Abstract
Presentation of exogenous antigens on MHC class I molecules, termed cross-presentation, is essential for the induction of CD8 T-cell responses and is carried out by specialized dendritic cell (DC) subsets. The mechanisms involved remain unclear. It has been proposed that antigens could be transported by endocytic receptors, such as the mannose receptor (MR) in the case of soluble ovalbumin, into early endosomes in which the cross-presentation machinery would be recruited. In these endosomal compartments, peptides would be trimmed by the aminopeptidase IRAP before loading onto MHC class I molecules. Here, we have investigated the contribution of this pathway to cross-presentation by steady-state CD8+ DC and inflammatory monocyte-derived DC (moDC) generated in vivo. We demonstrate that IRAP and MR are dispensable for cross-presentation by CD8+ DC and for cross-priming. Moreover, we could not find any evidence for diversion of endocytosed antigen into IRAP-containing endosomes in these cells. However, cross-presentation was impaired in moDC deficient in IRAP or MR, confirming the role of these two molecules in inflammatory DC. These results demonstrate that the mechanisms responsible for cross-priming by steady-state and inflammatory DC are different, which has important implications for vaccine design.
Keywords: antigen presentation, inflammation
Priming of CD8 T-cell responses requires the presentation of exogenous antigen on MHC class I molecules by antigen-presenting cells, a process called cross-presentation. Dendritic cells (DC) are the main cross-presenting cells in vivo (1, 2), but only certain subsets of DC have this ability, primarily the CD8+ resident DC found in the spleen and lymph nodes and the CD103+ migratory DC found in lymph nodes (2–4). Monocyte-derived DC (moDC) that differentiate during inflammation (5) have also been shown to mediate cross-presentation and activation of memory T cells (6, 7), but their contribution to CD8 T-cell priming in the absence of inflammation is unclear.
Several mechanisms for cross-presentation have been proposed. The ‘cytosolic pathway’ involves the transfer of internalized antigens to the cytosol where they are degraded by the proteasome. The resulting peptides are translocated into the endoplasmic reticulum (ER) by TAP transporters and loaded onto MHC class I molecules (8). It has also been proposed that some antigens are degraded in endosomal compartments for loading onto MHC class I molecules in a TAP-independent manner (9). A variation of this ‘endosomal pathway’ has been recently proposed based on the study of soluble ovalbumin (OVA). In this model, OVA is transported into specialized early endosomes by the mannose receptor (MR) and can be stored in those compartments for several hours (10, 11). The MHC class I presentation machinery (including TAP) seems to be selectively recruited to these early endosomes, where peptides are trimmed by the insulin-regulated aminopeptidase (IRAP) for direct loading onto MHC class I molecules without trafficking to the ER (12, 13).
In this study, we have investigated the involvement of the endosomal cross-presentation pathway in splenic CD8+ DC and in vivo-generated moDC. We show that cross-presentation of OVA is independent of IRAP and MR in CD8+ DC. We analyzed the subcellular trafficking of OVA in splenic DC and could not find any evidence for its diversion and storage into specialized early endosomal compartments. However, we observed that cross-presentation was impaired in IRAP−/− moDC generated in vivo and that uptake of OVA was diminished in MR−/− moDC leading to decreased antigen presentation. These results show that the mechanisms of cross-presentation differ between steady-state and inflammatory DC.
Results
IRAP Is Dispensable for Cross-Presentation by Splenic CD8+ DC.
