Summary
Previous work has shown that mature B cells depend upon survival signals delivered to the cells by their antigen receptor (BCR). To identify the molecular nature of this survival signal, we have developed a genetic approach in which ablation of the BCR is combined with the activation of specific, BCR dependent signaling cascades in mature B cells in vivo. Using this system, we provide evidence that the survival of BCR deficient mature B cells can be rescued by a single signaling pathway downstream of the BCR, namely PI3K signaling, with the FOXO1 transcription factor playing a central role.
Introduction
Mature B cells in the peripheral immune system depend on two central survival determinants, the B cell antigen receptor (BCR) and the receptor for the tumor necrosis factor (TNF) family cytokine, BAFF (Lam et al., 1997; Ng et al., 2005). Whereas the BCR controls the response of the cells to cognate antigen, the BAFF-R senses BAFF produced by neighboring cells in the B cell habitat and is thus critically involved in controlling B cell numbers. In BAFF or BAFF-R deficient mice, mature B cells scarcely develop, and BAFF depletion in adult animals leads to rapid apoptosis of these cells (Batten et al., 2000; Thompson et al., 2000; Yan et al., 2001). In the case of the BCR, using systems of induced and stage-specific gene targeting, Lam et al. (1997) and Kraus et al. (2004) demonstrated that mature B cells undergo apoptosis upon in vivo BCR ablation or mutation of one of its signaling units, the Igα polypeptide chain, and disappear from the body with a half-life of 3–6 days. This indicated that the BCR on resting, mature B cells transmits “tonic” survival signals into the cells, either upon interaction with ligands in the environment or spontaneously. The notion of ”tonic” BCR signals keeping mature B cell alive is supported by additional, more indirect evidence (Bannish et al., 2001; Grande et al., 2007; Stadanlick et al., 2008).
Which pathways downstream of the BCR and BAFF-R signal B cell survival? Upon BAFF engagement, the BAFF-R signals mainly through the alternative NF-κB pathway, and there is evidence that BAFF-R mediated activation of the latter is critical for mature B cell survival, although additional factors may be involved (Mecklenbrauker et al., 2004; Sasaki et al., 2006). However, in the case of the BCR, which can activate multiple signaling cascades, the nature of the survival signal has remained unresolved. One recent hypothesis is based on evidence that BCR engagement can activate the canonical NF-κB signaling pathway and mature B cells require canonical NF-κB signals for their in vivo maintenance (Cariappa et al., 2000; Pasparakis et al., 2002). This led Stadanlick et al. (2008) to propose a model in which mature B cell survival is based on an NF-κB mediated crosstalk between BCR and BAFF-R. In this model, BCR mediated canonical NF-κB signals enhance BAFF-R signaling by transcriptional up-regulation of the genes encoding BAFF-R on the one hand and p100, a critical component of the alternative NF-κB pathway, on the other (Bonizzi and Karin, 2004). However, while this mechanism may well play a role upon B cell activation, its role in B cell maintenance remained speculative and is not easily compatible with other experimental evidence (Ruland et al., 2001; Xue et al., 2003).
In an attempt to directly assess the molecular nature of the BCR mediated survival signal for mature B cells, we have developed a system, in which conditional ablation of the BCR in mature B cells in vivo is combined with the conditional activation of candidate signaling cascades in the same cells. Using this approach, we find that mature B cells losing their BCR are fully rescued by activation of the PI3K signaling pathway, but not that of canonical NF-κB. Thus, PI3K signaling, which has recently emerged as a critical determinant of B cell development (Aiba et al., 2008; Deane and Fruman, 2004; Herzog et al., 2008; Omori et al., 2006; Verkoczy et al., 2007) also provides the critical survival signal for mature B cells downstream of the BCR.
Results
Experimental design
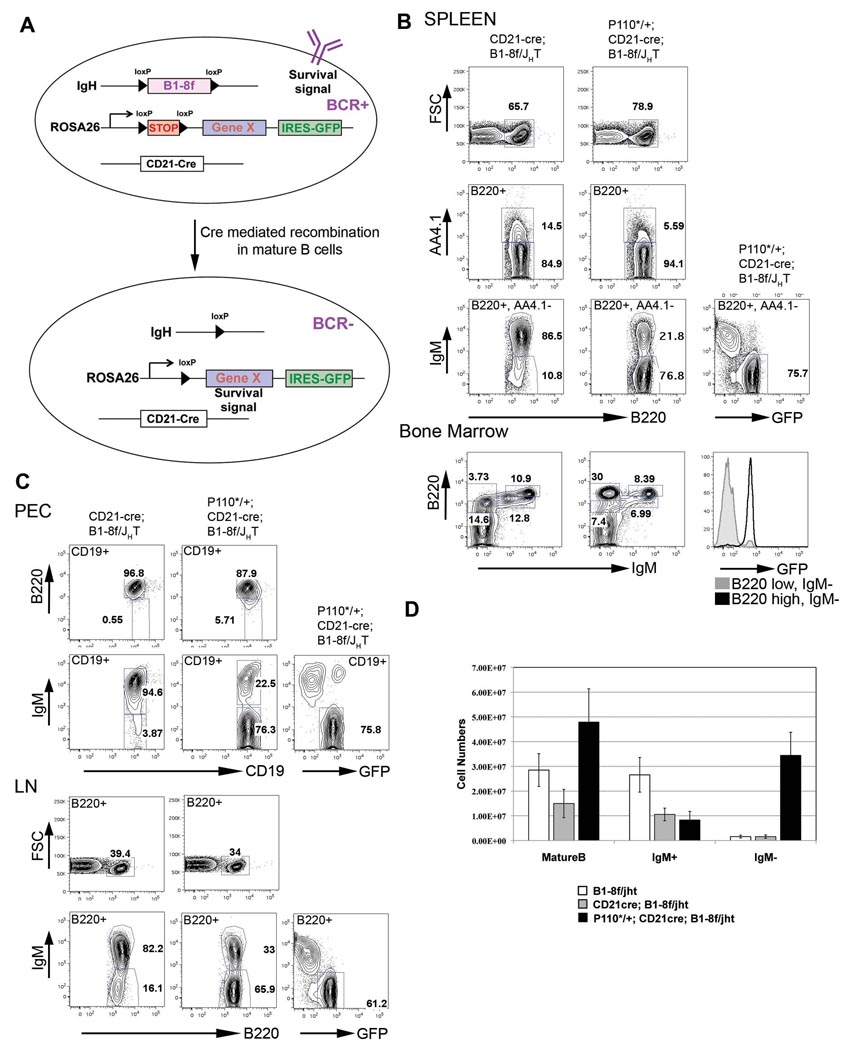
For targeted deletion of the BCR in mature B cells, we used the CD21-cre transgene, expressed when B cells differentiate from transitional to mature cells (Kraus et al., 2004), in combination with the B1-8f allele, in which the B1-8 VDJ gene segment, sitting in its physiological position in the IgH locus, is flanked by loxP sites (Lam et al., 1997). We complemented this system by a third genetic element, which would rescue the BCR deficient cells by concomitant, Cre-mediated activation of a survival signal mimicking the one normally provided by the BCR. Following a strategy which we had previously developed (Sasaki et al., 2006), we generated a series of ROSA26 alleles harboring cDNAs of constitutively active signaling molecules, preceded by a loxP flanked STOP cassette and marked by a GFP gene under the control of an internal ribosomal entry site downstream of the inserted cDNA. In combination with CD21-cre and B1-8f, cDNA and GFP expression would coincide with BCR deletion in mature B cells, allowing us to determine whether a signaling pathway is activated which rescues BCRneg mature B cells (Figure 1A).
Figure 1. Constitutively active PI3K rescues BCR negative B cells.
(A) Schematic representation of the experimental strategy. (B) Representative FACS analysis of spleen and bone marrow lymphocytes. Numbers represent percentages of cells within the lymphocyte gate or of cells in the gate indicated in the FACS plot. Expression of P110* leads to accumulation of BCRneg mature B cells (B220+, AA4.1−) in the spleen that are GFP positive. The histogram plot in the bone marrow section compares GFP expression in the progenitor gate (B220 low, IgMneg) and the IgMneg mature B cell gate (B220 high, IgMneg) from P110*/+; CD21-cre; B1-8f/JHT mice. (C) FACS analysis of lymphocytes from peritoneal cavity (PEC, top two rows) and lymph nodes (LN, bottom two rows). (D) Comparison of B cell numbers from the spleen of B1-8f/JHT (white bars), CD21-cre; B1-8f/JHT mice (grey bars) and P110*/+; CD21-cre; B1-8f/JHT mice (filled bars), mean and SD of a minimum of 6 mice per genotype.
