Abstract
Background
Food allergy is a disorder in which antigenic food proteins elicit immune responses. Animal models of food allergy have several limitations that influence their utility, including failure to recapitulate several key immunologic hallmarks. Consequently, little is known regarding the pathogenesis and mechanisms leading to food allergy. Staphylococcus aureus–derived enterotoxins, a common cause of food contamination, are associated with antigen responses in atopic dermatitis.
Objective
We hypothesized that S aureus–derived enterotoxins might influence the development of food allergy. We examined the influence of administration of staphylococcal enterotoxin B (SEB) with food allergens on immunologic responses and compared these responses with those elicited by a cholera toxin–driven food allergy model.
Methods
Oral administration of ovalbumin or whole peanut extract with or without SEB was performed once weekly. After 8 weeks, mice were challenged with oral antigen alone, and the physiologic and immunologic responses to antigen were studied.
Results
SEB administered with antigen resulted in immune responses to the antigen. Responses were highly TH2 polarized, and oral challenge with antigen triggered anaphylaxis and local and systemic mast cell degranulation. SEB-driven sensitization induced eosinophilia in the blood and intestinal tissues not observed with cholera toxin sensitization. SEB impaired tolerance specifically by impairing expression of TGF-b and regulatory T cells, and tolerance was restored with high-dose antigen.
Conclusions
We demonstrate a new model of food allergy to oral antigen in common laboratory strains of mice that recapitulates many features of clinical food allergy that are not seen in other models. We demonstrate that SEB impairs oral tolerance and permits allergic responses.
Keywords: Food allergy, Staphylococcus aureus enterotoxin B, TH2, anaphylaxis, murine, peanut, ovalbumin, tolerance, mast cells, eosinophils
Food allergy affects more than 11 million Americans and doubled in incidence between 1997 and 2002. Despite this, little is known about risk factors, disease pathogenesis, or the cellular and molecular processes involved in food allergy.
The availability of appropriate animal models is necessary to pursue the mechanisms and potential treatments. Currently available models have several limitations that significantly diminish their utility.
Oral dosing of several (1-10 mg) milligram quantities of peanut extract has been used routinely to generate allergic sensitization in mice.1-6 In addition, several of these approaches require antigen to be administered repeatedly within a short time frame (eg, 3-4 times weekly).
Different routes of sensitization have also been used. Clearly, approaches with intraperitoneal injection7 or genetically modified bacteria that express food antigens8 do not reflect the pathogenic mechanisms leading to food allergy. Sensitization through the skin has also been proposed9 and shown to be experimentally viable.10 However, allergenicity can vary depending on the route of administration and the fate of the allergen. Most allergens in food share similar chemical properties (ie, heat, acid, and enzymatic stability).11 Food allergens are considerably more stable in simulated gastric fluid than nonantigenic proteins,12 and reducing protein breakdown by acid neutralization increased antigen immunogenicity in mice.13 Also, the foods to which individuals are allergic often reflect regional diets. For example, in 107 cases of severe anaphylaxis to foods in Paris, France, 5 cases were to snails.14 Thus these reports are consistent with dietary antigen exposure.
Many studies have used the C3H/HeJ mouse strain (reviewed by Knippels et al15). However, these mice carry a mutation in the gene encoding Toll-like receptor 4, and as such, C3H/HeJ mice have a profound hyporesponsiveness to LPS.16,17 The requirement for Toll-like receptor 4 in food allergy responses in C3H/HeJ mice has been both supported18 and refuted.19
Cholera toxin (CT) has also been required to overcome oral tolerance when antigen has been administered through the oral route (reviewed by Berin and Shreffler20). However, as we demonstrate, the CT model fails to recapitulate many of the hallmark features of food allergy, and the mechanisms through which CT promotes immune responses remain controversial and unclear. Multiple processes, including disruption of intestinal fluid balance, alteration of dendritic cell and macrophage processes of antigen recognition, and alteration of T-cell responses (reviewed by Cox et al21), have been proposed. In addition, several reports have shown that CT-driven sensitization also promotes an IgG2a (TH1-associated) response in addition to the TH2-associated responses.22-24
Although there is no clinical association between cholera and atopy, Staphylococcus aureus enterotoxins have an established association with several atopic conditions. Approximately 90% of patients with atopic dermatitis (AD) have skin colonization with toxin-producing strains of S aureus versus only 5% to 10% of healthy individuals. Despite the presence of other toxins, correlations between AD and S aureus toxin–specific immunoglobulins are unique to staphylococcal enterotoxin B (SEB).25,26 Topical application of SEB triggered responses to allergens in patients with AD.27,28 In a murine model of AD, SEB alone promoted a TH2-associated skin inflammation to SEB but also to concurrently applied antigen.29,30 Further studies have demonstrated SEB-producing S aureus in the nasal tissues of patients with chronic rhinosinusitis31-33 that was linked to the incidence of food allergy in a subpopulation of these patients.34
S aureus contamination of food is one of the most prevalent causes of food poisoning in the United States,35 and S aureus–derived toxins are most frequently found in foods, such as milk, dairy products, eggs, meats, and shellfish/fish, all food allergens.36 SEB is resistant to breakdown by stomach acid and is transcytosed across the intestinal epithelium.37,38
In healthy individuals ingestion of food antigens promotes oral tolerance.39 Low antigen doses promote regulatory T (Treg) cell–mediated control of immune responses characterized by TGF-b and IL-10 expression,40,41 whereas high antigen doses induce anergy and deletion of reactive T-cell subsets.40 Children with food allergy have reduced numbers of TGF-b–producing T cells in their intestinal mucosa42 and IL-10–producing T cells in the circulation.43 Our findings show that oral tolerance is broken by an adjuvant influence of oral SEB. This promotes allergic responses and anaphylaxis to food antigens. Unlike other food allergy models, the SEB model elicits antigen-specific responses that bear striking similarity to clinical symptoms of food allergy. This report presents a new model to investigate food allergy, and the findings suggest that S aureus might be an environmental factor in food allergy.
