Abstract
We conducted a case–control study to evaluate the association between paternal smoking and childhood leukemia and to evaluate potential modification by polymorphisms in CYP1A1. Histologically confirmed childhood leukemia cases (n = 164) and non-cancer controls (n = 164) were recruited from three teaching hospitals in Seoul, Korea. Five single nucleotide polymorphisms in CYP1A1 (–17961T>C, –9893G>A, I462V, 1188C>T (*2A), and 11599C>G) were genotyped and haplotypes were estimated by the expectation-maximization method. We also conducted a meta-analysis of 12 studies that have reported the association between paternal smoking and childhood leukemia risk. Paternal smoking at home was associated with all leukemias (OR = 1.8, 95% CI = 1.1–2.8) and acute lymphoblastic leukemia (ALL) (2.0, 1.2–3.4). An increasing trend in risk was observed for pack-years smoked after birth (Ptrend = 0.06 and 0.02, respectively) and the number of smokers in the home during the child's life (Ptrend = 0.05 and 0.03, respectively). Among those without the CGACC haplotype, ALL risk was significantly increased by the father's smoking at home (2.8, 1.5–5.3) and the presence of at least one smoker in the home (2.3, 1.2–4.4), and the test for interaction was significant (Pinteraction = 0.03 and 0.02, respectively). The meta-analysis showed that overall paternal smoking (1.13, 1.04–1.24) and smoking before the pregnancy of the child (1.12, 1.04–1.21) were significantly associated with childhood leukemia risk. Our results suggest that paternal smoking is a risk factor for childhood leukemia and the effect may be modified by CYP1A1 genotype.
Keywords: Childhood leukemia, Paternal smoking, CYP1A1, Interaction, Haplotype
1. Introduction
Despite decreasing mortality rates over the past two decades, childhood cancer is the second most frequent cause of death after accidents and poisonings, among children aged 1 or older in Korea (about 4 per 100,000 person-years as of 2003). Although childhood leukemia is the most common childhood cancer with an incidence of 3.8 cases per 100,000 person-years in Korean children [1], no studies in Korea have evaluated environmental exposures as potential risk factors. Likewise, the risk factors in other study populations have not been well characterized with the exception of ionizing radiation.
Children may be particularly vulnerable to environmental toxicants because of their greater relative exposure, immature metabolism, and higher level of cell division and growth [2]. Cigarette smoke is a common environmental exposure for children, containing various carcinogens such as nitrosamines, polycyclic aromatic hydrocarbons (PAHs), heterocyclic amines, and radioactive compounds [3]. However, epidemiological evidence for a relationship between childhood leukemia and tobacco smoke exposure in utero or during the postnatal period is inconclusive [4,5].
Although several case–control studies suggested that maternal smoking during pregnancy increases risk of childhood leukemia [6,7], cohort studies have found inverse associations between maternal smoking during pregnancy and childhood leukemia [8], or no association [9–11]. Inverse associations were also observed between maternal smoking more than 10 cigarettes per day during pregnancy and childhood leukemia in case–control studies conducted in German and the U.K. [12,13]. Paternal smoking, especially in the prenatal period, has been shown to increase childhood leukemia risk in a number of studies [7,14–17]. However, other studies found no significantly increased risk [12,13,18–21].
In terms of postnatal smoking exposure, genetic variants of the child's enzymes (i.e., CYP1A1, CYP2D6, GSTM1/T1/P1, NAT1, and NAT2) involved in the oxidation activation and subsequent conjugation detoxification of carcinogens, such as PAHs and aromatic amines, may play a role in susceptibility to leukemogenesis in children. Presently, CYP1A1*2A [22–24], CYP2E1*5 [25], CYP2D6*3 [25], NAT1*4 [23], NAT2 slow acetylator [23], GSTM1 null genotype [23–26], and GSTP1*B [27], have been suggested to have effects on childhood leukemia risk. Furthermore, the most widely investigated polymorphism, the CYP1A1*2A (rs4646903, 1188C>T) which is potentially functional [28], has been reported to have a possible interactive effect with exposure to parental smoking [29,30].
To better understand the role of smoking exposure and potential interactive effect with CYP1A1 polymorphisms in the development of childhood leukemia, we conducted a case–control study in Korea population using a haplotype approach to evaluate the association between CYP1A1 polymorphisms and childhood leukemia. We also conducted a meta-analysis to evaluate the association between paternal smoking and childhood leukemia risk.
2. Materials and methods
2.1. Subjects
We conducted a hospital-based case–control study in Seoul, Korea. The protocol was reviewed and approved by the three participating hospitals (Seoul National University Hospital, Asan Medical Center and Samsung Medical Center). Histologically confirmed incident childhood leukemia cases (n = 176) aged 0–18 were recruited from these three teaching hospitals between May 2003 and May 2005. The histological subtypes of cases included acute lymphoblastic leukemia (ALL) (65%), acute myeloid leukemia (24%), acute biphenotypic leukemia (6%), and juvenile myelomonocytic leukemia (5%). Control subjects (n = 298) ages 0–18 were recruited from the patients admitted to the Department of Pediatrics, Seoul National University Hospital without a medical history of childhood cancer. The control group consisted of acute gastroenteritis (11%), hernia (10%), pneumonia (8%), Legg-Calve-Perthes disease (5%) and the other infectious or orthopedic diseases each of which was less than 5% of the total controls. Informed consent was obtained and interviews were conducted with the mother (93.5%), father (5.9%) or other legal guardian (0.6%) of the child. Participation rates were 80% for cases and 70% for controls. The type of interviewee was not related to education or smoking status of the fathers.
Interviewers used a structured questionnaire to obtain information about characteristics of the child including birth weight (<3.25, 3.25–3.70, and >3.70 kg) and duration of breast feeding (not breastfed, 1–6, 6–12, and >12 months). They also obtained a paternal and maternal lifetime smoking history including the usual number of cigarettes smoked per day, starting age and quitting age, and the number of smokers in the home during the child's life. The proportion of mothers who smoked was too small (6.1% in controls) to be evaluated in relation to childhood leukemia risk and is not considered further.