Constitutively active PI3K rescues BCR negative B cells
One of the constitutively active signaling molecules that we conditionally expressed from the ROSA26 locus was MP110*, a constitutively active form of P110α, the catalytic subunit of PI3K (Klippel et al., 1996). For simplicity we will refer to this allele, which is described in Figure S1, as P110* throughout the paper.
As compound mutant mice expressing P110* in mature B cells died soon after birth, possibly due to the expression of CD21-cre, and consequently P110*, in the forebrain (Schmidt-Supprian and Rajewsky, 2007), we studied the requirement of PI3K activity for the survival of BCRneg B cells in chimeric mice, generated by transfer of fetal liver cells of the compound mutants into syngeneic RAG2/common γ chain deficient recipients, which are lymphocyte deficient (Cao et al., 1995). Lymphocyte development was reconstituted in the chimeric mice, and no lethality was observed in recipients of either CD21-cre;B1-8f/JHT or P110*/+;CD21-cre;B1-8f/JHT fetal liver cells. JHT is a null allele of the IgH locus (Gu et al., 1993), introduced into the system to limit IgH expression to the B1-8f allele.
In accord with our previous work (Kraus et al., 2004), IgMneg B cells were detectable in spleens from CD21-cre; B1-8f/JHT fetal liver chimeric mice, but did not accumulate beyond around 10% of total mature B cells in steady state (Figure 1B). In striking contrast, 77% of splenic mature B cells (B220+, AA4.1−) from P110*/+; CD21-cre; B1-8f/JHT chimeras were IgMneg. Essentially all and only those cells expressed GFP, indicating that Cre-mediated recombination occurred at the IgH and the ROSA26 locus with equal efficiency, and consistent with a requirement of P110* expression for the survival of these cells. The increased percentage of mature (B220+, AA4.1−) B cells in the chimeras carrying the P110* allele is consistent with the counter selection of such cells when they loose their BCR in the absence of P110* expression (Kraus et al., 2004).
Rescue of BCRneg mature B cells by P110* was also seen in other lymphoid organs. Thus, a large fraction of mature (B220 high) B cells in the bone marrow of P110*/+; CD21-cre; B1-8f/JHT chimeras were IgMneg and expressed GFP, whereas in the absence of the P110* allele all B220 high cells were IgMpos (Figure 1B, bottom row). Large numbers of GFP expressing IgMneg B cells also accumulated in the peritoneal cavity and lymph nodes of compound mutant chimeras carrying the P110* allele (Figure 1C).
Numerically, the rescue of BCRneg mature B cells by P110* was extremely effective. P110*/+; CD21-cre; B1-8f/JHT chimeras had more than twice the number of splenic B cells of their P110*-negative counterparts, with almost all of the increase being due to the accumulation of IgMneg cells (Figure 1D). In comparison to mice without ongoing BCR ablation in mature B cells (B1-8f/JHT), the P110* rescue of IgMneg B cells generated a somewhat expanded peripheral B cell compartment (Figure 1D), partly due to a moderate expansion of the Marginal Zone (MZ) B cell subset (Figure S2).
P110* rescued BCR negative B cells resemble normal mature B cells
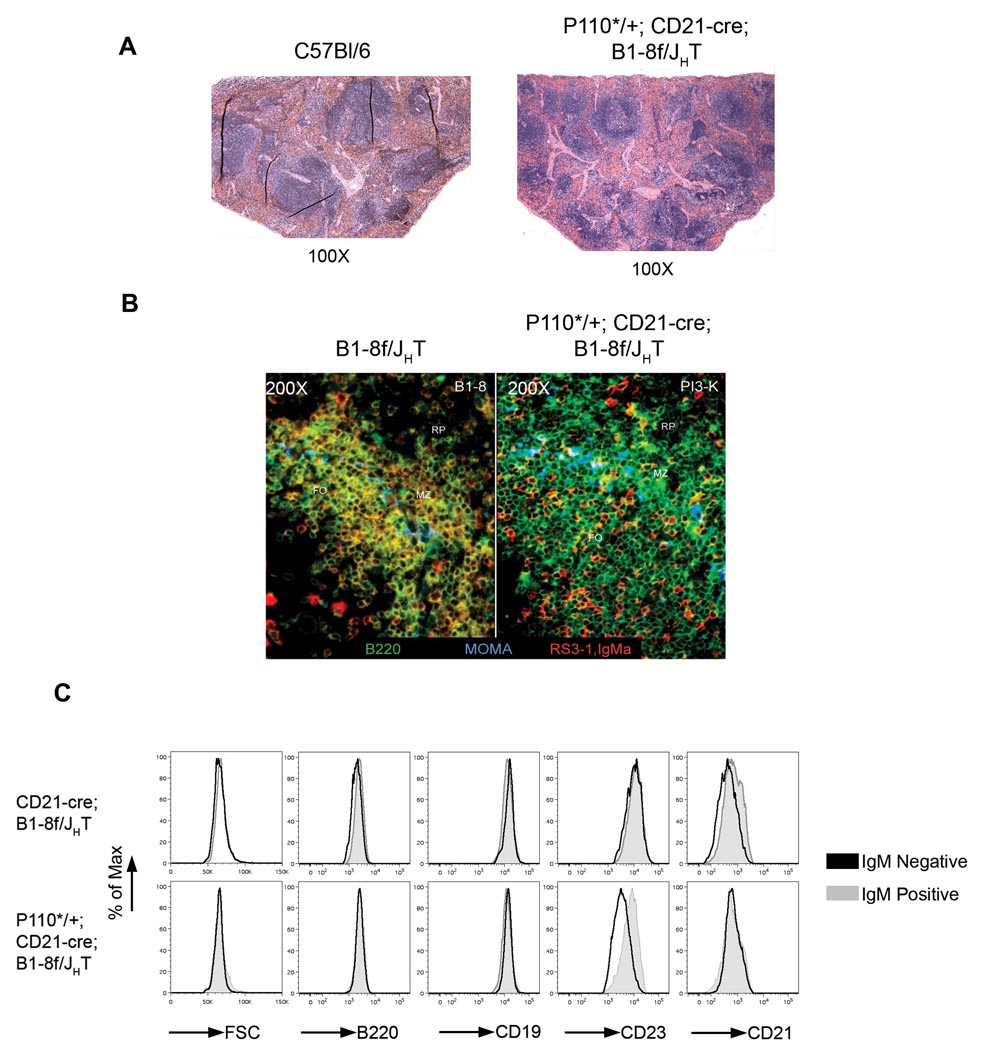
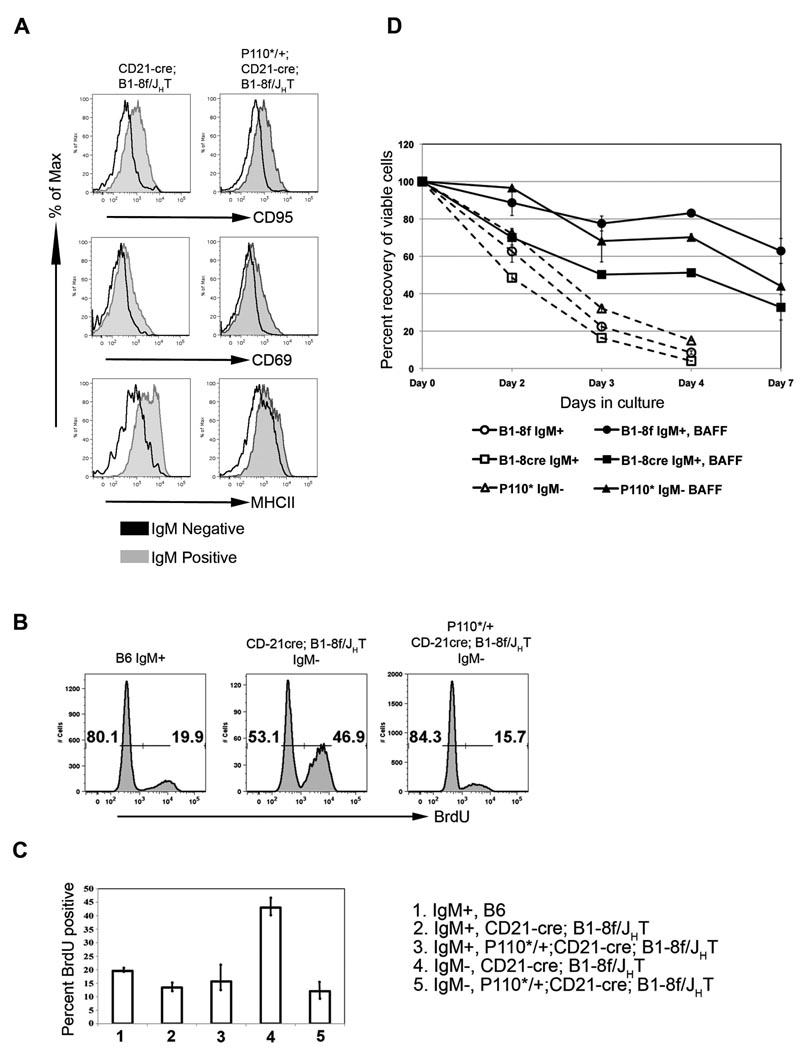
Histologically, a normal organization of BCRneg B cells into follicles and adjacent MZ was seen in the spleen, with a majority of BCRneg, B220+ cells intermingled with a minority of BCRpos, B220+ cells that had not undergone Cre mediated recombination (Figure 2 A, B). When analyzed by flow cytometry, mutant and control cells were identical in forward scatter analysis and expressed similar levels of the B cell markers B220, CD19 and CD21 (Figure 2C). CD23 was about 2-fold down regulated on the cells compared to controls, a phenomenon that will be further discussed below. Significantly, no upregulation of the activation markers CD95, CD69 and MHC II was seen in the rescued cells, suggesting that they were in a resting state like normal, mature B cells (Figure 3A). Accordingly, 84% of these cells did not incorporate bromodeoxyuridine (BrdU) into their DNA over a 20-day feeding period, similar to the 80% unlabelled cells in the controls (Figure 3B,C; Forster and Rajewsky, 1990). In contrast, 50% of the IgMneg B cells in compound mutants lacking the P110* allele incorporated BrdU, indicative of their rapid turnover (Kraus et al., 2004). An unexpected finding was the low degree of BrdU incorporation in the population of IgMpos B cells in the CD21-cre; B1-8f/JHT mutant chimeras (Figure 3C). Apparently, many of these cells escaped Cre-mediated recombination by an unknown mechanism.