METHODS
Reagents
SEB (Sigma-Aldrich, St Louis, Mo), covered under federal select agents requirements, was used as approved by the Northwestern University Office of Research Safety under the requirements of the Centers for Disease Control and Prevention and the Department of Agriculture and according to necessary containment and use reporting mandates. CT was obtained from List Biological Laboratories (Campbell, Calif). Ovalbumin (OVA; Grade V) was obtained from Sigma-Aldrich, and whole peanut extract (WPE) was prepared from unsalted uncooked peanuts by using 20 mmol/L Tris buffer, as previously described.44 Total protein concentration of WPE was determined with BCA (Pierce, Rockford, Ill).
Animals
Four- to 8-week-old BALB/c mice (female; Taconic Farms, Hudson, NY) or C57Bl/6 mice (female; Jackson Laboratories, Bar Harbor, Me) were housed under specific pathogen-free conditions and maintained on an OVA and peanut-free diet. All experiments were approved by the Northwestern University Animal Care and Use Committee.
Antigen sensitization
Mice were administered 100 mg of OVA with either 10 mg of CT or various concentrations of SEB in a final volume of 100 mL by using a ball-ended mouse feeding needle once a week for 8 weeks. Sensitization to WPE was performed by using 100 mg of WPE combined with 10 mg of SEB.
Antigen challenge
At week 9, all mice received a bolus challenge with oral antigen (5 mg). Symptom scoring was performed in a blind fashion by 2 independent investigators according to previously described parameters of symptoms for determining IgE-mediated responses in murine food allergy.2,45 Briefly, 0 was assigned if no symptoms were evident, and 1 through 5 was assigned if symptoms were observed, where 1 represents mild scratching, rubbing, or both of the nose, head, or feet; 2 and 3 represent intermediate symptoms (eg, edema around the eyes or mouth, pilar erection, and/or labored breathing); 4 represents significantly reduced motility, tremors, and/or significant respiratory distress; and 5 represents death. One hour after challenge, mice were bled for plasma histamine levels. Twenty-four hours later, mice were killed, and tissues were collected for analysis. Blood pressure was determined in groups of 3 SEB/OVA-sensitized BALB/c mice that were placed in a Coda 1 noninvasive blood pressure system (Kent Scientific, Torrington, Conn) for 5 minutes to establish baseline parameters. The mice were then challenged with 5 mg of OVA or PBS, and parameters were measured every 30 seconds.
Serum immunoglobulin levels
Serum was collected, and specific antibody levels were determined by means of sandwich ELISA, as detailed in the Methods section of this article's Online Repository at www.jacionline.org. OVA-specific IgG1, IgG2a, and IgE levels were quantified as previously described.46 SEB-specific IgE levels were determined by using biotin-labeled SEB as a secondary reagent to detect IgE captured with anti-IgE. WPE-specific IgE levels were determined by coating ELISA plates with 1 mg/mL WPE and detecting bound antigen-specific immunoglobulins with isotype-specific antibodies (BD PharMingen, San Jose, Calif).
Blood eosinophil quantification
Blood was collected into EDTA-coated tubes, and absolute eosinophil numbers were determined after staining with Discombe fluid, as previously described.47
Cytokine production
Single-cell suspensions of splenocytes were prepared as previously described47 and were cultured for 48 hours at a concentration of 2 3 106/mL in the presence or absence of OVA (100 mg/mL) or WPE (1 mg/mL). Additionally, separate cultures were prepared on anti-mouse CD3e (BD PharMingen)–coated plates (1 mg/mL). Cytokine concentrations in the culture supernatants were determined by using mouse TH1/TH2 CBA assays (BD PharMingen). The limit of detection was less than 2 pg/mL for each cytokine.
Histology
Tissue was collected, fixed in formalin, and then embedded in paraffin and stained with hematoxylin and eosin or pinacyanol erythrosinate for mast cells by Histo-Scientific Research Laboratories (Jackson, Va). Mast cell numbers and activation status were determined by counting cells with dense metachromatic granules and compact shape versus those with dispersed granules extending clearly outside the cell body. The average number of mast cells from 20 high-powered fields ( 3 400 magnification) was determined for each sample.