To control potential confounding factors related with age and sex, the interviewed cases and controls were frequency-matched on age (0, 1–2, 3–4, 5–6, 7–8, 9–10, 11–12, 13–14, 15+) and sex. A total of 164 cases and 164 controls were randomly selected for genotyping and these were the basis of the analyses presented here.
2.2. Genotyping
Peripheral blood samples (2 ml) were collected in EDTA tubes and stored at –70°C until DNA extraction using Puregene DNA Kits (Minneapolis, MN).
Ten single nucleotide polymorphisms (SNPs) on CYP1A1 (rs2472299, rs2470893, rs2606345, rs2198843, rs4646421, rs4646903, rs146432, rs17861115, rs7495708, and rs1048903) were selected on the basis of >10% minor allele frequency as determined by public web-based databases (SNP500Cancer: http://snp500cancer.nci.nih.gov, NCBI SNP: http://www.ncbi.nlm.nih.gov, and JSNP: http://snp.ims.u-tokyo.ac.jp/index_ja.html). Single base extension assays (SNaPshot) were developed for 10 SNPs and 47 samples were genotyped to check the validity of the assays and the minor allele frequencies. Two SNPs (rs4646421 and rs7495708) were not successfully genotyped. Five haplotype tagging SNPs were selected out of eight SNPs using the tagSNPs program (version 2: http://www-rcf.usc.edu/~stram/tagSNPs.html) based on a RSQ value of 1.00. Thus, the –17961T>C (rs2472299), –9893G>A (rs17861115), Ex7+13A>G (I462V) (a.k.a. *2C: rs1048943), 1188C>T (a.k.a. *2A: rs4646903), and 11599C>G (rs2198843) were genotyped further for all selected subjects by single base extension assay in ABI 3700 DNA Analyzer (Applied Biosystems, Foster City, CA) according to the SNaPshot Multiplex Kit Protocol [31].
Hardy–Weinberg equilibrium fitted all genotype distributions of five SNPs among controls (P>0.05) and strong linkage disequilibrium (LD) was found between –9893G>A (rs17861115) and Ex7+13A>G (I462V) (rs1048943) (D′ = 0.98 and r2 = 0.92). The concordance rate among five replicates of five quality control samples which were randomly placed was 96% for two assays (–9893G>A and Ex7+13A>G (I462V)) and 100% for the other three assays.
2.3. Statistical analysis
All statistical procedures were conducted using SAS version 9.1.3 (SAS Institute, Cary, NC) unless otherwise indicated. We defined ever-smokers as fathers who smoked ≥400 cigarettes in their lifetime. Among smokers, we calculated pack-years of smoking before the pregnancy, and after the birth of the index child. We categorized pack-years into three groups (0, ≤10 and >10).
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by unconditional logistic regression, adjusting for age as a continuous variable, gender, father's education (high school, university, graduate school), and birth weight (<3.25, 3.25–3.70, >3.70 kg). ORs were not calculated if there were fewer than five subjects in any cell of a frequency table. For variables with three or four categories, tests for trend were conducted by assigning ordinal scores to the categories and modeling the scores as a continuous variable. In addition to conducting analyses of all childhood leukemia, we calculated histologic subtype-specific ORs for ALL (n = 106), the most common subtype of childhood leukemia. We also conducted stratified analysis to see if the association with paternal smoking or CYP1A1 SNPs varied by age group and gender.
Individual haplotypes for five CYP1A1 tagging SNPs were estimated by expectation-maximization method and the omnibus test was conducted to assess differences in overall haplotype frequency profiles between cases and controls. Diplotype analysis included only haplotype pairs with greater than 95% probability (17 were excluded) and all the haplotype pairs were categorized into two groups (CGACC (+) and CGACC (–)) based on the presence or absence of the lowest risk haplotype (CGACC) (12.3% in cases and 15.0% in controls). Interactions between paternal smoking and diplotypes were tested on a multiplicative scale by adding a product term of smoking exposure variable and diplotype (CGACC (+) vs. CGACC (–)) into the logistic regression model. To confirm the results from the diplotype analysis, a Bayesian method was also applied using PHASE ver. 2.0.2 (http://www.stat.washington.edu/stephens/software.html) [32]. Both analytic methods resulted in similar findings.
A meta-analysis was conducted to summarize previous reports on the associations between paternal smoking status (ever vs. never) and childhood leukemia risk. Our meta-analysis included 12 case–control studies including our study [7,12–21,29]. In addition to lifetime paternal smoking status (N = 6; 4830 cases and 5010 controls), paternal smoking status before pregnancy (N = 11; 5540 cases and 10311 controls) and after birth of the child (N = 4; 1280 cases and 1362 controls) were also evaluated for the association with childhood leukemia risk. Only two studies reported the association between paternal smoking status during pregnancy and childhood leukemia [19,21]. When there were several reports from the same study, most recent one was included in the analysis. All meta-analytic procedures were performed using STATA version 10 (College Station, TX). The data were combined using a random-effects model to estimate the summary OR and 95% confidence interval. Heterogeneity among the studies was evaluated by means of the Cochrane Q test and publication bias was assessed by Begg's and Egger's test. In addition to conducting analyses of all childhood leukemias, we also estimated the ALL-specific summary OR and 95% CI.
3. Results
Selected characteristics of the cases and controls are shown in Table 1. Childhood leukemia was more common in males (61.6%) compared to females (38.4%). The age distribution peaked at ages 3–4 (20.7%). Increasing birth weight was associated with increased childhood leukemia risk (Ptrend = 0.001). Duration of breast feeding, father's educational attainment, and family history of cancer were not significantly associated with childhood leukemia risk.
Table 1.