Figure 2. P110* rescued IgMneg B cells are arranged in follicles in the spleen and are similar to IgMpos B cells.
(A) Hematoxylin and Eosin staining of sections of spleen from a wild type C57Bl/6 (B6) and P110*/+; CD21-cre; B1-8f/JHT (PI3K, Fetal liver chimera) mice. (B) Immuno-fluorescent staining of sections of spleen showing part of a single follicle from B1-8f/JHT and P110*/+; CD21-cre; B1-8f/JHT mice. B220, marking all B cells is stained in green, IgM is in red and MOMA1, marking metallophilic macrophages is in blue. (C) Comparison of cell size (FSC), and surface expression of B cell markers B220, CD19, CD23 and CD21 between IgMpos (grey) and IgMneg (black) follicular B cells from spleen of CD21-cre; B1-8f/JHT (top row) and P110*/+; CD21-cre; B1-8f/JHT (bottom row) mice.
Figure 3. P110* rescued IgMneg B cells are resting B cells dependent on BAFF for survival.
(A) Comparison of surface expression of activation markers on IgMpos (grey histograms) and IgMneg (black histograms) mature follicular B cells (gated on B220+, AA4.1− and CD23 high) from the spleen of CD21-cre; B1-8f/JHT (left column) and P110*/+; CD21-cre; B1-8f/JHT (right column) mice. (B) Representative FACS plots showing BrdU incorporation in B lymphocytes from spleen after 20 days of BrdU feeding of mice of indicated genotype. The numbers indicate the percentage of BrdU positive or negative cells within the mature B cell fraction (B220+, AA4.1−). (C) Graphical representation of data in B. Also shown is incorporation of Brdu in the IgMpos mature B cells from CD21-cre; B1-8f/JHT and P110*/+; CD21-cre; B1-8f/JHT fetal liver chimeric mice. (D) P110* rescued IgMneg B cells are dependent on BAFF for survival in vitro. IgMpos and IgMneg mature follicular B cells from B1-8f/JHT (B1-8f), CD21-cre; B1-8f/JHT (B1-8cre) and P110*/+; CD21-cre; B1-8f/JHT (P110*) mice were cultured in vitro in the presence (filled markers) or absence (open markers) of BAFF. Data is plotted as percent recovery of viable cells.
As the P110* rescued BCRneg cells are well controlled in terms of overall cell numbers, one would predict that they retain the dependence of normal mature B cells on the survival factor BAFF. On the other hand, BAFF:BAFF-R induced activation of the alternative NF-kB pathway leads to the phosphorylation of Akt, which is a main downstream target of PI3K (Patke et al., 2006; Otipoby et al., 2008). We therefore assayed the BAFF dependence of P110* rescued BCRneg B cells in cell culture experiments (Batten et al., 2000; Gross et al., 2001) and found that the survival of P110* rescued and control cells was equally dependent on BAFF (Figure 3D).
The P110* rescued BCRneg B cells are thus resting, long-lived cells, living in their normal habitats in the peripheral immune system and subject to normal homeostatic control.
Failure of canonical NF-κB signaling and constitutive MEK1 and Rac1 to efficiently rescue BCR negative B cells
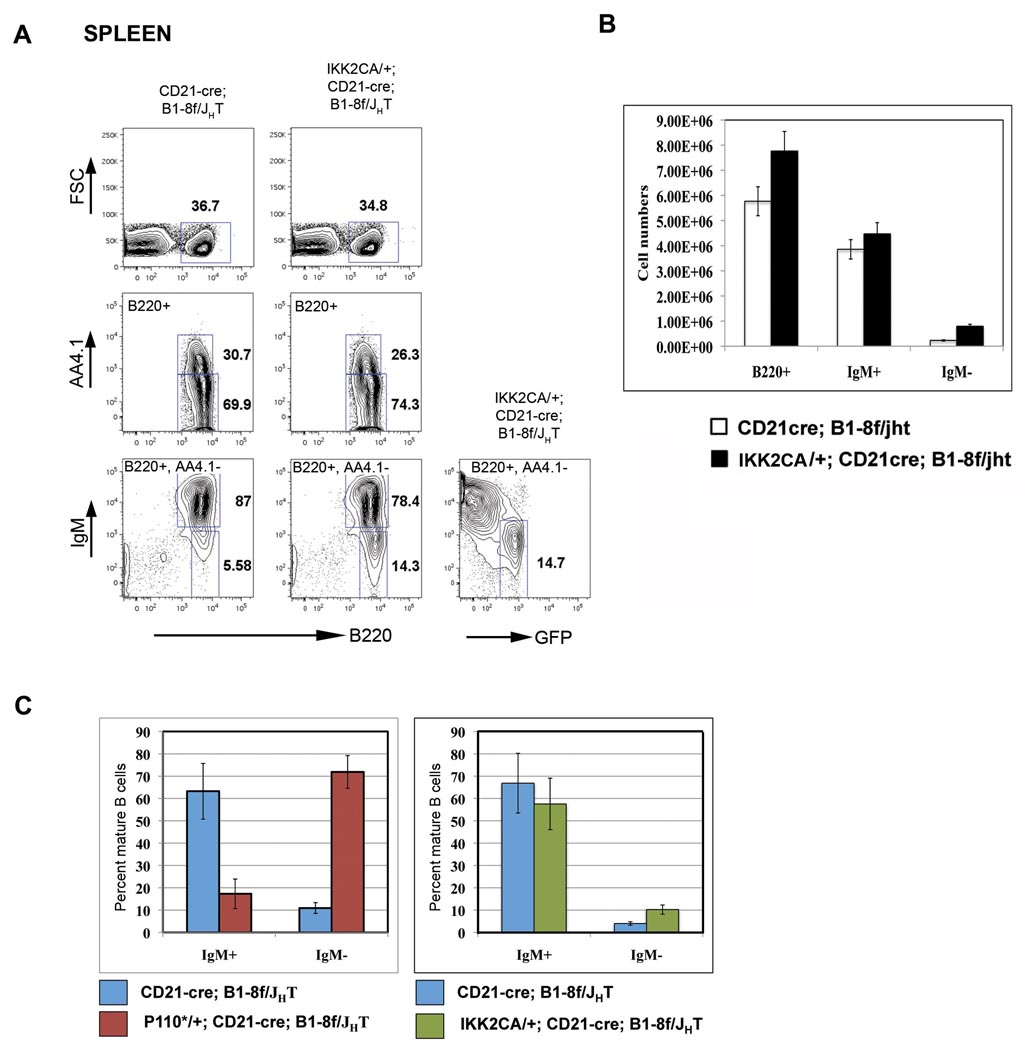
The efficient rescue of BCRneg B cells by P110* contrasted with our failure to rescue such cells by activation of the canonical NF-κB pathway. We used for this purpose the conditional IKK2ca allele, from which constitutively active IκB kinase 2 (IKK2) is expressed, activating the canonical NF-κB pathway and rescuing B cells from BAFF dependency (Sasaki et al., 2006). Compound mutant mice of the genotypes CD21-cre; B1-8f/JHT and IKK2ca/+; CD21-cre; B1-8f/JHT developed normally, so that the analysis of the B cell compartment did not require the generation of fetal liver chimeras. As shown in Figure 4, IKK2ca expression led to only a very limited rescue of BCRneg B cells in the spleen. This was also the case in other lymphoid organs (data not shown). Essentially all IgMneg cells expressed GFP, indicating that the cells had activated the IKK2ca transgene. The inefficient rescue of IgMneg cells by IKK2ca is not due to inefficient Cre mediated recombination in this system, because 80% of mature B cells in IKK2ca/+;CD21-cre mice expressed GFP, indicative of efficient excision of the STOP cassette in the IKK2ca allele (Figure S3).