Plasma histamine levels
Plasma histamine levels were determined by using an EIA kit from Becton Dickenson (Franklin Lakes, NJ), as per the manufacturer's instructions.
Real-time RT-PCR
Total RNA was isolated with the Qiagen RNeasy kit (Qiagen, Hilden, Germany), and cDNA was isolated with an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, Calif). Gene expression was determined by means of PCR with an ABI 7500 Thermal cycler (Applied Biosystems, Foster City, Calif) and specific Taqman probes (Applied Biosystems) for each gene of interest. b-Actin was used as a housekeeping gene for analysis of changes in cycle threshold values. The fold induction above SEB alone was determined based on changes in the D cycle threshold values.
Statistics
Statistics were performed on GraphPad Prism 4 software (Alexa, San Diego, Calif) by using the 2-tailed Student t test to determine significance.
RESULTS
Oral administration of SEB promotes a dominant IgE antibody response to antigen
We investigated the generation of antigen-specific immunoglobulins after weekly administration of oral antigen with or without SEB and compared responses elicited to the common mucosal adjuvant CT. SEB with OVA promoted a robust increase in OVA-specific IgE and IgG1 levels that was not observed with SEB or OVA alone (Table I). The levels of IgE and IgG1 produced were similar to those elicited by OVA administered with CT. However, although CT also promoted significant OVA-specific IgG2a responses, SEB/OVA-sensitized animals had significantly lower IgG2a levels. Both mice treated only with SEB and SEB/OVA-treated mice had increased SEB-specific IgE levels, and there was no significant difference between these groups.
TABLE I.
SEB promotes the generation of OVA-specific immunoglobulins
| OVA specific |
SEB specific |
|||
|---|---|---|---|---|
| IgE (ng/mL) | IgG1 (AU/mL) | IgG2a (AU/mL) | IgE (AU/mL) | |
| CT | ND | ND | ND | ND |
| OVA | 18.8 6 2.6 | 8.6 6 8.6 | ND | ND |
| SEB | ND | ND | ND | 1222.2 6 578 |
| CT/OVA | 596.3 6 35.5* | 258.3 6 47.7* | 903.1 6 223.9* | ND |
| SEB/OVA | 594.1 6 63.2* | 176.0 6 29.5* | 162.1 6 44.7* | 2149 6 851 |
Mice were orally exposed to 100 mL of OVA (100 mg), CT (10 mg), SEB (50 mg), or OVA mixed with CT (CT/OVA) or SEB (SEB/OVA) once weekly for 8 weeks. At week 9, serum immunoglobulin levels were determined by means of ELISA. Values represent the mean 6 SEM.
ND, None detected.
P < .01 compared with OVA alone, Student t test.
P < .01 compared with CT/OVA, Student t test.
SEB-driven sensitization primes for anaphylaxis and mast cell activation on antigen challenge
We next investigated the responses to oral challenge with antigen alone after sensitization by using a scoring method previously used in CT-driven murine models of food-induced anaphylaxis.45 Before challenge and during the sensitization period, all mice were apparently healthy and showed no weight loss or illness (data not shown). However, on oral challenge with OVA, sensitized mice exhibited a robust pattern of physiologic symptoms in the CT/OVA- and SEB/OVA-treated groups (Fig 1, A). Symptoms occurred within 15 minutes, peaked at 30 minutes (the time point shown in Fig 1, A), and began to resolve at 60 minutes after antigen challenge, which is indicative of an early-phase anaphylactic response. Responses were antigen specific because challenge with an unrelated protein, BSA, failed to elicit symptoms (Fig 1, B). The levels of histamine in plasma after challenge were increased in both the CT/OVA and SEB/OVA groups (Fig 1, C), with levels 10 to 20 times higher in SEB/OVA-treated mice than in CT/OVA-treated mice, indicating a strong anaphylactic response. We next used histologic staining with pinacyanol erythrosinate to determine mast cell numbers and granulation status in the skin and intestine. Ear skin was used to quantify mast cell phenotypes because their frequency and distribution is more homogeneous in skin than in intestinal tissues and because responses would be indicative of a systemic response to oral antigen. There was a modest increase in total numbers and a significant increase in the percentage of degranulated mast cells in both the CT/OVA and SEB/OVA groups (Fig 1, D). The number of granulated mast cells remaining in CT/OVA skin was statistically higher (P < .05, Student t test) than that seen in SEB/OVA-treated animals. The intestinal tissue also showed increased mast cell numbers and degranulation (Fig 1, E), as well as considerable vasodilation and capillary congestion (Fig 1, F). Noninvasive tail cuff measurements demonstrated that blood pressure also decreased within this early period of response to antigen challenge (Fig 1, G). These data demonstrate that mast cell–associated anaphylactic and allergic reactions occurred in response to oral OVA challenge after SEB-driven sensitization.
FIG 1.
