Abstract
Epstein–Barr, virus (EBV) is a ubiquitous human herpesvirus that is causally implicated in the development of lymphoid and epithelial tumors. Entry of virus requires fusion of virus envelopes and cell membranes. Fusion with B lymphocytes requires virus glycoprotein gB and a 3-part complex of glycoproteins, gHgLgp42. It is triggered by interactions between glycoprotein 42 (gp42) and HLA class II. However, fusion with epithelial cells is impeded by gp42 and instead is triggered by interactions between an unknown epithelial protein and a 2-part complex of gHgL. We report here that gHgL binds with high affinity to epithelial cells and that affinity of binding is increased by 3 orders of magnitude in the presence of Mn2+. Binding and infection can be reduced by fibronectin and vitronectin, by down-regulation of integrin αv, or by a peptide corresponding to 13 aa of gH which include a KGDE motif. Fusion of cells expressing gB and gHgL can be blocked by vitronectin or triggered by addition of soluble truncated integrins αvβ6 and αvβ8. We conclude that the direct interaction between EBV gHgL and integrins αvβ6 and αvβ8 can provide the trigger for fusion of EBV with an epithelial cell.
Epstein–Barr virus (EBV) is carried by >90% of the adult population worldwide. Many individuals are infected asymptomatically in childhood, but, although primary infection in adolescence or later often is accompanied by infectious mononucleosis, the major impact of the virus results from its role as a tumor initiator or tumor progressor. EBV is associated with both lymphoid and epithelial malignancies, reflecting its primary tropism for these 2 cell types (1).
The proteins involved in EBV penetration of a B cell are more clearly defined than those required for epithelial cells (reviewed in ref. 2). Attachment is mediated by an interaction between envelope glycoprotein gp350 and the complement receptor type 2 (CR2) or CD21. Fusion, as for all herpesviruses, requires the core fusion machinery (3) of glycoproteins gB and gH and its partner gL, which is essential to the folding and transport of gH. Triggering of the core fusion machinery from a metastable to an active state requires an interaction with HLA class II, which functions as a coreceptor or entry mediator on the B-cell surface. The interaction is mediated by an additional glycoprotein, gp42, which, like gL, binds directly to gH to form a 3-part complex, gHgLgp42.
Attachment of EBV to epithelial cells can be mediated by gp350, but on CR2-negative cells it also can be mediated by a 2-part complex, gHgL. A soluble truncated form of the complex, gHtgL, can bind specifically to epithelial cells but not to B cells (4), and a gH-null virus, which can still use gp350 to bind to CR2-positive cells, loses the ability to bind to a CR2-negative epithelial cell (5, 6). In addition, HLA class II, which is not constitutively expressed on epithelial cells, is unavailable to trigger fusion. Instead, fusion with an epithelial cell requires an unknown coreceptor, gB, and the gHgL complex that lacks gp42. Virus carries both 3-part gHgLgp42 complexes and 2-part gHgL complexes to accommodate infection of 2 cell types. Only 3-part complexes can mediate fusion with B cells, but only 2-part gHgL complexes can mediate entry of epithelial cells (7), and changes in the levels of gp42 by sequestration and degradation in an HLA class II-positive B cell, but not in an HLA class II-negative epithelial cell, switch virus tropism (8). The presence of gp42 blocks the ability of gHgL to mediate virus binding to an epithelial cell that lacks CR2 (4) and also blocks the ability of virus bound via gp350 to a CR2-positive cell to infect (7).
These observations, together with the findings that a monoclonal antibody to gHgL not only blocked gHgL binding and virus binding to a CR2-negative cell but also blocked entry of bound virus into a CR2-positive epithelial cell (4, 5), suggested that the molecule that serves as an epithelial gHgL receptor might also serve as the missing coreceptor needed to trigger epithelial cell fusion by EBV glycoproteins. We previously have speculated that this triggering might in fact be the primary function of the gHgL receptor, because, although the binding of the virus to the molecule is relatively robust, the ability of the virus to enter the cell when using the molecule, rather than CR2, for attachment is not (4). We report here that 1 set of proteins that can function as gHgL receptors are αv-containing integrins. Downregulation of αv expression reduced binding and infection, as did a peptide corresponding to residues of 184–196 of the gH precursor, which include a putative integrin-binding motif, KGDXXXL. Further, soluble forms of human integrins αvβ6 and αvβ8 triggered epithelial cell fusion mediated by EBV gB and gHgL. We conclude that integrins are able to serve both for attachment and fusion of EBV with epithelial cells, although their most important role is likely to be in fusion.
Results
Binding of gHtgL to Epithelial Cells Is Characteristic of Integrin Binding.