Characteristics of childhood leukemia cases and controls
| Cases (n = 164) | Controls (n = 164) | OR (95% CI)a | |
|---|---|---|---|
| Sex | |||
| Male | 101 (61.6) | 101 (61.6) | |
| Female | 63 (38.4) | 63 (38.4) | |
| Age | |||
| 0 | 9 (5.5) | 9 (5.5) | |
| 1–2 | 25 (15.3) | 25 (15.3) | |
| 3–4 | 34 (20.7) | 34 (20.7) | |
| 5–6 | 22 (13.4) | 22 (13.4) | |
| 7–8 | 21 (12.8) | 21 (12.8) | |
| 9–10 | 14 (8.5) | 14 (8.5) | |
| 11–12 | 19 (11.6) | 19 (11.6) | |
| 13–14 | 14 (8.5) | 14 (8.5) | |
| 15–17 | 6 (3.7) | 6 (3.7) | |
| Mean (±S.D.) | 6.96 (±4.34) | 7.09 (±4.42) | 0.80 |
| Birth weight (kg) | |||
| <3.25 | 62 (37.8) | 94 (57.3) | Ref. |
| 3.25–3.70 | 54 (32.9) | 45 (27.4) | 1.8 (1.1–3.0) |
| >3.70 | 48 (29.3) | 25 (15.3) | 3.0 (1.6–5.5) |
| Ptrend | 0.001 | ||
| Mean (±S.D.) | 3.56 (±0.24) | 3.46 (±0.24) | 0.001 |
| Duration of breast feeding (month) | |||
| 0 | 62 (38.0) | 58 (35.4) | Ref. |
| 0–6 | 67 (41.0) | 74 (45.1) | 0.9 (0.6–1.6) |
| 6–12 | 22 (13.5) | 20 (12.2) | 0.9 (0.4–1.9) |
| >12 | 12 (7.4) | 12 (7.3) | 0.7 (0.3–1.9) |
| Ptrend | 0.57 | ||
| Paternal education | |||
| High school | 68 (41.5) | 56 (34.6) | Ref. |
| University | 84 (51.2) | 91 (56.2) | 0.7 (0.4–1.1) |
| Graduate school | 12 (7.3) | 15 (9.3) | 0.7 (0.3–1.7) |
| Ptrend | 0.15 | ||
| Family history of cancer | |||
| No | 91 (55.8) | 95 (59.0) | Ref. |
| Yes | 72 (44.2) | 66 (40.1) | 1.1 (0.7–1.7) |
Adjusted for other covariates in the table.
The lifetime smoking status of the father (never vs. ever) was not associated with childhood leukemia risk (Table 2). However, paternal smoking at home was associated with all leukemia (OR = 1.8, 95% CI = 1.1–2.8) and acute lymphoblastic leukemia (ALL) (2.0, 1.2–3.4) and results were similar when we used never-smokers as the referent. For all leukemias and ALL, we observed a significant increasing trend in risk for pack-years smoked after birth of the child (Ptrend = 0.06 and 0.02, respectively) and the number of smokers in the home during the child's life (Ptrend = 0.05 and 0.03, respectively). On the other hand, the elevated risk associated with smoking at home during pregnancy or pack-years before pregnancy did not reach statistical significance for both all leukemias and ALL.
Table 2.
Smoking exposure and childhood leukemia risk
| Controls (n = 164) | All leukemia |
ALL |
|||
|---|---|---|---|---|---|
| Cases (n = 164) | OR (95% CI)a | Cases (n = 106) | OR (95% CI)a | ||
| Paternal smoking | |||||
| Smoking statusb | |||||
| Never-smoker | 41 (25.2) | 32 (19.5) | Ref. | 22 (20.8) | Ref. |
| Ever-smoker | 122 (74.9) | 132 (80.5) | 1.4 (0.8–2.4) | 84 (79.2) | 1.3 (0.7–2.4) |
| Smoking at home | |||||
| No | 105 (66.0) | 87 (53.4) | Ref. | 53 (50.5) | Ref. |
| Yes | 54 (34.0) | 76 (46.6) | 1.8 (1.1–2.8) | 76 (46.6) | 2.0 (1.2–3.4) |
| No | |||||
| Never-smoker | 41 (25.8) | 32 (19.6) | Ref. | 22 (21.0) | Ref. |
| Ever-smoker | 64 (40.3) | 55 (33.7) | 1.1 (0.6–2.0) | 31 (29.5) | 0.9 (0.5–1.8) |
| Yes | 54 (34.0) | 76 (46.6) | 1.8 (1.0–3.4) | 52 (49.5) | 1.9 (1.0–3.8) |
| Smoking at home during pregnancy | |||||
| No | 135 (86.0) | 130 (79.8) | Ref. | 53 (50.5) | Ref. |
| Yes | 22 (14.0) | 33 (20.3) | 1.6 (0.8–3.0) | 22 (21.0) | 1.8 (0.9–3.7) |
| No | |||||
| Never-smoker | 41 (26.1) | 32 (19.6) | Ref. | 22 (21.0) | Ref. |
| Ever-smoker | 94 (59.9) | 98 (60.1) | 1.3 (0.7–2.3) | 31 (29.5) | 1.2 (0.6–3.1) |
| Yes | 22 (14.0) | 33 (20.3) | 1.9 (0.9–4.1) | 22 (21.0) | 2.1 (0.9–4.9) |
| PYc before pregnancy | |||||
| 0 | 41 (30.6) | 32 (21.6) | Ref. | 22 (22.5) | Ref. |
| ≥10 | 60 (44.8) | 73 (49.3) | 1.6 (0.9–2.9) | 48 (49.0) | 1.6 (0.8–3.1) |
| >10 | 33 (24.6) | 43 (29.1) | 1.7 (0.9–3.3) | 28 (28.5) | 1.6 (0.8–3.5) |
| 0.13 | 0.22 | ||||
| PYc after birth | |||||
| 0 | 55 (38.2) | 45 (28.5) | Ref. | 27 (26.5) | Ref. |
| ≤10 | 77 (53.5) | 95 (60.1) | 1.5 (0.9–2.5) | 64 (62.8) | 1.7 (0.9–3.1) |
| >10 | 11 (8.3) | 18 (11.4) | 2.2 (0.8–5.5) | 11 (10.8) | 3.0 (1.0–8.8) |
| Ptrend | 0.06 | 0.02 | |||
| Number of smokers in the home | |||||
| 0 | 81 (59.6) | 76 (49.4) | Ref. | 47 (48.0) | Ref. |
| 1 | 51 (37.5) | 68 (44.2) | 1.4 (0.9–2.4) | 44 (44.9) | 1.5 (0.9–2.7) |
| ≥2 | 4 (2.9) | 10 (6.4) | 2.9 (0.8–10.2) | 7 (7.1) | 3.5 (0.9–13.3) |
| Ptrend | 0.04 | 0.03 | |||
| ≥1 | 55 (40.4) | 78 (50.6) | 1.5 (0.9–2.5) | 51 (52.0) | 1.7 (1.0–2.9) |
Adjusted for age of child, gender, father's education, and birth weight.