Antigen-driven anaphylaxis and mast cell activation in SEB/OV A-sensitized mice. After sensitization, mice received a bolus dose of 5 mg of OVA by mouth. A, Symptom scores elicited in CT-driven or SEB-driven sensitization were determined. B, SEB/OVA-sensitized mice responded to OVA but not to an irrelevant antigen, BSA. C, Plasma histamine levels were determined 60 minutes after antigen challenge. D, Numbers and granulation status of mast cells in ear skin were determined by means of histologic analysis. E, A representative section showing pinacyanol erythrosinate staining on jejunum tissue from an SEB/OVA mouse after challenge is shown. Mast cells are stained with a deep blue/purple color. F, Hematoxylin and eosin staining of jejunum , illustrating vasodilation. G, Blood pressure was measured in OVA/SEB-treated mice to determine systemic responses immediately after antigen challenge with a Coda 1 noninvasive blood pressure system . In Fig 1, C and D, data represent the means 6 SEMs (n 5 6-12). *P < .05 and **P < .01, Student t test.
SEB-driven sensitization promotes eosinophilia
We compared oral sensitization driven by CT or SEB on the generation of eosinophilia. CT failed to change circulating eosinophil numbers (Fig 2, A), results consistent with those observed in untreated mice (data not shown). Conversely, SEB and OVA promoted a significant increase in eosinophils. Eosinophilia was dependent on the SEB dose (Fig 2, B) and was statistically significant compared with that seen in untreated levels at the concentrations indicated (determined by using Student t test, as well as by means of multigroup comparison analysis [P < .05, ANOVA] on the dose-response curve). Importantly, the eosinophilia also extended into the intestinal tissue, as well as the circulation. Fig 2, C, shows that after challenge, the jejunum of SEB/OVA-sensitized animals had a significant inflammatory cell infiltrate that contained large numbers of eosinophils. Although the eosinophils were predominately located in the lamina propria and around the villi base, numerous eosinophils were also observed within the villi and toward the villi tips (data not shown).
FIG 2.
SEB-driven sensitization promotes increased eosinophilia, whereas CT does not. Peripheral blood eosinophil numbers were determined in treated mice. A, Peripheral blood eosinophils were increased in SEB/OVA-treated mice but not in CT/OVA-treated mice. B, Eosinophilia correlated with the amount of SEB administered during sensitization. C, Representative history of tissue stained with hematoxylin and eosin demonstrating a robust eosinophilic infiltration into the jejunum tissues. Black arrows indicate eosinophils. *P < .05 and **P < .01, Student t test (n 5 6-12).
SEB-driven sensitization promotes TH2 responsiveness
Fig 3, A, demonstrates that splenocyte production of both IL-4 and IL-5 was significantly enhanced in the SEB/OVA-treated mice. This was observed with both anti-CD3 stimulation and OVA stimulation (P 5 .0017 and .04 for IL-4 and P 5 .02 and .04 for IL-5, respectively). Levels in unstimulated cultures were not significantly different when compared with levels in cultures stimulated with either SEB or OVA alone (data not shown). The production of IFN-g was increased on anti-CD3 stimulation in the SEB/OVA group, but this was marginally not statistically significant from that seen in the control groups (P 5 .054, 2-tailed Student t test). The IFN-g response to OVA stimulation was relatively modest, even compared with those we observed previously in an OVA-induced asthma model,47 and was also not significant (P 5 .255, 2-tailed Student t test). Expression of TH2-associated IL-5 and IL-13 was also increased in the jejunum tissue from SEB/OVA-treated mice, whereas expression of IFN-g was not (Fig 3, B). Conversely, the expressions of the Treg cell–associated transcription factor foxhead box protein 3 and TGF-b were significantly reduced, whereas expression of IL-10 was unaltered (Fig 3, C). These data suggest that SEB-driven oral sensitization promotes a predominantly TH2-biased systemic cytokine response with a concomitant loss of Treg cell–associated responses.
FIG 3.
A TH2-skewed antigen-dependent cytokine profile develops after SEB-driven sensitization. Splenocytes from mice previously sensitized with SEB alone, OVA alone, or SEB/OVA were stimulated for 48 hours in the presence of 100 mg of OVA or with plate-bound anti-mouse CD3 (1 mg/mL). A, Cytokine levels in the supernatants were determined by using the cytometric bead assay. Gene expression levels were determined for TH/TH2 (B) and Treg cell–associated (C) mediators. *P < .05 and **P < .01, Student t test (n 5 5-6). NS, No significant difference.
SEB promotes responses to peanut antigens by impairing low dose tolerance
In H-2d strains of mice, such as BALB/c mice, OVA is recognized by T cells expressing Vb8,48 the T-cell receptor haplotype that also recognizes SEB.49 We observed a modest but significant decrease in the Vb8 repertoire of CD41 T cells and no change in the Vb4 or Vb6 repertoires in SEB/OVA-sensitized mice (see Fig E1 in this article's Online Repository at www.jacionline.org). Therefore we investigated whether SEB could elicit responses to antigens that do not solely use Vb8. We used an unrefined extract prepared from freshly ground peanuts to investigate whether SEB could induce responses to a complex mixture of antigens. In addition, we investigated whether low-dose, high-dose, or both phases of oral tolerance were affected by oral administration of SEB with antigen.