Figure 4. Expression of constitutively active IKK2 (IKK2CA) causes limited rescue of IgMneg B cells.
(A) Representative FACS analysis of lymphocytes from Spleens of CD21-cre; B1-8f/JHT (left column) and IKK2CA/+; CD21-cre; B1-8f/JHT mice. Numbers represent percentages of cells within the lymphocyte gate or of cells in the gate indicated in the FACS plot. (B) Comparison of B cell numbers from CD21-cre; B1-8f/JHT (open bars) and IKK2CA/+; CD21-cre; B1-8f/JHT (filled bars) mice showing limited rescue of IgMneg B cells by the IKK2CA transgene, mean and SD of 4 mice per genotype. (C) Comparison of rescue of IgMneg B cells by activation of PI3K signaling (left panel) or canonical NF-kB signaling (right panel) in the spleen. Data is plotted as percent of mature B cells (B220+, AA4.1−).
The inefficient rescue by IKK2ca suggests that the rescue of BCRneg B cells by P110* is not mediated by NF-κB activation. Indeed, when we analyzed the expression of genes of the NF-κB pathway in such cells by quantitative RT-PCR, we saw little change in comparison to BCRpos controls or BCRneg cells lacking P110* (Figure S4). Only c-Rel transcripts were about 2-fold down regulated in the latter cells compared to their BCRpos counterparts, and this downregulation was not seen in the P110* rescued BCRneg cells. This result is in agreement with Matsuda et al. (2009), who reported that PI3K activity maintains the expression of the NF-kB proteins c-Rel and RelA.
We also analyzed the ability of two additional signaling molecules, which are rapidly activated upon BCR engagement, to rescue BCRneg B cells, namely MEK1, a member of the Raf-MEK-Erk MAP Kinase pathway, and the Rho GTPase Rac1 (Genot and Cantrell, 2000). Activation of Rac1 upon BCR engagement leads to rapid actin polymerization and cytoskeletal rearrangements, and ablation of Rac1 and 2 blocks B cell development (Walmsley et al., 2003). Activation of the MAP Kinase pathway and Rac1 was done with the help of newly constructed ROSA26 alleles, from which constitutively active MEK1, MEK1DD (Cowley et al., 1994), or dominant active Rac1, RacDA (Kobayashi et al., 1998) are expressed upon Cre-mediated excision of a STOP cassette. When we used these alleles in our experimental in vivo system, they failed to rescue BCRneg B cells in the spleen, despite their ability to increase phospho-Erk levels and PAK1 binding activity, respectively, in splenic B cells (Figure S5; Bokoch, 2003).
Rescue of BCRneg B cells by Pten knockout
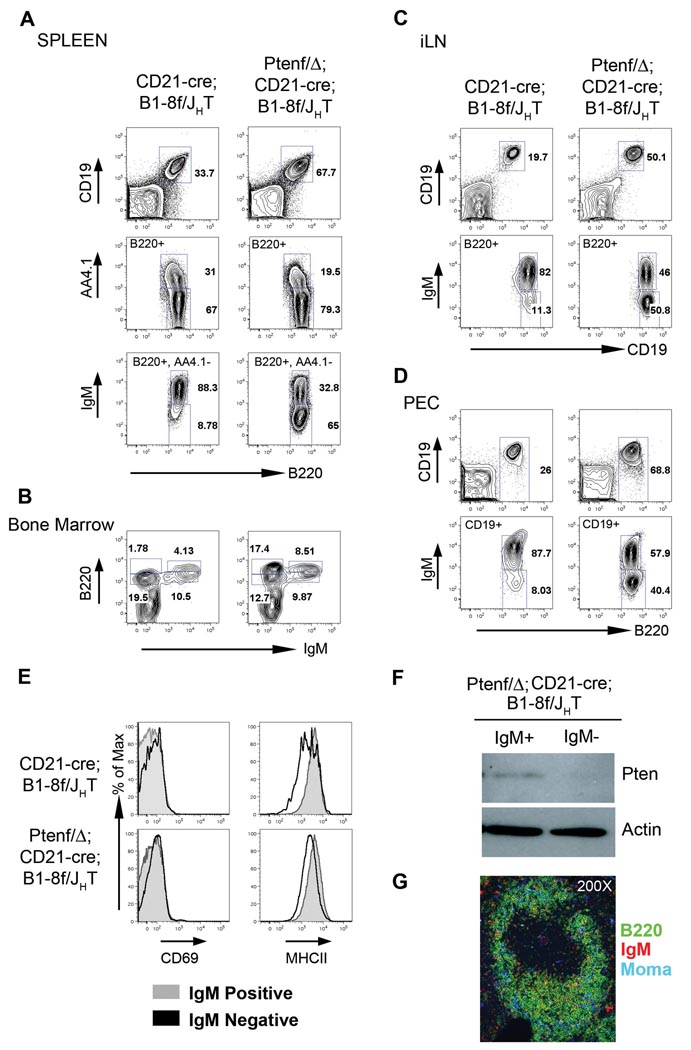
To corroborate that PI3K signaling is indeed the central survival signal in mature B cells downstream of the BCR, we assessed whether rescue of BCRneg B cells can also be achieved by deletion of the Pten tumor suppressor, the key negative regulator of the PI3K pathway via its phosphatase directed conversion of PIP3 to PIP2 (Suzuki et al., 2008). Pten is expressed in the B cell lineage and known to play a role in B cell differentiation (Anzelon et al., 2003; Suzuki et al., 2003; Omori et al., 2006). Because Pten deletion by CD21-cre, like activation of P110*, produces lethality in the compound mutant mice (data not shown), chimeric mice were generated, whose lymphocytes had either the CD21-cre; B1-8f/JHT or Ptenf/Δ; CD21-cre; B1-8f/JHT genotype. The analysis of these mice revealed that Pten ablation rescued BCRneg B cells almost as efficiently as the P110* transgene (Figure 5). Spleens from Ptenf/Δ; CD21-Cre; B1-8f/JHT mice had more B cells (Figure 5A, top row), a decreased fraction of immature B cells (middle row) and a substantial increase in the percentage of mature IgMneg B cells (bottom row) in comparison to controls deleting the BCR in the absence of Pten ablation. In the bone marrow, the Pten deleted, rescued IgMneg (B220 high) B cells constituted 17% of all lymphocytes (Figure 5B). Substantial fractions of rescued BCRneg B cells were also found in lymph nodes (Figure 5C), and peritoneal cavity (Figure 5D). As in the case of P110*, there was no upregulation of the activation markers CD69 and MHC II in the rescued cells (Figure 5E). They were also again identical to their IgMpos counterparts in forward scatter analysis and expressed similar levels of the B cell markers B220, CD19 and CD21, whereas CD23 was down regulated about 2-fold (Figure S6). No Pten protein was detectable in the rescued cells by Western blot (Figure 5F). Histological analysis of the spleens of the compound mutants revealed a normal organization of B cells into follicles and MZ B cells, with the majority of the cells being negative for IgM (Figure 5G).