Never-smoker: <400 cigarettes/lifetime; ever-smoker: ≥400 cigarettes/lifetime.
Pack-years.
None of the five individual SNPs was significantly associated with childhood leukemia risk (Table 3) except for several suggested positive associations in subgroup analyses (data not shown). Further, the overall distribution of CYP1A1 haplotypes composed of five SNPs did not differ between childhood leukemia cases and controls.
Table 3.
CYP1A1 single nucleotide polymorphisms (SNPs) and childhood leukemia risk
| All leukemia |
ALL |
||||
|---|---|---|---|---|---|
| Controls (n = 164) | Cases (n = 164) | OR (95% CI)a | Cases (n = 106) | OR (95% CI)a | |
| –17961T>C | |||||
| CC | 81 (50.0) | 68 (43.3) | Ref. | 40 (39.2) | Ref. |
| CT | 61 (37.7) | 66 (42.0) | 1.3 (0.8–2.1) | 49 (48.0) | 1.6 (0.9–2.8) |
| TT | 20 (12.3) | 23 (14.7) | 1.3 (0.7–2.8) | 13 (12.8) | 1.4 (0.6–3.1) |
| CT/TT | 81 (50.0) | 89 (56.7) | 1.3 (0.8–2.1) | 62 (60.9) | 1.6 (0.9–2.6) |
| –9893G>A | |||||
| GG | 87 (54.4) | 90 (57.0) | Ref. | 59 (58.4) | Ref. |
| GA | 64 (40.0) | 59 (37.3) | 0.9 (0.6–1.4) | 36 (35.6) | 0.8 (0.5–1.4) |
| AA | 9 (5.6) | 9 (5.7) | 1.0 (0.4–2.8) | 6 (5.9) | 1.0 (0.3–3.0) |
| GA/AA | 73 (45.6) | 68 (43.0) | 0.9 (0.6–1.4) | 42 (41.6) | 0.8 (0.5–1.4) |
| Ex7+131A>G (I462V) | |||||
| AA | 85 (53.5) | 90 (56.3) | Ref. | 60 (57.1) | Ref. |
| AG | 65 (40.9) | 61 (38.1) | 0.9 (0.5–1.4) | 39 (37.1) | 0.9 (0.5–1.5) |
| GG | 9 (5.7) | 9 (5.6) | 1.0 (0.4–2.7) | 6 (5.7) | 0.9 (0.3–2.7) |
| AG/GG | 74 (46.5) | 70 (43.8) | 0.9 (0.6–1.4) | 45 (42.9) | 0.9 (0.5–1.5) |
| 1188C>T | |||||
| TT | 56 (34.6) | 58 (36.2) | Ref. | 34 (33.3) | Ref. |
| TC | 74 (45.6) | 80 (50.0) | 0.9 (0.6–1.6) | 56 (54.9) | 1.1 (0.6–2.0) |
| CC | 32 (19.8) | 22 (13.8) | 0.7 (0.3–1.3) | 12 (11.8) | 0.6 (0.3–1.3) |
| TC/CC | 106 (65.4) | 102 (63.8) | 0.9 (0.5–1.4) | 68 (66.7) | 1.0 (0.6–1.7) |
| 11599C>G | |||||
| CC | 53 (32.5) | 39 (24.4) | Ref. | 25 (24.3) | Ref. |
| CG | 69 (42.3) | 80 (50.0) | 1.6 (0.9–2.8) | 54 (52.4) | 1.8 (1.0–3.3) |
| GG | 41 (25.2) | 41 (25.6) | 1.4 (0.7–2.5) | 24 (23.3) | 1.3 (0.6–2.7) |
| CG/GG | 110 (67.5) | 121 (75.6) | 1.5 (0.9–2.5) | 78 (75.7) | 1.6 (0.9–2.8) |
| Haplotypeb | All leukemia |
ALL |
|||
|---|---|---|---|---|---|
| Controls (%) | Cases (%) | OR (95% CI)a | Cases (%) | OR (95% CI)a | |
| TGATG | 29.5 | 34.4 | Ref. | 35.2 | Ref. |
| CAGCC | 23.8 | 22.0 | 0.8 (0.5–1.2) | 21.4 | 0.7 (0.4–1.2) |
| CGATG | 17.9 | 16.6 | 0.8 (0.5–1.4) | 14.8 | 0.7 (0.4–1.3) |
| CGACC | 14.7 | 12.1 | 0.7 (0.4–1.2) | 12.2 | 0.6 (0.3–1.2) |
| CGATC | 10.9 | 11.4 | 1.0 (0.5–1.7) | 12.2 | 0.9 (0.5–1.7) |
| Rare haplotypes | 3.2 | 3.5 | 4.2 | ||
| Pomnibusc | 0.61 | 0.46 | |||
Adjusted for age of child, gender, father's education, and birth weight.
Composed of –17961T>C, –9893G>A, Ex7+13A>G (I462V), 1188C>T, and 11599C>G.
Omnibus test for overall distribution of haplotypes.
The association between paternal smoking and all leukemias and ALL was modified by CYP1A1 diplotype (Table 4). For all leukemias, among the CGACC (–) group, father's smoking at home significantly increased risk by 2.1-fold (95% CI = 1.2–3.7) and the presence of at least one smoker in the home during the child's life increased risk by 1.8-fold (95% CI = 1.0–3.2); whereas, there was no association among the CGACC (+) group. However, the test for interactions was not significant (Pinteraction = 0.13 and 0.14, respectively). We observed stronger evidence for effect modification when the analyses were restricted to cases with ALL (Table 4). Among the CGACC (–) group, ALL risk was significantly increased 2.8-fold (95% CI = 1.5–5.3) by father's smoking at home and 2.3-fold (95% CI = 1.2–4.4) by presence of at least one smoker in the home during the child's life. The test for interaction was significant (Pinteraction = 0.03 and 0.02, respectively).
