Abstract
Currently, treatment with the relatively low-affinity NMDA receptor antagonist memantine provides limited benefit in Alzheimer's disease (AD). One probable dose-limiting factor in the use of memantine is the inhibition of NMDA receptor-dependent synaptic plasticity mechanisms believed to underlie certain forms of memory. Moreover, amyloid-β protein (Aβ) oligomers that are implicated in causing the cognitive deficits of AD potently inhibit this form of plasticity. Here we examined if subtype-preferring NMDA receptor antagonists could preferentially protect against the inhibition of NMDA receptor-dependent plasticity of excitatory synaptic transmission by Aβ in the hippocampus in vivo. Using doses that did not affect control plasticity, antagonists selective for NMDA receptors containing GluN2B but not other GluN2 subunits prevented Aβ1–42 -mediated inhibition of plasticity. Evidence that the proinflammatory cytokine TNFα mediates this deleterious action of Aß was provided by the ability of TNFα antagonists to prevent Aβ1–42 inhibition of plasticity and the abrogation of a similar disruptive effect of TNFα using a GluN2B-selective antagonist. Moreover, at nearby synapses that were resistant to the inhibitory effect of TNFα, Aβ1–42 did not significantly affect plasticity. These findings suggest that preferentially targeting GluN2B subunit-containing NMDARs may provide an effective means of preventing cognitive deficits in early Alzheimer's disease.
Keywords: Alzheimer's disease, amyloid-β protein oligomers, glutamate
Glutamatergic processes are strongly implicated in causing and mediating the symptoms of Alzheimer's disease (AD) (1). Early studies found that AD-associated amyloid β-protein (Aβ) promoted glutamatergic excitotoxicity. More recently Aβ was discovered to form soluble oligomers that rapidly and potently disrupt glutamatergic synapses and plasticity mechanisms underlying cognitive function, including long-term potentiation (LTP), in the absence of cell death, providing an explanation for the cognitive deficits in AD (2–4).
Apart from anticholinesterases, memantine, a low-affinity NMDA receptor (NMDAR) antagonist (5), is the only currently approved treatment for clinical dementia of the Alzheimer type. Although memantine can partially protect against Aβ-mediated disruption of LTP at synapses that requires NMDAR activation for its induction, it also inhibits LTP over an overlapping dose range, presumably because of a relatively poor discrimination between antagonism of physiological and disruptive NMDAR activation (6). Newer subtype selective NMDAR antagonists (7) potentially could increase the dose range over which a beneficial effect is obtained if the LTP-disrupting actions of Aβ and of NMDAR antagonists are preferentially mediated by different NMDARs. Indeed the GluN2B (formerly known as NR2B or NMDA-R2B) (8) subunit has been implicated in regulating the actions and localization of Aβ oligomers, and Aβ oligomers have been reported to promote endocytosis of GluN2B-containing receptors (9–13), whereas both synaptic GluN2A- and GluN2B- containing NMDARs play key roles in LTP induction (14–17). On the other hand, in cultured cells expressing cloned NMDARs, Aβ-induced effects can be selectively mediated through GluN2A over GluN2B subunits (18) and memantine can preferentially block GluN2C/D- over GluN2A/B-containing NMDARs (19, 20), but see ref. 21.
In the light of these findings we postulated that protection against Aβ inhibition of NMDAR-dependent LTP might be achieved with doses of GluN2 subtype selective agents below the threshold for impairing such plasticity on their own. Furthermore, since deleterious effects of Aß in vitro are dependent on TNFα action (22) and NMDAR-TNFα synergism (23) we also investigated TNFα's role in the synaptic plasticity impairing effects of Aß in vivo.
Results
Abrogation of Aβ-Mediated Disruption of Hippocampal Synaptic Plasticity in Vivo by Antagonists Selective for GluN2B-Containing NMDARs.
The role of different NMDAR subtypes in mediating the inhibitory effect of Aβ on high frequency stimulation (HFS) induction of LTP at hippocampal CA1 synapses was assessed in vivo, using antagonists for different GluN2 subunits. We compared the effect of the antagonist NVP-AAM077 with approximately 10-fold selectivity for GluN2A over GluN2B and approximately 2-fold over GluN2C/D, the antagonist ifenprodil which has > approximately 200-fold selectivity for GluN2B over other GluN2 subunits, and the antagonist UBP141 with > approximately 5-fold selectivity for GluN2C/D over GluN2A/B (7, 24). First we titrated the agents against LTP to find doses that were approximately half the threshold for inhibition of NMDAR-dependent synaptic plasticity (Fig. S1). Intracerebroventricular injection of NVP-AAM077 (125 pmol, 129.5 ± 4.3% pre-HFS mean baseline EPSP amplitude ± SEM, at 3 h post-HFS, n = 5), ifenprodil (3 nmol, 133.9 ± 5.3%, n = 5) or UBP141 (6.25 nmol, 133.8 ± 6.5%, n = 4) had no significant effect alone on LTP induction (P > 0.05 compared with vehicle-injected controls; P < 0.05 compared with baseline; two-way ANOVA with repeated measures and paired Student's t tests) (Fig. 1). Importantly, using these relatively low doses, of the three compounds tested only the GluN2B-selective agent ifenprodil prevented the inhibition of LTP by soluble Aβ1–42. In animals that were coinjected with ifenprodil and Aβ1–42 (80 pmol, i.c.v.), the conditioning HFS induced LTP (125.7 ± 6.5%, n = 6, P < 0.05 compared with baseline; P < 0.05 compared with Aβ1–42 alone, 102.1 ± 2.2%, n = 6) that was similar in magnitude to vehicle-injected controls (133.1 ± 5.5%, n = 6; P > 0.05). In contrast, coinjection of Aβ1–42 with the GluN2A-selective NVP-AAM077 (125 pmol i.c.v.) (98.6 ± 2.6%, n = 6; P > 0.05 compared with Aβ1–42 -treated animals) or the GluN2C/D preferring UBP141 (6.25 nmol i.c.v.) (106.0 ± 6.1%, n = 4; P > 0.05 compared with Aβ1–42 treated animals) completely inhibited LTP (P > 0.05 compared with pre-HFS baseline). Similar results were obtained when the higher doses of NVP-AAM077 (250 pmol, n = 4) and UBP141 (12.5 nmol, n = 4) that inhibited LTP on their own, were injected before Aß1–42 (Fig. 2A and Fig. S1).