FIG E1.
T-cell receptor repertoire use after oral SEB exposure. The percentage of each Vb subset of T cells was determined by using flow cytometry on spleen cells. Data represent the average percentage of Vb subsets for the entire CD41 T-cell population. **P < .01, Student t test (n 5 5 per group).
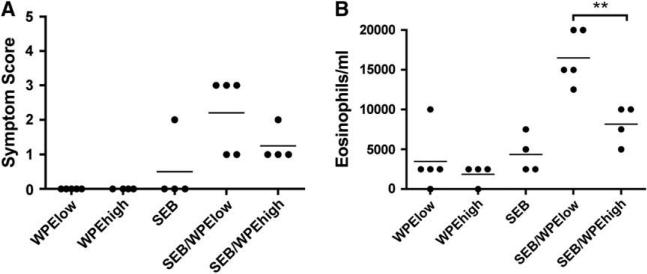
Using 100 mg of WPE as a low dose and a 10-fold higher amount as a high dose of antigen, SEB promoted anaphylaxis only to 100 mg of WPE (SEB/WPElow), whereas responses to 1 mg (SEB/WPEhigh) were lower (Fig 4, A). This was true of all time points and not due to a delay in the timing of reactivity (data not shown). In further experiments this response was associated with a decrease in core body temperature (see Fig E2 in this article's Online Repository at www.jacionline.org). Similarly, mice receiving SEB/WPElow showed both increased peripheral blood eosinophilia (Fig 4, B) and WPE-specific IgE levels (Fig 4, C), whereas SEB/WPEhigh failed to elicit these responses. Splenocytes from SEB/WPElow-treated mice produced significantly higher amounts of IL-4 (Fig 4, D) and IL-5 (Fig 4, E) in response to either anti-CD3 stimulation or WPE. Although we observed a trend toward increased IFN-g production in splenocytes from OVA-sensitized mice, there was no such change in IFN-g production with WPE-sensitized cells (Fig 4, F). Conversely, cytokine levels from SEB/WPEhigh-treated mice were similar to those seen in the control groups. SEB/WPElow mice also had increased serum IL-5 levels (31.4 6 17.3 pg/mL) compared with the other groups, in which IL-5 levels were less than the detection limit. Finally, we recapitulated these results in a C57BL/6J background strain that is H2b (see Fig E3 in this article's Online Repository at www.jacionline.org), suggesting that the priming effect of SEB is not MHC class II restricted. Together, these results support a specific effect on low dose tolerance.
FIG 4.
SEB promotes allergic responses to peanut at a low dose but not at a high dose. Responses to oral administration of 100 mg of WPE (WPElow) or 1 mg of WPE (WPEhigh) 6 10 mg of SEB were determined. Symptom scoring in response to challenge with 5 mg of WPE (A), peripheral blood eosinophilia (B), and WPE-specific IgE levels (C) was determined. Finally, in vitro cytokines on stimulation of splenocytes with 1 mg of WPE or anti-mouse CD3 (1 mg/mL) for 48 hours were studied. IL-4 (D), IL-5 (E), and IFN-g (F) levels are shown. *P < .05 and **P < .01. Student t test (n 5 5 per group).
FIG E2.
Core temperatureresponses after antigen challenge. Rectal temperature was measured in WPE/SEB-treated mice to determine systemic responses immediately after antigen challenge. Rectal temperatures were measured concurrently in 7 animals by using an automated PhysioTemp mouse rectal temperature system (PhysioTemp Instruments, Inc, Clifton, NJ) and shown as a change in baseline for each individual mouse, and these were not significantly different (36.78C 6 0.48C for control animals and 36.28C 6 0.48C for sensitized mice).
FIG E3.
SEB promotes allergic responses to peanut in C57BL/6J mice. Responses to oral administration of 100 mg of WPE (WPElow) or 1 mg of WPE (WPEhigh) 6 10 mg of SEB were determined. Symptom scoring in response to challenge with 5 mg of WPE (A) and peripheral blood eosinophilia (B) are shown. **P < .01, Student t test.
DISCUSSION
Establishing better models for food allergy is important to improve our understanding of underlying mechanisms and for establishing therapeutic potential for developing therapies. We show that SEB elicits sensitization to low doses of OVA or WPE in mice. Our data also demonstrate that SEB elicits an immunologic profile that includes some hallmark responses seen in patients with food allergy. For example, the profound eosinophilia in the blood and tissues of our mice is not seen with CT. This is an important distinction because eosinophils have recently been shown to be important regulators of T-cell responses in allergic airway inflammation.50 We have shown effective responses in both BALB/c and C57BL/6J strains, opening potential investigation of a myriad of genetically manipulated mice. Studies on the C3H/HeJ stain also demonstrated strong anaphylaxis, including death (5 score), that was not observed in either the BALB/c or C57BL/6J strains (data not shown). We are further investigating this strain-specific difference in the magnitude of anaphylactic responses.
