Abstract
Recent studies in humans and animals raise the possibility that actively maintaining a detailed memory of a scene within working memory may require the hippocampus, a brain structure better known for its role in long-term memory. We show that the hippocampus is behaviorally and functionally critical for configural-relational (CR) maintenance by orchestrating the synchrony of occipital and temporal brain regions in the theta-frequency range. Using magnetoencephalography in healthy adults and patients with bilateral hippocampal sclerosis, we distinguish this hippocampus-dependent theta-network from one that is independent of the hippocampus and used for non-CR scene maintenance. This non-CR theta-network involved frontal and parietal brain regions. We also show that the functional and topographical dissociation between these two networks cannot be accounted for by perceptual difficulty or the amount of information to be maintained (“load”). Also, we confirm in healthy adults that active maintenance of the CR arrangement of objects within a scene is impaired by task-interference during the delay in a manner akin to working-memory maintenance processes. Together, these findings demand reconsideration of the classical functional-anatomical distinctions between long- and short-term memory.
Keywords: working memory, long-term memory, short-term memory, phase-coupling
Working memory allows information from transient events to persist in the brain as active representations. This active persistence, or maintenance, enables goal-directed behaviors such as decision making and learning to use and manipulate information beyond its transient sensory availability. Recently, it has been shown that patients with bilateral lesions in the hippocampus, a structure located in the medial temporal lobes, are impaired in their ability to maintain associative forms of information while performing normally for nonassociative information (1–3). After a brief presentation of a topographical scene for example, these patients are unable to keep in mind the configural relation between multiple objects in the scene (1, 2). Although a relational memory (4) deficit was evident even at very short delays of just a few seconds, it is still unclear to what extent this impairment reflects a deficit of active maintenance akin to working memory.
Recent physiological studies in animals raise the possibility that one key functional contribution of the hippocampus toward the active maintenance of relational visual relationships is to coordinate the activity of cortical representations through slow network oscillations at a frequency of 4–8 Hz (so-called theta frequency) (5–7). This role for theta oscillations in the active maintenance of visual scenes is supported by the observation that the neural firing of monkey's visual area V4 is patterned by the local theta phase during working memory delays (8). Studies in rodents show that, in principle, this theta modulation could be orchestrated by the hippocampus (6, 7, 9, 10). For example, it has been demonstrated that theta oscillations generated by the hippocampus (9, 10) can modify neuronal activity outside limbic areas (7). During goal-directed behavior in rodents, medial prefrontal cortical neuronal firing is phase-locked to the hippocampal theta rhythm and this phase-locking is accompanied by synchronization of the hippocampal and prefrontal theta rhythms (6). Together, these disparate pieces of evidence converge toward the hypothesis that hippocampus-dependent theta oscillations are critical for working memory of configural-relational (CR) aspects of the visual scene, either through facilitation of the functional coupling between brain regions that represent the multiple scene elements and their locations in space (11, 12), or through facilitation of the reciprocal orchestration of these regions with the hippocampus (7) or both. Both of these functional roles would be apparent with hippocampus-dependent theta synchrony between brain regions during the delay period of a relational maintenance task.
Here, we investigated this hypothesis using an integrative approach that combines the study of working memory maintenance in patients who have selective hippocampal lesions with functional measures of theta synchrony under experimental conditions in which* CR and non-CR forms of maintenance demands are tested (for term definitions, see Methods). We obtained whole-head magnetoencephalography (MEG) recordings of theta activity during delayed-match-to-sample (DMS) tasks where the CR aspects of visual scenes needed to be maintained over a period of 5 s. We contrasted behavioral performance and theta coupling in this condition with those obtained during non-CR maintenance of visual scenes and a control condition that had no maintenance requirements but was matched in perceptual difficulty with the CR DMS condition. In an additional MEG study with healthy adults, we establish whether or not CR maintenance is functionally equivalent to increasing working-memory load. Last, characteristic of active stimulus maintenance in working memory, both CR and non-CR (high-load) forms of maintenance in our task were determined to be impaired by delay interference.
Results
Experiment 1: CR DMS in Healthy Participants.
We recorded whole-head MEG while participants engaged in three variants of a DMS task. In the CR DMS task, the nonmatching probe differed from the matching probe only in the relative location or omission of scene elements. To detect the match, it was necessary to maintain a detailed record of all objects in the scene as well as their relational binding within the scene. In the non-CR version, the nonmatching probe displayed a completely different scene allowing detection of the match by maintaining just one element of the sample scene. The control condition was matched to the CR condition in perceptual difficulty at the probe phase, but did not require any maintenance of sample information (Fig. 1A).