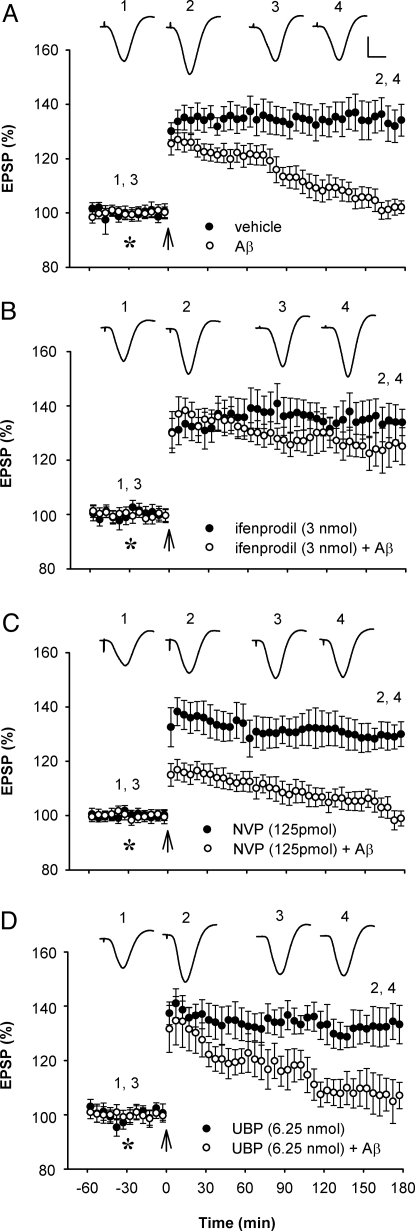
Fig. 1.
Low-dose NMDAR antagonist selective for GluN2B but not GluN2A or GluN2C/D subunits abrogates Aβ1–42-mediated inhibition of LTP in vivo. (A) Intracerebroventricular (i.c.v., asterisk) injection of soluble Aβ1–42 (80 pmol) inhibited high frequency stimulation (arrow) -induced LTP (n = 6; P < 0.05 compared with vehicle, n = 6; P > 0.05 compared with baseline; two-way ANOVA with repeated measures and paired t tests). (B) A low dose (3 nmol, i.c.v.) of the GluN2B selective antagonist ifenprodil that did not affect LTP on its own (n = 5), prevented the inhibition of LTP by Aβ1–42 (n = 6; P < 0.05 compared with Aβ1–42 alone). (C) A relatively low dose (125 pmol, i.c.v.) of the GluN2A selective antagonist NVP-AAM077 that did not affect LTP on its own (n = 5), failed to prevent the inhibition of LTP by Aβ1–42 (n = 6; P > 0.05). (D) Similarly, a relatively low dose (6.25 nmol, i.c.v.) of the GluN2C/D selective antagonist UBP141 that did not affect LTP on its own (n = 4), failed to prevent the inhibition of LTP by Aβ1–42 (n = 4; P > 0.05). Values are the mean percentage of pre-HFS baseline EPSP amplitude (±SEM). Insets show representative EPSP traces at the times indicated. Calibration bars: vertical, 2 mV; horizontal, 10 ms.
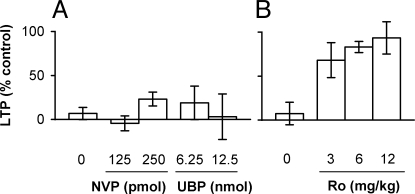
Fig. 2.
Dose-dependence of the effects of subtype-selective NMDAR antagonists on the inhbition of LTP by Aβ1–42. (A) Neither pretreatment with the GluN2A antagonist NVP-AAM077 (125 pmol, n = 5; and 250 pmol, n = 4, i.c.v.) nor the GluN2C/D antagonist UBP141 (6.25, n = 4; and 12.5 pmol, n = 4, i.c.v.) significantly affected the inhibition of LTP by Aβ1–42 (80 pmol, i.c.v., n = 6 for Aβ1–42 alone) (P > 0.05, one-way ANOVA). (B) In contrast, pretreatment with the GluN2B antagonist Ro 25–6981 (3 mg/kg, n = 4; 6 mg/kg, n = 6; and 12 mg/kg, n = 4, i.p.) significantly (P < 0.05) reduced the Aβ1–42-mediated inhibition of LTP (n = 7 for Aβ1–42 alone). LTP values are expressed as the mean (±SEM) % control magnitude of LTP at 3 h after high frequency conditioning stimulation.