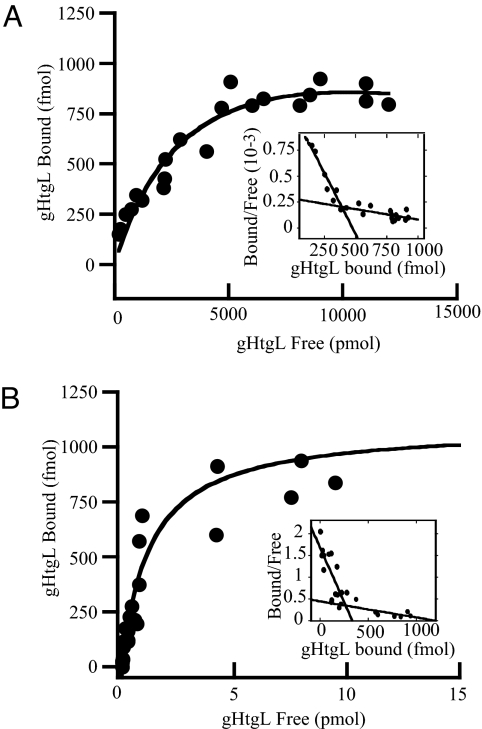
The CR2-negative AGS gastric carcinoma cell line and the SVKCR2 keratinocyte cell line engineered to express CR2 have been used extensively to study epithelial cell infection with cell-free virus. On AGS cells, gHgL is essential for both virus attachment and fusion; on SVKCR2 cells, gp350 can be used for attachment, and gHgL is essential only for fusion (5). To characterize gHgL binding to each cell line, gHtgL, which carries high levels of mannose sugars as a result of production in insect cells, was isolated by affinity chromatography on Lentil Lectin Sepharose. Biological activity of gHtgL was monitored during isolation by flow cytometric analysis of protein bound to cells on ice and stained with monoclonal antibody CL59 to gH. Binding was saturable, fitted well to a hyperbolic curve [see supporting information (SI) Fig. S1], and could inhibit virus infection (Fig. S2). Isolated protein then was labeled with 125I and used for Scatchard analysis of binding to AGS and SVKCR2. On AGS cells, 2 binding sites with affinities differing by 1 order of magnitude were detected (Fig. 1 and Table 1); only the high-affinity sites were found on SVKCR2 cells (Table 1).
Fig. 1.
Manganese increases the affinity of gHtgL binding to AGS cells without affecting the number of binding sites. Scatchard analysis of 125I-labeled gHtgL binding to AGS cells in the absence (A) or presence (B) of 200 μM MnCl2.
Table 1.
Affinity and number of gHtgL binding sites on AGS and SVKCR2 cells
| Cell line | Mn2+ addition | High-affinity binding sites |
Low-affinity binding sites |
||
|---|---|---|---|---|---|
| KD (M) | Bmax (cell−1) | KD (M) | Bmax (cell−1) | ||
| AGS | none | (0.61 ± 0.90*) × 10−9 | (286 ± 1.00) × 103 | (6.0 ± 0.05) × 10−9 | (8.41 ± 1.40) × 106 |
| 200 μM | (0.26 ± 0.06) × 10−12 | (297 ± 36.00) × 103 | (0.97 ± 0.22) × 10−12 | (6.03 ± 0.01) × 105 | |
| SVKCR2 | none | (0.91 ± 0.14) × 10−9 | (51 ± 2.00) × 103 | none | none |
| 200 μM | (0.18 ± 0.02) × 10−12 | (49 ± 1.00) × 103 | none | none | |
*Standard deviations were calculated from three separate experiments.
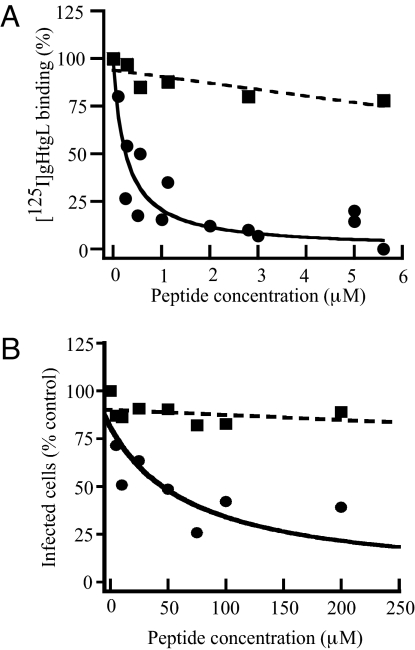
The predicted 706-aa sequence of gH includes a KGD motif at residues 188–191 that potentially could serve as a ligand for integrins. Integrins can exist in an open or a closed conformation, and the difference in affinity of the 2 conformations for ligand can be as much as 9,000-fold (9). Because Mn2+ locks integrins in an open conformation, its effects on the affinity of gHtgL binding were measured. Addition of 200 μM Mn2+ had no effect on the number of binding sites on AGS or SVKCR2 cells but increased their affinity of binding by approximately 3 orders of magnitude (Fig. 1 and Table 1). To determine if the region of gH that included the KGD motif might serve to bind gHtgL to the cells, binding was repeated in the presence of a peptide including residues 184–196 of gH (RVTEKGDEHVLSL). A cysteine and a tyrosine residue were added to facilitate determination of peptide concentration. The cognate peptide, but not a scrambled form containing the same residues, blocked gHtgL binding (Fig. 2). The cognate peptide also reduced the ability of virus to infect SVKCR2 cells, although, as might be expected for a peptide in competition with a multivalent ligand, considerably more peptide was required to reduce infection as much as 70% than was required to inhibit gHtgL binding.
Fig. 2.
A peptide corresponding to residues 184–196 of gH blocks gHtgL binding to and infection of SVKCR2 cells, but a scrambled peptide does not. (A) Binding of 125I-labeled gHtgL to cells preincubated with increasing concentrations of cognate peptide including the KGD motif (solid line) or a scrambled form of the same residues (dotted line). The data were fit using Kaleida Graph (Synergy Software). The plot of the scrambled peptide obeys a linear equation (r = 0.82), and that for the cognate peptide obeys an equation for competitive inhibition of binding (r = 0.91). Initial slopes of the 2 conditions are significantly different (P < 0.0001). (B) Infection of cells preincubated with the indicated amounts of the cognate KGD peptide (solid line) or a scrambled form of the same peptide (dotted line). The plot of the scrambled peptide obeys a linear equation (r = 0.73), and that for the cognate peptide obeys an equation for competitive inhibition of binding (r = 0.63). Initial slopes of the 2 conditions are significantly different (P = 0.0003). Plots in both panels are the averages of 3 independent experiments.