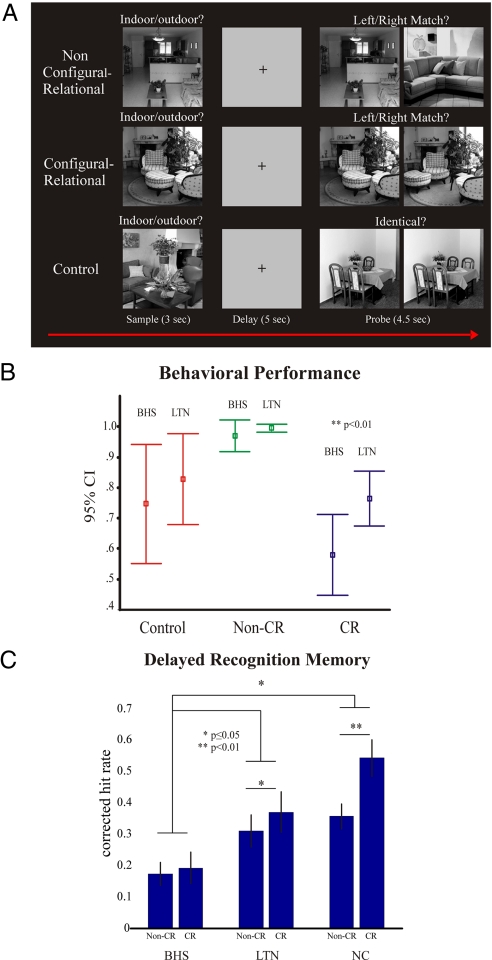
Fig. 1.
Experimental design and behavioral results for experiments 1 and 3. (A) A non-CR DMS trial where participants are instructed to make a deep encoding “indoor/outdoor” judgment at sample and maintain this information over the delay period to make a “left/right” match decision at test (Top). A CR DMS trial where the configural relationship of items within a scene must be maintained over the delay to make a correct decision at test (Middle). A control trial where participants are not required to maintain any stimulus information to make a discrimination judgment at test (Bottom). (B) Mean behavioral performance in DMS trials in experiment 3 for BHS patients and left temporal lobe epilepsy patients determined to be MRI-negative for hippocampal pathology (LTN). The data show a selective impairment for the BHS group for CR DMS performance (>95% confidence interval; error bars indicate SEM). (C) Mean corrected hit rates of delayed recognition memory for non-CR and CR stimuli in experiment 3 for BHS, LTN, and NC. P values denoting differences between conditions are indicated by asterisks (paired t test, one-tailed, SD).
Behavioral Performance.
The three conditions differed in participants' response accuracy during the probe phase [repeated-measures ANOVA; F(2,16) = 39.7; P < 0.001). Posthoc paired t tests (two-tailed, mean, SEM) showed that accuracy was better in the non-CR DMS condition (99.3 ± 0.4%) than the CR DMS [69.6 ± 2.7%, t(8) = 10.689, P < 0.001] and control condition [59.3 ± 4.6%, t(8) = 8.265, P < 0.001]. The CR and control conditions were well-matched for accuracy [69.6 ± 2.7% and 59.3 ± 4.6%, respectively, t(8) = 1.729, P = 0.122]. Reaction time data are not reported for the probe phase, because there was no response deadline in this paradigm.
Phase-Coupling Analysis.
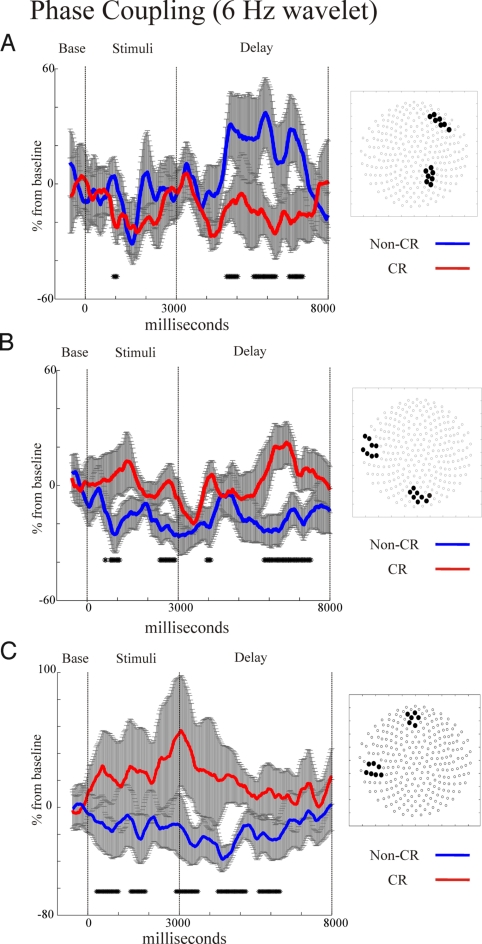
We performed an unbiased search for theta coupling (for all possible combinations of MEG sensors) lasting at least three successive theta cycles (threshold of P < 0.05). Compared with the control condition, CR DMS showed stronger coupling of left occipital and temporal sensors, whereas non-CR DMS showed stronger coupling of right frontal and parietal sensors.