Having found that the inhibition of LTP by Aβ1–42 was prevented by ifenprodil but not NVP-AAM077 or UBP141, we next assessed the ability of systemic treatment with the NMDAR antagonist Ro 25–6981, which has a >3,000-fold selectivity for GluN2B over other GluN2 subunits, and which has a much higher selectivity than ifenprodil for NMDARs (7, 25), to prevent the effect of Aβ1–42. Systemic injection of Ro 25–6981 (6 mg/kg, i.p.) 60 min before the HFS completely prevented the inhibition of LTP caused by Aβ1–42 (80 pmol, i.c.v.) (125.9 ± 2.0%, n = 6; P < 0.05 compared with Aβ alone, 102.3 ± 4.0%, n = 7; P > 0.05 compared with vehicle controls, 131.2 ± 3.0%, n = 5; P < 0.05 compared with baseline) (Fig. 3). Injection of this dose of Ro 25–6981 alone had no significant effect on LTP (129.0 ± 7.5%, n = 5; P > 0.05 compared with vehicle controls; P < 0.05 compared with baseline). Further experiments in animals pretreated with either a lower (3 mg/kg, n = 4) or higher (12 mg/kg, n = 4) dose of Ro 25–6981 indicated that the prevention of the inhibitory effect of Aβ1–42 by Ro 25–6981 was dose-dependent in this dose range (Fig. 2B). By way of comparison, we also assessed the effects of doses of memantine in combination with Aβ1–42 above and below that tested previously (6) (Fig. S2)
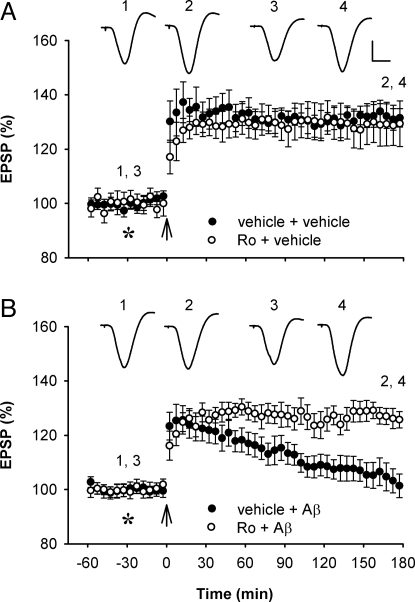
Fig. 3.
Systemic treatment with the GluN2B subunit-selective NMDAR antagonist Ro 25–6981 prevents Aβ1–42-mediated inhibition of LTP. (A) Systemic administration of Ro 25–6981 (6 mg/kg, i.p.) did not significantly affect LTP (n = 5; P > 0.05 compared with vehicle-injected controls, n = 5). (B) Pretreatment with Ro 25–6981 prevented the inhibition of LTP caused by Aß1–42 (80 pmol, i.c.v., asterisk) (n = 7; P < 0.05 compared with Aβ1–42 alone, n = 6). Values are the mean percentage of pre-HFS baseline EPSP amplitude (±SEM). Calibration bars for EPSP traces: vertical, 2 mV; horizontal, 10 ms.
Prevention of the Disruptive Effects of Aß on Synaptic Plasticity by Agents That Reduce TNFα Availability.
Because the inhibitory effect of Aβ on LTP in vitro is dependent on endogenous release of TNFα (22), we hypothesized that the GluN2B-dependence of LTP inhibition may be indirectly mediated through TNFα. We examined the effects of agents that reduce TNFα availability (infliximab, a chimaeric IgG1κ monoclonal antibody, and a TNFα peptide antagonist with specific and high affinity binding to TNFα) or production (the CNS penetrant inhibitor thalidomide). Injection of either infliximab (25 μg in 5 μL, i.c.v.) or the TNFα peptide antagonist (2 nmol in 5 μL, i.c.v.) 10 min before Aβ1–42 completely prevented the inhibition of LTP (125.3 ± 1. 4%, n = 5, and 126.7 ± 2.5%, n = 5, respectively, P < 0.05 compared with 99.5 ± 7.2% after Aβ1–42 alone; P > 0.05 compared with 130.5 ± 4% in vehicle-treated animals; P < 0.05 compared with baseline) using doses that alone did not significantly affect the magnitude of LTP (128.8 ± 3.9%, n = 4, and 129.9 ± 2.1%, n = 5, respectively, P < 0.05 compared with baseline; P > 0.05 compared with vehicle) (Fig. S3). Similarly, systemic administration of a dose of thalidomide (45 mg/kg, i.p.) that did not significantly affect LTP induction alone (124.6 ± 2.9%, n = 6, P > 0.05 compared with vehicle; P < 0.05 compared with baseline), abrogated the inhibition of LTP caused by Aβ1–42 (128.5 ± 8.2%, n = 4; P < 0.05 compared with Aß1–42 alone or baseline; P > 0.05 compared with vehicle). Because these findings support a requirement for TNFα in the inhibitory effect of Aβ, we next examined the effect of TNFα alone. Like Aβ, pretreatment with TNFα (1.5 pmol, i.c.v.) completely inhibited LTP (98.9 ± 3.4%, n = 5, P > 0.05 compared with baseline; P < 0.05 compared with vehicle, 130.5 ± 3.4% n = 8) (Fig. S4).