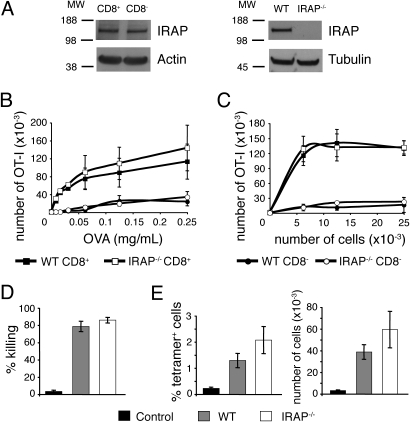
To analyze the contribution of the endosomal pathway to cross-presentation by CD8+ DC, we first examined the role of the aminopeptidase IRAP. IRAP was similarly expressed in CD8+ and CD8− DC subsets (Fig. 1A). To address its role in cross-presentation, purified wild-type or IRAP−/− CD8+ or CD8− DC were incubated with soluble OVA (Fig. 1B) or with OVA-coated irradiated splenocytes (a model of cell-associated antigen), and cocultured with CFSE-labeled OVA-specific transgenic CD8 OT-I T cells. T-cell proliferation was measured 3 days later as a read-out for cross-presentation (Fig. 1C). Wild-type and IRAP−/− CD8+ DC cross-presented soluble or cell-associated OVA with similar efficiency. We also investigated the role of IRAP in the in vivo induction of cytotoxic T lymphocytes (CTL), a process mainly dependent on cross-presentation by CD8+ DC (2, 14). Wild-type and IRAP−/− mice were immunized with OVA-coated irradiated splenocytes and the differentiation of effector CD8 T cells was assessed 5 days later. In vivo killing of target cells by CTL (Fig. 1D) and proliferation of endogenous specific CD8 T cells in the spleen (Fig. 1E) were similar in both groups. These results show that IRAP is not required for cross-presentation of antigens by splenic CD8+ DC.
Fig. 1.
IRAP is dispensable for cross-presentation of OVA by splenic dendritic cells. (A) Total cell lysates of wild-type CD8+ and CD8− DC or wild-type and IRAP−/− total splenic DC were analyzed by Western blot for the presence of IRAP, actin, or tubulin. Molecular weights are indicated (kDa). (B–C) Purified wild-type or IRAP−/− CD8+ and CD8− DC were incubated with soluble OVA and washed (B) or were cultured with OVA-coated splenocytes (C) and cocultured with CFSE-labeled OT-I T cells. After 3 days, proliferation of OT-I T cells was analyzed by flow cytometry. The number of OT-I T cells that had undergone at least one division is shown (mean ± SEM of three independent experiments). (D–E) wild-type or IRAP−/− mice were immunized with OVA-coated splenocytes. Control wild-type mice were injected with uncoated splenocytes. After 5 days, CD45.1+ CFSE-labeled target cells were injected and spleens were analyzed the next day. (D) The percentage of killing was determined by comparing the number of remaining SIINFEKL-pulsed target cells and unpulsed target cells (mean ± SEM, n = 6 in two independent experiments). (E) The proliferation of OVA-specific endogenous T cells was analyzed by tetramer staining. The percentage among CD8+ T cells and total number of tetramer+ CD8+ T cells in the spleen are shown (mean ± SEM, n = 6 in two independent experiments).
MR Is Dispensable for Cross-Presentation of Soluble OVA by Splenic CD8+ DC.
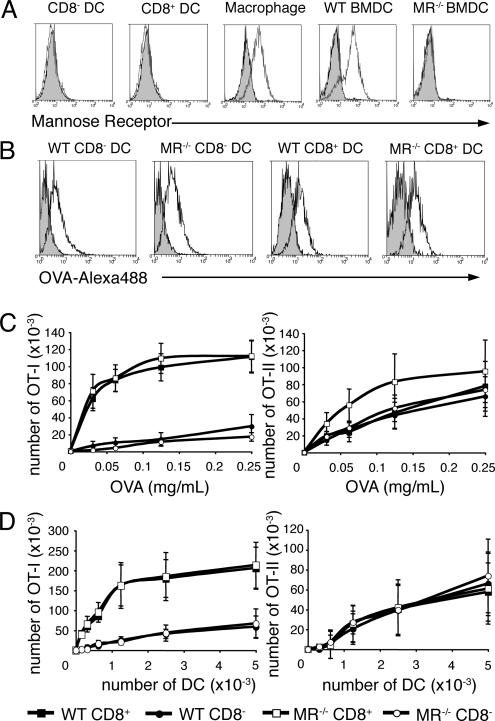
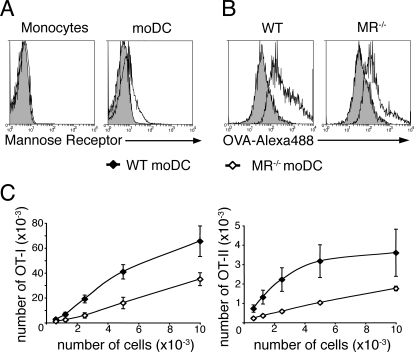