SEB is a potent T-cell superantigen and has been studied extensively in animal models. It elicited local inflammation and also enhanced epicutaneous sensitization to concurrently applied OVA in an eczema model.29,30 In this model the profile of cytokines elicited was seen as both TH1 and TH2 associated. Our data show that SEB administered through the oral route promotes a dominant TH2 response, although only when administered with antigen. SEB alone showed no response in our model. Importantly, we demonstrate that SEB alone produced anti-SEB–specific IgE responses (Table I). However, these mice did not display TH2 cytokine responses and were similar to mice treated with antigen alone in every other regard, including eosinophilia. Therefore the IgE produced against SEB itself does not significantly contribute to the responses observed on antigen challenge.
Recently, SEB has been shown by Yang et al51 to modulate T-cell immunoglobulin and mucin domain molecule (TIM) 4 expression on intestinal dendritic cells. More recent work has shown TIM-4 to be a receptor for phosphatidylserine on apoptotic cells52,53 and that it can promote T-cell activation through binding to TIM-1.54 This process might explain the strong TH2 bias that we observed in our model. However, the model of “intestinal” allergy that Yang et al51 used involved intraperitoneal injection with antigen and SEB followed by an oral challenge and thereby avoided the issue of oral tolerance. Our data suggest that SEB influences this important checkpoint. Indeed, we propose that the development of TH2 immunity to oral antigen likely occurs as a result of a loss in immune suppression.
Mechanistically, we observed loss of the expression of TGF-b and no change in IL-10 expression. This potentially indicates a selective influence on the TH3-type Treg cells but not on the IL-10–secreting TR1-type cells.55 Patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome, who lack Treg cells, have severe food allergies.56-58 Patients with food allergy also have fewer TGF-b–producing cells in their intestines.42 In studies of patients with AD, SEB diminished the suppressive capacity of CD41 CD251 Treg cells in vitro.59 Additionally, Treg cell–mediated suppression was indirectly inhibited by SEB through the upregulation of glucocorticoid-induced TNF receptor–related protein ligand on monocytes.60 Our data demonstrate that oral SEB might impair the functions of Treg responses in vivo.
In conclusion, our data establish that SEB exposure leads to allergic sensitization to food allergens, perhaps through a break-through of oral tolerance. SEB-driven sensitization elicits a spectrum of responses to food antigens that is highly reminiscent of the response in human subjects and is achieved in standard laboratory strains of mice. This model might be valuable in further investigations into the mechanisms of food allergy.
Clinical implications: Oral exposure to SEB, through colonization or food contamination, might promote the generation of allergic responses to food antigens.
Acknowledgments
Supported by the Food Allergy Project. R.P.S. was supported by National Institutes of Health (NIH) grants RO1 HL068546, HL078860, and AI072570. P.J.B. was supported by NIH grants RO1 AI076456 and R21 AI078525.
Abbreviations used
- AD
Atopic dermatitis
- CT
Cholera toxin
- OVA
Ovalbumin
- SEB
Staphylococcal enterotoxin B
- TIM
T-cell immunoglobulin and mucin domain molecule
- Treg
Regulatory T
- WPE
Whole peanut extract
METHODS
Antigen-specific immunoglobulin ELISA
With the exception of OVA-specific IgE, sandwich ELISA assays were used to quantify OVA-specific or WPE-specific immunoglobulins. Ninety-six–well flat-bottom plates (Immulon II; Nunc, Roskilde, Denmark) were coated with either 10 mg/mL OVA or 1 mg/mL WPE overnight at 48C. Plates were washed 3 times with PBS containing 0.05% Tween-20. Blocking of nonspecific binding was achieved by means of addition of 3% BSA for 2 hours, followed by washing as before. Appropriate dilutions of serum were prepared in PBS and studied in duplicate wells. Sera pooled from 5 previously immunized mice, for which an arbitrary unit value was given, was used to determine a standard curve for all assays performed. Samples and standards were incubated overnight at 48C before being washed 3 times, as before. Appropriate biotin-labeled secondary antibodies against murine immunoglobulin isotypes (2 mg/mL) were added for 1 hour, washed 4 times as before, and followed by addition of horseradish peroxidase–conjugated streptavidin (1:1000 dilution; Zymed, South San Francisco, Calif). After 30 minutes, plates were washed 5 times as before, and ABTS substrate (Zymed) was added. The intensity of color was determined at 405 nm, and the values of each sample were determined as an arbitrary unit based on the standard curve generated from the standards after correction for blank wells. The linearity of the standard curve was an r2 value of greater than 0.9 for all experiments, and sample values were calculated only when they fell within the linear region of each standard curve.
OVA-specific IgE levels were determined by using anti-mouse IgE as the capture antibody and biotin-labeled OVA (prepared with the NHS-biotin labeling assay [Pierce, Cheshire, United Kingdom], as per the manufacturer's instructions) as a secondary reagent. The quantity of OVA-specific IgE was determined by comparing values to a standard curve by using purified mouse OVA-specific IgE from the TOe hybridoma.