Elevated left occipito-temporal theta synchrony for CR DMS and fronto-parietal theta synchrony for the non-CR DMS also held when directly contrasting CR and non-CR DMS conditions (Fig. 2 A and B). Right fronto-parietal synchrony emerged ≈1,500 ms after sample offset and continued for the rest of the delay period in the non-CR condition, but remained constant relative to baseline throughout the delay in the CR DMS condition (Fig. 3A). However, occipito-temporal theta synchrony was elevated throughout much of the sample and delay period for the CR DMS condition, whereas non-CR levels remained at baseline or below (Fig. 3B). Also, there was augmented theta-phase coupling for mid-frontal and left temporal sensors in the CR DMS condition (Fig. 3C), which peaked soon after stimulus offset. Importantly, these synchrony patterns were not artifacts of theta amplitude differences between DMS conditions (Fig. S1 A–C).
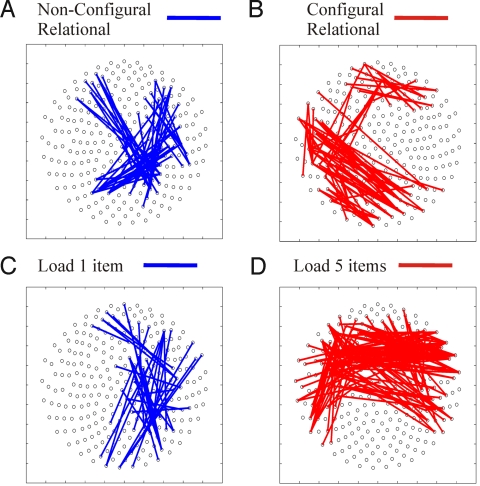
Fig. 2.
Phase-coupling analysis (6-Hz wavelet) contrasting experimental conditions during delay periods in sensor space for experiment 1 comparing CR and non-CR DMS (Upper), and experiment 2 comparing non-CR DMS load conditions (Lower). In experiment 1, non-CR delay maintenance (A) display increased right frontal and parietal theta-band sensor coupling (blue) compared with the CR DMS delays (B) that increased left occipito-temporal theta-coupling (red). In experiment 2, one-item delay maintenance (C) displayed a similar right frontal and parietal theta-phase coupling (blue) as the non-CR task in experiment 1. The five-item delay (D) increased theta-phase coupling of bifrontal sensor groups (red), a pattern that is nonoverlapping with the CR maintenance sensor coupling of experiment 1.
Fig. 3.
Serial measures t test comparisons of 6-Hz phase coupling on selected sensor groups (shown on right-side insets) for non-CR (in blue) vs. CR (in red) DMS conditions in experiment 1 (significance at P < 0.05 indicated by markings on x axis, see Methods). Non-CR DMS significantly increased theta synchrony of right fronto-parietal sensor groups during delay periods (A). Alternatively, CR DMS engaged a network of left occipito-temporal (B) and fronto-temporal theta synchrony (C) during stimulus encoding and delay periods.
Experiment 2: Non-CR DMS Load.
The distinct occipital-temporal coupling found in the CR DMS condition of experiment 1 may reflect difficulty due to the requirements of maintaining a higher number of single elements within a scene and may not be driven by the specific demand of maintaining a CR memory linking these elements in DMS tasks. To address this alternative account, we investigated the theta-coupling patterns associated with increasing the non-CR DMS “load” from 1 to 3 or 5 scenes to be maintained without the additional demands of CR maintenance (Fig. S2, see also SI Methods).
Behavioral Performance.
As expected, the ability to identify the matching probe (accuracy) decreased with load (see SI Results).
Phase-Coupling Analysis.
Compared with high load 5, load 1 (corresponds to the non-CR DMS of experiment 1) showed enhanced right frontal-parietal theta synchrony (same statistical threshold as in experiment 1) (Fig. 2C), very much like non-CR maintenance in experiment 1. In contrast, load 5, when compared with load 1, showed enhanced coupling between bilateral frontal and temporal sensors (Fig. 2D), a strikingly different pattern from the left occipito-temporal synchrony, which distinguished CR from non-CR maintenance in experiment 1. For the time course of synchrony patterns, see Fig. S3.
Experiment 3: CR DMS in Patients with Bilateral Hippocampal Sclerosis (BHS).
