Abstract
The acute impairing effects of alcohol on inhibitory control have been well documented in healthy drinkers. By contrast, little is known about alcohol effects in individuals with disorders characterized by poor impulse control, such as those with ADHD. Alcohol could produce greater inhibitory impairment in these individuals. The present study tested this hypothesis in adults with ADHD (N = 10) and controls (N = 12) using the cued go/no-go task. The task requires quick responses to go targets and suppression of responses to no-go targets following the presentation of cues. Prior research on healthy adults shows that valid cues can protect against alcohol impairment (Marczinski & Fillmore, 2003). Performance was tested under 3 doses of alcohol: 0.65 g/kg, 0.45 g/kg, and 0.0 g/kg (placebo). Alcohol dose-dependently increased inhibitory failures in controls in the invalid, but not the valid, cue condition. By contrast, those with ADHD displayed significant alcohol impairment regardless of cue condition. Thus, unlike controls, valid cues offered little protection from the disinhibiting effects of alcohol in drinkers with ADHD, suggesting an increased sensitivity to alcohol-impairment of inhibitory control.
Keywords: ADHD, alcohol, inhibitory control, cues
Alcohol and other drug abuse disorders are considered by many investigators to be symptomatic of some disinhibitory psychopathology (Cloninger, 1987; Finn, Kessler, & Hussong 1994; Sher & Trull, 1994; Widiger & Smith, 1994). This argument is based on findings from studies examining drug abuse in relation to impulsivity/disinhibition as a central characteristic of a psychopathology. For example, several studies have examined the link between DSM personality disorder clusters and drug abuse. The general finding from this research is that substance abuse disorders have a high comorbidity with cluster B personality disorders (e.g., Grekin, Sher, & Wood, 2006; Trull, Waudby, & Sher, 2004). This cluster includes antisocial, borderline, and histrionic disorders, which are all characterized by under-controlled, disinhibited, and impulsive patterns of behavior. It is also well established that externalizing disorders, such as attention deficit/hyperactivity disorder (ADHD) and conduct disorder (CD), pose risk for developing substance abuse disorders (Barkley, 2006; Flory, Milich, & Lynam, 2003; Flory & Lynam, 2003; Molina, Smith, & Pelham, 1999). A hallmark characteristic of externalizing disorders, such as ADHD, is disinhibited or undercontrolled behavior.
Although it is important to characterize the behavioral correlates of drug abuse in terms of complex traits and personality, there is also a need to identify the specific behavioral mechanisms that increase risk for alcohol abuse in individuals with disinhibitory disorders, such as ADHD. Cognitive neuroscience approaches the concept of impaired self-control with the aim of identifying and characterizing the basic neurocognitive mechanisms that underlie the regulation of behavior (Goschke, 2003; Miller & Cohen, 2001). Theoretically based tasks that model the joint contribution of inhibitory and activational mechanisms in the control of behavior have been utilized to study the ability to inhibit inappropriate behavioral responses (Fillmore, 2003; 2007). Two of these tasks are the stop-signal and the cued go/no-go task (Logan, 1994; Miller, Schaffer, & Hackley, 1991). The tasks model behavioral control using a reaction-time scenario that measures the countervailing influences of inhibitory and activational mechanisms. Individuals are required to activate a response to a go-signal quickly and to inhibit a response when a stop-signal occasionally occurs. Activation is typically measured as the speed of responding to go-signals, and inhibition to stop-signals is assessed by the probability of suppressing the response or by the time needed to suppress the response. In these models, inhibition of a response is usually required in a context in which there is a strong tendency to respond to a stimulus (i.e., a pre-potency), thus making inhibition difficult.
Stop-signal and cued go/no-go tasks have demonstrated inhibitory deficits in children with ADHD for many years (Alderson, Rapport, & Kofler, 2007; Barkley, 1997; Lijffijit, Kenemans, Verbaten, & Van Engeland, 2005; Oosterlaan, Logan, & Sergeant, 1998; Tannock, 1998). More recently, these tasks have been used to examine inhibitory deficits in adults with ADHD. For example, Bekker and colleagues (2005) administered a stop-signal task to adults with ADHD and comparison controls. The authors found that stop-signal reaction time was significantly slower than the go-stimulus reaction time in adults with ADHD, indicating deficits in inhibitory mechanisms relative to activational mechanisms that were not observed in controls. Additionally, individuals with ADHD committed more errors, which the authors suggested might reflect more premature responses and decreased response inhibition.
These same models of inhibitory control also have been used to detect acute impairing effects of drugs, such as alcohol (de Wit, Crean, & Richards, 2000; Fillmore & Vogel-Sprott, 1999; Mulvihill, Skilling, & Vogel-Sprott, 1997). Studies using stop-signal and cued go/no-go tasks find that moderate doses of alcohol selectively reduce the drinker's ability to inhibit behavior at doses that leave the ability to activate behavior relatively unaffected (Marczinski & Fillmore, 2003; Mulvihill et al., 1997). What is particularly remarkable about these findings is the robust impairment that is evident despite the relatively modest doses examined. The impairing effects on inhibitory control are often observed at blood alcohol concentrations (BACs) at or below 80 mg/100 ml (Fillmore, 2003; 2007), the legal limit of intoxication throughout most of the United States. The findings suggest that activities requiring the quick suppression of actions might be particularly vulnerable to the disruptive influences of alcohol. Moreover, the acute impairments produced by alcohol closely resemble those inhibitory deficits observed in individuals with ADHD (Fillmore, 2007; Fillmore & Vogel-Sprott, 1999). This raises an intriguing possibility that alcohol temporarily disrupts cognitive functioning in a manner similar to the enduring cognitive disturbances that are characteristic of disorders like ADHD.
