower upon presentation of the contralateral noise relative to the NoN condition. This would result in a shift of DPOAE frequency toward higher frequencies as well as a widening of fine structure spacing. Fine structure spacing has been reported to remain unchanged upon efferent activation (Abdala et al., 2009), a result supported observationally in our data even though we did not quantify fine structure spacing.
A shift toward the higher frequencies can also be caused if the overall phase of the CF component shows a lead due to the contralateral noise. For a frequency shift to occur, this lead has to be greater than any lead seen in the phase of the overlap component. This appears to be the case as displayed in the bottom panel of Fig. 7. While both the overlap and CF component phases show a lead at all levels of contralateral noise, the change is statistically significantly larger for the CF component (except for the noise condition at 80 dB SPL). This shift in phase without a change in the slope of the phase results in a shift of DPOAE fine structure toward higher frequencies without a change in spacing. A change in the slope of the CF component phase was observed in one subject (MOC089, right panel of Fig. 6). The slope of the CF component phase became steeper with the introduction of the noise but frequency shifts in the fine structure pattern were not evident, especially between 1100 and 1300 Hz where the change in slope was most evident.
MOC effect and MEM contamination
All reports of modulation of OAEs by acoustic stimulation of the efferents must carefully treat the issue of accidental activation of the MEMR. We attempted to control for this by choosing a group of subjects with high MEMR thresholds and then making additional measurements to ensure that the MEMR was not activated for the levels of contralateral noise used (see Figs. 12). Based on these precautions, we are fairly confident that the effects demonstrated here are dominated by the MOC efferents. Consistent with this assertion, we did not observe any abrupt discontinuities in the trajectories of change in either DPOAE level or frequency with increasing stimulus level. Such a change in trajectory has been associated with the activation of the MEMR (Guinan et al., 2003).
CONCLUSIONS
Contralateral stimulation with BBN causes changes in both DPOAE level and frequency of fine structure patterns. The most consistent reduction is observed by tracking the change in DPOAE level maxima, taking into account the shifts in the frequency of these patterns with the introduction of contralateral noise. The oft-reported enhancement at minima is caused not only by a greater reduction in the DPOAE component from the CF region but also by a shift in DPOAE fine structure patterns toward higher frequencies. Both the level and the phase of the CF component are affected to a greater extent by the contralateral noise. The effective input for the generation of the CF component is the portion of the overlap component that travels inward rather than outward toward the ear canal. This input is smaller in magnitude than the stimulus tones that serve as the input for the generation of the overlap component. Whether this greater effect on the CF component is because of a lower level input or reflective of mechanistic differences in the generation of the two DPOAE components remains an open question. Since both the level and phase of DPOAE components are affected by the contralateral noise, tracking true change in DPOAE level is best achieved by tracking the magnitude of a level maximum or minimum as it shifts in frequency. This ensures that the observed change in DPOAE magnitude is due mostly to changing magnitudes of DPOAE components as changes in the relative phase of the two components are accounted for.
ACKNOWLEDGMENTS
We would like to thank Dashiell Oatman-Stanford and Deepika Sriram for assistance with data collection. Helpful discussions with Wei Zhao, Renee Banakis, Hwa Jung Son, Mead Killion, Carolina Abdala, and Jonathan Siegel shaped our thinking about the issues presented here. This research was funded by Grant Nos. R01DC008420 and R01DC003552 (subcontract to Northwestern from the House Ear Institute) from the NIDCD, and the Hugh Knowles Center for Hearing Research at Northwestern University. Parts of this work were presented at the 2009 Annual Convention of the American Auditory Society.