We then addressed the role of MR in splenic DC subsets. As reported previously (15), MR was not detected on splenic CD8+ or CD8− DC, although it was detected on macrophages copurified from the same spleens (Fig. 2A). By contrast, MR was highly expressed by bone-marrow derived DC (BM-DC) generated in the presence of GM-CSF (Fig. 2A), consistent with a previous report (10). However, we could not discard the possibility that MR was expressed on splenic DC at low levels that were undetectable by flow cytometry. To definitively address the requirement for MR for uptake and presentation of soluble OVA by splenic DC, we analyzed purified CD8+ and CD8− DC from WT or MR−/− mice. DC were incubated with OVA-Alexa488 and OVA uptake was assessed by flow cytometry (Fig. 2B). MR−/− and WT DC subsets internalized similar amounts of OVA. CD8+ and CD8− DC were also incubated with OVA, washed, and cocultured with CFSE-labeled transgenic OVA-specific OT-I or OT-II T cells (Fig. 2C). MR−/− DC were as efficient as WT DC at inducing T-cell proliferation, both for cross-presentation and MHC II-restricted presentation. To rule out the possibility that the binding of MR to its ligand was affected by our splenic DC purification process, we injected OVA intravenously into WT or MR−/− mice and splenic DC were purified 3 h later to assess ex vivo presentation. We obtained similar results (Fig. 2D), demonstrating that MR is not required for uptake and presentation of soluble OVA by splenic DC.
Fig. 2.
The mannose receptor is dispensable for presentation of OVA by splenic dendritic cells. (A) Splenocytes were stained for CD11c, MR, CD11b, and either F4/80 or CD8. MR expression is shown for CD8− DC (gated as CD11c+CD11b+CD8−), CD8+ DC (gated as CD11c+CD11b−CD8+), and splenic macrophages (gated as CD11c−CD11b+F4/80+). Bone marrow-derived DC (BMDC) from GM-CSF cultures of wild-type or MR−/− bone marrow were stained for MR. Gray shaded histograms represent control isotype staining. (B) CD8+ or CD8− DC from wild-type or MR−/− mice were incubated with OVA-Alexa488 for 30 min at 4 °C (gray shaded histogram) or 37 °C (black histogram) and analyzed by flow cytometry. (C) Purified wild-type or MR−/− CD8+ and CD8− DC were incubated with soluble OVA, washed and cultured with CFSE-labeled OT-I or OT-II T cells. T-cell proliferation was assessed by flow cytometry after 3 days of culture. The number of OT-I or OT-II cells that have undergone at least one division is shown (mean ± SEM of four independent experiments). (D) Wild-type or MR−/− mice were injected i.v with 2 mg OVA and DC were isolated 3 h later. Serial dilutions of sorted CD8+ or CD8− DC were cultured with CFSE-labeled OT-I or OT-II T cells and T-cell proliferation was assessed by flow cytometry after 3 days of culture. The number of OT-I or OT-II cells that have undergone at least one division is shown (mean ± SEM, n = 3 in three independent experiments).
OVA Is Not Stored in IRAP-Containing Endosomal Compartments in Splenic DC.