Figure 5. Deletion of Pten rescues BCRneg B cells.
Representative FACS analysis of lymphocytes from spleen (A), bone marrow (B), inguinal lymph node (iLN, C) and peritoneal cavity (PEC, D) from CD21-cre; B1-8f/JHT and PtenΔ/f; CD21-cre; B1-8f/JHT mice showing rescue of IgMneg mature B cells by Pten deletion. Numbers represent percentages of cells within the lymphocyte gate or of cells in the gate as indicated in the FACS plot. (E) IgMneg cells rescued by Pten deletion are not activated. Comparison of surface expression of activation markers CD69 and MHC class II on IgMpos (grey histograms) and IgMneg (black histograms) cells from the spleen of CD21-cre; B1-8f/JHT and PtenΔ/f; CD21-cre; B1-8f/JHT mice. (F) Western blot analysis for Pten (top panel) and Actin (bottom panel) in sorted IgMpos and IgMneg mature B cells from spleen of PtenΔ/f; CD21-cre; B1-8f/JHT mice. (G) Immuno-fluorescent staining of sections of spleen showing a single follicle from PtenΔ/f; CD21-cre; B1-8f/JHT mice. B220, marking all B cells is stained in green, IgM is in red and MOMA1, marking metallophilic macrophages is in blue.
The PI3K-FOXO pathway mediates survival of BCRneg B cells
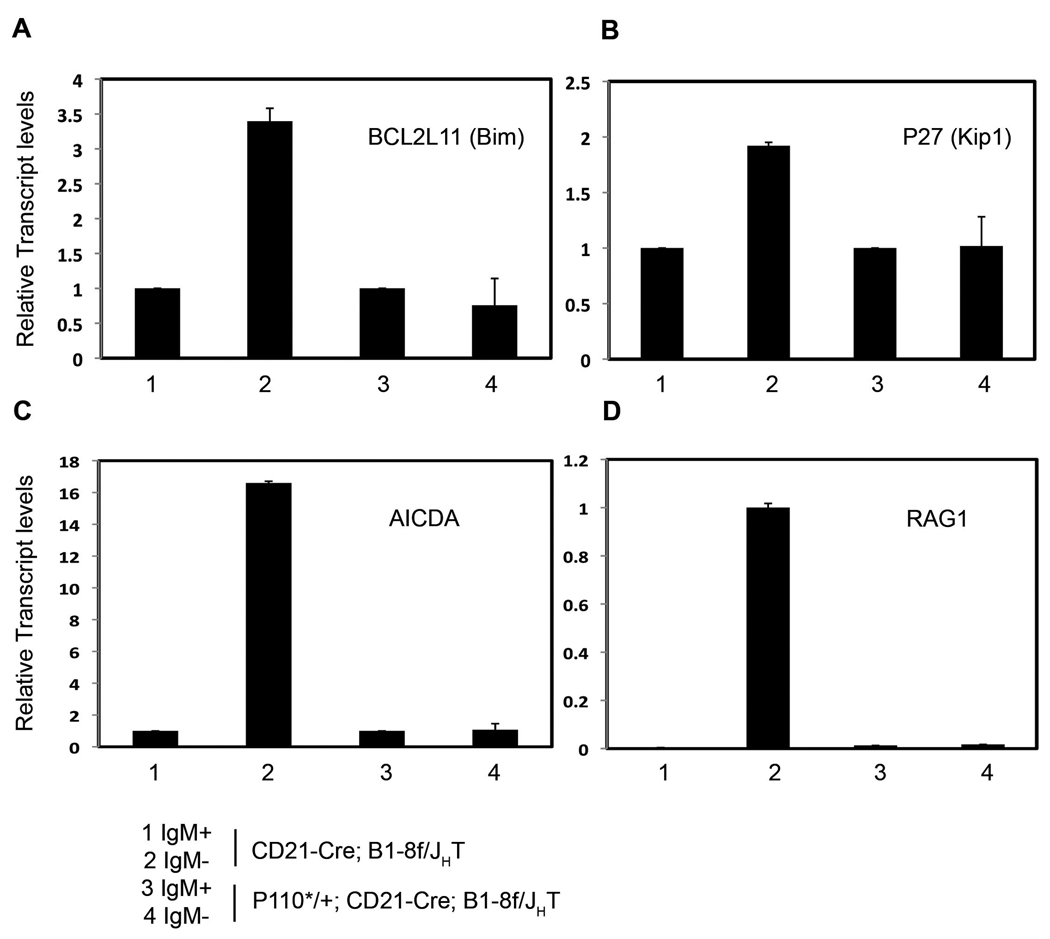
The negative regulation of FOXO transcription factors through the serine/threonine kinase Akt, a key target of PI3K signaling, has recently emerged as a central regulator of B cell development and differentiation (Herzog et al., 2009; Chen et al., 2006; Yusuf et al., 2004). Analyzing in vivo generated BCRneg B cells in the absence of any rescue, we indeed found upregulation of transcripts of the pro-apoptotic FOXO target genes BCL2L11 (encoding the Bcl2 family member Bim) and p27/Kip1 (Dansen and Burgering, 2008; Figure 6), and of two additional recently identified direct or indirect FOXO target genes, AICDA (encoding activation induced cytidine deaminase (AID)) and recombination activating gene 1 (RAG1; Amin and Schlissel, 2008; Dengler et al., 2008; Herzog et al, 2008; Figure 6). In all four cases, upregulation was reversed upon P110* rescue. These results are consistent with a role for FOXO in the control of BCR-mediated B cell survival and also indicate that P110* is active in the rescued cells by controlling gene expression through FOXO transcription factors.
Figure 6. P110* rescued IgMneg cells do not up-regulate FOXO targets.
Quantitative RT-PCR analysis of FOXO targets in sorted IgMpos and IgMneg mature B cells from spleens of CD21-cre; B1-8f/JHT and P110*/+; CD21-cre; B1-8f/JHT mice. Transcripts shown include BCL2L11 (A), P27/KIP1 (B), AICDA(C) and Rag1 (D). Lanes as indicated in the figure, mean and SD of triplicate experiment are shown.