We expected that patients with BHS would display impaired performance in the CR DMS condition and a selective loss of occipito-temporal theta synchronization, whereas the fronto-parietal theta synchrony seen in the non-CR DMS condition from experiment 1 should be unaffected. Patients with left temporal lobe epilepsy determined to be “MRI-negative” for hippocampal volume reductions and signal abnormalities (MRI negative left temporal lobe epilepsy, LTN), served as a control group to match the effects of recurrent epileptic seizures and antiepileptic medication in BHS patients, but without hippocampal sclerosis (see SI Methods and Fig. S4).
Behavioral Performance.
BHS patients were selectively impaired in CR DMS performance. In contrast, the LTN patients were unimpaired relative to normal controls (NC) in both conditions (Fig. 1B). A 2 × 3 repeated-measures ANOVA with condition (CR and non-CR DMS) as the within-subjects factor and group as the between subjects factor (BHS, LTN, and NC) revealed main effects for condition [F(1,19) = 136.19, P < 0.001] and group [F(2,19) = 16.84, P < 0.001], as well as a condition by group interaction [F(2,19) = 8.85, P < 0.005]. Follow up ANOVAs showed that LTN patients' scores were comparable with NC in both DMS conditions, with no interaction [F(1,14) = 1.719, P = 0.211] or group effects [F(1,14) = 1.77, P = 0.204], whereas there was an interaction of condition and group between the BHS and NC groups [F(1,15) = 15.61, P < 0.005]. ANOVAs showed a nearly significant interaction between BHS and LTN and condition [F(1,9) = 4.827, P = 0.056] and posthoc independent t test comparisons (one-tailed, mean, SEM) revealed lower accuracy for CR DMS in BHS patients (58.0 ± 5.1%) compared with LTN patients [76.4 ± 3.2%; t(9) = −2.790, P < 0.01]. In contrast, no differences were found between groups for non-CR [t(9) = −1.083, P = 0.15] or control [t(9) = −0.841, P = 0.21] condition accuracy, thus confirming a selective impairment within the BHS group for CR DMS performance. An additional posthoc paired t test comparison (one-tailed, mean, SEM), showed lower accuracy in BHS patients for CR DMS (58.0 ± 5.2%) compared with the control condition [74.7 ± 7.6%; t(5) = 2.464, P < 0.05] (Table S1).
Delayed Recognition Performance.
In 50% all trials, there were no probe stimuli. Scenes from these trials were later used to test long-term recognition memory (see SI Methods). Both NC and LTN participants displayed increased delayed memory recognition for CR stimuli compared with non-CR DMS condition, whereas BHS patients showed no such difference. A 2 × 3 repeated-measures ANOVA of corrected recognition (hit) rates with condition (CR and non-CR samples) as the within-subjects factor and group (LTN, NC, and BHS) as the between-subjects factor, revealed main effects for condition [F(1,20) = 9.26, P < 0.01] and group [F(2,20) = 7.92, P < 0.005], as well as a group by condition interaction [F(2,20) = 3.55, P < 0.05]. Compared with LTN, BHS patients were impaired in both recognition memory tests [main effect of group, F(1,10) = 5.26, P < 0.05]. Posthoc paired t test comparisons (one-tailed, mean, SD) showed increased delayed memory recognition for CR stimuli compared with non-CR stimuli for the NC [0.54 ± 0.20 vs. 0.36 ± 0.13, t(10) = −3.65, P < 0.01] and for the LTN [0.370 ± 0.07 vs. 0.310 ± 0.05, t(5) = −1.974, P = 0.05], but not for the BHS [0.19 ± 0.11 vs. 0.17 ± 0.08, t(5) = −0.484, P = 0.649] group (Fig. 1C).
Phase-Coupling Analysis.
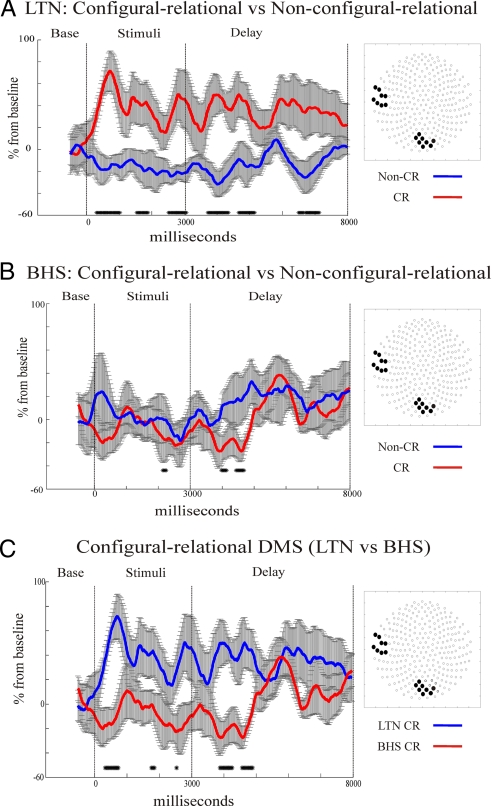
To test our hypothesis that hippocampal integrity is necessary for the occipito-temporal theta synchrony during the CR DMS condition, we analyzed LTN and BHS patients' 6-Hz phase coupling from similar sensor regions displaying significant synchrony in NC (experiment 1). Consistent with this hypothesis, we expected reduced occipito-temporal synchrony for CR DMS in BHS, whereas fronto-parietal synchrony would be functionally intact during non-CR DMS.