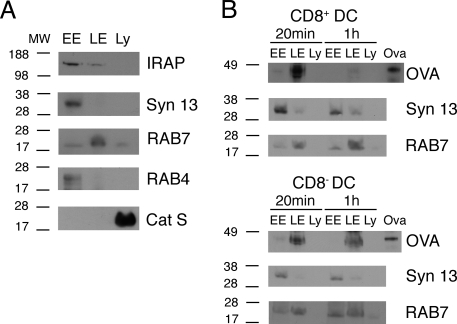
We then investigated whether exogenous antigen is sequestered and stored in early endosomal compartments in cross-presenting splenic DC. Trafficking of soluble OVA along the endocytic route was examined by subcellular fractionation. CD8+ and CD8− DC were incubated with OVA, washed, and subcellular fractions were prepared at different time points. As shown by the presence of classical markers, intracellular organelles were separated into early and recycling endosomes [RAB4+ syntaxin13+ fraction (16)], late endosomes (RAB7+ fraction) and lysosomes (Cathepsin S+ fraction) (Fig. 3A). IRAP was predominantly detected in the early and recycling endosomes fraction (Fig. 3A), suggesting that this fraction contained the proposed cross-presenting early endosomal compartment (12). However, we did not observe any accumulation of internalized OVA in early endosomes in CD8+ DC and there was no major difference in the trafficking of OVA between CD8+ and CD8− DC (Fig. 3B). These results argue against the diversion and storage of OVA into specialized cross-presenting endosomal compartments in splenic CD8+ DC.
Fig. 3.
Internalized OVA does not accumulate in IRAP-containing endosomes in splenic dendritic cells. (A) Subcellular fractions containing early endosomes (EE), late endosomes (LE) or lysosomes (Ly) were prepared from splenic CD8+ DC. Fractions were analyzed by Western blot for the presence of RAB4, RAB7, cathepsin S (Cat S), syntaxin 13 (Syn 13), or IRAP. (B) Splenic CD8+ or CD8− DC were incubated with soluble OVA for 45 min, washed, and further incubated for 20 min or 1 h. Subcellular fractions were prepared and the presence of OVA was analyzed by Western blot. Syntaxin 13 and RAB7 stainings are shown as a loading control. Results are representative of three independent experiments.
IRAP and MR Are Involved in Cross-Presentation of Soluble OVA by moDC.
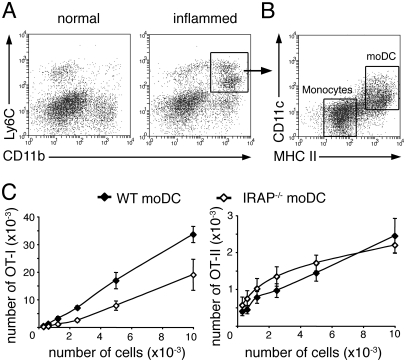
Most of the experiments supporting a model for cross-presentation occurring in specialized early endosomes have been conducted on in-vitro-generated BM-DC, and our results show that these findings do not apply to CD8+ DC. Because BM-DC are thought to be the in vitro equivalent of inflammatory moDC (17, 18), we explored the relevance of this cross-presentation model in in-vivo-generated moDC. To obtain these cells, we used a model of GM-CSF dependent inflammatory peritonitis that allows the differentiation of monocytes into moDC in the spleen (19, 20). The spleen of inflamed mice contained a population of Ly6C+CD11b+ cells that was absent from normal spleen (Fig. 4A). Within this population, moDC were characterized by high expression of MHC class II molecules and intermediate levels of CD11c (20, 21) (Fig. 4B). To assess the role of IRAP in antigen presentation by moDC, inflammatory peritonitis was induced in wild-type or IRAP−/− animals and mice were injected intravenously with soluble OVA. Splenic moDC were isolated 3 h later and cocultured with CFSE-labeled OT-I or OT-II T cells. Cross-presentation of soluble OVA was impaired in IRAP−/− moDC, but MHC II-restricted presentation was not affected (Fig. 4C) indicating that IRAP is involved in cross-presentation by moDC. We also sought to address the role of MR in OVA presentation by moDC. Unlike monocytes, in-vivo-generated moDC expressed MR (Fig. 5A). MR has been shown to mediate the internalization of OVA by BM-DC (10), so we analyzed the role of MR in OVA uptake by incubating splenocytes from wild-type or MR−/− inflamed mice with OVA-Alexa488. Internalization of OVA was decreased in MR−/− moDC as compared to wild-type moDC (Fig. 5B). Consistent with diminished uptake, cross-presentation and MHC II-restricted presentation of OVA were impaired in MR−/− moDC (Fig. 5C). These results show that MR is involved in the uptake and presentation of OVA by moDC.