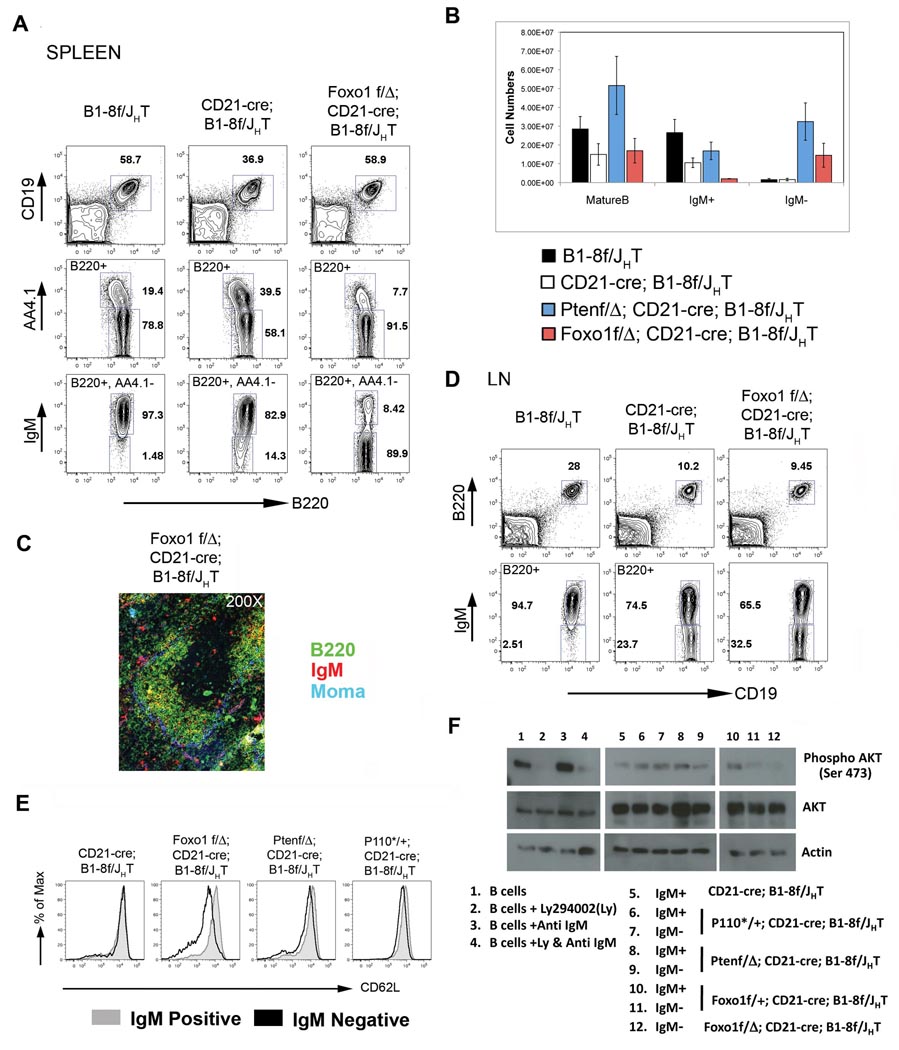
In the light of these results, we sought to provide direct genetic evidence for a role for FOXO in the rescue of BCRneg B cells. While FOXO transcription factors are encoded by four different genes (FOXO 1, FOXO 3, FOXO 4 and FOXO 6), at least two of which (FOXO 1 and FOXO 3) are expressed in B cells (Kerdiles et al., 2009), recent experiments indicate that FOXO1 is the dominant player in B cell differentiation (Amin and Schlissel, 2008; Dengler et al., 2008; Herzog et al., 2008). We therefore used a conditional FOXO1 allele (Paik et al., 2007) to delete FOXO1 in mature B cells concomitantly with the BCR through the CD21-cre transgene. As can be seen in Figure 7A, FOXO1 deletion in mature B cells in Foxo1f/Δ; CD21-cre; B1-8f/JHT mice led to a striking accumulation of IgMneg B cells in the spleen, with most of the cells displaying a mature (AA4.1−) phenotype. Indeed, in these mice 90% of the mature splenic B cells were IgMneg, with a strong reduction of the fraction of immature (AA4.1−) cells compared to controls. Efficient rescue of BCRneg B cells was also seen in bone marrow and peritoneal cavity (Figure S7). Comparison of B cell numbers upon rescue by Pten or FOXO1 revealed that while in the case of Pten a larger fraction of IgMpos cells persisted in the mice, the numbers of rescued IgMneg cells were almost equivalent in the two mutants (Figure 7B).
Figure 7. Survival of resting B cells through BCR is mediated by PI3K-FOXO pathway.
(A) FACS analysis of lymphocytes from spleens of B1-8f/JHT (left column), CD21-cre; B1-8f/JHT (middle column) and Foxo1Δ/f; CD21-cre; B1-8f/JHT mice (right column) showing rescue of IgMneg mature B cells by deletion of Foxo1. (B) Comparison of splenic B cell numbers of the mice as indicated in the figure, mean and SD of a minimum of 4 mice per genotype are shown. (C) Immuno-fluorescent staining of splenic sections from Foxo1Δ/f; CD21-cre; B1-8f/JHT mice. (D) FACS analysis of lymphocytes from lymph nodes of the mice as indicated in the figure showing limited rescue of IgMneg B cells in the lymph node upon deletion of Foxo1. (E) Comparison of surface expression of CD62L on IgMpos (grey histogram) and IgMneg (black histogram) B cells from the lymph nodes of mice as indicated in the figure. (F) Western blot analysis of sorted IgMpos and IgMneg B cells from mice as indicated in the figure (Lanes 5–12) for Phospho- Akt (Ser473, Top panel), Akt (middle panel) and Actin (bottom panel). Lanes 1–4 represents mature B cells sorted from B1-8f/JHT mice and contains two fold less protein than lanes 5–12. For a detailed description of this experiment see the Experimental Procedures section.
However, the BCRneg B cells rescued by FOXO1 deletion lacked a number of properties of normal B cells, quite distinct from what we had observed in the case of P110* and Pten. Histological analysis of the spleen from Foxo1f/Δ; CD21-Cre; B1-8f/JHT revealed the efficient rescue of such cells in the follicles, but lack of MZ B cells (Figure 7C), which was also confirmed by flow cytometric analysis (Figure S8A). Furthermore, only a minor fraction of BCRneg B cells was detected in lymph nodes (Figure 7D). This correlated with a strong decrease of L-selectin (CD62L) expression on the surface of these cells (Figure 7E). CD62L controls the migration of lymphocytes into lymph nodes, and in T cells FOXO1 indeed controls this process as well a CD62L expression (Kerdiles et al., 2009). In addition, the rescued FOXO1 deficient cells had drastically reduced levels of CD23 on their surface, and were slightly smaller than normal B cells or BCRneg B cells rescued by Pten knockout or P110* (Figure S8B)
We gained some understanding of the abnormal properties of the rescued FOXO1 knockout cells when we analyzed FOXO1 protein levels in these cells and BCRneg B cells rescued by P110* expression or Pten knockout. While FOXO1 was undetectable in the former cells (Figure S9A), in the cells rescued by P110* expression or Pten knockout FOXO1 levels were reduced to a level roughly equivalent to that in heterozygous FOXO1 knockouts, correlating with a slight down regulation of CD62L on the surface of the rescued cells, which was equally seen in BCRneg B cells rescued by P110* (Figure 7E). This indicated that the PI3K pathway indeed controls FOXO1 levels in mature B cells and suggested that small changes of the concentration of FOXO1 in the cells have major effects on their physiology. Indeed, when we analyzed the rescue of BCRneg B cells in a situation where the BCR was conditionally deleted in mature B cells on a FOXO1 +/− background, we found a 2–4 fold increase of BCRneg B cells in both lymph nodes and spleen, compared to FOXO+/+ controls (Figure S9B).
While these results indicate that the control of FOXO1 by PI3K signaling is a central element of the BCR controlled B cell survival signal, they also reveal the subtlety of this control mechanism, which seems to require the modulation of this transcription factor within a narrow range.
Loss of PI3K-mediated Akt phosphorylation upon BCR ablation and its reconstitution by P110* or deletion of Pten
Sufficient BCRneg cells could be isolated from FOXO1f/Δ;CD21-cre;B1-8f/JHT mice to allow biochemical analysis. We focused on the activation of Akt through phosphorylation of serine 473, which is known to be under PI3K control. Accordingly, Akt S473 phosphorylation, detectable in resting and, more strongly, in activated mature B cells, was abolished by treatment of the cells with the PI3K inhibitor Ly294002 (Deane and Fruman, 2004; Figure 7F, lanes 1–4). In BCRneg B cells rescued by partial or complete FOXO1 ablation, Akt phosphorylation on S473 was strongly reduced and indeed undetectable in 2/3 experiments (Figure 7F, lanes 10–12; Figure S10A). The data in Figure 7 and Figure S10A (repeat experiments) also show that the extent of Akt S473 phosphorylation induced in the cells upon rescue by P110* or Pten ablation is moderate and closer to that seen in resting mature B cells than B cells activated by BCR engagement or even just exposed to serum containing culture medium. We also did not see significantly increased phosphorylation of the Akt targets GSK3α and β in the rescued cells (Figure S10B). Finally, control and rescued cells did not exhibit any detectable phosphorylation of the mTOR target S6 kinase (S6K), which is strongly induced through this branch of the PI3K pathway upon B cell activation (Engelman et al., 2006; Figure S10C). We conclude that BCR expression mediates the moderate PI3K activity seen in mature B cells, and that, in the light of the genetic rescue experiments, this activity is critical for the survival of the cells.
Discussion
Using concomitant BCR deletion and activation of candidate signaling pathways in mature B cells in vivo, we have identified the PI3K signaling pathway as the critical survival determinant in mature, resting B cells downstream of the BCR. The central evidence for this conclusion is the efficient rescue of mature B cells losing their BCR by either transgenic expression of constitutively active PI3K or knockout of the inhibitor of PI3K signaling, Pten. In both cases, the rescue involves, as determined through the phosphorylation of the PI3K target Akt and further downstream components of the pathway, reconstitution of PI3K activity to levels only modestly above those in resting BCRpos B cells, and much below PI3K activity induced by B cell activation. Our results also show that in resting mature B cells the PI3K-mediated phosphorylation of Akt largely depends on BCR expression.