To date, studies of the acute impairing effects of alcohol on inhibitory control have examined primarily healthy drinkers and no studies have tested these effects in individuals with disorders characterized by poor inhibitory control, such as those with ADHD. Considering that disinhibition is thought to play an important role in the development of drug abuse problems, it is important to determine if individuals with ADHD might be more sensitive to the acute disinhibiting effect of alcohol. Given that adults with ADHD demonstrate preexisting deficits involving inhibitory control, it seems reasonable to assume that alcohol could produce greater disruption of inhibitory control in these individuals compared with adults with no history of ADHD.
The present study was designed to test this hypothesis using the cued go/no-go task as a measure of inhibitory control. The cued go/no-go task was chosen because the task has been well-documented for its sensitivity to the disinhibiting effects of moderate doses of alcohol (e.g., Fillmore & Weafer, 2004; Marczinski & Fillmore, 2003), and its sensitivity to inhibitory deficits associated with ADHD (Derefinko, Adams, Milich, Fillmore, Lorch, & Lynam, 2008). Moreover, the task also examines the degree to which antecedent environmental cues can contribute to, or provide protection from, the impairing effects of alcohol. Response preparation is critical for behavioral control (Miller et al., 1991). The task cues provide information concerning the probability that a go or no-go target will be presented. The cue-target relationship is manipulated so that cues have a high probability of correctly signaling a go or no-go target (valid cues), and a low probability of incorrectly signaling a target (invalid cues). Correct (i.e., valid) cues facilitate response execution and response inhibition. For example, responses to go targets are faster when they are preceded by a go cue. Similarly, the likelihood of suppressing a response to a no-go target is greater when it is preceded by a no-go cue. Moreover, studies using the cued go/no-go task show that valid cues also protect the drinker from the impairing effects of moderate alcohol doses (Fillmore, 2004; Marczinski & Fillmore, 2003). In a study of healthy adults, Marczinski and Fillmore (2003) showed that alcohol produced a dose-dependent increase in inhibitory failures following invalid go cues but had no effect on inhibitory failures following the valid no-go cues. The same cue-dependent pattern of results was observed for response execution. Alcohol slowed reaction time in a dose-dependent manner following the invalid no-go cues, but had no effect on reaction time following the valid go cues. Such protective effects of valid cues have been attributed to covert preparation to either execute or suppress an action before the actual go or no-go target is presented (Fillmore, 2004).
To date, the protective effects of valid cues from the impairing effects of alcohol have been well-documented in studies of healthy adults. However, no research has examined the effects of the cues in adults with pre-existing deficits of behavioral control, such as those with ADHD. The present study compared adults with ADHD to healthy controls in terms of their performance on the cued go/no-go task in response to three doses of alcohol (placebo, 0.45 g/kg, and 0.65 g/kg). Based on previous studies it was predicted that inhibitory control would be impaired in response to active doses of alcohol. Further, it was predicted that this impairment would be most pronounced in the context of invalid cues and among those with ADHD.
Method
Participants
Ten participants with ADHD (8 men and 2 women; M age = 22.8 years, SD = 1.8) and 12 control participants (9 men and 3 women; M age = 22.8 years, SD = 1.1) with no history of ADHD took part in this study. The racial make-up of the ADHD group was Caucasian (N = 10) and the racial make-up of the control group was Caucasian (N = 10) and African American (N = 2). Volunteers were recruited by flyers, posters, and newspaper advertisements seeking adults for studies of the effects of alcohol on cognitive functions. The study was approved by the university Medical Institutional Review Board. Volunteers completed questionnaires that provided demographic information and alcohol use history. All potential volunteers had to be between the ages of 21 and 26 years, and have no history of alcohol dependence, as determined by a Short-Michigan Alcoholism Screening Test (Selzer, Vinokur, & Van Rooijen, 1975) score of 5 or higher. To ensure that participants in both groups were social drinkers, only those who reported consuming alcohol at least twice a month were asked to participate.
Volunteers were carefully screened using health questionnaires and a medical history interview. These measures gathered information about volunteers’ histories of serious physical disease, current physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure, head trauma, CNS tumors, or past histories of psychiatric disorder, (i.e., Axis I, DSM-IV). No volunteers reported any history of serious physical illness, neurological disorder, head injury, mental illness, or substance use disorder.
Participants with ADHD responded to study advertisements specifically seeking adults with a diagnosis of ADHD for studies of cognitive and behavioral tasks. Individuals who indicated having a medical diagnosis of ADHD or attention deficit disorder (ADD) were asked a series of questions about their diagnosis and current treatment status. After providing informed consent, medical records of the participants were obtained to verify the medical diagnoses.