Table 4.
Interactive effects between smoking exposure and CYP1A1 diplotypes on risk of childhood leukemia
| All Leukemia |
ALL |
|||||||
|---|---|---|---|---|---|---|---|---|
| CGACC (+) |
CGACC (–)a |
CGACC (+) |
CGACC (–)a |
|||||
| Cases/controls | OR (95% CI)b | Cases/controls | OR (95% CI)b | Cases/controls | OR (95% CI)b | Cases/controls | OR (95% CI)b | |
| Paternal smoking | ||||||||
| Smoking statusc | ||||||||
| Never-smoker | 8/7 | Ref. | 22/31 | Ref. | 6/7 | Ref. | 15/31 | Ref. |
| Ever-smoker | 24/39 | 0.5 (0.2–1.7) | 98/81 | 1.8 (0.9–3.4) | 16/39 | 0.5 (0.1–1.8) | 62/81 | 1.7 (0.8–3.6) |
| Pinteraction | 0.05 | 0.07 | ||||||
| Smoking at home | ||||||||
| No | 20/24 | Ref. | 63/78 | Ref. | 15/24 | Ref. | 35/78 | Ref. |
| Yes | 12/20 | 0.8 (0.3–2.3) | 56/32 | 2.1 (1.2–3.7) | 7/20 | 0.6 (0.2–2.1) | 41/32 | 2.8 (1.5–5.3) |
| Pinteraction | 0.12 | 0.03 | ||||||
| Smoking at home during pregnancy | ||||||||
| No | 26/36 | Ref. | 96/94 | Ref. | 18/36 | Ref. | 60/94 | Ref. |
| Yes | 6/8 | 1.4 (0.4–4.9) | 23/14 | 1.4 (0.7–3.0) | 4/8 | 1.5 (0.4–6.5) | 16/14 | 1.7 (0.7–3.8) |
| Pinteraction | 0.78 | 0.78 | ||||||
| PYd after birth | ||||||||
| 0 | 9/13 | Ref. | 33/39 | Ref. | 6/13 | Ref. | 19/39 | Ref. |
| ≤10 | 20/19 | 1.6 (0.5–5.0) | 69/56 | 1.4 (0.8–2.6) | 14/19 | 1.9 (0.5–6.7) | 46/56 | 1.6 (0.8–3.3) |
| >10 | 2/5 | NCe | 15/7 | 2.9 (0.9–8.9) | 1/5 | NCe | 10/7 | 4.9 (1.3–17.5) |
| Ptrend | 0.07 | 0.02 | ||||||
| Pinteraction | 0.35 | 0.27 | ||||||
| Number of smokers in the home | ||||||||
| 0 | 19/22 | Ref. | 54/57 | Ref. | 15/22 | Ref. | 30/57 | Ref. |
| ≥1 | 10/18 | 0.8 (0.3–2.3) | 60/34 | 1.8 (1.0–3.2) | 5/18 | 0.5 (0.1–1.7) | 42/34 | 2.3 (1.2–4.4) |
| Pinteraction | 0.12 | 0.02 | ||||||
Other than CGACC haplotype.
Adjusted for age of child, gender, father's education, and birth weight.
Never-smoker: <400 cigarettes/lifetime; ever-smoker: ≥400 cigarettes/lifetime.
Pack-years.
Not calculated due to sparse data.
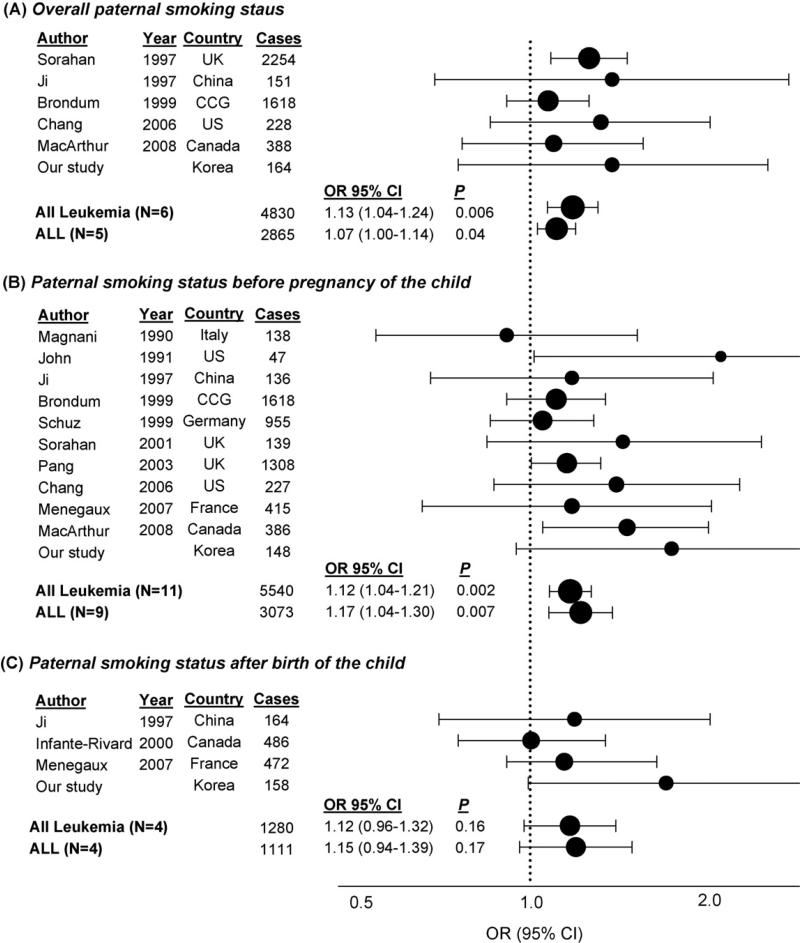
The meta-analysis of lifetime paternal smoking (ever vs. never) and childhood leukemia showed an elevated risk for all childhood leukemias (OR = 1.13, 95% CI = 1.04–1.24; P = 0.006) and ALL (OR = 1.07, 95% CI = 1.00–1.14; P = 0.04) (Fig. 1). Paternal smoking status before pregnancy of the child also showed significant association with all childhood leukemias (OR = 1.12, 95% CI = 1.04–1.21; P = 0.002) and ALL (OR = 1.17, 95% CI = 1.04–1.30; P = 0.007). However, we did not observe a significant association of paternal smoking status after birth with all childhood leukemias (OR = 1.12, 95% CI = 0.96–1.32; P = 0.16) or ALL (OR = 1.15, 95% CI = 0.94–1.39; P = 0.17). There was no evidence of significant heterogeneity among studies (P > 0.3). In terms of publication bias, all P-values from Begg's and Egger's tests were >0.08 and >0.18, respectively, except for the association between paternal smoking status before pregnancy of the child and all childhood leukemias (P = 0.03 and 0.05, respectively), due to the significant result from the smallest study [7]).