The rescued BCRneg cells resemble normal mature B cells almost perfectly: They are resting, long-lived cells that populate the same compartments in the peripheral immune system as wild type B cells, with B2 and MZ B cells located in their respective habitats in spleen and LNs. (The experimental system precludes the analysis of B1 cells, which are not generated in B1-8f mice; Lam and Rajewsky, 1999). In both B cell follicles and the MZ, the BCRneg cells intermix with B cells that have escaped BCR ablation, emphasizing their physiologically normal behavior. The cells also resemble normal mature B cells in terms of surface marker expression, with a few exceptions discussed below. The rescued BCRneg cells comprise 60–90% of the total peripheral B cells, whose overall number is similar to that seen in wild type mice, with the exception of a slightly increased MZ B cell compartment. This indicates that the rescued cells are subject to the same numerical control as wild type B cells. Indeed, the BCRneg cells are as dependent on BAFF-BAFF-R interactions as wild type B cells.
The equal rescue of BCRneg B cells by constitutively active PI3K and Pten knockout implies that mature B cells display some PI3K activity irrespective of whether they express a BCR or not. Modulation of PI3K signaling thus appears to be the key element in the control of mature B cell survival by the BCR. This notion is in accord with the general view that the quantitative control of the PI3K pathway determines its manifold roles in cellular physiology. Thus, heterozygous Pten knockout mice, which exhibit a 50% reduction in Pten protein levels in T cells (DiCristofano et al 1999; Xiao et al., 2008), develop autoimmunity, and T cells from these mice have a reduced response to Fas ligation. Monoallelic loss of Pten also contributes to tumor growth in the context of other somatic mutations, with Pten protein levels correlating with disease severity (Salmena et al., 2008). Along the same lines, the graded expression of FOXO transcription factors, which are targets of PI3K signaling, determines the balance between the proliferation and differentiation of B cell progenitors (Amin and Schlissel, 2008; Dengler et al., 2008; Herzog et al., 2008).
The present results demonstrate that the FOXO 1 transcription factor is similarly crucial for the control of mature B cell survival by the BCR. However, the B cells rescued by FOXO1 ablation have a number of aberrant properties compared to the properties of normal mature B cells and BCRneg B cells rescued by constitutive PI3K activity or Pten knockout. Therefore, the role of FOXO1 in the control of mature B cell survival by BCR mediated PI3K signaling is subtler than modeled by our conditional loss-of-function experiment. Perhaps the FOXO transcription factors expressed in mature B cells play redundant and non-exclusive roles in this process.
In contrast to the efficient rescue of BCRneg cells by manipulation of the PI3K signaling pathway we saw little rescue upon activation of the canonical NF-κB pathway in mature B cells losing their BCR. The IKK2ca allele used in these experiments induces strong canonical NF-κB signaling in mature B cells, making these cells BAFF independent and enhancing their response to mitogenic stimuli (Sasaki et al., 2006). The limited rescue of BCRneg B cells by IKK2ca is in accord with our previous demonstration that transgenic Bcl2 rescues BCRneg B cells only transiently (Lam et al., 1997). Taken together, these results indicate that canonical NF-κB activity, while essential for mature B cell persistence under conditions of cellular competition (Pasparakis et al., 2002), is unable to substitute for loss of the BCR in these cells and thus does not by itself represent the critical survival signal downstream of the BCR. This conclusion is in accord with the presence of a large compartment of mature, resting B cells in Bcl10 knockout mice, where BCR engagement does not elicit canonical NF-κB activity (Ruland et al., 2001; Xue et al., 2003). It is also supported by our findings that PI3K activation in mature B cells did not significantly impact the transcription of NF-κB components and target genes, except for an expected 2-fold up-regulation of c-Rel mRNA; and that FOXO1 appears to be the major downstream target of PI3K mediated mature B cell survival. With respect to the work of Stadanlick et al. (2008), one might speculate that the postulated crosstalk between BCR mediated canonical and BAFF-R mediated NF-κB activity may apply to certain processes of B cell activation rather than tonic BCR signaling in resting B cells.
While our failure to rescue BCRneg B cells by constitutively active Rac1 was not unexpected, it is noteworthy that this was also the case for MEK1DD, despite our demonstration that the expression of this molecule in mature B cells in vivo mediated substantial ERK phosphorylation. As the latter appeared to be in the same range as ERK phosphorylation in control B cells, this result does not support the possibility that BCR mediated MAP kinase activation either through PI3K signaling or independent of it (Brummer et al., 2002; Dye et al., 2007), is part of the survival signal downstream of the BCR.
How PI3K signaling through the BCR is initiated in resting mature B cells is not fully understood. If low affinity interactions of the BCR with self-antigens are involved in this process, PI3K signaling would likely be induced through the phosphorylation of the adapter protein BCAP and the CD19 co-receptor by protein tyrosine kinases, with src family kinases and Syk playing a central role (Kurosaki, 1999). It is interesting in this context that the inactivation of a negative regulator of src family and other kinases, c-src tyrosine kinase (Csk), in developing T cells results in a quasi normal compartment of peripheral CD4+ T cells that lack T cell receptor expression (Schmedt et al., 1998). In a different model emerging from very recent evidence (Delgado et al., 2009), the antigen receptors in resting T and B cells are connected to the PI3K pathway by the GTPase TC21 and perhaps other members of the family, which directly recruits the catalytic subunit of PI3K to the non-phosphorylated immuno-tyrosine activation motifs (ITAMs) of these receptors. In this scenario, the “tonic”, PI3K-mediated survival signal is independent of BCR engagement by an external ligand.
While the present data indicate a critical role of PI3K signaling in BCRneg mediated mature B cell survival, they clearly do not define the survival signal in all its complexity. The slight alterations in CD23 and CD62L expression in the cells rescued by P110* or Pten ablation likely reflect the reduced FOXO1 levels in these cells, due to the modestly increased PI3K activity in the rescued, BCRneg compared to BCRpos control cells. However, PI3K likely signals B cell survival through additional targets, and we also cannot exclude minor contributions of other signaling pathways to this process. Additional candidate PI3K targets include the regulators of cell size and metabolism, mTOR (Edinger and Thompson, 2002), in line with the failure of FOXO1 deletion to rescue BCRneg cell size.
Perhaps the biggest surprise in the present study was the finding that despite the ability of the BCR to activate a variety of different signaling pathways in parallel (Kurosaki, 1999), a single one of them seems to signal cellular survival. Furthermore, it is through modulation of the activity of the PI3K pathway, a pathway that is of central importance for the homeostasis and survival of higher cells in general (Engelman et al., 2006), that BCR deficient mature B cells can be rescued. While a contribution of an ER stress response upon loss of BCR expression remains a possibility, our results consistently support the concept that the survival of mature B cells depends upon BCR-mediated activation of the PI3K pathway. We have shown in a separate study that BCR mediated PI3K activation also controls the proliferative response of mature B cells to mitogens through Toll like receptors (Otipoby, Waisman et al., unpublished). We speculate that the BCR has subverted the PI3K signaling pathway in order to integrate the control of cellular survival and responsiveness to pathogens through innate and cognate immune receptors. The exact mechanism of this subversion remains to be elucidated. It will also be interesting to investigate, to which extent PI3K signaling controls the maintenance of B cell lymphomas, which in the human mostly derive from mature B cells and seem to often depend on BCR expression.
Experimental Procedures
Genetically modified mice
To generate tissue-specific P110*-transgenic mice we cloned a loxP-flanked neoR-stop cassette into a modified version of pROSA26-1. We then added a cDNA encoding P110*, a frt-flanked IRES-eGFP cassette and a bovine polyadenylation sequence (R26StopFLP110*). B6 ES cells (Artemis) were transfected, cultured, and selected as previously described for Bruce 4 ES cells (Sasaki et al., 2006). Pten conditional knockout mice were purchased from the Jackson Laboratory (stock number 006068). Other mouse strains used in this study include Cd21-cre (Kraus et al., 2004), B1-8f (Lam et al., 1997), JHT (Gu et al., 1993) and Foxo1f (Paik et al., 2007). Mice were analyzed at the age of 8–12 weeks. Fetal liver chimeras were generated by transferring fetal liver cells from 14.5 DPC embryos into sub-lethally irradiated (600rads) Rag2/common gamma chain knockout mice purchased from Taconic farms (stock number 004111-MM). Chimeric mice were analyzed 10–12 weeks after transfer. All mice were bred and maintained in specific pathogen free conditions. Mouse protocols were approved by the Harvard University Institutional Animal Care and Use Committee and by the Immune Disease Institute
Flow Cytometry
Single-cell suspensions prepared from various lymphoid organs were stained with the following monoclonal antibodies: anti-CD19 (ID3), anti-CD23 (B3B4), anti-CD1d (1B1), anti-CD95 (Fas), anti-CD69 (H1.2F3), anti CD62L (MEL-14), anti B220 (RA3-6B2), anti-CD93 (AA4.1), anti-MHCII (all from eBioscience), anti-CD21 (Biolegend) and Goat anti mouse IgM (Fab fragment, Jackson Immunoresearch). All samples were acquired either on a FACSCalibur or FACSCanto II (BD Pharmingen), and results analyzed using Flow-Jo software (Tree star inc).