For individuals whose medical records could not be obtained, ADHD diagnosis was confirmed by meeting symptom-based criteria on two of the three following scales: the Conners Adult ADHD Rating Scale-Long Form (CAARS-S:L; Conners et al., 1999), the ADD/H Adolescent Self-Report Scale-Short Form (Robin & Vandermay, 1996), and an ADHD Symptom Checklist of 12 ADHD symptoms that serve as diagnostic criteria according to the DSM-IV (American Psychiatric Association, 1994). All diagnoses were confirmed by a licensed clinical psychologist with over twenty years of experience in diagnosing ADHD. Three of the CAARS-S:L scales are based on well-established DSM-IV criteria of ADHD and have been used for adult ADHD diagnostic purposes in other research (e.g., Adler et al., 2006; Rybak, McNeely, Mackenzie, Jain, & Levitan, 2006). The items in this scale provide information on any experience of ADHD symptoms throughout adulthood. The diagnostic criterion for the CAARS-S:L is a T-score of 65 or higher on the ADHD symptoms scale. The ADD/H Adolescent Self-Report Scale-Short Form is specific to symptoms experienced in the past month, thus providing evidence that participants are currently experiencing the symptoms of ADHD. The diagnostic criterion for this scale is a score of 11 or higher. Sufficient psychometric properties have been demonstrated in both of these measures, and both have demonstrated criterion validity for identifying individuals with ADHD (Erhardt, Epstein, Conners, Parker, & Sitarenios, 1999; Robin & Vandermay, 1996). Furthermore, these scales were chosen because of their emphasis on adult symptoms. The ADHD Symptom Checklist was created using DSM-IV symptoms and items that loaded highly on the ADHD symptoms factor on the YAQ-S (Young, 2004). The scale emphasizes symptoms present as an adult and includes six inattentive and six hyperactive symptoms. Participants rated the frequency of symptom occurrence as Not at all, Sometimes, Often, and Very Often. Any symptom occurrence rated as Often or Very Often was counted and a symptom count of four or greater was required to meet criterion for ADHD.
Diagnoses of 5 of the 10 ADHD participants were confirmed through medical records. The remaining 5 ADHD participants, whose medical records could not be obtained, met diagnostic criteria for inclusion on the symptom-based scales. The control participants also completed each of the ADHD symptom-based scales, and none of these participants met criteria for ADHD.
Seven individuals with ADHD reported current prescriptions to Adderall ™, and one individual with ADHD reported current prescriptions to both Adderall and Adderall XR™. In order to examine performance of individuals with ADHD in an unmedicated state, the 8 participants currently prescribed medication for ADHD were asked to refrain from taking their medication for 24 hours prior to each study session. This allowed for the examination of actual deficits associated with ADHD, as opposed to studying the effects of medication on performance. Compliance with this request was verified by self-report and urine analysis at the beginning of each session. Recent use of barbiturates, amphetamines, benzodiazepines, cocaine, opiates, and tetrahydrocannabinol was also assessed by means of urine analysis. Any volunteer who tested positive for the presence of any of these drugs was excluded from participation. No female volunteers who were pregnant or breast-feeding, as determined by self-report and urine human chorionic gonadotrophin levels, participated in this study.
Apparatus and Materials
Cued Go/No-Go Task
Inhibitory control was measured by a cued go/no-go reaction time task used in other research to measure the disinhibiting effects of alcohol (e.g., Fillmore, Marczinski, & Bowman, 2005; Marczinski & Fillmore, 2003). Cues provide preliminary information regarding the type of imperative target stimulus (i.e., go or no-go) that is likely to follow, and the cues have a high probability of signaling the correct target.
The task was operated using E-Prime experiment generation software (Schneider, Eschman, & Zuccolotto, 2002) and was performed on a PC. A trial involved the following sequence of events: (a) presentation of a fixation point (+) for 800 ms; (b) a blank white screen for 500 ms; (c) a cue, displayed for one of five stimulus onset asynchronies (SOAs = 100, 200, 300, 400, and 500 ms); (d) a go or no-go target, which remained visible until a response occurred or 1000 ms had elapsed; and (e) an inter-trial interval of 700 ms.
The cue was a rectangle (7.5 cm × 2.5 cm) framed in a 0.8-mm black outline that was presented in the center of the computer monitor against a white background. The cue was presented in either a horizontal (2.5 cm tall × 7.5 cm wide) or vertical (7.5 cm tall × 2.5 cm wide) orientation. The go and no-go targets were colored green and blue, respectively, and they were displayed on the monitor as a solid hue that filled the interior of the rectangle cue. Participants were instructed to press the forward slash (/) key on the keyboard as soon as a go (green) target appeared and to suppress the response when a no-go (blue) target was presented. Key presses were made with the right index finger.
The orientation of the cue (vertical or horizontal) signaled the probability that a go or no-go target would be displayed. Cues that were presented vertically preceded the go target on 80% of the trials and preceded the no-go target on 20% of the trials. Cues that were presented horizontally preceded the no-go target on 80% of the trials and preceded the go target on 20% of the trials. Therefore, on the basis of cue-target pairings, vertical and horizontal cues operated as go and no-go cues, respectively. The different SOAs (100, 200, 300, 400, and 500 ms) between cues and targets encouraged participants to pay attention to the cues, and the variability and randomness of the SOAs prevented the participants from anticipating the exact onset of the targets.
A test consisted of 250 trials that presented the four possible cue-target combinations. An equal number of vertical (125) and horizontal (125) cues were presented, and an equal number of go (125) and no-go (125) target stimuli were presented. Each cue-target combination was presented at each of the five SOAs, and an equal number of SOAs separated each cue-target combination. The presentation of cue-target combinations and SOAs was random. For each trial, the computer recorded whether a response occurred and, if so, the reaction time (RT) in milliseconds was measured from the onset of the target until the key was pressed. To encourage quick and accurate responding, feedback was presented to the participant during the inter-trial interval by displaying the words correct or incorrect along with the RT in milliseconds. A test required approximately 15 minutes to complete.