Differential Vulnerability of Apical and Basal Synapses to the Plasticity Disruptive Effects of TNFα and Aβ.
Previous studies using TNFR1 knockout mice indicate that deleterious TNFα-dependent effects of Aβ are mediated through TNFR1 (26), including inhibition of LTP by Aβ in the dentate gyrus of hippocampal slices (22). In view of known regional variations in the expression of TNFRs (27) and in different forms of LTP (28), we also examined the effects of TNFα and Aβ on LTP of synaptic transmission at basal dendrites in the stratum oriens. Administration of the same dose of TNFα that completely inhibited LTP at apical dendrites did not significantly affect LTP at basal dendrites. Thus, in animals that were administered an i.c.v. injection of TNFα (1.5 pmol) the HFS induced LTP (136.4 ± 4.1%, n = 6; P < 0.05 compared with baseline) that was similar in magnitude to that found in vehicle-injected animals (143.4 ± 4.4%, n = 6; P < 0.05 compared with baseline; P > 0.05 compared with TNFα) (Fig. 4). Importantly, LTP at basal dendrites was also resistant to the inhibitory effect of Aβ1–42. Aß1–42 (320 pmol, i.c.v.) pretreatment did not significantly affect the magnitude of LTP (142.5 ± 5.8%, n = 6; P < 0.05 compared with baseline; P > 0.05 compared with vehicle). Similar to apical dendrite LTP (29), LTP induction at basal dendrites was NMDAR-dependent, being completely blocked by D-AP5 (100 nmol, i.c.v., 102.1 ± 5.0%, n = 6; P > 0.05 compared with baseline; P < 0.05 compared with vehicle).
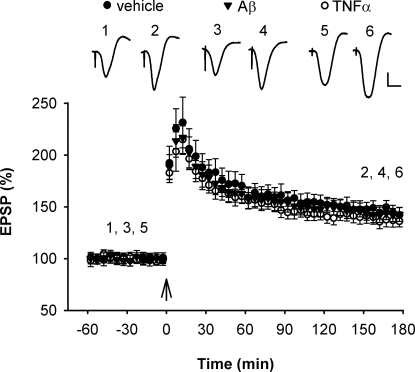
Fig. 4.
Resistance of LTP induction at basal dendrites to the inhibitory effect of TNFα and Aβ1–42. High frequency stimulation (arrows) induced robust LTP of synaptic transmission at basal dendrites of CA1 pyramidal cells in the stratum oriens of animals injected i.c.v. with either vehicle (5 μL, n = 6, P < 0.05) (closed circles), TNFα (1.5 pmol, n = 6, P < 0.05) (open circles) or Aß1–42 (320 pmol, n = 6, P < 0.05) (triangles). Values are the mean percentage of pre-HFS baseline EPSP amplitude (±SEM). Calibration bars for EPSP traces: vertical, 0.5 mV; horizontal, 10 ms.
GluN2B-Selective Antagonist Prevents the Inhibition of Synaptic Plasticity by TNFα.
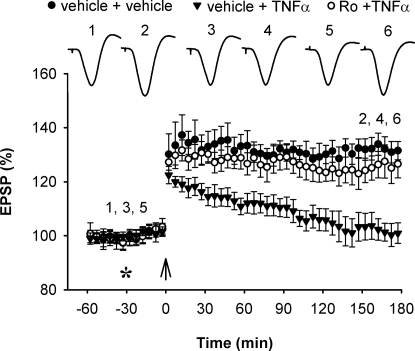
Because the inhibition of LTP by Aβ1–42 was dependent on GluN2B, we next assessed the ability of systemic treatment with Ro 25–6981 to prevent the inhibitory effect of TNFα. Whereas TNFα (1.5 pmol, i.c.v.) alone inhibited HFS-induced LTP (100.6 ± 3.6%, n = 5; P < 0.05 compared with vehicle; P > 0.05 compared with baseline), it failed to inhibit LTP in animals that had been systemically pretreated with Ro 25–6981 (6 mg/kg, i.p.) (125.8 ± 5.0%, n = 6; P < 0.05 compared with baseline and compared with TNFα alone; P > 0.05 compared with vehicle) (Fig. 5).
Fig. 5.
Systemic treatment with the GluN2B subunit-selective NMDAR antagonist Ro 25–6981 prevents TNFα-mediated inhibition of LTP. Pretreatment with Ro 25–6981 prevented the inhibition of LTP caused by TNFα (1.5 pmol, i.c.v.) (n = 6; P < 0.05 compared with TNFα alone, n = 5; P > 0.05 compared with vehicle-injected controls, n = 5). Values are the mean percentage of pre-HFS baseline EPSP amplitude (±SEM). Calibration bars for EPSP traces: see Fig. 1.
Discussion
The present results clearly show that targeting GluN2B subunit-containing NMDARs with a selective antagonist prevents the synaptic plasticity-disrupting effects of Aβ in vivo. Importantly, protection was achieved using systemic treatment with a dose below that affecting normal synaptic plasticity. The additional findings that TNFα also inhibited LTP in a GluN2B-dependent manner and that the inhibitory effect of Aβ required TNFα action provide strong evidence of a critical role for TNFα promotion of activation of GluN2B-containing NMDARs in mediating the disruption of mechanisms underlying cognition by Aβ. The present results support further clinical investigation of the potential benefit of targeting both TNFα and GluN2B subunits in cognitive impairment, particularly in early AD.