As expected, the increased occipito-temporal synchrony for CR vs. non-CR conditions was maintained in patients with LTN (Fig. 4A), but was abolished in the BHS group (Fig. 4B). Between group t test comparisons of CR DMS conditions (independent samples t test, same threshold as above) revealed increased 6-Hz synchrony during the encoding and delay periods for LTN compared with BHS for CR DMS (Fig. 4C). Also, within the BHS cohort, t test comparisons showed preserved increases in fronto-parietal theta coupling for non-CR DMS during maintenance and encoding when compared with the control condition (Fig. S5A). In fact, t tests directly comparing CR and non-CR DMS in the BHS group displayed identical time courses of fronto-parietal theta-synchrony enhancement (Fig. S5B). Last, LTN patients displayed stronger left fronto-temporal synchrony during CR maintenance than BHS patients (Fig. S6).
Fig. 4.
Serial measures t test comparisons of 6-Hz phase coupling on selected sensor groups (shown on right-side insets) for non-CR (in blue) vs. CR (in red) DMS conditions in experiment 3. Increased occipito-temporal theta synchrony for CR DMS during encoding and maintenance was significantly enhanced in temporal lobe epilepsy patients without hippocampal lesions (LTN) (A). This theta synchrony increase was absent for identical sensor groups in BHS patients during CR DMS (B). In the encoding and maintenance phase of CR DMS, the LTN (blue) group showed stronger occipito-temporal theta synchrony than the BHS group (red) (C).
Experiment 4: Delay Period Interference.
Behavioral experiment in 17 NCs showed that memory performance in the CR DMS and the five-item load non-CR DMS condition was significantly impaired when an interference task was introduced during the 5-s delay period [reduction of five-item load DMS performance from 83.00 ± 2.45% to 76.24 ± 3.03%, t(16) = 2.439, P = 0.027; reduction of CR DMS performance from 78.71 ± 2.47% to 67.18 ± 2.38%, t(16) = 5.229, P = 0.0001] (see SI Results).
Discussion
Our findings provide converging evidence for a critical role of hippocampus-dependent cortical theta synchrony in the active maintenance of CR visual information. We also demonstrate that this theta-synchrony, coupling occipital and temporal sensors, is functionally and anatomically dissociated from non-CR visual maintenance, which engaged theta synchrony between frontal and parietal sensors. Furthermore, increasing the working memory load in the absence of CR maintenance demands engaged bilateral frontal theta synchrony. This topographic difference indicates that occipito-temporal theta synchrony in CR maintenance cannot be accounted for by the additional demands of maintaining more scene elements.
In patients with BHS occipito-temporal, theta synchrony was selectively abolished (Fig. 4B) and left fronto-temporal theta synchrony was reduced (Fig. S6), while being preserved in our patients with LTN and normal appearing hippocampi (Fig. 4A). Importantly, BHS patients also displayed a selective memory impairment in the CR DMS condition, whereas their accuracy for non-CR DMS tasks was comparable with that of the NC and LTN group. This convergence of structural, functional, and behavioral results indicates a critical role of hippocampus-dependent theta oscillations in coordinating the encoding and subsequent active maintenance of CR information. Previous work has predicted such a possibility (7, 13–15), but the combined hippocampal lesion and functional coupling approach necessary to demonstrate this process has so far been missing.
Our findings in the BHS group are consistent with recent behavioral studies that patients with medial temporal lobe amnesia (caused by hypoxic brain injury or encephalitis) have intact working memory performance for object and location, but significant impairments for object-location conjunctions (16, 17). Likewise, bilateral hippocampal atrophy caused by hypoxia has recently been reported to cause a selective deficit in CR working memory performance (1). Occipito-temporal theta synchrony during CR maintenance may indicate the coordination of cortical representations along the visual ventral stream processing hierarchy. In fact, there is abundant evidence for the possibility that theta oscillations can be generated neocortically in humans (18–20). However, a recent study in rodents also suggested that cortically recorded theta oscillations may be volume conducted from the hippocampus (7). Although we cannot exclude this possibility, the fact that the task differences in occipito-temporal theta synchrony were not accompanied by corresponding changes in theta amplitudes argues against a volume conduction account (Fig. S1).