Barratt Impulsiveness Scale (BIS-10; Patton, Stanford, & Barratt, 1995)
Participants completed the BIS in order to provide additional criterion-related validity for the group classification. Impulsivity is a core characteristic associated with ADHD in adults and so the scale was administered to verify differences in impulsivity between the ADHD and control groups. This 34-item self-report questionnaire measures the personality dimension of impulsivity. Sample items include “I plan tasks carefully,” “I am self-controlled,” and “I act ‘on impulse’.” Participants indicated how typical each of the statements is for them on a 4-point Likert scale (“rarely/never,” “occasionally,” “often,” or “almost always/always”). Scores range from 34-136, with higher scores indicating greater total levels of impulsiveness. In addition to a total score, six factors can be obtained from the questionnaire that assess different aspects of impulsivity, including attention (focusing on the task at hand), motor impulsiveness (acting on the spur of the moment), self-control (planning and thinking carefully), cognitive complexity (enjoying challenging mental tasks), perseverance (a consistent life style), and cognitive instability (thought insertions and racing thoughts).
Personal Drinking Habits Questionnaire (PDHQ; Vogel-Sprott, 1992)
This questionnaire yielded three measures of a drinker's current, typical drinking habits: (a) frequency (the number of drinking occasions per week), (b) quantity (the number of standard alcoholic drinks (e.g., 1.5 oz of liquor) typically consumed per occasion); and (c) duration (time span in hours of a typical drinking occasion). The questionnaire also provided a measure of history of alcohol use (number of months of regular drinking).
Blood Alcohol Concentrations (BACs) were determined from breath samples measured by an Intoxilyzer, Model 400 (CMI, Inc., Owensboro, KY, USA).
Procedure
Familiarization session
This session served to acquaint volunteers with the laboratory and to gather background information. All participants were tested individually. After providing informed consent, participants’ heights and weights were measured. Participants were then interviewed and completed questionnaires concerning their health status, drug use, impulsivity, and demographic characteristics. Intellectual functioning was also assessed by the Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990). Those who reported a diagnosis of ADHD provided a signed release of their medical records. Proof of age was obtained in order to verify that all participants were at least 21 years old. Task instructions for the cued go/no-go task were then explained, and participants completed one 15-minute practice trial, which is sufficient to become familiar with the task (Marczinski & Fillmore, 2003).
Test sessions
Performance on the cued go/no-go task was tested under three doses of alcohol: 0.65 g/kg, 0.45 g/kg, and 0.0 g/kg (placebo). Each dose was administered on a separate test session. Sessions were separated by a minimum of 24 hours and a maximum of one week. Dose order was randomized across participants, and doses were calculated based on body weight. The 0.65 g/kg dose produces an average peak BAC of 75 mg/100 ml and was chosen on the basis of prior research that showed that response inhibition is reliably impaired at this BAC (e.g., Fillmore & Vogel-Sprott, 1999; Marczinski & Fillmore, 2003). The 0.45 g/kg dose produces an average peak BAC of 50 mg/100 ml, and was included in this study because this represents the minimal threshold blood alcohol level that reliably impairs psychomotor and RT performance in laboratory tasks (e.g., Holloway, 1995).
The alcohol beverage was served as one part alcohol and three parts carbonated mix, divided equally into two glasses. Participants had two minutes to finish each glass, and the two glasses were served four minutes apart. This dosing procedure produces a mean rate of rise in BAC of 1.0 mg/100 ml/minute (Fillmore & Vogel-Sprott, 1998). Thus, the peak BACs after the 0.45 g/kg and 0.65 g/kg doses were expected to occur approximately 50 and 75 min, respectively, after drinking began. The placebo beverage consisted of four parts carbonated mix and was served in the same manner. Five ml of alcohol was floated on the top of each glass and the glasses were sprayed with an alcoholic mist, which resembled condensation and provided a strong alcoholic odor. Previous research has shown that individuals report that these beverages contain alcohol (e.g., Fillmore & Blackburn, 2002).
Participants’ cued go/no-go task performance was tested 45 minutes after drinking began. Breath samples were collected at 45 and 60 minutes after drinking (immediately preceding and immediately following the testing period), during both the placebo and alcohol test sessions. Once the testing was finished, participants remained at leisure in the lounge area until their BACs, which were monitored at 20-minute intervals, reached 20 mg/100 ml or below. Participants were provided with a meal during this leisure time and were allowed to watch movies and read magazines.
Criterion Measures and Data Analyses
The two primary measures of interest were the participants’ failures to inhibit responses to no-go targets (failure of response inhibition) and their speed of responding to go targets (response execution).
Failure of response inhibition
Failure of response inhibition was measured as the proportion of no-go targets in which a participant failed to inhibit a response. These p-inhibition failure scores were calculated for each cue condition (go and no-go). Group and dose effects on p-inhibition failures in the go and no-go cue conditions were analyzed by two separate 2 (group) × 3 (dose) ANOVAs.
Response execution
Response execution was measured by the RT to go targets. Shorter RTs indicated greater facilitation of response execution. A mean RT score for a participant was calculated for each cue. Responses with RTs less than 100 ms and greater than 1000 ms were excluded. These outliers were infrequent, occurring on average less than 0.25% of the trials for which a response was observed (i.e., less than one trial per test). Group and dose effects on RT scores in the go and no-go cue conditions were analyzed by two separate 2 (group) × 3 (dose) ANOVAs. Omission errors were also recorded. These errors occurred when participants failed to respond to go targets. Omission errors were infrequent and occurred on less than 2% of go target trials (~two trials per test).