Fig. 1.
Meta-analysis of the association between paternal smoking (ever vs. never) and childhood leukemia risk: (A) Overall paternal smoking and childhood leukemia elevated the risk for all childhood leukemias (OR = 1.13; 95% CI = 1.04–1.24; P = 0.006) and ALL (OR = 1.07; 95% CI = 1.00–1.14; P = 0.04); (B) Paternal smoking status before pregnancy of the child also showed significant association with all childhood leukemia (OR = 1.12; 95% CI = 1.04–1.21; P = 0.002) and ALL (OR = 1.17; 95% CI = 1.04–1.30; P = 0.007); (C) However, significant association was not found between paternal smoking status after birth and all childhood leukemia (OR = 1.12; 95% CI = 0.96–1.32; P = 0.16) or ALL (OR = 1.15; 95% CI = 0.94–1.39; P = 0.17). There was no evidence of significant heterogeneity among studies (P > 0.3). In terms of publication bias, all P-values from Begg's and Egger's tests were >0.08 and >0.15, respectively, except for the association between paternal smoking status before pregnancy of the child and all childhood leukemias (P = 0.03 and 0.05, respectively), due to the significant result from the smallest study [7]). Note: CCG = Childhood Oncology Group.
4. Discussion
Our study suggests that paternal smoking increases the risk of childhood leukemia, especially ALL, and the effect may be modified by polymorphisms in CYP1A1, an enzyme which is responsible for activation of PAHs.
Many previous epidemiological studies found that prenatal paternal smoking is associated with increased risk of childhood leukemia [7,14–17]. Our meta-analysis of previous reports [7,12–21,29], showed that lifetime paternal smoking and paternal smoking before pregnancy of the child was significantly associated with increased risk of childhood leukemia. A plausible biological mechanism for the association with paternal smoking before the pregnancy could be cigarette smoke-induced oxidative DNA damage in human semen, which causes chromosome breaks that ultimately lead to translocations in utero and childhood leukemia development [33]. Support for this comes from studies that found that the level of DNA damage [34], or benzo[a]pyrene diol epoxide (BPDE)-DNA adducts [35], was higher in the sperm of smokers compared with nonsmoking men.
The results of our study suggest that postnatal paternal smoking may also play a role in the development of childhood leukemia and that paternal smoking at home, rather than paternal smoking itself, significantly increases risk of childhood leukemia. Pack-years smoked after birth and the number of smokers in the home during the child's life showed a moderate association with childhood leukemia risk. According to the “two hit” hypothesis proposed by Greaves [36], for ALL, the initiation step includes chromosome translocations originating in utero whereas, postnatal exposures provide the second hit. Thus, postnatal exposure to cigarette smoke may increase risk of ALL by causing a secondary genetic event. The interactive effect between CYP1A1 genotype of the child and paternal smoking that we observed in our study also supports a role for children's postnatal exposure to cigarette smoke in the etiology of leukemia risk. We note that our meta-analysis for paternal smoking after birth of the child included only four studies with relatively small number of subjects, and that more studies need to be conducted to evaluate the association between smoking exposure after birth and childhood leukemia.
Environmental exposure to cigarette smoking among children has been related to the level of biomarkers of genetic damage such as sister chromatid exchange [37], and hemoglobin adducts with BPDE or ethylene oxide which can serve as surrogates for DNA adducts induced by the same chemicals [37,38]. Cigarette smoke has been causally related to adduct formation between BPDE and DNA or protein [39], and increases aromatic hydrocarbon hydroxylase (AHH) activity [40], and CYP1A1 expression [41]. AHH activity level has been associated with the CYP1A1*2A allele [28], and 462Val [42], whereas the mRNA level of CYP1A1 was associated only with 462Val [42]. Given this experimental evidence, it has been hypothesized that a CYP1A1 haplotype including these two SNPs may play a role as an effect modifier for the association between postnatal smoking exposure and childhood leukemia. However, individual SNP analysis does not support a role of these SNPs in childhood leukemia risk and further investigation is needed.
The primary limitation of our study is the small sample size and, as a consequence, low statistical power to detect associations. In addition, the small sample size may have resulted in false positive result by chance due to the increased likelihood of a false positive finding, particularly if the risk factor has a low prior probability [43]. The suggestive interactive effect found between CYP1A1 diplotype and paternal smoking while plausible also needs cautious interpretation. Thus, it is important to attempt to replicate the findings in other studies of childhood leukemia.
We evaluated possible selection bias since controls were recruited from only one hospital (Seoul National University Hospital), whereas cases were recruited from three hospitals in Seoul including SNUH. Although all three hospitals are considered to be among the top ranking hospitals in Seoul, the socio-economic status of the patients of SNUH tended to be somewhat lower compared to those of the other two hospitals. Smoking rates are higher among lower socio-economic groups in Korea [44]. However, restricting the analyses to cases and controls from SNUH, we found similar associations for paternal smoking and smoking at home to those for the overall study population; thus, there seems little evidence for selection bias.