Fig. 4.
IRAP is involved in cross-presentation of OVA by monocyte-derived dendritic cells. Inflammatory peritonitis was induced in mice by two successive intradermal injections of mBSA emulsified in CFA followed by i.p. injection of mBSA. (A) Splenocytes from normal or inflamed mice were stained for Ly6C and CD11b. (B) Ly6C+CD11b+ cells from inflamed mouse spleen were analyzed for their expression of CD11c and MHC class II molecules. (C) Inflamed wild-type or IRAP−/− mice were injected i.v with 2 mg OVA and splenocytes were harvested 3 h later. Serial dilutions of purified monocyte-derived DC were cultured with CFSE-labeled OT-I or OT-II T cells and T-cell proliferation was assessed by flow cytometry after 3 days of culture. The number of OT-I or OT-II cells that have undergone at least one division is shown (mean ± SEM, n = 6 in two independent experiments).
Fig. 5.
The mannose receptor is involved in uptake of OVA by monocyte-derived dendritic cells. (A) Splenocytes from inflamed mice were stained for Ly6C, CD11b, CD11c, MHC class II molecules, and MR. MR expression is shown for monocytes (gated as Ly6C+CD11b+MHCII−CD11c−) and monocyte-derived DC (moDC) (gated as Ly6C+CD11b+MHCII+CD11c+). Gray shaded histograms represent control isotype staining. (B) Splenocytes from wild-type or MR−/−inflamed mice were incubated with OVA-Alexa488 for 30 min at 4 °C (gray shaded histogram) or 37 °C (black histogram) and analyzed by flow cytometry. Alexa488 fluorescence is shown for monocyte-derived DC. (C) Inflamed wild-type or MR−/− mice were injected i.v with 2 mg OVA and splenocytes were harvested 3 h later. Serial dilutions of purified monocyte-derived DC were cultured with CFSE-labeled OT-I or OT-II T cells and T-cell proliferation was assessed by flow cytometry after 3 days of culture. The number of OT-I or OT-II cells that have undergone at least one division is shown (mean ± SEM, n = 5 in three independent experiments).
Discussion
Cross-presentation by DC plays a crucial role in anti-viral and anti-tumor immunity (cross-priming) and in the inactivation of autoreactive T cells (cross-tolerance) (22). Moreover, vaccine formulations designed to target cross-presenting DC can result in tolerance or immunity (23, 24), providing opportunities for clinical exploitation of this pathway. Cross-presentation can also contribute to disease, allowing DC to induce autoimmunity, hyperresponsiveness, graft rejection, tumor immunoescape, and immunopathology (22). To fully characterize the role of cross-presentation in vivo, identify potential targets for pharmacological intervention and design effective vaccines, it is important to define which DC type(s) mediate this function and which mechanisms are involved. In this manuscript we demonstrate that the endosomal cross-presentation pathway requiring MR and IRAP is functional in inflammatory moDC but not in steady-state CD8+ DC.
Our results are largely in agreement with previous reports on the role of IRAP and MR in cross-presentation, which examined primarily BM-DC (10–12), but some discrepancies were also apparent. First, Saveanu et al. reported a small, albeit significant, reduction in the number of endogenous CD8 T cells that responded to cross-presented OVA in IRAP−/− mice as compared to control mice (12), whereas we did not detect significant differences (Fig. 1E). We also compared the induction of CTL activity by cross-priming in WT and IRAP−/− mice, which supported our conclusion that IRAP played little or no role in cross-priming by steady-state DC (Fig. 1D). Second, the report by Burgdorf et al. (11) did not detect reduced MHC II presentation of soluble OVA by MR−/− DC, but we did (Fig. 5C). These discrepancies may be due to subtle differences in the genetic background of the mice used or in the protocols used by each laboratory.