Results
Demographics, Drinking Habits, and Impulsivity Measures
Table 1 summarizes the demographic data, drinking habits, and impulsivity scores for the groups. A Chi-square analysis showed that gender make-up was independent of group, Chi Square (1, N = 22) = 0.1, p = .78. The groups also did not differ significantly in age, t(20) = 0.1, p = .94, or in verbal, non-verbal, and full-scale IQ (ps > 0.48). Table 1 shows that the groups did not differ on any measure of drinking habits as reported on the PDHQ (ps > .14). As expected, the ADHD group reported higher levels of total impulsivity as measured by the total BIS score, t(20) = 4.8, p < .01, as well as higher scores on each of the following subscales: attention, t(20) = 5.9, p < .01, motor impulsiveness, t(20) = 3.0, p < .01, self-control, t(20) = 3.8, p < .01, cognitive complexity, t(20) = 4.3, p < .01, and cognitive instability, t(20) = 4.8, p < .01.
Table 1.
Mean Demographics, PDHQ and BIS Scores by Group
| Group |
Contrasts |
||||
|---|---|---|---|---|---|
| ADHD |
Controls |
||||
| M | SD | M | SD | ||
| Demographics | |||||
| Age | 22.8 | 1.8 | 22.8 | 1.1 | ns. |
| IQ: Verbal | 104.2 | 6.2 | 105.8 | 3.8 | ns. |
| IQ: Non-verbal | 109.3 | 7.1 | 110.6 | 8.0 | ns. |
| IQ: Full-scale | 107.3 | 5.5 | 109.0 | 5.4 | ns. |
| PDHQ | |||||
| Frequency | 2.0 | 1.3 | 1.2 | 1.0 | ns. |
| Quantity | 4.8 | 2.2 | 4.0 | 1.9 | ns. |
| Duration | 3.6 | 1.3 | 3.4 | 1.3 | ns. |
| History | 93.5 | 41.4 | 70.8 | 30.7 | ns. |
| BIS | |||||
| Total | 67.4 | 8.9 | 49.9 | 8.2 | Sig*** |
| Attention | 10.6 | 1.4 | 6.5 | 1.8 | Sig*** |
| Motor | 18.5 | 3.5 | 15.2 | 1.5 | Sig** |
| Self-control | 16.2 | 2.4 | 11.8 | 2.9 | Sig** |
| Cognitive complexity | 9.0 | 1.7 | 6.1 | 1.5 | Sig*** |
| Perseveration | 5.8 | 1.5 | 5.0 | 1.1 | ns. |
| Cognitive instability | 7.3 | 1.8 | 5.4 | 1.3 | Sig** |
Note. Group contrasts were tested by one-way between subjects ANOVAs. Sig** indicates a significance value of p < .01 and Sig*** indicates a significance value of p < .001.
Blood Alcohol Concentrations
No detectable BACs were observed in the placebo condition in either the ADHD or control group. In order to test for any group differences in BACs in the active dose conditions, t tests were conducted. No group differences were found at pre- or post-test under either dose of alcohol (ps > .11). Based on the entire sample, the mean BACs at pre- and post-test under the 0.45 g/kg dose were 55.0 mg/100 ml (SD = 10.2) and 53.6 mg/100 ml (SD = 8.1), respectively. For the 0.65 g/kg dose, the mean BACs at pre- and post-test were 81.3 mg/100 ml (SD = 17.0) and 86.4 mg/100 ml (SD = 11.5), respectively.
Cued Go/No-Go Task Performance
Failure of response inhibition following invalid (go) cues
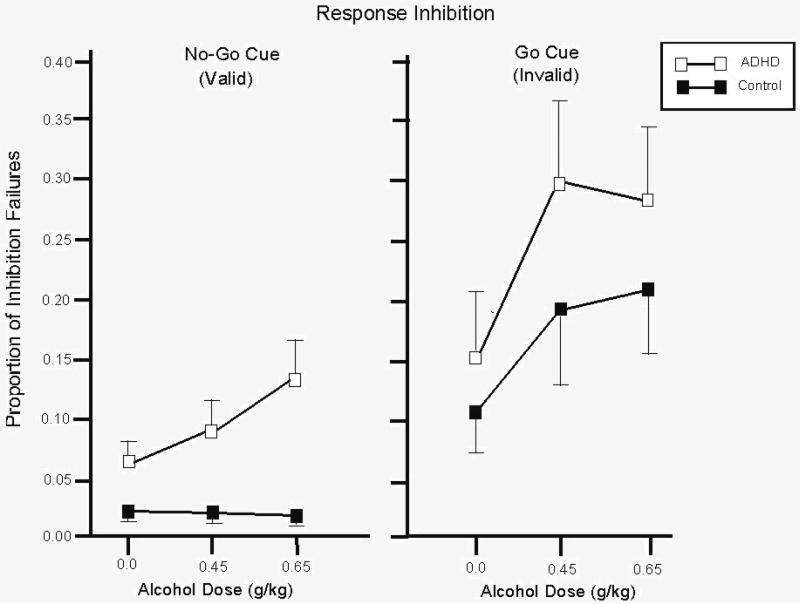
In the go cue condition, there was a significant main effect of dose, F(2, 40) = 6.9, p < .01. Figure 1 (right panel) illustrates this effect. The figure shows that p-inhibition failures increased as a function of dose in both groups. A priori one-tailed t tests confirmed these observations. Compared with placebo both groups committed significantly more inhibitory failures in response to both active doses of alcohol (ps < .05). No main effect of group or interaction was observed (ps > .30).