References
- Abdala, C., Mishra, S. K., and Williams, T. L. (2009). “Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex,” J. Acoust. Soc. Am. 125, 1584–1594. 10.1121/1.3068442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association (ASHA) (2005). Guidelines for Manual Pure-Tone Threshold Audiometry, ASHA, Rockville, MD. [Google Scholar]
- Bassim, M. K., Miller, R. L., Buss, E., and Smith, D. W. (2003). “Rapid adaptation of the 2f1-f2 DPOAE in humans: Binaural and contralateral stimulation effects,” Hear. Res. 182, 140–152. 10.1016/S0378-5955(03)00190-4 [DOI] [PubMed] [Google Scholar]
- Dallos, P. (2008). “Cochlear amplification, outer hair cells and prestin,” Curr. Opin. Neurobiol. 18, 370–376. 10.1016/j.conb.2008.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar, S., and Abdala, C. (2007). “A comparative study of distortion-product-otoacoustic-emission fine structure in human newborns and adults with normal hearing,” J. Acoust. Soc. Am. 122, 2191–2202. 10.1121/1.2770544 [DOI] [PubMed] [Google Scholar]
- Dhar, S., Long, G. R., Talmadge, C. L., and Tubis, A. (2005). “The effect of stimulus-frequency ratio on distortion product otoacoustic emission components,” J. Acoust. Soc. Am. 117, 3766–3776. 10.1121/1.1903846 [DOI] [PubMed] [Google Scholar]
- Dhar, S., and Shaffer, L. A. (2004). “Effects of a suppressor tone on distortion product otoacoustic emissions fine structure: Why a universal suppressor level is not a practical solution to obtaining single-generator DP-grams,” Ear Hear. 25, 573–585. 10.1097/00003446-200412000-00006 [DOI] [PubMed] [Google Scholar]
- Dhar, S., Talmadge, C. L., Long, G. R., and Tubis, A. (2002). “Multiple internal reflections in the cochlea and their effect on DPOAE fine structure,” J. Acoust. Soc. Am. 112, 2882–2897. 10.1121/1.1516757 [DOI] [PubMed] [Google Scholar]
- Giraud, A. L., Collet, L., Chery-Croze, S., Magnan, J., and Chays, A. (1995). “Evidence of a medial olivocochlear involvement in contralateral suppression of otoacoustic emissions in humans,” Brain Res. 705, 15–23. 10.1016/0006-8993(95)01091-2 [DOI] [PubMed] [Google Scholar]
- Goodman, S. S., and Keefe, D. H. (2006). “Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions,” J. Assoc. Res. Otolaryngol. 7, 125–139. 10.1007/s10162-006-0028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga, M. P., Neely, S. T., Ohlrich, B., Hoover, B., Redner, J., and Peters, J. (1997). “From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss,” Ear Hear. 18, 440–455. 10.1097/00003446-199712000-00003 [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr. (2006). “Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans,” Ear Hear. 27, 589–607. 10.1097/01.aud.0000240507.83072.e7 [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr., Backus, B. C., Lilaonitkul, W., and Aharonson, V. (2003). “Medial olivocochlear efferent reflex in humans: Otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs,” J. Assoc. Res. Otolaryngol. 4, 521–540. 10.1007/s10162-002-3037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan, J. J., Jr., and Gifford, M. L. (1988). “Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. III. Tuning curves and thresholds at CF,” Hear. Res. 37, 29–45. 10.1016/0378-5955(88)90075-5 [DOI] [PubMed] [Google Scholar]
- Harrison, W. A., and Burns, E. M. (1993). “Effects of contralateral acoustic stimulation on spontaneous otoacoustic emissions,” J. Acoust. Soc. Am. 94, 2649–2658. 10.1121/1.407349 [DOI] [PubMed] [Google Scholar]
- Heitmann, J., Waldmann, B., Schnitzler, H. U., Plinkert, P. K., and Zenner, H. P. (1998). “Suppression of distortion product otoacoustic emissions (DPOAE) near f1-f2 removes DP-gram fine structure—Evidence for a secondary generator,” J. Acoust. Soc. Am. 103, 1527–1531. 10.1121/1.421290 [DOI] [Google Scholar]
- Kalluri, R., and Shera, C. A. (2001). “Distortion-product source unmixing: A test of the two-mechanism model for DPOAE generation,” J. Acoust. Soc. Am. 109, 622–637. 10.1121/1.1334597 [DOI] [PubMed] [Google Scholar]
- Kawase, T., Delgutte, B., and Liberman, M. C. (1993). “Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones,” J. Neurophysiol. 70, 2533–2549. [DOI] [PubMed] [Google Scholar]
- Kemp, D. T. (1978). “Stimulated acoustic emissions from within the human auditory system,” J. Acoust. Soc. Am. 64, 1386–1391. 10.1121/1.382104 [DOI] [PubMed] [Google Scholar]
- Kim, D. O., Dorn, P. A., Neely, S. T., and Gorga, M. P. (2001). “Adaptation of distortion product otoacoustic emission in humans,” J. Assoc. Res. Otolaryngol. 2, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman, M. C., Puria, S., and Guinan, J. J., Jr. (1996). “The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1-f2 distortion product otoacoustic emission,” J. Acoust. Soc. Am. 99, 3572–3584. 10.1121/1.414956 [DOI] [PubMed] [Google Scholar]
- Lisowska, G., Smurzynski, J., Morawski, K., Namyslowski, G., and Probst, R. (2002). “Influence of contralateral stimulation by two-tone complexes, narrow-band and broad-band noise signals on the 2f1-f2 distortion product otoacoustic emission levels in humans,” Acta Oto-Laryngol. 122, 613–619. 10.1080/000164802320396286 [DOI] [PubMed] [Google Scholar]