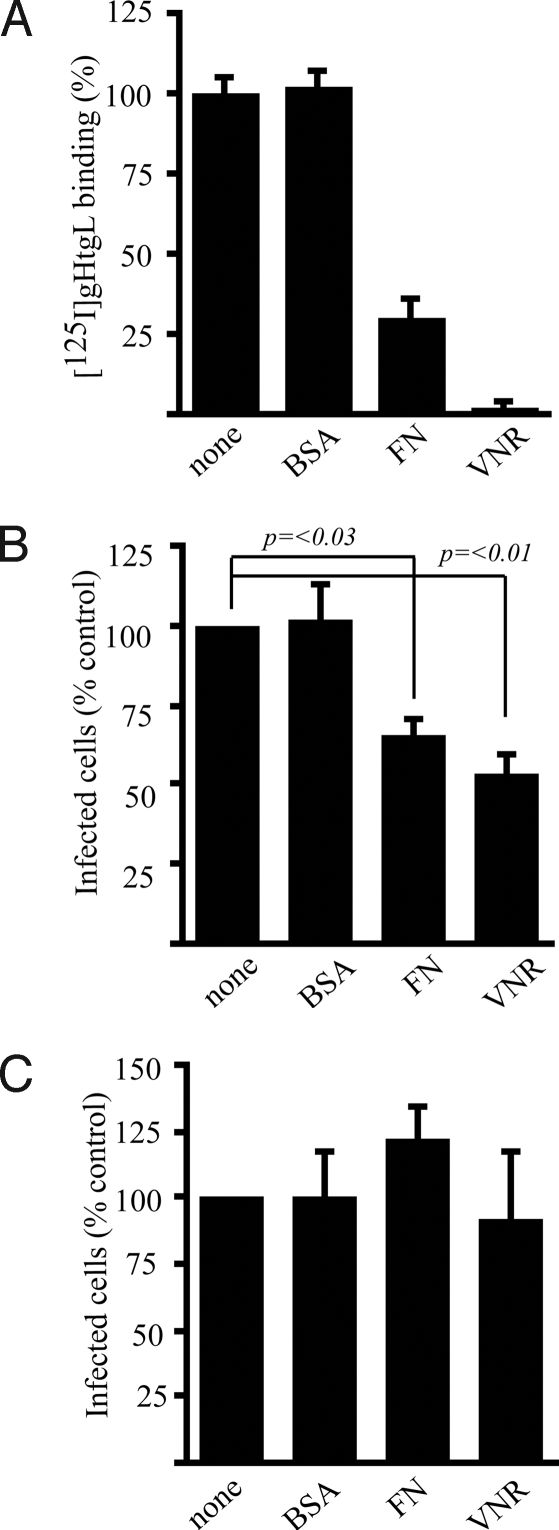
Fibronectin and Vitronectin Reduce Binding and Infection, and Vitronectin Inhibits Fusion.
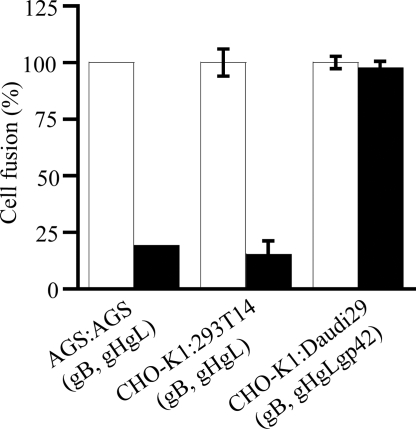
The natural ligands of integrins are matrix proteins that also might be expected to compete with gHtgL for binding. Preincubation of cells with fibronectin and vitronectin, but not with BSA, competitively reduced gHtgL binding and infection of SVKCR2 cells when gHgL was used only for fusion (Fig. 3 and Fig. S3). Neither fibronectin nor vitronectin had any effect on infection of B cells, which do not bind gHtgL (Fig. 3). Vitronectin blocked fusion of AGS cells transfected with plasmids expressing gB and gHgL and examined visually (Fig. 4). It also blocked fusion of CHO-K1 cells transfected with plasmids encoding gB, gH, and gL. In this assay cells were cotransfected with a plasmid-expressing luciferase under control of the T7 promoter and overlaid with 293T14 cells that express T7 polymerase. Fusion is measured in terms of luciferase activity. In contrast, vitronectin had no effect on the ability of CHO-K1 cells transfected with plasmids encoding gB, gHgLgp42, and luciferase to fuse with Daudi29 B cells expressing T7 polymerase.
Fig. 3.
Fibronectin and vitronectin block gHtgL binding and infection. (A) Binding of 125I-labeled gHtgL to AGS cells in medium alone (none) or in the presence of 40 μg/mL of BSA (BSA), fibronectin (FN), or vitronectin (VNR). (B) Infection of SVKCR2 cells in the presence of medium alone or 40 μg/mL BSA, fibronectin, or vitronectin. (C) Infection of EBV-negative Akata B cells in the presence of medium alone or 40 μg/mL BSA, fibronectin, or vitronectin. Error bars indicate SD of 3 infections and duplicate binding assays.
Fig. 4.
Vitronectin blocks fusion of epithelial cells but not of B cells. Vitronectin was added immediately after transfection of AGS cells with gB and gHgL (AGS:AGS), after transfection of CHO-K1 cells with gB, gHgL, and a luciferase construct and subsequently overlaid with 293T14 cells (CHO:293T14), or after transfection of CHO-K1 cells with gB, gHgL, gp42, and a luciferase construct, subsequently overlaid with Daudi29 cells (CHO:Daudi29) (black bars). Fusion is expressed as a percentage of that occurring in the absence of vitronectin (white bars). Error bars indicate SD of 3 experiments; the SD of AGS :AGS fusion in the presence of vitronectin is too small to visualize.