Figure 1.
Mean proportion of failures to inhibit responses to no-go targets after go and no-go cues for the control and ADHD groups under three alcohol doses: 0.0 g/kg (placebo), 0.45 g/kg, and 0.65 g/kg. Capped vertical lines show standard errors of the mean.
Failure of response inhibition following valid (no-go) cues
For the no-go cue condition, a significant main effect of group, F(1, 20) = 15.6, p < .01, and significant dose × group interaction, F(2, 40) = 3.9, p = .03 was observed. These effects are illustrated in Figure 1 (left panel). The figure shows that p-inhibition failures in the control group were infrequent and appeared unaffected by dose. By contrast, p-inhibition failures were more frequent in the ADHD group and increased as a function of dose. A priori t tests confirmed these observations. For the controls, inhibitory failures were not significantly increased by either 0.45 g/kg or 0.65 g/kg alcohol (ps > .68). By contrast, compared with placebo those with ADHD displayed significantly more inhibition failures in response to the 0.65 g/kg dose, t(9) = 2.1, p = .03, d = 0.68, and the increase in response to the 0.45 g/kg alcohol approached statistical significance, t(9) = 1.4, p = .08, d = 0.45. Additionally, between-groups t tests revealed that the ADHD group displayed significantly more inhibition failures than did controls under placebo and both active dose conditions (ps < .02).
Response execution following valid (go) cues
Group and dose effects on mean RTs to go targets in the go and no-go cue conditions were analyzed by two separate 2 (group) × 3 (dose) ANOVAs. For the go cue condition, no main effects or interaction were found (ps > .14). Mean RT in the go cue condition for the entire sample was 268.3 (SD = 31.1).
Response execution following invalid (no-go) cues
For the no-go cue condition, a main effect of dose was obtained, F(2, 40) = 6.3, p < .01. No main effect of group or interaction was observed (ps > .72). Mean RTs for the entire sample under the 0.0, 0.45, and 0.65 g/kg doses were 293.5 (SD = 29.3), 302.7 (SD = 27.8), and 317.0 (SD = 43.3), respectively. A priori one-tailed t tests confirmed that RT was significantly slowed in response to both active doses of alcohol compared with placebo (ps < .05).
Discussion
This study used a cued go/no-go task to measure the degree to which alcohol impaired inhibitory control and response execution in adults with ADHD and healthy controls in a context where cues signaled the likelihood that a response should be executed or suppressed. With respect to inhibitory control, the results showed that alcohol dose-dependently increased inhibitory failures in control subjects when the target was preceded by an invalid (i.e., go) cue. When the cue was valid (i.e., no-go) control drinkers displayed no significant impairment of inhibitory control following alcohol. Both of these findings are consistent with previous research that used this task to examine alcohol effects in healthy adults (e.g., Marczinski & Fillmore, 2003). By contrast, the present study showed that those with ADHD displayed significant impairment of inhibitory control following alcohol regardless of cue condition. Following either valid or invalid cues, those with ADHD displayed dose-dependent increases in inhibitory failures in response to alcohol. Thus, unlike controls, valid, no-go cues offered little protection from the disinhibiting effects of alcohol in drinkers with ADHD. With regard to response execution, both those with ADHD and controls were similarly impaired (i.e., slowed) by alcohol when go targets were preceded by invalid (i.e., no-go) cues. Neither group was impaired by alcohol when go responses were preceded by valid (i.e., go) cues. Thus, impairment in inhibitory control cannot be due to a speed-accuracy trade-off in either group. Further, the group differences observed in the disinhibiting effects of alcohol cannot be accounted for by group differences in the BACs at the time of testing. Nor can group differences be attributed to drinking habits, age, gender make-up, or IQ, because the two groups were similar on these measures.
Evidence that individuals with ADHD were impaired by alcohol regardless of whether or not targets were preceded by valid cues is particularly noteworthy given prior evidence for the protective effects of valid cues from alcohol impairment in this task (Fillmore, 2004; Marczinski & Fillmore, 2003). Previous work showed that in the presence of valid (i.e., no-go) cues these doses of alcohol do not impair inhibitory control in healthy adults. Nor did the controls in the present study demonstrate any impairment of inhibitory control in this cue condition. Cued go/no-go task performance involves three stages of processing: perceptual, decision (central), and response output (Miller et al., 1991). It is argued that valid cues offer protection from the disinhibiting effects of alcohol because the cue allows the drinker to prepare the appropriate response (i.e., to inhibit) in advance before the actual response is required. This “head-start” reduces the amount of information that needs to be processed when the actual response is required (Fillmore, 2004; Marczinski & Fillmore, 2003). The failure of the valid cues to protect against alcohol impairment of inhibitory control in those with ADHD suggests that this advanced processing for response inhibition might have failed to occur in response to the no-go cue. Consequently, responding might have remained somewhat “pre-potent” for the ADHD group, even in the no-go cue condition. These individuals might have been able to control this prepotent response tendency when sober, but not following active doses of alcohol. With regard to response execution, the findings show that those with ADHD did benefit from the protective effects of the valid cue (i.e., the go cues). Like controls, those with ADHD displayed no significant impairment (i.e., slowing) of response execution when go targets were preceded by valid go cues. Again, a general increased prepotency to respond in this group would be beneficial against the response-slowing effects of the drug.