The hippocampus-dependent occipito-temporal theta synchronization may contribute to integrating representations of complex conjunctions of scene elements in more rostral portions of the ventral stream, such as the rhinal cortex, with representations of component features in more posterior regions (e.g., visual areas such as V4) (13–15, 21). It has been suggested that, through theta oscillations, the hippocampus may drive the reciprocal exchange of information with neocortical areas (7). According to this suggestion, the hippocampus may actively control the transfer of neocortical information to the hippocampus itself via theta-phase biasing of neocortical network dynamics (7). With our findings, one possibility is that the hippocampus drives this reciprocal transfer of information with both occipital and temporal brain regions. Another possibility is that only one of these neocortical regions is entrained by the hippocampus, whereas the other is entrained through cortico-cortical theta synchrony (for discussion of the role of cortico-cortical coordination in cognition, see refs. 22 and 23). Both of these possibilities are compatible with our observation that the occipito-temporal theta synchrony is abolished with bilateral hippocampal injury.
Our results show that the active maintenance of non-CR visual information enhances theta synchrony between right frontal and parietal sensors (Fig. 2A), compatible with earlier studies showing fronto-parietal engagement during working memory (24–27). In patients with hippocampal atrophy (BHS), this network remained functionally intact (Fig. S5A), and was thus unaffected by hippocampal integrity. This observation of hippocampally-independent neocortical theta coupling is physiologically plausible given the evidence for mechanisms responsible for the local neocortical generation of theta oscillations (28). Also, the behavioral functionality of this network was shown to be spared in these patients, because no performance differences for non-CR DMS conditions were found between BHS, LTN, or NC (Fig. 1B; see Results). Comparisons of CR and non-CR maintenance conditions in the BHS group showed identical time courses of fronto-parietal theta-synchrony enhancement (Fig. S5B), suggesting that when hippocampal integrity is compromised, the fronto-parietal theta network is used also under CR task demands, but this theta network cannot functionally support the maintenance requirements of CR representations.
Increasing the number of scene images to be retained during the delay from one to five was associated with increased theta coupling of bilateral frontal and temporal sensors (Fig. 2D). This pattern is very similar to increases in frontal theta coupling during working-memory loading tasks found previously in EEG studies (25). Most importantly, the bifrontal theta synchrony due to increased non-CR DMS load was nonoverlapping with the occipito-temporal theta synchrony of CR maintenance (Fig. 2B), indicating that dissociable networks are responsible for supporting the separate maintenance demands of item loading and CR representations. This topographic difference strongly argues against the possibility that functional differences between the CR and non-CR maintenance conditions are due to increases in non-CR item loading in the CR condition. Our current results are compatible with recent studies showing that patients with medial temporal lobe amnesia showed a selective impairment in the ability to maintain relational conjunctions in working memory but had no memory load related impairment (3, 17). However, our data do not necessarily rule out the possibility that maintenance under high non-CR DMS load may also be compromised by bilateral hippocampal lesions (29).
Our study also addressed the relationship between working memory and long-term memory by testing memory for scene images (for those trials that had no probe images) from each condition in a delayed (30 min) recognition memory test. Here, the samples had to be discriminated from previously unseen scenes. In NC and LTN patients, delayed recognition memory for scenes from the non-CR DMS trials was enhanced when compared with scenes from the non-CR DMS trials (Fig. 1C). Importantly, delayed recognition memory for both types of samples was at chance in BHS patients, despite the fact that these patients were unimpaired in the DMS performance for non-CR stimuli (Fig. 1B) and also displayed intact fronto-parietal synchrony (Fig. S5A). These findings suggest that the hippocampus-dependent theta coordination of occipital and temporal regions during CR DMS also contributed to encoding into long-term memory, but this was not the case for the hippocampus-independent fronto-parietal theta synchrony. This account is compatible with the suggestion that hippocampal maintenance operations may contribute to long-term memory encoding (14–16).
It has recently been argued that a hallmark of working memory is its susceptibility to interference during delay periods (30), and also, that the hippocampus is not critical for this form of active maintenance or working memory. Behaviorally (see experiment 4 in SI Methods), not just the high working memory load condition but also the CR condition of our DMS task was shown to be sensitive to delay interference supporting the possibility raised by our theta-coupling data that the CR condition also required an active form of maintenance akin to working memory. Therefore, our findings show that the hippocampus is functionally and behaviorally critical for actively maintaining the configural relationships of visual stimuli in working memory. In agreement with other authors (for review see ref. 31) our data call for a reconsideration of the classical distinction between hippocampus-dependent long-term memory and hippocampus-independent active maintenance or working memory.