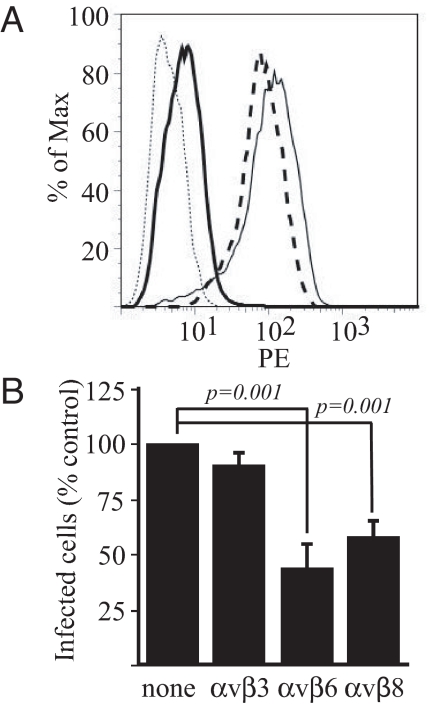
Silencing of αv Integrins as Well as Soluble Forms of Human Integrins That Interact with gHtgL Reduce gHtgL Binding and Infection.
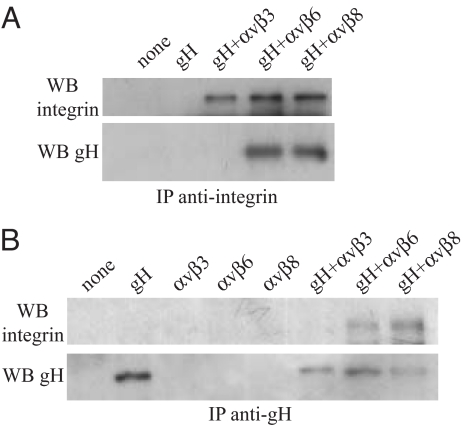
The ability of vitronectin or fibronectin to affect binding and fusion suggested that an αv integrin might be involved and the KGD motif in gH is followed by a leucine residue at the plus-4 position, as is characteristic of a ligand that binds αvβ6 (10) or αvβ8 (11), integrins expressed on epithelial cells (12) but not on B cells (13). SVKCR2 cells expressed 4 integrins known to bind vitronectin or fibronectin, α5β1, αvβ5, αvβ6, αvβ8. The mean fluorescence intensities (MFI) of antibody binding were 61.10, 27.56, 109.11, and 29.68, respectively (Fig. S4). A fraction of the cells also expressed αvβ3 (MFI 29.47). AGS cells expressed α5β1, αvβ5, and αvβ6 (MFI 44.82, 23.72, and 26.09, respectively) but not αvβ8 (MFI 4.02) or αvβ3 (MFI 3.78). Akata B cells, which do not bind gHtgL, also express α5β1 (4), so it seemed probable that gHtgL was binding to an αv integrin. To test this possibility, SVKCR2 cells were transfected with siRNA to αv. Expression of αv was reduced on cells transfected with cognate but not control siRNA (MFI reduced from 198.93 to 20.45; Fig. 5A), and binding of gHtgL also was reduced (MFI reduced from 46.69 to 28.14). Importantly, reduction of expression of αv also reduced infection by EBV expressing GFP, dropping the MFI of infected cells from 1115.47 to 163.64 (Fig. 5B). Although vitronectin blocked gHtgL binding well, gHtgL failed to bind to CHO cells expressing human αvβ3 (Fig. S5). Culture media of CHO cells engineered to secrete a soluble form of human αvβ6 blocked gHtgL binding to SVKCR2 cells, reducing the MFI from 126.87 to 8.97 (Fig. 6A), but culture media of CHO-K1 cells did not. Preincubation of virus with soluble forms of integrins αvβ6 and αvβ8 made in engineered 293 cells reduced infection, but a soluble form of αvβ3 made in the same cell type did not (Fig. 6B). An interaction between gHtgL and the soluble integrins αvβ6 and αvβ8, but not between gHtgL and αvβ3, also could be observed by reciprocal immunoprecipitation (Fig. 7).
Fig. 5.
gHtgL binding and infection are reduced by siRNA to αv. (A) SVKCR2 cells were nucleoporated with siRNA to αv (dashed line) or with control siRNA (solid line), and expression of αv (Left) or binding of gHtgL (Right) was measured by flow cytometry. The isotype controls are shown as dotted lines. (B) SVKCR2 cells were transfected with siRNA to αv (dashed line) or control siRNA (solid line), and expression of αv (Left) or infection (Right) was measured by flow cytometry. Isotype controls or uninfected cells are shown as dotted lines. Experiments are representative of 3 different nucleoporations/transfections.
Fig. 6.
gHtgL binding and infection are reduced by media containing soluble integrins. (A) Flow cytometric analysis of gHtgL binding to SVKCR2 cells in the presence of fresh tissue culture media (light solid line), concentrated spent tissue culture media from CHO-K1 cells (dashed line), or CHO-C5 cells secreting soluble human αvβ6 (heavy solid line). Cells were preincubated with supernatants before addition of gHtgL. The isotype control is shown as a dotted line. (B) Infection of SVKCR2 cells with virus preincubated with soluble αvβ3, αvβ6, or αvβ8. Error bars indicated the SD of triplicate infections.