Footnotes
Disclosure of potential conflict of interest: X. Luo has received research support from the National Institutes of Health and the Juvenile Diabetes Research Foundation. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Lee SY, Huang CK, Zhang TF, Schofield BH, Burks AW, Bannon GA, et al. Oral administration of IL-12 suppresses anaphylactic reactions in a murine model of peanut hypersensitivity. Clin Immunol. 2001;101:220–8. doi: 10.1006/clim.2001.5122. [DOI] [PubMed] [Google Scholar]
- 2.Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, et al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108:639–46. doi: 10.1067/mai.2001.118787. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171–8. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 4.van Wijk F, Hartgring S, Koppelman SJ, Pieters R, Knippels LM. Mixed antibody and T cell responses to peanut and the peanut allergens Ara h 1, Ara h 2, Ara h 3 and Ara h 6 in an oral sensitization model. Clin Exp Allergy. 2004;34:1422–8. doi: 10.1111/j.1365-2222.2004.02062.x. [DOI] [PubMed] [Google Scholar]
- 5.van Wijk F, Hoeks S, Nierkens S, Koppelman SJ, van Kooten P, Boon L, et al. CTLA-4 signaling regulates the intensity of hypersensitivity responses to food antigens, but is not decisive in the induction of sensitization. J Immunol. 2005;174:174–9. doi: 10.4049/jimmunol.174.1.174. [DOI] [PubMed] [Google Scholar]
- 6.van Wijk F, Wehrens EJ, Nierkens S, Boon L, Kasran A, Pieters R, et al. CD4( 1 )CD25( 1 ) T cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin Exp Allergy. 2007;37:572–81. doi: 10.1111/j.1365-2222.2007.02681.x. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh KY, Hsu CI, Lin JY, Tsai CC, Lin RH. Oral administration of an edible-mushroom-derived protein inhibits the development of food-allergic reactions in mice. Clin Exp Allergy. 2003;33:1595–602. doi: 10.1046/j.1365-2222.2003.01790.x. [DOI] [PubMed] [Google Scholar]
- 8.Adel-Patient K, Ah-Leung S, Creminon C, Nouaille S, Chatel JM, Langella P, et al. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin Exp Allergy. 2005;35:539–46. doi: 10.1111/j.1365-2222.2005.02225.x. [DOI] [PubMed] [Google Scholar]
- 9.Strid J, Strobel S. Skin barrier dysfunction and systemic sensitization to allergens through the skin. Curr Drug Targets Inflamm Allergy. 2005;4:531–41. doi: 10.2174/156801005774322199. [DOI] [PubMed] [Google Scholar]
- 10.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757–66. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 11.Breiteneder H, Mills EN. Molecular properties of food allergens. J Allergy Clin Immunol. 2005;115:14–24. doi: 10.1016/j.jaci.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14:1269–73. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 13.Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–23. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 14.Moneret-Vautrin DA, Kanny G, Morisset M, Rance F, Fardeau MF, Beaudouin E. Severe food anaphylaxis: 107 cases registered in 2002 by the Allergy Vigilance Network. Allerg Immunol (Paris) 2004;36:46–51. [PubMed] [Google Scholar]
- 15.Knippels LM, van Wijk F, Penninks AH. Food allergy: what do we learn from animal models? Curr Opin Allergy Clin Immunol. 2004;4:205–9. doi: 10.1097/00130832-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, et al. Endotoxintolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 19.Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, et al. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111:1122–8. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- 20.Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. 2008;121:1311–22. doi: 10.1016/j.jaci.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Cox E, Verdonck F, Vanrompay D, Goddeeris B. Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Vet Res. 2006;37:511–39. doi: 10.1051/vetres:2006014. [DOI] [PubMed] [Google Scholar]
- 22.Grdic D, Smith R, Donachie A, Kjerrulf M, Hornquist E, Mowat A, et al. The mucosal adjuvant effects of cholera toxin and immune-stimulating complexes differ in their requirement for IL-12, indicating different pathways of action. Eur J Immunol. 1999;29:1774–84. doi: 10.1002/(SICI)1521-4141(199906)29:06<1774::AID-IMMU1774>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Kroghsbo S, Christensen HR, Frokiaer H. Experimental parameters differentially affect the humoral response of the cholera-toxin-based murine model of food allergy. Int Arch Allergy Immunol. 2003;131:256–63. doi: 10.1159/000072137. [DOI] [PubMed] [Google Scholar]
- 24.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;153:647–57. [PubMed] [Google Scholar]
- 25.Breuer K, Wittmann M, Bosche B, Kapp A, Werfel T. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB). Allergy. 2000;55:551–5. doi: 10.1034/j.1398-9995.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- 26.Campbell DE, Kemp AS. Production of antibodies to staphylococcal superantigens in atopic dermatitis. Arch Dis Child. 1998;79:400–4. doi: 10.1136/adc.79.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langer K, Breuer K, Kapp A, Werfel T. Staphylococcus aureus-derived enterotoxins enhance house dust mite-induced patch test reactions in atopic dermatitis. Exp Dermatol. 2007;16:124–9. doi: 10.1111/j.1600-0625.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 28.Strange P, Skov L, Lisby S, Nielsen PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996;132:27–33. [PubMed] [Google Scholar]