NMDARs are normally composed of assemblies of two obligatory GluN1 with two GluN2 subunits, the latter consisting largely of GluN2A and GluN2B subtypes in the mammalian forebrain, including the mature rodent hippocampus (30). Depending on developmental stage and other as yet poorly defined factors, both GluN2A and GluN2B containing NMDARs have been shown to be critical in mediating NMDAR-dependent LTP at CA3-to-CA1 synapses (14–17, 31, 32). Remarkably and in contrast to the GluN2A- and GluN2C/D-subtype selective NMDAR antagonists NVP-AAM077 and UBP141, the GluN2B selective antagonists ifenprodil and Ro 25–6981 at concentrations that did not affect control LTP when administered alone, prevented the inhibition of LTP by Aβ1–42. This differential sensitivity to the different antagonists provides evidence for a selective role of GluN2B subunit-containing NMDARs in the synaptic plasticity-disrupting effects of Aβ in vivo.
The protection against the plasticity-disrupting action of Aβ by relatively low doses of the GluN2B-selective antagonists appears to be a significant advance on memantine, which only weakly abrogates the synaptic plasticity-disrupting action of Aβ (Fig. S2)(6). Systemic treatment with GluN2B selective agents including Ro 25–6981 has been reported to have minimal cognitive impairing effects at doses that are in the pharmacologically relevant range in rodents (33, 34) and man (35).
Excessive or inappropriate activation of NMDARs can block LTP (36), and under certain circumstances Aβ can selectively enhance NMDAR-mediated currents and synaptic transmission (18, 37–40), or promote increased Ca2+ influx and elevate the levels of potentially toxic reactive oxygen species in an NMDAR-dependent manner (37, 41). However, in contrast to the present findings, the inhibition of LTP by exogenous application of NMDA has been reported to be caused by Ca2+ entry following activation of GluN2A- rather than GluN2B-containing NMDARs (42). Intriguingly, in cultured neurons Aβ1–42 can increase GluN2B tyrosine phosphorylation and trigger an ifenprodil-sensitive transient activation of Akt (9). Activation of Akt causes GSK3ß phosphorylation, which can dramatically alter the induction of synaptic plasticity, including LTP (43, 44).
GluN2B subunits are found both synaptically and extrasynaptically and appear to be more mobile between these compartments than GluN2A subunits (45). Indeed, the anchoring and coupling of GluN2B subunits may differ at synaptic and extrasynaptic sites, with many apparently opposite or mutually exclusive effects on protein–protein interactions and signaling mechanisms that are known to be involved in regulating LTP induction (45, 46). If Aß acts preferentially to promote activation of extrasynaptic receptors these different properties of extrasynaptic NMDARs containing GluN2B subunits may account for the involvement of GluN2B subunits in the inhibition of LTP by Aβ. On the other hand evidence consistent with a synaptic action of Aβ comes from the finding that Aβ aggregates at synapses containing GluN2B subunits in an activity and ifenprodil-sensitive manner (10). Whether or not Aβ binds less efficiently to synapses lacking GluN2B subunits or if the larger aggregates are more disruptive to synaptic function, remains to be investigated.
While Aβ can directly bind to NMDARs or adjacent sites (41, 47, 48), the present data support a more indirect mechanism of promoting receptor activation. The findings that intracerebral or systemic treatment with agents that reduce free TNFα levels abrogated the inhibition of LTP in vivo by Aβ implicate TNFα in the synaptic plasticity-disrupting action of Aβ and confirm and extend previous in vitro studies (22, 49). Strong evidence supporting the importance of TNFα was the ability of i.c.v. injection of TNFα to mimic the inhibition of LTP by Aβ and the discovery that synapses that were resistant to the plasticity-disrupting effects of TNFα were equally resistant to the inhibitory effects of Aβ. Further corroboration is provided by the effectiveness of systemic treatment with the GluN2B selective NMDAR antagonist Ro 25–6981 to prevent this action of TNFα.
How might TNFα mediate the GluN2B-dependent action of Aβ? Aβ is known to trigger the release of TNFα (50, 51) and TNFα can increase extracellular glutamate concentration both by reducing glutamate transport into neurons and glia or by promoting glutamate release (52, 53). Consistent with this proposal Aβ also can inhibit glutamate uptake (11, 54) and enhance glutamate release (55–57) by glia and neurons although it is not known if TNFα is required for these effects. Since glutamate has a relatively high affinity for GluN2B subunit-containing NMDARs (58) and glutamate spillover can preferentially activate such receptors (59), this increase in glutamate concentration should cause an excessive or inappropriate activation of GluN2B subunit-containing NMDARs. Such indirect actions of Aβ via TNFα may synergize with more direct actions on the glutamatergic system (41, 47, 48).