Fig. 7.
gHtgL is reciprocally precipitated with αvβ6 and αvβ8 but not with αvβ3. Integrins or gHtgL were immunoprecipitated (IP) individually or together as indicated, with antibody to the alkaline phosphatase fused to the carboxy termini of each integrin (A) or with antibody to gH (B) and were Western blotted (WB) with antibody to αv (Upper) or gH (Lower).
Soluble Human Integrins That Interact with gHtgL Trigger Fusion of Cells That Express gB and gHgL but Lack Human Integrins.
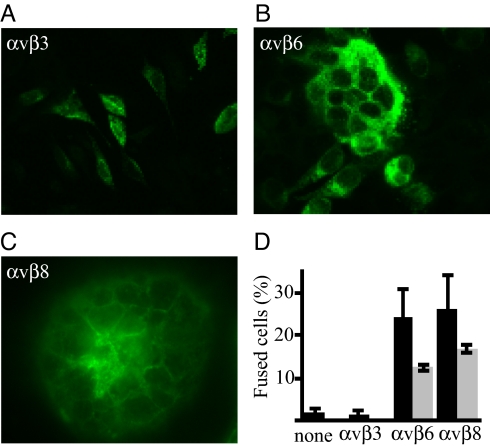
CHO-K1 cells, which do not bind gHtgL, are used for cell-based fusion assays because they do not fuse with each other when transfected with gB and gHgL. To determine if the interactions of integrins αvβ6 and αvβ8 with gHgL were able to trigger epithelial cell fusion, these integrins were added to CHO-K1 cells 4 h after transfection of cells with plasmids expressing gB and gHgL; 20 h later cells were inspected visually for evidence of fusion. Soluble αvβ6 and soluble αvβ8, triggered fusion but soluble αvβ3 did not, even in the presence of 1 mM Mn2+ (Fig. S6). Preincubation of αvβ6 and αvβ8 with cognate-blocking antibodies reduced the extent of fusion (Fig. 8).
Fig. 8.
Soluble αvβ6 and αvβ8 trigger fusion of CHO-K1 cells transfected with gB and gHgL but solubleαvβ3 does not. Cells were transfected with gB and gHgL, overlaid with integrins, and 20 h later were fixed and stained with monoclonal antibody to gB. (A) Cells overlaid with αvβ3. (B) Cells overlaid with αvβ6. (C) Cells overlaid with αvβ8. (D) Percentage of cells expressing gB that contained 4 or more nuclei (% fusion) after overlay with integrins (black bars) or integrins preincubated with cognate blocking antibody (gray bars). Error bars indicate SD of 5 experiments without antibody and 2 experiments with antibody.
Discussion
Many viruses, including herpesviruses, use integrins to facilitate entry into mammalian cells (14). The interactions can serve to attach virus to cells (15), to stimulate endocytosis (16), and to induce signaling pathways that are important to infection (17, 18). Interaction of an EBV envelope protein, BMRF2, with integrins α5β1 and α3 plays a role in the infection of polarized epithelial cells (19). We show here that the interaction with integrins of another, essential envelope protein, gH, is directly responsible for triggering fusion of epithelial cells by EBV gB and gHgL.
EBV and closely related gammaherpesviruses (20, 21) are not the only herpesviruses that carry potential integrin-binding motifs in gH. The gH homolog of the alphaherpesvirus, herpes simplex virus (HSV), includes an RGD sequence at residues 176–178 of the predicted 803-aa protein. Low-affinity binding assays confirmed that in this context the motif can bind to αvβ3 integrins (22), but mutation of the sequence had no effect on virus penetration (23). Instead, in all cell types currently examined, HSV fusion more closely resembles fusion of EBV with a B cell, in that it requires not only the core fusion machinery (gB and gHgL) but also gD. Although gD binds to several different cell proteins or sulfated sugars expressed on different cell types, gD has a triggering function analogous to that of gp42 (3).
Rather than broaden the spectrum of interactions of which gp42 is capable, as seems to have happened for gD, EBV has adopted a fundamentally different and more direct approach to activate fusion with an epithelial cell. The question that thus arises—how 2 different cell proteins, HLA class II and an integrin, result in activation of what is thought to be ultimately the same fusion machinery—is similar in some ways to that raised by the ability of so many different structures to have the same downstream effect when they interact with gD. However, gH, unlike HSV gD, is thought to play an intrinsic role in fusion by inducing hemi-fusion (24), and thus there presumably are even more functional constraints on the ways in which gH can interact with a trigger than there are for gD. The binding of gp42 to gH before fusion with a B cell (25, 26) suggests that it is gH that is used to transmit, to the entire fusion machinery, the conformational changes that are likely to occur following HLA class II binding to gp42 (27); this notion is consistent with the ability of a direct interaction between gH and an integrin to activate a fully functional fusion machinery. Mutational analysis has distinguished sequences in gH that are differentially important for fusion with a B cell and an epithelial cell (25, 28), suggesting that some events downstream of the integrin–gH interaction may be distinct from those initiated by a gp42-HLA class II complex. However, the assumption that the ultimate effects on gH are the same seems reasonable.