Enhanced occipito-temporal synchrony in the CR task was initially engaged during encoding and then extended into the delay period for NC (Fig. 3B) and patients with LTN and normal hippocampi (Fig. 4A). In BHS patients, in contrast, theta synchrony was abolished already during stimulus encoding (Fig. 4B). These findings suggest that bilateral hippocampal injury may impair cooperative binding of information distributed across occipital and temporal regions initially during encoding, and the deficits observed during the delay may be an extension of this problem. Such an account is compatible with recent fMRI findings that short-term memory for object-location relationships is accounted for by encoding related activation of the hippocampus (14). One question that may therefore arise is whether our data imply a role for the hippocampus also in the perceptual processing of the CR aspects of scenes during encoding (32, 33); for a discussion see ref. 34. Our finding that the BHS patients performed normally in the perceptual control condition (Fig. 1B), which involved the same amount of visual scene manipulations as in the CR DMS condition, does not support such a role of the hippocampus in perceptual feature integration; for similar observations, see ref. 35.
Here, we have provided evidence that hippocampus-dependent theta coordination is critical for the ability to maintain CR information in visual working memory. In contrast, non-CR maintenance was associated with hippocampus-independent theta synchrony between frontal and parietal regions. These data thus show the existence of hippocampus-dependent and hippocampus-independent cortical networks of theta synchronization for stimulus maintenance in working memory. Overall, these findings challenge the classical functional-anatomical distinctions between long-term and short-term memory.
Methods
Experiment 1: CR DMS in Healthy Participants.
Participants.
Eight right-handed healthy subjects (five male; mean age, 21.6; SD 3.2) participated in the experiment. Participants for all four studies gave written informed consent, and the study was approved by the ethics committee of the University College London Research Ethics Committee for human-based research. All participants were compensated at a rate of £6 per hour for their time.
Stimuli and task design.
The stimuli used were photographs of 260 indoor and 260 outdoor scenes. All pictures were gray scaled and normalized to a mean gray value of 127 (75 SD), and set at 300 × 300 pixels. The experiment consisted of three conditions: a non-CR DMS condition, a CR DMS condition, and a “control” condition. In all three conditions, the trial structure and stimulus-timing were identical (Fig. 1A). After a 2-s intertrial interval, an indoor or an outdoor scene was presented for 3 s, followed by a blank screen with a fixation cross for 5 s and then by two test stimuli for 4.5 s.
In all three conditions, participants were required to make a speeded indoor/outdoor discrimination for the sample image indicated by button press (right-hand index or middle finger). In the DMS conditions, the two test images after the delay interval were used to probe memory for the sample. In the non-CR DMS, one of the probes was an exact repetition of the sample and the other depicted an entirely different new scene of the same category (indoor or outdoor). In the CR DMS, one of the probes was again an exact repetition of the sample; however, the other depicted the same scene as the sample with components of the scene being either shifted in location or omitted. In both versions of the DMS conditions, participants were instructed to maintain the sample image in their memory to correctly identify the matching sample. The location of the matching probe and the nonmatching foil were counterbalanced across trials.
In the CR trial condition, stimuli were manipulated in a relatively similar fashion, as reported in refs. 15 and 36. Manipulated versions of the scenes involved changes in the relations among some elements of the scenes. The types of manipulations were (i) addition or deletion of a new object or (ii) a spatial shifting of an object within a scene. Manipulation type (i) was used in 56% of the trials, and type (ii) was used in 44% of the trials in the CR DMS conditions. Thus, during sample scene presentation in the CR condition, participants were required to encode the individual objects (e.g., a tree, a bench), as well as object-object (e.g., the tree among the benches) and object-location (e.g., the tree in the upper left-hand corner of the scene) associations. Because participants were not able to predict which type of sample manipulation would be used in any given trial, both of these types of relational associations needed to be retained during the maintenance period to detect the nonmatching sample (for a review of relational memory types, see ref. 4). Also, during the probe phase, participants could also use changes to the CR scene-layout caused by either type of manipulation to detect the nonmatching sample. In this type of paradigm, Ryan and Cohen (15) observed intact preserved memory performance (using eye movements as critical measure) over very short delays (≈1.77 s), but impaired memory over longer delays.
In the test phase of the control conditions, two scene images, both of which were completely different from the sample, were presented side-by-side, and subjects were instructed to indicate if the two pictures were the same or different by button press. In half of the trials, the pictures were identical, and the other half, they differed to the same degree as the probes in the CR DMS condition. The difficulty of discriminating between stimuli was designed to match the CR DMS condition so as to account for any anticipatory activity of difficult choices during the delay. Subjects were instructed that they should not maintain the sample stimulus in memory, because this maintenance would not help them to make the difficult same/different discrimination during the test phase.
All conditions were blocked with 20 trials each. Subjects were instructed before each block as to which condition would be tested. There were three blocks per condition, resulting in 60 trials per condition. Presentation of indoor/outdoor stimuli was counterbalanced across each block (for MEG recording and data analysis methods, see SI Methods).