Observations from studies of the brains of patients with AD are consistent with this general sequence of events. Thus, glutamate uptake is reduced (60, 61) and GluN2B-containing NMDAR distribution and density are abnormal (1). Moreover, TNFα and TNF receptors are extensively disrupted in AD (26, 62). Notably, elevated spontaneous TNFα production from peripheral mononuclear cells and increased soluble TNFR1 in cerebrospinal fluid in nondemented people are predictors of progress to clinical AD (63, 64). Although there are no clinically approved TNFα-based treatments for AD, in open-label trials perispinal administration of an antibody to TNFα has been reported to produce a rapid improvement in the cognitive status of patients (65). The present findings on the protective effect of administration of TNFα-neutralizing agents, including an antibody to TNFα, against the inhibition of LTP by Aβ in vivo provide support for controlled trials assessing this general approach. Because TNFα is considered a major factor in cognitive deficits both in AD and in other neurological and psychiatric illnesses (66) and TNFα disrupts the mechanisms underlying cognition in a GluN2B-dependent manner the development of systemic treatments with agents against these targets seems particularly attractive.
It is not clear why LTP at basal dendrites, in contrast to apical dendrites, is resistant to the inhibitory effects of Aβ and TNFα. Although both forms of LTP are NMDAR-dependent, LTP at basal synapses, unlike LTP at apical synapses, was characterized by the presence of a large initial decremental potentiation consistent with previous reports that this form of LTP has different properties (67–69). Interestingly, LTP at basal synapses does not use the same signaling mechanisms, some of which have been implicated in the synaptic plasticity disruptive actions of Aβ at apical synapses (68, 70–72). Since TNFR1 is essential for both Aβ- and TNFα- mediated inhibition of LTP (22), the potential differential regional expression of TNFRs and associated signaling mechanisms also warrants detailed investigation.
There is a growing realization of the involvement of aberrant excitatory activity in neuronal networks in the cognitive deficits of AD (73). The present in vivo data clearly support a mediating role for excessive GluN2B-containing NMDA receptor activation and the potential benefit of selectively blocking these receptors in AD.
Materials and Methods
Animals and Surgery.
Experiments were carried out on urethane (1.5–1.6 g/kg i.p.) -anesthetized male Wistar rats (250–300 g). The body temperature of the rats was maintained at 37 to 38 °C with a feedback-controlled heating blanket. The animal care and experimental protocol were approved by the Department of Health, Republic of Ireland.
Cannula Implantation.
A stainless-steel cannula (22 gauge, 0.7-mm outer diameter) was implanted above the right lateral ventricle (1 mm lateral to the midline and 4 mm below the surface of the dura). Intracerebroventricular (i.c.v.) injection was made via an internal cannula (28 gauge, 0.36-mm outer diameter). The solutions were injected in a 5-μL volume over a 3-min period. Verification of the placement of cannula was performed postmortem by checking the spread of ink dye after i.c.v. injection.
Electrode Implantation.
Electrodes were made and implanted as described in ref. 29. Briefly, twisted bipolar electrodes were constructed from Teflon-coated tungsten wires (62.5-μm inner core diameter, 75-μm external diameter). Field excitatory postsynaptic potentials (EPSPs) were recorded either from the stratum radiatum or stratum oriens in the CA1 area of the right hippocampus in response to stimulation of the ipsilateral Schaffer collateral-commissural pathway. Electrode implantation sites were identified using stereotaxic coordinates relative to bregma, with the recording site located 3.4 mm posterior to bregma and 2.5 mm lateral to midline, and stimulating site 4.2 mm posterior to bregma and 3.8 mm lateral to midline. The final placement of electrodes was optimized by using electrophysiological criteria and confirmed via postmortem analysis.
Electrophysiology.
Test EPSPs were evoked by square wave pulses (0.2 ms duration) at a frequency of 0.033 Hz and an intensity that triggered a 50% maximum response. LTP was induced using 200 Hz high frequency stimulation (HFS) consisting of either one set of 10 trains of 20 pulses (inter-train interval of 2 s) or three sets of 10 trains of 12 stimuli (interset interval of 5 min). The stimulation intensity was raised to trigger EPSPs of 75% maximum during the HFS.
Compounds.
Aβ42 (Bachem or Biopolymer Laboratory, University of California, Los Angeles Medical School) was prepared as a stock solution in 0.1% ammonium hydroxide, centrifuged at 100,000 × g, and the supernatant stored at −80 °C until required (74). Thalidomide (Sigma) and (αR,ßS)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidinepropanol hydrochloride (Ro 25–6981, Sigma) were dissolved in DMSO (dimethyl sulfoxide) and diluted in saline. (2R*,3S*)-1-(Phenanthrenyl-3-carbonyl)piperazine-2,3-dicarboxylic acid (UBP141, Ascent Scientific) was dissolved to 50 mM in 1eq. NaOH and diluted with water to the required concentration. Ifenprodil (Sigma), (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077, a generous gift from Yves Auberson, Novartis), infliximab (Centocar BV), TNFα (Sigma), and TNFα peptide antagonist (Bachem) were prepared in distilled water.
Pilot studies investigated the threshold dose for inhbition of LTP with intracerebroventricular injection of NVP-AAM077 (250 pmol), ifenprodil (6 nmol), and UBP141 (12.5 nmol). Half these doses of were tested in the investigation of Aβ-mediated inhibition of LTP.
Data Analysis.
The magnitude of LTP was expressed as the percentage of pre-HFS baseline EPSP initial amplitude or the percentage control LTP (±SEM). Two-way ANOVA with repeated measures was used to compare the magnitude of LTP over the 3 h post-HFS period between the experimental and control groups. One-way ANOVA was used to compare magnitude of LTP for the last 10 min (i.e., at 3 h) post-HFS between multiple groups. Student's t tests and posthoc Tukey's test were used for detailed statistical analysis where appropriate and P < 0.05 was considered as statistically significant.