Potential recall or reporting bias is common in case–control studies. However, this bias would likely be relatively small because both case and control subjects were admitted to the hospitals and their mothers would be expected to have similar recall [45]. We observed non-significant results for paternal smoking before pregnancy and significant results for paternal smoking after birth. These results may be partly explained by the fact that the information about smoking history of the father was collected by the mother of the index child, thus starting or quitting age might be relatively inaccurate compared to smoking information provided by the father after birth.
This study is one of several that have evaluated the interactive effects between postnatal smoking exposure and genetic polymorphisms in relation to risk of childhood leukemia and we found some suggestive results in support of effect modification. Additionally, the results of our meta-analysis support an association between prenatal smoking of the father and childhood cancer risk.
In conclusion, paternal smoking may increase the risk of childhood leukemia and the effect may be modified by the CYP1A1 genotype of the child. However, these findings need to be replicated in other populations using accurate smoking exposure data, molecular biomarkers, and complete coverage of tagging SNPs in CYP1A1 and other genes that play a role in the metabolism of carcinogens in cigarette smoke.
Acknowledgements
We gratefully acknowledge the individuals who participated in the research and the clinicians who gave permission for us to approach their patients. This research was funded by Korea Electrotechnology Research Institute, Ministry of. Knowledge Economy (MKE) and by a grant of the Korea Health 21 R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (A030001: 03-PJ10-PG13-GD01-0002).
Footnotes
Conflicts of Interest
None
References
- 1.National Cancer Registry, Korea . National Cancer Registry Report. Seoul; Korea: 2002. [Google Scholar]
- 2.Perera FP. Environment and cancer: who are susceptible. Science. 1997;278(5340):1068–73. doi: 10.1126/science.278.5340.1068. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;8(3):461–9. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 4.Kuper H, Boffetta P, Adami HO. Tobacco use and cancer causation: association by tumour type. J Intern Med. 2002;252(3):206–24. doi: 10.1046/j.1365-2796.2002.01022.x. [DOI] [PubMed] [Google Scholar]
- 5.California Environmental Protection Agency Office of Environmental Health Hazard Assessment Health effects of exposure to environmental tobacco smoke. Final Report. 1997. [DOI] [PMC free article] [PubMed]
- 6.Stjernfeldt M, Berglund K, Lindsten J, Ludvigsson J. Maternal smoking during pregnancy and risk of childhood cancer. Lancet. 1986;1(8494):1350–2. doi: 10.1016/s0140-6736(86)91664-8. [DOI] [PubMed] [Google Scholar]
- 7.John EM, Savitz DA, Sandler DP. Prenatal exposure to parents’ smoking and childhood cancer. Am J Epidemiol. 1991;133(2):123–32. doi: 10.1093/oxfordjournals.aje.a115851. [DOI] [PubMed] [Google Scholar]
- 8.Mucci LA, Granath F, Cnattingius S. Maternal smoking and childhood leukemia and lymphoma risk among 1,440,542 Swedish children. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1528–33. [PubMed] [Google Scholar]
- 9.Neutel CI, Buck C. Effect of smoking during pregnancy on the risk of cancer in children. J Natl Cancer Inst. 1971;47(1):59–63. [PubMed] [Google Scholar]
- 10.Pershagen G, Ericson A, Otterblad-Olausson P. Maternal smoking in pregnancy: does it increase the risk of childhood cancer. Int J Epidemiol. 1992;21(1):1–5. doi: 10.1093/ije/21.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Klebanoff MA, Clemens JD, Read JS. Maternal smoking during pregnancy and childhood cancer. Am J Epidemiol. 1996;144(11):1028–33. doi: 10.1093/oxfordjournals.aje.a008874. [DOI] [PubMed] [Google Scholar]
- 12.Schuz J, Kaatsch P, Kaletsch U, Meinert R, Michaelis J. Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol. 1999;28(4):631–9. doi: 10.1093/ije/28.4.631. [DOI] [PubMed] [Google Scholar]
- 13.Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer. 2003;88(3):373–81. doi: 10.1038/sj.bjc.6600774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji BT, Shu XO, Linet MS, Zheng W, Wacholder S, Gao YT, et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997;89(3):238–44. doi: 10.1093/jnci/89.3.238. [DOI] [PubMed] [Google Scholar]
- 15.Sorahan T, Prior P, Lancashire RJ, Faux SP, Hultén MA, Peck IM, et al. Childhood cancer and paternal use of tobacco: deaths from 1971 to 1976. Br J Cancer. 1997;76(11):1525–31. doi: 10.1038/bjc.1997.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorahan T, McKinney PA, Mann JR, Lancashire RJ, Stiller CA, Birch JM, et al. Childhood cancer and parental use of tobacco: findings from the inter-regional epidemiological study of childhood cancer (IRESCC). Br J Cancer. 2001;84(1):141–6. doi: 10.1054/bjoc.2000.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA. Parental smoking and the risk of childhood leukemia. Am J Epidemiol. 2006;163(12):1091–100. doi: 10.1093/aje/kwj143. [DOI] [PubMed] [Google Scholar]
- 18.Magnani C, Pastore G, Luzzatto L, Terracini B. Parent occupation and other environmental factors in the etiology of leukemias and non-hodgkin's lymphomas in childhood: a case–control study. Tumori. 1990;76:413–9. doi: 10.1177/030089169007600501. [DOI] [PubMed] [Google Scholar]
- 19.Brondum J, Shu XO, Steinbuch M, Severson RK, Potter JD, Robison LL. Parental cigarette smoking and the risk of acute leukemia in children. Cancer. 1999;85(6):1380–8. [PubMed] [Google Scholar]