Although multiple types of DC and non-DC are capable of cross-presentation under some circumstances (22, 25), the resident CD8+ DC subtype has been shown to play the dominant part in cross-priming against multiple viruses, bacteria and tumours, and in cross-tolerance (3, 26). Furthermore, vaccines targeting this subtype are more effective at inducing CTL responses by cross-priming than those targeting other subtypes (24, 27). The importance of CD8+ DC for cross-priming in vivo has been dramatically demonstrated with the analysis of mice deficient in the transcription factor Batf3, which lack CD8+ DC and are severely impaired in their capacity to elicit immune responses by cross-priming (2). Since our results indicate that the MR/IRAP-dependent mechanism of cross-presentation is not operative in CD8+ DC, our results suggest that pharmacological manipulation of this mechanism, or vaccines designed to exploit this pathway, would not work in steady-state conditions. It is during infection or inflammation, situations in which moDC are recruited to the infected or inflamed site and have been shown to contribute to cross-priming (6, 7), that the IRAP/MR-dependent pathway most likely plays a role in vivo. Although the human equivalent of mouse CD8+ DC has yet to be characterized, it is worth noting that human splenic DC seem to lack expression of MR (28) while human moDC have been shown to express MR (29), which can serve for uptake and presentation of antigen (30). These observations suggest that our findings in the mouse system have a correlate in the human dendritic cell network.
MoDC lacking MR or IRAP were not completely impaired in their capacity to cross-present, suggesting that they possess at least another mechanism, in addition to the endosomal pathway, to carry out this function. Presumably this is the same mechanism used by the CD8+ DC, namely the cytosolic pathway. Indeed, some of the mechanisms that are thought to play a role in cross-presentation are found in both moDC and CD8+ DC. For instance, in these two DC types endosomal compartments containing captured antigens are acidified slowly, favoring cross-presentation, and this mechanism is not found in noncross-presenting CD8− DC (31, 32). Further studies of this pathway in CD8+ DC will be required to fully characterize the mechanisms of cross-presentation under steady-state conditions in vivo, which may yield clues for the development of approaches for pharmacological intervention or vaccination.
Methods
Mice.
C57BL 6 (B6), B6.CH-2bm 1 (bm1), OT-I (33), OT-II (34), mannose receptor deficient (35), IRAP deficient mice (36), and wild-type littermates were bred and maintained under specific pathogen-free conditions at The Walter and Eliza Hall Institute or Howard Florey Institute animal breeding facilities according to institute guidelines. Where indicated, B6 mice were injected s.c. with 5 × 106 B16 melanoma cells secreting murine Fms-like tyrosine kinase ligand (Flt3-L) (37) and killed after 9–10 days.
Induction of Inflammatory Peritonitis.
Induction of inflammatory peritonitis was performed as described in ref. 19. Briefly, mice were injected intradermally at the base of the tail with 100 μg of mBSA (Sigma–Aldrich) emulsified in an equal volume of CFA containing 5 mg/mL heat-killed Mycobacterium tuberculosis (H37 Ra; Difco Laboratories) and were subjected 2 weeks later to the same treatment. Seven days later, mice were injected intraperitonally with 100 μg mBSA in PBS for the induction of inflammation. Mice were used 2 days later.
Cell Isolation.
OT-I and OT-II T cells were isolated from the lymph nodes of OT-I or OT-II transgenic mice as described in ref. 38. Total DC from normal mouse spleens were isolated as described (39, 40). Light-density splenocytes from inflamed mouse were isolated after digestion of the spleens with DNase I (0.1%, Roche Molecular Biochemicals) and collagenase (1 mg/mL, type II, Worthington Biochemical) and centrifugation in Nycodenz medium (density 1.082 g/cm3, 1700 × g for 10 min). Cells were further purified by cell sorting with a FACS Aria (Becton Dickinson) or Mo-Flo (Cytomation) instrument. For fractionation experiments, total DC were prepared from the spleens of Flt3-L treated mice and CD8+ DC were further isolated by positive selection using immuno-magnetic beads (MACS, Miltenyi Biotec) after staining with anti-CD8 (YTS 169.4) antibody. Remaining cells were depleted of CD205+CD24high DC precursors (38) after staining with anti-CD205 (NLDC-145) and anti-CD24 (M1/69) antibodies and MACS beads depletion, and finally CD8− DC were isolated by positive selection using MACS beads after staining with anti-CD11b (M1/70) antibody.