- 29.Laouini D, Kawamoto S, Yalcindag A, Bryce P, Mizoguchi E, Oettgen H, et al. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J Allergy Clin Immunol. 2003;112:981–7. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Savinko T, Lauerma A, Lehtimaki S, Gombert M, Majuri ML, Fyhrquist-Vanni N, et al. Topical superantigen exposure induces epidermal accumulation of CD8 1 T cells, a mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. J Immunol. 2005;175:8320–6. doi: 10.4049/jimmunol.175.12.8320. [DOI] [PubMed] [Google Scholar]
- 31.Conley DB, Tripathi A, Seiberling KA, Schleimer RP, Suh LA, Harris K, et al. Superantigens and chronic rhinosinusitis: skewing of T-cell receptor V beta-distributions in polyp-derived CD4 1 and CD8 1 T cells. Am J Rhinol. 2006;20:534–9. doi: 10.2500/ajr.2006.20.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiberling KA, Conley DB, Tripathi A, Grammer LC, Shuh L, Haines GK, 3rd, et al. Superantigens and chronic rhinosinusitis: detection of staphylococcal exotoxins in nasal polyps. Laryngoscope. 2005;115:1580–5. doi: 10.1097/01.mlg.0000168111.11802.9c. [DOI] [PubMed] [Google Scholar]
- 33.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Wang BQ, Zheng PY, He SH, Yang PC. Rhinosinusitis derived staphylococcal enterotoxin B plays a possible role in pathogenesis of food allergy. BMC Gastroenterol. 2006;6:24. doi: 10.1186/1471-230X-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003;2:63–76. [PubMed] [Google Scholar]
- 37.Shupp JW, Jett M, Pontzer CH. Identification of a transcytosis epitope on staphylococcal enterotoxins. Infect Immun. 2002;70:2178–86. doi: 10.1128/IAI.70.4.2178-2186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamad AR, Marrack P, Kappler JW. Transcytosis of staphylococcal superantigen toxins. J Exp Med. 1997;185:1447–54. doi: 10.1084/jem.185.8.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 40.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci U S A. 1994;91:6688–92. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuji NM, Mizumachi K, Kurisaki J. Interleukin-10-secreting Peyer's patch cells are responsible for active suppression in low-dose oral tolerance. Immunology. 2001;103:458–64. doi: 10.1046/j.1365-2567.2001.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Machado MA, Ashwood P, Thomson MA, Latcham F, Sim R, Walker-Smith JA, et al. Reduced transforming growth factor-beta1-producing T cells in the duodenal mucosa of children with food allergy. Eur J Immunol. 2003;33:2307–15. doi: 10.1002/eji.200323308. [DOI] [PubMed] [Google Scholar]
- 43.Scott-Taylor TH, Hourihane JB, Harper J, Strobel S. Patterns of food allergen-specific cytokine production by T lymphocytes of children with multiple allergies. Clin Exp Allergy. 2005;35:1473–80. doi: 10.1111/j.1365-2222.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 44.Koppelman SJ, Knol EF, Vlooswijk RA, Wensing M, Knulst AC, Hefle SL, et al. Peanut allergen Ara h 3: isolation from peanuts and biochemical characterization. Allergy. 2003;58:1144–51. doi: 10.1034/j.1398-9995.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 45.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–14. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 46.Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. J Clin Invest. 2006;116:1624–32. doi: 10.1172/JCI26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bryce PJ, Geha R, Oettgen HC. Desloratadine inhibits allergen-induced airway inflammation and bronchial hyperresponsiveness and alters T-cell responses in murine models of asthma. J Allergy Clin Immunol. 2003;112:149–58. doi: 10.1067/mai.2003.1616. [DOI] [PubMed] [Google Scholar]
- 48.Renz H, Bradley K, Larsen GL, McCall C, Gelfand EW. Comparison of the allergenicity of ovalbumin and ovalbumin peptide 323-339. Differential expansion of V beta-expressing T cell populations. J Immunol. 1993;151:7206–13. [PubMed] [Google Scholar]
- 49.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–64. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang PC, Xing Z, Berin CM, Soderholm JD, Feng BS, Wu L, et al. TIM-4 expressed by mucosal dendritic cells plays a critical role in food antigen-specific Th2 differentiation and intestinal allergy. Gastroenterology. 2007;133:1522–33. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–9. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–40. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, et al. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706–13. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J, et al. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood. 2007;109:383–5. doi: 10.1182/blood-2006-05-025072. [DOI] [PubMed] [Google Scholar]
- 57.Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–17. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 58.Wildin RS, Freitas A. IPEX and FOXP3: clinical and research perspectives. J Autoimmun. 2005;25(suppl):56–62. doi: 10.1016/j.jaut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004;113:756–63. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 60.Cardona ID, Goleva E, Ou LS, Leung DY. Staphylococcal enterotoxin B inhibits regulatory T cells by inducing glucocorticoid-induced TNF receptor-related protein ligand on monocytes. J Allergy Clin Immunol. 2006;117:688–95. doi: 10.1016/j.jaci.2005.11.037. [DOI] [PubMed] [Google Scholar]