- 20.Menegaux F, Ripert M, Hemon D, Clavel J. Maternal alcohol and coffee drinking, parental smoking and childhood leukaemia: a French population-based case–control study. Paediatr Perinat Epidemiol. 2007;21(4):293–9. doi: 10.1111/j.1365-3016.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- 21.Macarthur AC, McBride ML, Spinelli JJ, Tamaro S, Gallagher RP, Theriault G. Risk of childhood leukemia associated with parental smoking and alcohol consumption prior to conception and during pregnancy: the cross-Canada childhood leukemia study. Cancer Causes Control. 2008;19(3):283–95. doi: 10.1007/s10552-007-9091-8. [DOI] [PubMed] [Google Scholar]
- 22.Krajinovic M, Labuda D, Richer C, Karimi S, Sinnett D. Susceptibility to childhood acute lymphoblastic leukemia: influence of CYP1A1, CYP2D6, GSTM1, and GSTT1 genetic polymorphisms. Blood. 1999;93(5):1496–501. [PubMed] [Google Scholar]
- 23.Sinnett D, Krajinovic M, Labuda D. Genetic susceptibility to childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2000;38(56):447–62. doi: 10.3109/10428190009059264. [DOI] [PubMed] [Google Scholar]
- 24.Joseph T, Kusumakumary P, Chacko P, Abraham A, Radhakrishna Pillai M. Genetic polymorphism of CYP1A1, CYP2D6 GSTM1 and GSTT1 and susceptibility to acute lymphoblastic leukaemia in Indian children. Pediatr Blood Cancer. 2004;43(5):560–7. doi: 10.1002/pbc.20074. [DOI] [PubMed] [Google Scholar]
- 25.Aydin-Sayitoglu M, Hatirnaz O, Erensoy N, Ozbek U. Role of CYP2D6, CYP1A1, CYP2E1, GSTT1, and GSTM1 genes in the susceptibility to acute leukemias. Am J Hematol. 2006;81(3):162–70. doi: 10.1002/ajh.20434. [DOI] [PubMed] [Google Scholar]
- 26.Pakakasama S, Mukda E, Sasanakul W, Kadegasem P, Udomsubpayakul U, Thithapandha A, et al. Polymorphisms of drug-metabolizing enzymes and risk of childhood acute lymphoblastic leukemia. Am J Hematol. 2005;79(3):202–5. doi: 10.1002/ajh.20404. [DOI] [PubMed] [Google Scholar]
- 27.Krajinovic M, Labuda D, Sinnett D. Glutathione S-transferase P1 genetic polymorphisms and susceptibility to childhood acute lymphoblastic leukaemia. Pharmacogenetics. 2002;12(8):655–8. doi: 10.1097/00008571-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Petersen DD, McKinney CE, Ikeya K, Smith HH, Bale AE, McBride OW, et al. Human CYP1A1 gene: cosegregation of the enzyme inducibility phenotype and an RFLP. Am J Hum Genet. 1991;48(4):720–5. [PMC free article] [PubMed] [Google Scholar]
- 29.Infante-Rivard C, Krajinovic M, Labuda D, Sinnett D. Parental smoking, CYP1A1 genetic polymorphisms and childhood leukemia (Quebec, Canada). Cancer Causes Control. 2000;11(6):547–53. doi: 10.1023/a:1008976116512. [DOI] [PubMed] [Google Scholar]
- 30.Clavel J, Bellec S, Rebouissou S, Ménégaux F, Feunteun J, Bonaïti-Pellié C, et al. Childhood leukaemia, polymorphisms of metabolism enzyme genes, and interactions with maternal tobacco, coffee and alcohol consumption during pregnancy. Eur J Cancer Prev. 2005;14(6):531–40. doi: 10.1097/00008469-200512000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Applied Biosystems . Protocol for SNaPshot Multiplex Kit. Foster City, CA, US: 2005. [Google Scholar]
- 32.Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–9. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greaves MF, Wiemels J. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer. 2003;3(9):639–49. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- 34.Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res. 1996;351(2):199–203. doi: 10.1016/0027-5107(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 35.Horak S, Polanska J, Widlak P. Bulky DNA adducts in human sperm: relationship with fertility, semen quality, smoking, and environmental factors. Mutat Res. 2003;537(1):53–65. doi: 10.1016/s1383-5718(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 36.Greaves M. In utero origins of childhood leukaemia. Early Hum Dev. 2005;81(1):123–9. doi: 10.1016/j.earlhumdev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Tang D, Warburton D, Tannenbaum SR, Skipper P, Santella RM, Cereijido GS, et al. Molecular and genetic damage from environmental tobacco smoke in young children. Cancer Epidemiol Biomarkers Prev. 1999;8(5):427–31. [PubMed] [Google Scholar]
- 38.Bono R, Vincenti M, Schilirò T, Traversi D, Pignata C, Scursatone E, et al. Cotinine and N-(2-hydroxyethyl)valine as markers of passive exposure to tobacco smoke in children. J Expo Anal Environ Epidemiol. 2005;15(1):66–73. doi: 10.1038/sj.jea.7500344. [DOI] [PubMed] [Google Scholar]
- 39.Mollerup S, Berge G, Baera R, Skaug V, Hewer A, Phillips DH, et al. Sex differences in risk of lung cancer: expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer. 2006;119(4):741–4. doi: 10.1002/ijc.21891. [DOI] [PubMed] [Google Scholar]
- 40.Lagueux J, Pereg D, Ayotte P, Dewailly E, Poirier GG. Cytochrome P450 CYP1A1 enzyme activity and DNA adducts in placenta of women environmentally exposed to organochlorines. Environ Res. 1999;80(4):369–82. doi: 10.1006/enrs.1998.3920. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Sherman ME, Curriero FC, Guengerich FP, Strickland PT, Sutter TR. Expression of cytochromes P450 1A1 and 1B1 in human lung from smokers, non-smokers, and ex-smokers. Toxicol Appl Pharmacol. 2004;199(3):210–9. doi: 10.1016/j.taap.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Crofts F, Taioli E, Trachman J, Cosma GN, Currie D, Toniolo P, et al. Functional significance of different human CYP1A1 genotypes. Carcinogenesis. 1994;15(12):2961–3. doi: 10.1093/carcin/15.12.2961. [DOI] [PubMed] [Google Scholar]
- 43.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96(6):434–42. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim CS, Yun SC, Kim HR, Khang YH. A multilevel study on the relationship between the residential distribution of high class (power elites) and smoking in Seoul. J Prev Med Pub Health. 2006;39(1):30–8. (in Korean) [PubMed] [Google Scholar]
- 45.Infante-Rivard C. Hospital or population controls for case–control studies of severe childhood diseases. Am J Epidemiol. 2003;157:176–82. doi: 10.1093/aje/kwf174. [DOI] [PubMed] [Google Scholar]