The full panoply of integrins that can interact with EBV gH is not addressed here. It remains possible that integrins in addition to αvβ6 and αvβ8 can bind and function as fusion inducers. Attempts to identify these integrins by use of function-blocking antibodies have been only partially illuminating. Antibodies to β1 or α5β1, 2 other integrins (in addition to the non-interactive αvβ3) that are present on the epithelial cells used, did not affect binding (Fig. S7A). Antibody to αvβ5, αvβ6, αvβ8, and αv alone reduced binding by ≈50% or slightly more, suggesting that αvβ5 might serve as a trigger as well. However, the possibility that the effect was mediated by interference of this antibody with αv cannot be ruled out completely, because we know nothing about the nature of the interface of gH and any integrin. A combination of antibodies decreased binding beyond that mediated by antibody to αv alone, but the combination could not eliminate binding completely, possibly because the affinity of EBV gH for its binding partner is at least as high as the affinity of many antibodies, or perhaps because there are additional cell surface molecules with which gHgL can interact. Antibodies also could reduce virus infection only incompletely (Fig. S7B). We do know, however, in the case of integrin binding, that both α and β integrin subunits must be human, because gHtgL did not bind to CHO cells expressing hamster αv and human β6 (Fig. S8). Determination of the full range of integrins that can be used to trigger fusion will require generation of reagents that currently are unavailable. The extent to which infection of human cell lines can be reproduced in CHO-K1 cells by expression of pairs of human integrins together with the entry-competent attachment receptor CR2 also will require the generation of novel reagents. CR2 is expressed on relevant epithelial cells in vivo (29), and the extent of its dominant role in facilitating efficient infection is still unclear.
It was somewhat surprising that a soluble integrin alone was able to stimulate fusion, but we assume that cell–cell interactions were themselves sufficient to put gB and gHgL close enough to an adjacent cell that, once activated, they could mediate fusion. This assumption suggests that the binding of gH to an integrin results in a significant conformational change, a possibility that will be explored further. The fact that it was possible to block infection by prolonged pretreatment of virus with soluble integrins suggests that the conformational change must occur in proximity to a membrane to be productive.
In the context of EBV pathogenesis, the use of integrins αvβ6 and αvβ8, most particularly αvβ6, is intriguing. Expression of αvβ8 in epithelial cells is limited primarily to basal cells in normal airway epithelium (30). In contrast, αvβ6 is expressed at low levels on most normal epithelial tissues in vivo, and its expression is up-regulated during tissue remodeling, including that accompanying wound-healing, inflammation, and carcinogenesis (31, 32). Functionally, in addition to roles in mediating interactions with the extracellular matrix, αvβ6 and αvβ8 bind to TGF-β1 latency-associated peptide and in doing so cause local activation of endogenous TGF-β1 (11, 33). Up-regulation of αvβ6 or αvβ8 increases activation of TGF-β1, which in turn enhances matrix deposition and fibrosis. This effect may be of interest, given the association of EBV with idiopathic pulmonary fibrosis (34–36). Critically, however, TGF-β1 also induces EBV lytic reactivation in B cells (37–39). There thus are 2 reasons why up-regulation of αvβ6 on dysplastic cells might enhance the chances of virus transfer from any proximal B cell carrying EBV. If, as has been suggested, a role of EBV in development of tumors such as nasopharyngeal carcinoma is to induce progression in a cell that already has undergone molecular alterations (40, 41), the consequences may not be benign.
Materials and Methods
Cells.
AGS, SVKCR2, EBV-negative Akata, Akata-GFP, and Sf9 insect cells have been described (4, 42). Daudi29 cells that express T7 polymerase (27) were grown in RPMI MEDIUM 1640 with 10% heat-inactivated serum. CHO-K1 cells VNRC3 (CHO cells expressing human αvβ3) (43), F4B6 (CHO cells expressing human β6), CHO-C5 (cells that express a soluble form of human αvβ6) (44), 293T14 (cells that express T7 RNA polymerase) (45), and 293-B8 AVAP (11), 293-B6 AVAP, and 293-B3 AVAP (cells that, respectively, secrete truncated αvβ8, αvβ6, and αvβ3 conjugated to alkaline phosphatase) (46) were grown in DMEM supplemented with 10% heat-inactivated FBS and 1% nonessential amino acids.
Antibodies and Immunofluorescence.
Monoclonal antibodies used include CL59 to gH (5); E1D1 to gHgL (47); CL55 to gB (28); 37E1 to αvβ8 (11); L230 (44) to integrin αv; monoclonal antibody 13 to integrin β1 (a gift of Kenneth M. Yamada, National Institute of Dental and Craniofacial Research); BV3 to integrin αvβ3; Sc-8066 to alkaline phosphatase (Santa Cruz); andAV1 to integrin αv, R6G9 to integrin β6, 10D5 to integrin αvβ6, P1F6 to integrin αvβ5, and JBS5 to integrin α5β1 (all from Chemicon). CL59, E1D1, CL55, 37E1, and L230 were purified by affinity chromatography on protein A Agarose (RepliGen). Rabbit anti-peptide antibody to gH (48) and sheep anti-mouse IgG coupled to phycoerythrin (Jackson ImmunoResearch) or to fluorescein isothiocyanate (Cappel) were used also. Cells were prepared for flow cytometry (4) or indirect immunofluorescence as described in ref. 28. See SI Methods for more details.
Virus and Virus Infection.
EBV-GFP and baculovirus expressing gHtgL (49) were made as described in ref. 4. Infection of epithelial cells or B cells was done as described in ref. 4, and the effects of peptides and cell matrix proteins were examined by preincubation of cells with reagents for 1 h at 4 °C. See SI Methods for more details.
Isolation of Soluble gHtgL.
Soluble gHtgL was obtained from culture medium of Sf9 cells infected with recombinant baculovirus as described in ref. 4, concentrated by ultrafiltration, and dialyzed to remove sugars. gHtgL was isolated on Lentil Lectin Sepharose (Sigma) and eluted with 10 mM methyl-α-mannopyranoside. See SI Methods for more details.
Isolation of Soluble Truncated Integrins.