BrdU labeling and staining
Mice were fed with drinking water containing 0.8mg/ml BrdU (Sigma) for 20 days. Splenocytes were purified and stained using the FITC BrdU flow kit (BD bioscience) according to manufacturers instructions.
Cell sorting, qRT-PCR & immunoblotting
CD19+, AA4.1−, IgM +/− splenic mature B cells were sorted on a FACSVantage (BD biosciences). Total RNA was isolated from the sorted cells using Trizol (Invitrogen) and cDNA was synthesized using Superscript III cDNA synthesis kit (Invitrogen). qPCR reactions were performed on a Step One Plus real time PCR system using SYBR Green PCR Core reagents according to manufacturers instructions (Applied Biosystems). Primer sequences are listed in Table S1. Immunoblotting was carried out as previously described (Xiao et al., 2008). For details refer supplemental methods.
Histology
Tissues fixed with 10% formalin (Sigma) were stained with hematoxylin and eosin (H&E). For immunofluorescence analysis, spleens were frozen in OCT (Sakura Finetek) in liquid nitrogen and staining was done as described previously (Oliver et al., 1999). Antibodies used for staining include FITC anti-B220 conjugate (BD Biosciences), Alexa 488-RS3-1 (Specific for the IgMa allotype) and anti-Moma1.
In vitro cell survival assay
1× 106 Mature B cells (B220+, AA4.1−) were cultured in 0.5ml B cell medium (complete DMEM containing 10% FCS) with or without 200ng/ml recombinant mouse BAFF (R&D systems). The FCS was previously tested for it’s potential to induce CD69 and MHC class II expression that are sensitive markers for B cell activation. Viable cells were determined at each time point using the Guava -PCA96 cell counter (Guava technologies Inc).
Supplementary Material
Acknowledgements
We thank D. Ghitza, A. Pellerin, A. Monti, A.Tetreault, J. Grundy, J. Wang, J. Xia and W.F. Jia for expert technical assistance; E. Derudder for reagents; all Rajewsky lab members for discussions and M. Janz for critical reading of the manuscript; A. Klippel for the MP110* cDNA; and K. Kaibuchi for Rac1DA cDNA. K.R. is supported by NIH grants AI054636 and CA092625, the Leukemia and Lymphoma Society and the European Union through MUGEN; L.S. by the T32 training grant of the Joint Program in Hematology and Transfusion Medicine at Harvard Medical School; J.F.K. by NIH grant AI014782-31; D.P.C. by a postdoctoral fellowship from the Portuguese Foundation for Science and Technology (FCTMCES); B.Z. by a postdoctoral fellowship of the Leukemia and Lymphoma Society; and RAD by the NIH and the Robert A. and Renee E. Belfer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat Immunol. 2008;9:613–622. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001;194:1583–1596. doi: 10.1084/jem.194.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Brummer T, Shaw PE, Reth M, Misawa Y. Inducible gene deletion reveals different roles for B-Raf and Raf-1 in B-cell antigen receptor signalling. EMBO J. 2002;21:5611–5622. doi: 10.1093/emboj/cdf588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Liou HC, Horwitz BH, Pillai S. Nuclear factor kappa B is required for the development of marginal zone B lymphocytes. J Exp Med. 2000;192:1175–1182. doi: 10.1084/jem.192.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yusuf I, Andersen HM, Fruman DA. FOXO transcription factors cooperate with delta EF1 to activate growth suppressive genes in B lymphocytes. J Immunol. 2006;176:2711–2721. doi: 10.4049/jimmunol.176.5.2711. [DOI] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Dansen TB, Burgering BM. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- Delgado P, Cubelos B, Calleja E, Martínez-Martín N, Ciprés A, Mérida I, Bellas C, Bustelo XR, Alarcón B. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nat Immunol. 2009 doi: 10.1038/ni.1749. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- Dye JR, Palvanov A, Guo B, Rothstein TL. B cell receptor cross-talk: exposure to lipopolysaccharide induces an alternate pathway for B cell receptor-induced ERK phosphorylation and NF-kappa B activation. J Immunol. 2007;179:229–235. doi: 10.4049/jimmunol.179.1.229. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Forster I, Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci U S A. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genot E, Cantrell DA. Ras regulation and function in lymphocytes. Curr Opin Immunol. 2000;12:289–294. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- Grande SM, Bannish G, Fuentes-Panana EM, Katz E, Monroe JG. Tonic B-cell and viral ITAM signaling: context is everything. Immunol Rev. 2007;218:214–234. doi: 10.1111/j.1600-065X.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Herzog S, Hug E, Meixlsperger S, Paik JH, DePinho RA, Reth M, Jumaa H. SLP-65 regulates immunoglobulin light chain gene recombination through the PI(3)K-PKB-Foxo pathway. Nat Immunol. 2008;9:623–631. doi: 10.1038/ni.1616. [DOI] [PubMed] [Google Scholar]
- Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9:195–205. doi: 10.1038/nri2491. [DOI] [PubMed] [Google Scholar]
- Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kuroda S, Fukata M, Nakamura T, Nagase T, Nomura N, Matsuura Y, Yoshida-Kubomura N, Iwamatsu A, Kaibuchi K. p140Sra-1 (specifically Rac1-associated protein) is a novel specific target for Rac1 small GTPase. J Biol Chem. 1998;273:291–295. doi: 10.1074/jbc.273.1.291. [DOI] [PubMed] [Google Scholar]
- Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555–592. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- Lam KP, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J Exp Med. 1999;190:471–477. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Mikami Y, Ohtani M, Fujiwara M, Hirata Y, Minowa A, Terauchi Y, Kadowaki T, Koyasu S. Critical role of class IA PI3K for c-Rel expression in B lymphocytes. Blood. 2009;113:1037–1044. doi: 10.1182/blood-2008-06-163725. [DOI] [PubMed] [Google Scholar]
- Mecklenbrauker I, Kalled SL, Leitges M, Mackay F, Tarakhovsky A. Regulation of B-cell survival by BAFF-dependent PKCdelta-mediated nuclear signalling. Nature. 2004;431:456–461. doi: 10.1038/nature02955. [DOI] [PubMed] [Google Scholar]
- Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Mol Immunol. 2005;42:763–772. doi: 10.1016/j.molimm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Otipoby KL, Sasaki Y, Schmidt-Supprian M, Patke A, Gareus R, Pasparakis M, Tarakhovsky A, Rajewsky K. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc Natl Acad Sci U S A. 2008;105:12435–12438. doi: 10.1073/pnas.0805460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Schmidt-Supprian M, Rajewsky K. IkappaB kinase signaling is essential for maintenance of mature B cells. J Exp Med. 2002;196:743–752. doi: 10.1084/jem.20020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruland J, Duncan GS, Elia A, del Barco Barrantes I, Nguyen L, Plyte S, Millar DG, Bouchard D, Wakeham A, Ohashi PS, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 1998;394:901–904. doi: 10.1038/29802. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Nakano T, Mak TW, Sasaki T. Portrait of PTEN: messages from mutant mice. Cancer Sci. 2008;99:209–213. doi: 10.1111/j.1349-7006.2007.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, MacKay F, Bixler SA, Zafari M, Liu ZY, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Morris SW, Orihuela C, Tuomanen E, Cui X, Wen R, Wang D. Defective development and function of Bcl10-deficient follicular, marginal zone and B1 B cells. Nat Immunol. 2003;4:857–865. doi: 10.1038/ni963. [DOI] [PubMed] [Google Scholar]
- Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, Cancro M, Grewal IS, Dixit VM. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- Yusuf I, Zhu X, Kharas MG, Chen J, Fruman DA. Optimal B-cell proliferation requires phosphoinositide 3-kinase-dependent inactivation of FOXO transcription factors. Blood. 2004;104:784–787. doi: 10.1182/blood-2003-09-3071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.