Supplementary Material
Acknowledgments.
We thank Dr. Yves Auberson for the generous supply of NVP-AAM077. Supported by the Health Research Board of Ireland and Science Foundation Ireland.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908083106/DCSupplemental.
References
- 1.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 3.Lambert MP, et al. Diffusible, nonfibrillar ligands derived from A beta(1–42) are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raina P, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: Evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 6.Klyubin I, et al. Protection against Aß-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.005. In press. [DOI] [PubMed] [Google Scholar]
- 7.Paoletti P, Neyton J. NMDA receptor subunits: Function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Collingridge GL, Olsen R, Peters JA, Spedding M. Ligand gated ion channels. Neuropharmacology. 2009;56:1. doi: 10.1016/j.neuropharm.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Abbott JJ, Howlett DR, Francis PT, Williams RJ. Abeta(1–42) modulation of Akt phosphorylation via alpha7 nAChR and NMDA receptors. Neurobiol Aging. 2008;29:992–1001. doi: 10.1016/j.neurobiolaging.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande A, Kawai H, Metherate R, Glabe CG, Busciglio J. A role for synaptic zinc in activity-dependent Abeta oligomer formation and accumulation at excitatory synapses. J Neurosci. 2009;29:4004–4015. doi: 10.1523/JNEUROSCI.5980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roselli F, et al. Soluble beta-amyloid1–40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J Neurosci. 2005;25:11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett TE, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Berberich S, Jensen V, Hvalby O, Seeburg PH, Kohr G. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology. 2007;52:77–86. doi: 10.1016/j.neuropharm.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Fox CJ, Russell KI, Wang YT, Christie BR. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus. 2006;16:907–915. doi: 10.1002/hipo.20230. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 18.Domingues A, Almeida S, da Cruz e Silva EF, Oliveira CR, Rego AC. Toxicity of beta-amyloid in HEK293 cells expressing NR1/NR2A or NR1/NR2B N-methyl-D-aspartate receptor subunits. Neurochem Int. 2007;50:872–880. doi: 10.1016/j.neuint.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer's drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrighton DC, Baker EJ, Chen PE, Wyllie DJA. Mg2+ and memantine block of rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. 2008;586:211–225. doi: 10.1113/jphysiol.2007.143164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bresink I, et al. Effects of memantine on recombinant rat NMDA receptors expressed in HEK 293 cells. Br J Pharmacol. 1996;119:195–204. doi: 10.1111/j.1476-5381.1996.tb15971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Wu J, Rowan MJ, Anwyl R. Beta-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. Eur J Neurosci. 2005;22:2827–2832. doi: 10.1111/j.1460-9568.2005.04457.x. [DOI] [PubMed] [Google Scholar]
- 23.Floden AM, Li S, Combs CK. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J Neurosci. 2005;25:2566–2575. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morley RM, TH, Feng B, Miller JC, Monaghan DT, Jane DE. Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem. 2005;48:2627–2637. doi: 10.1021/jm0492498. [DOI] [PubMed] [Google Scholar]
- 25.Mony L, Kew JN, Gunthorpe MJ, Paoletti P. Allosteric modulators of NR2B-containing NMDA receptors: Molecular mechanisms and therapeutic potential. Br J Pharmacol. 2009;157:1301–1317. doi: 10.1111/j.1476-5381.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, et al. Tumor necrosis factor death receptor signaling cascade is required for amyloid-beta protein-induced neuron death. J Neurosci. 2004;24:1760–1771. doi: 10.1523/JNEUROSCI.4580-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraft AD, McPherson CA, Harry GJ. Heterogeneity of microglia and TNF signaling as determinants for neuronal death or survival. Neurotoxicology. 2009 doi: 10.1016/j.neuro.2009.07.001. doi: 10.1016/j.neuro.2009.1007.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lisman JE. The pre/post LTP debate. Neuron. 2009;63:281–284. doi: 10.1016/j.neuron.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Hu NW, Smith IM, Walsh DM, Rowan MJ. Soluble amyloid-beta peptides potently disrupt hippocampal synaptic plasticity in the absence of cerebrovascular dysfunction in vivo. Brain. 2008;131:2414–2424. doi: 10.1093/brain/awn174. [DOI] [PubMed] [Google Scholar]
- 30.Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;8334:8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romberg C, et al. Induction and expression of GluA1 (GluR-A)-independent LTP in the hippocampus. Eur J Neurosci. 2009;29:1141–1152. doi: 10.1111/j.1460-9568.2009.06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Engelhardt J, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 33.Higgins GA, Ballard TM, Enderlin M, Haman M, Kemp JA. Evidence for improved performance in cognitive tasks following selective NR2B NMDA receptor antagonist pre-treatment in the rat. Psychopharmacology (Berl) 2005;179:85–98. doi: 10.1007/s00213-005-2203-9. [DOI] [PubMed] [Google Scholar]
- 34.Mathur P, Graybeal C, Feyder M, Davis MI, Holmes A. Fear memory impairing effects of systemic treatment with the NMDA NR2B subunit antagonist, Ro 25–6981, in mice: Attenuation with ageing. Pharmacol Biochem Behav. 2009;91:453–460. doi: 10.1016/j.pbb.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merchant RE, et al. A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci. 1999;890:42–50. doi: 10.1111/j.1749-6632.1999.tb07979.x. [DOI] [PubMed] [Google Scholar]