Together, the findings highlight the vulnerability of inhibitory mechanisms versus activational mechanisms of behavioral control. Indeed, investigators point to inhibitory deficits as a core symptom of ADHD (Barkley, 2006) and studies using stop-signal and cued go/no-go tasks in adults with ADHD find greater deficits involving inhibitory mechanisms relative to activational mechanisms (e.g., Bekker et al., 2005). Greater vulnerability of inhibitory versus activational mechanisms of behavior is also evident in the disruptive effects of alcohol. Prior research shows that it is response inhibition, and not response execution, that is particularly vulnerable to the disruptive effects of alcohol at the range of doses examined in the present research (Fillmore, 2003; 2007). Taken together, inhibitory control might be particularly vulnerable to the disruptive effects of alcohol in individuals with poor inhibitory control. Moreover, such vulnerability might be especially pronounced for such individuals in environmental situations that normally afford protection against the disinhibiting effects of the drug (e.g., advanced cues for response inhibition).
It is reasonable to assume that impairments of attentional processes also might have contributed to the group differences observed in response to alcohol. ADHD is characterized by a deficit in the ability to maintain focused attention for prolonged lengths of time (Tannock, 1998). As such, these individuals might be periodically less attentive to the cues throughout the task. Sporadic lapses of their attention to valid cues might result in failures to prepare appropriate responses to the targets. However, the results do not support a failure of this group to learn or to respond to the cue-target associations. Results showed that those with ADHD appeared to learn and attend to the cue-target relationships to a similar degree as controls. For example, with respect to reaction times to go targets, both individuals with ADHD and controls responded faster to targets that followed go cues versus those that followed no-go cues, and this difference was confirmed by ad hoc pairwise t tests of RTs between cue conditions. Evidence for this “cue-dependent” responding among those with ADHD suggests that their increased impairment of inhibitory control cannot be simply attributed to a lack of attention to task stimuli in general, but instead appears specific to how these individuals suppress responses to inhibitory signals.
Increased sensitivity to the disinhibiting effects of alcohol among drinkers with ADHD might also represent greater behavioral risks associated with alcohol consumption. In addition to the current evidence of greater impairment as BAC peaked under each active dose, it is also likely that this disinhibited state would continue under the dose for a considerable time even when BAC begins to decline. Indeed, studies have demonstrated that cognitive functions show little or no acute recovery from alcohol-induced impairment during a dose, even when BAC begins to decline (for a review see, Schweizer & Vogel-Sprott, 2008). This lack of acute recovery during the declining limb of the BAC curve is also evident in inhibitory control, which remains impaired even though the ability to execute responses recovers to sober performance levels (e.g., Fillmore et al., 2005). Taken together, such evidence suggests that for individuals with ADHD, inhibitory control could be particularly vulnerable to the disruptive effects of alcohol, both in terms of sensitivity and duration of impairment.
A potential limitation of the present study is the relatively small sample size utilized. Caution should be taken in generalizing these results to all ADHD patients, and future research utilizing larger samples should be conducted to replicate these results. However, it is also important to note that the effect sizes reported in this study were large, demonstrating robust dose and group effects.
In conclusion, the present findings demonstrate that adults with ADHD exhibited a deficit in inhibitory control compared to healthy controls as measured by the cued go/no-go task, and that this deficit was exacerbated in response to alcohol. The group differences in impairment of inhibitory control were evident in the valid cue condition, when overall levels of disinhibition were low. The current study suggests that adults with ADHD exhibit an increased sensitivity to alcohol-impairment of basic acts of inhibitory control, and this may contribute to the high incidence of impulsive behaviors observed in individuals with this disorder, especially in response to alcohol. Additional work is needed to examine the potential behavioral consequences of an increased sensitivity to the acute disinhibiting effects of alcohol in adults with ADHD, including increased alcohol consumption and risky behavior while intoxicated.
Acknowledgments
This research was supported by Award Number R21 DA021027 and Award Number DA005312 from the National Institute on Drug Abuse, and by Award Number R01 AA12895 from the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Alcohol Abuse And Alcoholism or the National Institutes of Health.