Experiment 3: CR DMS in Patients with BHS.
Participants.
Six right-handed patients with temporal lobe epilepsy and BHS (two female; mean age, 43.2; SD 9.8) participated in the MEG experiment as the testing group. The MEG control group consisted of six right-handed patients with left temporal lobe epilepsy (LTN) (two female; mean age, 37.2; SD 11.4) with normal structural MRI scans. The LTN group was chosen to match the effects of recurrent epileptic seizures and antiepileptic medication in the BHS cohort, while comparing structurally intact hippocampi (LTN) to patients with BHS (Table S2). All patients were attending clinics at the Department of Clinical and Experimental Epilepsy of University College London Institute of Neurology and the National Hospital for Neurology and Neurosurgery. Also, 11 NC participated in a behavioral version of the same experiment (four male; mean age, 24.7; SD 3.9).
Stimuli and Task Design.
A slightly modified DMS paradigm from experiment 1 was used for this study (see SI Methods).
Supplementary Material
Acknowledgments.
This work was funded by the Brain Research Trust and was undertaken at University College London Hospitals/University College London, who received a portion of funding from the Department of Health National Institute for Health Research Biomedical Research Centre's funding scheme, and additional support from the German Research Council (KFG 163) and the Volkswagen Foundation. Additional funding was provided by the Wellcome Trust (WT080568MA).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
The term “configural” processing has been used in face-perception literature to denote holistic relational processing. Here, the term configural-relational refers to the relational (spatial and object) aspects of visual scenes in an associative manner that may (though not necessarily) include holistic representation of these relations.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904823106/DCSupplemental.
References
- 1.Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartley T, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson IR, et al. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konkel A, et al. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 6.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirota A, et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45:147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 10.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 11.O'Keefe J. Hippocampus, theta, and spatial memory. Curr Opin Neurobiol. 1993;3:917–924. doi: 10.1016/0959-4388(93)90163-s. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: Why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- 13.Aggleton JP, Sanderson DJ, Pearce JM. Structural learning and the hippocampus. Hippocampus. 2007;17:723–734. doi: 10.1002/hipo.20323. [DOI] [PubMed] [Google Scholar]
- 14.Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan JD, Cohen NJ. Processing and short-term retention of relational information in amnesia. Neuropsychologia. 2004;42:497–511. doi: 10.1016/j.neuropsychologia.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Ezzyat Y, Olson IR. The medial temporal lobe and visual working memory: Comparisons across tasks, delays, and visual similarity. Cogn Effect Behav Neurosci. 2008;8:32–40. doi: 10.3758/cabn.8.1.32. [DOI] [PubMed] [Google Scholar]
- 17.Finke C, et al. The human hippocampal formation mediates short-term memory of colour-location associations. Neuropsychologia. 2008;46:614–623. doi: 10.1016/j.neuropsychologia.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caplan JB, et al. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghavachari S, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458:632–635. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bressler SL, Coppola R, Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature. 1993;366:153–156. doi: 10.1038/366153a0. [DOI] [PubMed] [Google Scholar]
- 23.Bressler SL, Kelso JA. Cortical coordination dynamics and cognition. Trends Cogn Sci. 2001;5:26–36. doi: 10.1016/s1364-6613(00)01564-3. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JD, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 25.Deiber MP, et al. Distinction between perceptual and attentional processing in working memory tasks: A study of phase-locked and induced oscillatory brain dynamics. J Cognit Neurosci. 2007;19:158–172. doi: 10.1162/jocn.2007.19.1.158. [DOI] [PubMed] [Google Scholar]
- 26.Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. NeuroImage. 2000;11:145–156. doi: 10.1006/nimg.1999.0527. [DOI] [PubMed] [Google Scholar]
- 27.Postle BR, Stern CE, Rosen BR, Corkin S. An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. NeuroImage. 2000;11:409–423. doi: 10.1006/nimg.2000.0570. [DOI] [PubMed] [Google Scholar]
- 28.Blatow M, et al. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 29.Axmacher N, et al. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. J Neurosci. 2008;28:4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Mem Cognit. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- 33.Graham KS, et al. Abnormal categorization and perceptual learning in patients with hippocampal damage. J Neurosci. 2006;26:7547–7554. doi: 10.1523/JNEUROSCI.1535-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki WA, Baxter MG. Memory, perception, and the medial temporal lobe: A synthesis of opinions. Neuron. 2009;61:678–679. doi: 10.1016/j.neuron.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Shrager Y, Gold JJ, Hopkins RO, Squire LR. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J Neurosci. 2006;26:2235–2240. doi: 10.1523/JNEUROSCI.4792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.