Serum-free spent culture medium from CHO- or 293-cell derivatives was concentrated by ultrafiltration. Fractions containing soluble integrins from 293-cell derivatives were isolated by size-exclusion chromatography on Superdex 200(GE Healthcare). The concentrations of these integrins were equalized by Western blotting dilutions of protein with antibody to αv and secondary antibody coupled to HRP, detected with ECL reagent (GE Healthcare) and fluorography and scanned into ImageQuant (GE Healthcare).
gHtgL Binding and Scatchard Analysis.
Binding was done as described in ref. 4. Flow cytometric analysis of gHtgL binding used monoclonal antibody CL59 to gH. Binding also was measured after gHtgL was radiolabeled with 125I. Specific binding of radiolabeled protein was determined by subtracting the amount that bound to cells following preincubation with a saturating amount of unlabeled gHtgL. Nonspecific binding never exceeded 7% of total binding. The range of gHtgL concentrations used for Scatchard analysis was chosen to cover 0.1/10 KD after calculation of an approximate KD from saturation curves obtained by flow cytometry. Data obtained from repeat experiments were plotted together without normalizing. Numeric data were obtained using EnzFitter 1 (Biosoft). See SI Methods for more details.
Silencing.
SVKCR2 cells, as indicated, either were nucleofected with 15 nmol of siRNAs to human αv or negative control siRNA obtained from Ambion using an Amaxa nucleoporator (Lonza), the V kit, and program T20 or were transfected with 2 μM siRNA using Dharmafect 1 reagent (Dharmacon) according to the manufacturer's instructions. gHtgL binding, integrin expression, and infection were measured by flow cytometry.
Cell Fusion Assays.
Cell fusion assays were done as described by transfection of AGS cells and visual examination (28) or by transfection of CHO-K1 cells, overlaying with 293T14 cells or Daudi29 cells, and measurement of luciferase activity (28, 45). Triggering of CHO-K1 cell fusion without overlaying cells was achieved by adding isolated integrins 4 h after transfection followed by visual examination 20 h later. In some experiments integrins were preincubated with 20 μg cognate antibody for 1 h before being added to cells. See SI Methods for more details.
Immunoprecipitation and Western Blotting.
Proteins were immunoprecipitated, electrophoresed, and transferred to PVDF membranes as described in ref. 5, except that proteins were run under nonreducing conditions. The membranes were reacted with primary antibody and secondary antibody coupled to HRP (Amersham) for detection by ECL (GE Healthcare) and fluorography.
Supplementary Material
Acknowledgments.
We thank Susan Turk for excellent technical assistance, Richard Longnecker (Northwestern University) for the gift of Daudi29 and 293T14 cells, Mark Ginsberg (University of California, San Diego) for CHO cells expressing human αvβ3, Dean Sheppard (University of California, San Francisco) for CHO cells expressing human β6 and soluble human αvβ6 and for helpful discussion, and Kenneth M. Yamada (National Institute of Dental and Craniofacial Research) for the gift of monoclonal antibody 13. This work was supported by National Institute for Dental and Craniofacial Research Grants DE016669 (to L.M.H.-F) and HL63993 and NS04415 (to S.L.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907508106/DCSupplemental.
References
- 1.Rickinson AB, Kieff E. In: Fields Virology. Knipe DM, Howley PM, editors. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2655–2700. [Google Scholar]
- 2.Hutt-Fletcher LM. Epstein–Barr virus entry. J Virol. 2007;81:7825–7832. doi: 10.1128/JVI.00445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spear PG, Longnecker R. Herpesvirus entry: An update. J Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borza CM, Morgan AJ, Turk SM, Hutt-Fletcher LM. Use of gHgL for attachment of Epstein–Barr virus to epithelial cells compromises infection. J Virol. 2004;78:5007–5014. doi: 10.1128/JVI.78.10.5007-5014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molesworth SJ, Lake CM, Borza CM, Turk SM, Hutt-Fletcher LM. Epstein–Barr virus gH is essential for penetration of B cell but also plays a role in attachment of virus to epithelial cells. J Virol. 2000;74:6324–6332. doi: 10.1128/jvi.74.14.6324-6332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oda T, Imai S, Chiba S, Takada K. Epstein–Barr virus lacking glycoprotein gp85 cannot infect B cells and epithelial cells. Virology. 2000;276:52–58. doi: 10.1006/viro.2000.0531. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Kenyon WJ, Li QX, Mullberg J, Hutt-Fletcher LM. Epstein–Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol. 1998;72:5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein–Barr virus. Nat Med. 2002;8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- 9.Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;31:485–516. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- 10.Burman A, et al. Specificity of the VP1 GH loop of foot-and-mouth disease virus for αv integrins. J Virol. 2006;80:9798–9810. doi: 10.1128/JVI.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mu D, et al. The integrin αvβ8 mediates epithelial homeostasis through MT-1-MMP–dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard D. Airway epithelial integrins: Why so many? Am J Respir Cell Mol Biol. 1998;19:349–351. doi: 10.1165/ajrcmb.19.3.f144. [DOI] [PubMed] [Google Scholar]