References
- Adler LA, Sutton VK, Moore RJ, Dietrich AP, Reimherr FW, Sangal RB, et al. Quality of life assessment in adult patients with attention-deficit/hyperactivity disorder treated with atomoxetine. Journal of Clinical Psychopharmacology. 2006;26:648–652. doi: 10.1097/01.jcp.0000239797.21826.70. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Barkley RA. Attention-deficit/hyperactivity disorder. In: Wolfe DA, Mash EJ, editors. Behavioral and emotional disorders in adolescents: Nature, assessment, and treatment. Guilford Publications; New York, NY: 2006. pp. 91–152. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Overtoom CC, Kenemans JL, Kooij JJ, De Noord I, Buiterlaar JK, Verbaten MN. Stopping and changing in adults with ADHD. Psychological Medicine. 2005;35:807–816. doi: 10.1017/s0033291704003459. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, Sparrow E. Self-ratings of ADHD symptoms in adults: I. Factor structure and normative data. Journal of Attention Disorders. 1999;3:131–151. [Google Scholar]
- Derefinko KJ, Adams ZW, Milich R, Fillmore MT, Lorch EP, Lynam DR. Response style differences in the inattentive and combined subtypes of attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36:745–758. doi: 10.1007/s10802-007-9207-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behavioral Neuroscience. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Erhardt D, Epstein JN, Conners CK, Parker JDA, Sitarenios G. Self-ratings of ADHD symptoms in adults: II. Reliability, validity, and diagnostic sensitivity. Journal of Attention Disorders. 1999;3:153–158. [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: Current approaches and findings. Behavioral and Cognitive Neuroscience Reviews. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Environmental dependence of behavioral control mechanisms: Effects of alcohol and information processing demands. Experimental and Clinical Psychopharmacology. 2004;12:216–223. doi: 10.1037/1064-1297.12.3.216. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Acute alcohol-induced impairment of cognitive functions: Past and present findings. International Journal of Disability and Human Development. 2007;6:115–125. [Google Scholar]
- Fillmore MT, Blackburn J. Compensating for alcohol-induced impairment: Alcohol expectancies and behavioral disinhibition. Journal of Studies on Alcohol. 2002;63:237–246. doi: 10.15288/jsa.2002.63.237. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. Journal of Studies on Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Behavioral impairment under alcohol: Cognitive and pharmacokinetic factors. Alcoholism: Clinical and Experimental Research. 1998;22:1476–1482. [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Experimental and Clinical Psychopharmacology. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–1246. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kessler DN, Hussong AM. Risk for alcoholism and classical conditioning to signals for punishment: Evidence for a weak behavioral inhibition system? Journal of Abnormal Psychology. 1994;103:293–301. doi: 10.1037//0021-843x.103.2.293. [DOI] [PubMed] [Google Scholar]
- Flory K, Lynam DR. The relation between attention deficit hyperactivity disorder and substance abuse: What role does conduct disorder play? Clinical Child and Family Psychology Review. 2003;6:1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Flory K, Milich R, Lynam DR. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: Individuals with symptoms of attention deficit/hyperactivity disorder are uniquely at risk. Psychology of Addictive Behaviors. 2003;17:151–158. doi: 10.1037/0893-164x.17.2.151. [DOI] [PubMed] [Google Scholar]
- Goschke T. Voluntary action and cognitive control from a cognitive neuroscience perspective. In: Massen S, Prinz W, Roth G, editors. Voluntary action: Brains, minds, and sociality. Oxford University Press; New York, NY: 2003. pp. 49–85. [Google Scholar]
- Grekin ER, Sher KJ, Wood PK. Personality and substance dependence symptoms: modeling substance-specific traits. Psychology of Addictive Behaviors. 2006;20:415–424. doi: 10.1037/0893-164X.20.4.415. [DOI] [PubMed] [Google Scholar]
- Holloway FA. Low-dose alcohol effects on human behavior and performance. Alcohol, Drugs, and Driving. 1995;11:39–56. [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. Manual. American Guidance Service; Circle Pines, MN: 1990. [Google Scholar]
- Lijffijit M, Kenemans JL, Verbaten MN, Van Engeland H. A meta-analytic review of stopping performance in ADHD: deficient inhibitory control or inattention? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A user's guide to the stop-signal paradigm. In: Dagenback D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego, CA: 1994. pp. 189–239. [Google Scholar]
- Marczinski CA, Fillmore MT. Preresponse cues reduce the impairing effects of alcohol on the execution and suppression of responses. Experimental and Clinical Psychopharmacology. 2003;11:110–117. doi: 10.1037//1064-1297.11.1.110. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller J, Schaffer R, Hackley SA. Effects of preliminary information in a go versus no-go task. Acta Psychologica. 1991;76:241–292. doi: 10.1016/0001-6918(91)90022-r. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Smith BH, Pelham WE. Interactive effects of attention deficit/hyperactivity disorder and conduct disorder on early adolescent substance use. Psychology of Addictive Behaviors. 1999;13:348–358. [Google Scholar]
- Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. Journal of Studies on Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. Journal of Child Psychology and Psychiatry. 1998;39:411–425. [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Robin AL, Vandermay SJ. Validation of a measure for adolescent self-report of attention deficit disorder symptoms. Journal of Developmental and Behavioral Pediatrics. 1996;17:211–215. [PubMed] [Google Scholar]
- Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. Journal of Clinical Psychiatry. 2006;67:1527–1535. doi: 10.4088/jcp.v67n1006. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-prime user's guide. Psychology Software Tools; Pittsburgh, PA: 2002. [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: A review of acute tolerance and recovery of cognitive performance. Experimental and Clinical Psychopharmacology. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST). Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Tannock R. Attention deficit hyperactivity disorder: Advances in cognitive, neurobiological, and genetic research. Journal of Child Psychology and Psychiatry. 1998;39:65–99. [PubMed] [Google Scholar]
- Trull TJ, Waudby CJ, Sher KJ. Alcohol, tobacco, and drug use disorders and personality disorder symptoms. Experimental and Clinical Psychopharmacology. 2004;12:65–75. doi: 10.1037/1064-1297.12.1.65. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M. Alcohol tolerance and social drinking: Learning the consequences. Guilford Press; New York: 1992. [Google Scholar]
- Widiger TA, Smith GT. Substance use disorder: Abuse, dependence and dyscontrol. Addiction. 1994;89:267–282. doi: 10.1111/j.1360-0443.1994.tb00889.x. [DOI] [PubMed] [Google Scholar]
- Young S. The YAQ-S and YAQ-I: The development of self and informant Questionnaires reporting on current adult ADHD symptomatology, comorbid and associated problems. Personality and Individual Differences. 2004;36:1211–1223. [Google Scholar]