BM-DC Culture.
After lysis of red blood cells, bone marrow cells were cultured for 7 days in RPMI medium 1640 supplemented with 10% FCS, 50 μM 2-mercaptoethanol, 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin (DC medium) in the presence of 20 ng/mL GM-CSF (PeproTech).
Subcellular Fractionation.
DC were incubated with OVA (1 mg/mL) for 45 min at 37 °C in DC medium. After three washes in PBS, cells were further incubated for 20 min or 1 h at 37 °C in DC medium. Cells were homogenized with a cell-cracker (HGM Laboratory Equipment) in homogenization buffer [PBS, 0.25 M sucrose, 10 mM Tris, 1 mM EDTA supplemented with proteases inhibitors (Roche), pH 6.8)]. Postnuclear supernatant was prepared by centrifugation (1,000 × g for 10 min) and loaded on top of a 10% Percoll (GE Healthcare) solution in homogenization buffer. After ultracentrifugation (50,000 × g for 45 min), 1 mL fractions were collected. The top three fractions (early endosomes) were pooled and concentrated by ultracentrifugation (130,000 × g for 1 h). The bottom two fractions were pooled and loaded on top of a 45% Percoll solution in homogenization buffer. After ultracentrifugation (50,000 × g for 45 min), the top three fractions (late endosomes) ant the bottom two fractions (lysosomes) were pooled and concentrated by ultracentrifugation (130,000 × g for 1 h).
Western Blot Analysis.
Total cell lysates were run on a 4–12% SDS/PAGE gel (Invitrogen) and transferred onto nitrocellulose membrane. Membranes were stained with serum against mouse actin (Sigma), IRAP (41), antibodies against mouse tubulin (Abcam), syntaxin 13 (15G2, Stressgen), or polyclonal antibodies against RAB4 (Stressgen) or Cathepsin S (M-19, Santa Cruz). Horseradish peroxidase (HRP)–conjugated anti-rabbit IgG antibody (Sigma), anti-mouse IgG (Pierce), anti-goat IgG (Abcam) were used for protein detection.
Flow Cytometry Analysis.
The following antibodies were used and produced in-house unless otherwise indicated: anti-CD11c (N418), CD8 (YTS 169.4), CD4 (GK1.5), CD11b (M1/70), CD45.1 (A20–1.1), MHC class II (M5/114), F4/80, Ly6C (5075–3.6), H-2Kb gB497–505 tetramer, mannose receptor (MR5D3, AbD Serotec), and Vα2 (B20.1, BD). Cells were labeled with propidium iodide (PI) to assess viability and analyzed on a FACSCalibur or LSR instrument (Becton Dickinson). Flow cytometry data were analyzed with the in-house software WEASEL.
Antigen-Presentation Assay.
For in vitro presentation of soluble OVA (Sigma), splenic DC populations (5 × 103 cells per well) were incubated in 96-well plates (Costar-Corning) with different concentrations of OVA at 37 °C in DC medium. After 45 min, cells were washed twice in DC medium. For presentation of cell-associated antigen, bm1 spleen cells were γ-irradiated (1500 rads), washed, incubated with 10 mg/mL OVA in RPMI medium 1640 for 10 min at 37 °C, and washed three times with RPMI medium 1640 supplemented with 3% FCS. Splenic DC populations (25 × 103 cells per well) were plated with different numbers of bm1 OVA-coated splenocytes in DC medium. For ex vivo presentation, mice were injected intravenously with 2 mg of OVA. After 3 h, splenic DC populations were sorted and different numbers of DC were plated in 96-well plates in DC medium. Fifty thousand CFSE-labeled OT-I or OT-II cells were added in each well in DC medium supplemented with 10 ng/mL GM-CSF. Proliferation was analyzed by flow cytometry after 60–65 h of culture as described in ref. 40. Each determination was performed in duplicate.