- 36.Coan EJ, Irving AJ, Collingridge GL. Low-frequency activation of the NMDA receptor system can prevent the induction of LTP. Neurosci Lett. 1989;105:205–210. doi: 10.1016/0304-3940(89)90038-4. [DOI] [PubMed] [Google Scholar]
- 37.Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- 38.Molnar Z, et al. Enhancement of NMDA responses by beta-amyloid peptides in the hippocampus in vivo. Neuroreport. 2004;15:1649–1652. doi: 10.1097/01.wnr.0000134471.06244.d2. [DOI] [PubMed] [Google Scholar]
- 39.Szegedi V, Juhasz G, Budai D, Penke B. Divergent effects of Abeta1–42 on ionotropic glutamate receptor-mediated responses in CA1 neurons in vivo. Brain Res. 2005;1062:120–126. doi: 10.1016/j.brainres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, Anwyl R, Rowan MJ. beta-Amyloid selectively augments NMDA receptor-mediated synaptic transmission in rat hippocampus. Neuroreport. 1995;6:2409–2413. doi: 10.1097/00001756-199511270-00031. [DOI] [PubMed] [Google Scholar]
- 41.De Felice FG, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 42.Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci. 2006;26:7181–7188. doi: 10.1523/JNEUROSCI.1258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooper C, et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 44.Peineau S, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Groc L, Bard L, Choquet D. Surface trafficking of N-methyl-D-aspartate receptors: Physiological and pathological perspectives. Neuroscience. 2009;158:4–18. doi: 10.1016/j.neuroscience.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Martel MA, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158:334–343. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cowburn RF, Wiehager B, Trief E, Li-Li M, Sundstrom E. Effects of beta-amyloid-(25–35) peptides on radioligand binding to excitatory amino acid receptors and voltage-dependent calcium channels: Evidence for a selective affinity for the glutamate and glycine recognition sites of the NMDA receptor. Neurochem Res. 1997;22:1437–1442. doi: 10.1023/a:1021942109490. [DOI] [PubMed] [Google Scholar]
- 48.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- 50.Meda L, et al. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 51.Song DK, et al. Central beta-amyloid peptide-induced peripheral interleukin-6 responses in mice. J Neurochem. 2001;76:1326–1335. doi: 10.1046/j.1471-4159.2001.00121.x. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi H, et al. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 53.Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: Neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Matos M, Augusto E, Oliveira CR, Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: Involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 55.Bobich JA, Zheng Q, Campbell A. Incubation of nerve endings with a physiological concentration of Abeta1–42 activates CaV2.2(N-Type)-voltage operated calcium channels and acutely increases glutamate and noradrenaline release. J Alzheimers Dis. 2004;6:243–255. doi: 10.3233/jad-2004-6305. [DOI] [PubMed] [Google Scholar]
- 56.Kabogo D, Rauw G, Amritraj A, Baker G, Kar S. beta-Amyloid-related peptides potentiate K(+)-evoked glutamate release from adult rat hippocampal slices. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.009. doi: 10.1016/j.neurobiolaging.2008.1008.1009. [DOI] [PubMed] [Google Scholar]
- 57.Puzzo D, et al. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kutsuwada T, et al. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 59.Scimemi A, Fine A, Kullmann DM, Rusakov DA. NR2B-containing receptors mediate cross talk among hippocampal synapses. J Neurosci. 2004;24:4767–4777. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacob CP, et al. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer's disease. J Alzheimers Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- 61.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 62.Tweedie D, Sambamurti K, Greig NH. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: New drug candidates and targets. Curr Alzheimer Res. 2007;4:375–378. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 63.Buchhave P, et al. Soluble TNF receptors are associated with Abeta metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.012. In press. [DOI] [PubMed] [Google Scholar]
- 64.Tan ZS, et al. Inflammatory markers and the risk of Alzheimer disease: The Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 65.Tobinick E. Perispinal etanercept for neuroinflammatory disorders. Drug Discov Today. 2009;14:168–177. doi: 10.1016/j.drudis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaibara T, Leung LS. Basal versus apical dendritic long-term potentiation of commissural afferents to hippocampal CA1: A current-source density study. J Neurosci. 1993;13:2391–2404. doi: 10.1523/JNEUROSCI.13-06-02391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kramar EA, Lynch G. Developmental and regional differences in the consolidation of long-term potentiation. Neuroscience. 2003;118:387–398. doi: 10.1016/s0306-4522(02)00916-8. [DOI] [PubMed] [Google Scholar]
- 69.Leung LS, Shen B. N-methyl-D-aspartate receptor antagonists are less effective in blocking long-term potentiation at apical than basal dendrites in hippocampal CA1 of awake rats. Hippocampus. 1999;9:617–630. doi: 10.1002/(SICI)1098-1063(1999)9:6<617::AID-HIPO2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Wang Q, et al. alphav integrins mediate beta-amyloid induced inhibition of long-term potentiation. Neurobiol Aging. 2007;29:1485–1493. doi: 10.1016/j.neurobiolaging.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Wang Q, Rowan MJ, Anwyl R. Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci. 2004;24:6049–6056. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.