- Long, G. R., and Talmadge, C. L. (1997). “Spontaneous otoacoustic emission frequency is modulated by heartbeat,” J. Acoust. Soc. Am. 102, 2831–2848. 10.1121/1.420339 [DOI] [PubMed] [Google Scholar]
- Long, G. R., Talmadge, C. L., and Lee, J. (2008). “Measuring distortion product otoacoustic emissions using continuously sweeping primaries,” J. Acoust. Soc. Am. 124, 1613–1626. 10.1121/1.2949505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutman, M. E., and Deeks, J. (1999). “Correspondence amongst microstructure patterns observed in otoacoustic emissions and Bekesy audiometry,” Audiology 38, 263–266. [DOI] [PubMed] [Google Scholar]
- Maison, S. F., and Liberman, M. C. (2000). “Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength,” J. Neurosci. 20, 4701–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott, J. B., Norton, S. J., Neely, S. T., and Warr, W. B. (1989). “Changes in spontaneous otoacoustic emissions produced by acoustic stimulation of the contralateral ear,” Hear. Res. 38, 229–242. 10.1016/0378-5955(89)90068-3 [DOI] [PubMed] [Google Scholar]
- Mountain, D. C. (1980). “Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics,” Science 210, 71–72. 10.1126/science.7414321 [DOI] [PubMed] [Google Scholar]
- Müller, J., Janssen, T., Heppelmann, G., and Wagner, W. (2005). “Evidence for a bipolar change in distortion product otoacoustic emissions during contralateral acoustic stimulation in humans,” J. Acoust. Soc. Am. 118, 3747–3756. 10.1121/1.2109127 [DOI] [PubMed] [Google Scholar]
- Murugasu, E., and Russell, I. J. (1996). “The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea,” J. Neurosci. 16, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, D. W., Butler, B. E., Saunders, T. J., and Allen, P. (2008). “Distortion product otoacoustic emission contralateral suppression functions obtained with ramped stimuli,” J. Acoust. Soc. Am. 124, 2133–2148. 10.1121/1.2973192 [DOI] [PubMed] [Google Scholar]
- Puria, S., Guinan, J. J., Jr., and Liberman, M. C. (1996). “Olivocochlear reflex assays: Effects of contralateral sound on compound action potentials versus ear-canal distortion products,” J. Acoust. Soc. Am. 99, 500–507. 10.1121/1.414508 [DOI] [PubMed] [Google Scholar]
- Relkin, E. M., Sterns, A., Azeredo, W., Prieve, B. A., and Woods, C. I. (2005). “Physiological mechanisms of onset adaptation and contralateral suppression of DPOAEs in the rat,” J. Assoc. Res. Otolaryngol. 6, 119–135. 10.1007/s10162-004-5047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, K., and Hammershoi, D. (2006). “Distortion product otoacoustic emission fine structure analysis of 50 normal-hearing humans,” J. Acoust. Soc. Am. 120, 270–279. 10.1121/1.2205130 [DOI] [PubMed] [Google Scholar]
- SAS (2008). “SPSS for Mac OS X,” SPSS Inc., Chicago, IL.
- Shaffer, L. A., and Dhar, S. (2006). “DPOAE component estimates and their relationship to hearing thresholds,” J. Am. Acad. Audiol. 17, 279–292. 10.3766/jaaa.17.4.6 [DOI] [PubMed] [Google Scholar]
- Shera, C. A. (2003). “Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves,” J. Acoust. Soc. Am. 114, 244–262. 10.1121/1.1575750 [DOI] [PubMed] [Google Scholar]
- Siegel, J. H., and Kim, D. O. (1982). “Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity,” Hear. Res. 6, 171–182. 10.1016/0378-5955(82)90052-1 [DOI] [PubMed] [Google Scholar]
- Stover, L. J., Neely, S. T., and Gorga, M. P. (1996). “Latency and multiple sources of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 99, 1016–1024. 10.1121/1.414630 [DOI] [PubMed] [Google Scholar]
- Sun, X. M. (2008a). “Contralateral suppression of distortion product otoacoustic emissions and the middle-ear muscle reflex in human ears,” Hear. Res. 237, 66–75. 10.1016/j.heares.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Sun, X. M. (2008b). “Distortion product otoacoustic emission fine structure is responsible for variability of distortion product otoacoustic emission contralateral suppression,” J. Acoust. Soc. Am. 123, 4310–4320. 10.1121/1.2912434 [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Long, G. R., Tubis, A., and Dhar, S. (1999). “Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 105, 275–292. 10.1121/1.424584 [DOI] [PubMed] [Google Scholar]
- Wagner, W., Heppelmann, G., Muller, J., Janssen, T., and Zenner, H. P. (2007). “Olivocochlear reflex effect on human distortion product otoacoustic emissions is largest at frequencies with distinct fine structure dips,” Hear. Res. 223, 83–92. 10.1016/j.heares.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Zhang, F., Boettcher, F. A., and Sun, X. M. (2007). “Contralateral suppression of distortion product otoacoustic emissions: Effect of the primary frequency in Dpgrams,” Int. J. Audiol. 46, 187–195. 10.1080/14992020601164162 [DOI] [PubMed] [Google Scholar]
- Zwicker, E. (1990). “On the frequency separation of simultaneously evoked otoacoustic emissions’ consecutive extrema and its relation to cochlear traveling waves,” J. Acoust. Soc. Am. 88, 1639–1641. 10.1121/1.400324 [DOI] [PubMed] [Google Scholar]