In Vivo CTL Assay.
WT or IRAP−/− mice were immunized intravenously with 20 × 106 OVA-coated bm1 splenocytes and LPS (Sigma) (1 μg/mouse). Control WT mice were injected with 20 × 106 uncoated bm1 splenocytes and LPS (1 μg/mouse). Five days later, mice were injected intravenously with 5 × 106 CD45.1+ splenocytes that had been pulsed with 33 ng/mL SIINFEKL peptide (Genscript) for 40 min and labeled with 2.5 μM CFSE, and 5 × 106 unpulsed CD45.1+ splenocytes labeled with 0.25 μM CFSE. The next day, spleens were analyzed for the presence of CD45.1+ target cells.
OVA Uptake Assay.
OVA was conjugated to Alexa488 using Alexa488-labeling kit (Invitrogen) according to manufacturer's instructions. Cells were incubated with 30 μg/mL OVA-Alexa488 in DC medium for 30 min either on ice or at 37 °C. Cells were washed three times, then stained for FACS analysis.
Acknowledgments.
We thank Annemarie van Nieuwenhuijze for help with the inflammatory peritonitis experiments and Michel Nussenzweig for providing MR-deficient mice. This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (to J.A.V.), the Leukemia and Lymphoma Society (to J.A.V.), a NHMRC Fellowship (to S.Y.C.) and Marie Curie Fellowships from the European Commission (to E.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segura E, Villadangos JA. Antigen presentation by dendritic cells in vivo. Curr Opin Immunol. 2009;21:105–110. doi: 10.1016/j.coi.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 5.Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. ImmunolCell Biol. 2008;86:320–324. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- 6.Le Borgne M, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 8.Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86:353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 9.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 11.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 12.Saveanu L, et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 2009;325:213–217. doi: 10.1126/science.1172845. [DOI] [PubMed] [Google Scholar]
- 13.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 14.Lin ML, et al. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proc Natl Acad Sci USA. 2008;105:3029–3034. doi: 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linehan SA, Martinez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 19.Cook AD, Braine EL, Hamilton JA. Stimulus-dependent requirement for granulocyte-macrophage colony-stimulating factor in inflammation. J Immunol. 2004;173:4643–4651. doi: 10.4049/jimmunol.173.7.4643. [DOI] [PubMed] [Google Scholar]
- 20.Naik SH, et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 21.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 23.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shortman K, Lahoud MH, Caminschi I. Improving vaccines by targeting antigens to dendritic cells. Exp Mol Med. 2009;41:61–66. doi: 10.3858/emm.2009.41.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 27.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 28.Pack M, et al. DEC-205/CD205+ dendritic cells are abundant in the white pulp of the human spleen, including the border region between the red and white pulp. Immunology. 2008;123:438–446. doi: 10.1111/j.1365-2567.2007.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avrameas A, et al. Expression of a mannose/fucose membrane lectin on human dendritic cells. Eur J Immunol. 1996;26:394–400. doi: 10.1002/eji.1830260219. [DOI] [PubMed] [Google Scholar]
- 30.Newman SL, Holly A. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect Immun. 2001;69:6813–6822. doi: 10.1128/IAI.69.11.6813-6822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savina A, et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 36.Albiston AL, et al. Gene knockout of insulin-regulated aminopeptidase: Loss of the specific binding site for angiotensin IV and age-related deficit in spatial memory. neurobiology of learning and memory. 2009 doi: 10.1016/j.nlm.2009.07.011. In press. [DOI] [PubMed] [Google Scholar]
- 37.Mach N, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 38.Bedoui S, et al. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J Immunol. 2009;182:4200–4207. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- 39.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 40.Wilson NS, et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 41.Fernando RN, Larm J, Albiston AL, Chai SY. Distribution and cellular localization of insulin-regulated aminopeptidase in the rat central nervous system. J Comp Neurol. 2005;487:372–390. doi: 10.1002/cne.20585. [DOI] [PubMed] [Google Scholar]