- 13.Luo B-H, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart PL, Nemerow GR. Cell integrins: Commonly used receptors for diverse pathogens. Trends Microbiol. 2007;15:500–507. doi: 10.1016/j.tim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Duque H, LaRocco M, Golde WT, Baxt B. Interactions of foot-and-mouth disease virus with soluble bovine αvβ3 and αvβ6 integrins. J Virol. 2004;78:9773–9781. doi: 10.1128/JVI.78.18.9773-9781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemerow GR, Stewart PL. Roles of αv integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma-Walia N, Naranatt P, Krishnan HH, Zeng L, Chandran B. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-src-phophatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Huang DY, Huong SM, Huang ES. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat Med. 2005;11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao J, Palefsky JM, Herrera R, Tugizov SM. Characterization of the Epstein–Barr virus glycoprotein BMRF2. Virology. 2007;359:382–396. doi: 10.1016/j.virol.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 20.Rivailler P, Cho Y-G, Wang F. Complete genomic sequence of an Epstein–Barr virus related herpesvirus naturally infecting a new world primate: A defining point in the evolution of oncogenic lymphocryptoviruses. J Virol. 2002;76:12055–12068. doi: 10.1128/JVI.76.23.12055-12068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivailler P, Jiang H, Cho Y-G, Quink C, Wang F. Complete nucleotide sequence of the rhesus lymphocryptovirus: Genetic validation for an Epstein–Barr virus animal model. J Virol. 2002;76:421–426. doi: 10.1128/JVI.76.1.421-426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parry C, Bell S, Minson T, Browne H. Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3 integrins. J Gen Virol. 2005;86:7–10. doi: 10.1099/vir.0.80567-0. [DOI] [PubMed] [Google Scholar]
- 23.Galdiero M, et al. Site-directed and linker insertion mutagenesis of herpes simplex virus type 1 glycoprotein H. J Virol. 1997;71:2163–2170. doi: 10.1128/jvi.71.3.2163-2170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian RP, Geraghty RJ. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc Natl Acad Sci USA. 2007;104:2903–2908. doi: 10.1073/pnas.0608374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Hutt-Fletcher LM. Point mutations in EBV gH that abrogate or differentially affect B cell and epithelial cell fusion. Virology. 2007;363:148–155. doi: 10.1016/j.virol.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschner AN, Lowrey AS, Longnecker R, Jardetzky TS. Binding site interactions between Epstein–Barr virus fusion proteins gp42 and gH/gL reveal a peptide that inhibits both epithelial and B-cell membrane fusion. J Virol. 2007;81:9216–9229. doi: 10.1128/JVI.00575-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva AL, Omerovic J, Jardetzky TS, Longnecker R. Mutational analysis of Epstein–Barr virus glycoprotein gp42 reveals functional domains not involved in receptor binding but required for membrane fusion. J Virol. 2004;78:5946–5956. doi: 10.1128/JVI.78.11.5946-5956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Borza CM, Hutt-Fletcher LM. Mutations of Epstein–Barr virus gH that are differentially able to support fusion with B cells or epithelial cells. J Virol. 2005;79:10923–10930. doi: 10.1128/JVI.79.17.10923-10930.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang R, Gu X, Nathan C, Hutt-Fletcher L. Laser-capture microdissection of oropharyngeal epithelium indicates restriction of Epstein–Barr virus receptor/CD21 mRNA to tonsil epithelial cells. J Oral Pathol Med. 2008;37:626–633. doi: 10.1111/j.1600-0714.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheppard D. Functions of pulmonary epithelial integrins: From development to disease. Physiol Rev. 2003;83:673–686. doi: 10.1152/physrev.00033.2002. [DOI] [PubMed] [Google Scholar]
- 31.Thomas GJ, Nystrom ML, Marshall JF. αvβ6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 32.Breuss JM, et al. Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 33.Munger JS, et al. The integrin αvβ6 binds and activates latent TGFβ1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 34.Egan JJ, et al. Epstein–Barr virus replication within pulmonary epithelial cells in cryptogenic fibrosing alveolitis. Thorax. 1995;50:1234–1239. doi: 10.1136/thx.50.12.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein–Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:510–513. doi: 10.1164/rccm.2103058. [DOI] [PubMed] [Google Scholar]
- 36.Tsukamoto K, et al. Involvement of Epstein–Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax. 2000;55:958–961. doi: 10.1136/thorax.55.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahmi H, Cochet C, Hmama Z, Opolon P, Joab I. Transforming growth factor beta 1 stimulates expression of the Epstein–Barr virus BZLF1 immediate-early gene product ZEBRA by an indirect mechanism which requires the MAPK kinase pathway. J Virol. 2000;74:5810–5818. doi: 10.1128/jvi.74.13.5810-5818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.di Renzo L, Altiok A, Klein G, Klein E. Endogenous TGF-beta contributes to the induction of the EBV lytic cycle in two Burkitt lymphoma cell lines. Int J Cancer. 1994;57:914–919. doi: 10.1002/ijc.2910570623. [DOI] [PubMed] [Google Scholar]
- 39.Liang C-L, Chen J-L, Hsu Y-PP, Ou JT, Chang Y-S. Epstein–Barr virus BZLF1 gene is activated by transforming growth factor-β through cooperativity of Smads and c-Jun/c-Fos proteins. J Biol Chem. 2002;277:23345–23357. doi: 10.1074/jbc.M107420200. [DOI] [PubMed] [Google Scholar]
- 40.Moody CA, Scott RS, Su T, Sixbey JW. Length of Epstein–Barr virus termini as a determinant of epithelial cell clonal emergence. J Virol. 2003;77:8555–8561. doi: 10.1128/JVI.77.15.8555-8561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo KW, Huang DP. Genetic and epigenetic changes in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:451–462. doi: 10.1016/s1044579x02000883. [DOI] [PubMed] [Google Scholar]
- 42.Turk SM, Jiang R, Chesnokova LS, Hutt-Fletcher LM. Antibodies to gp350/220 enhance the ability of Epstein–Barr virus to infect epithelial cells. J Virol. 2006;80:9628–9633. doi: 10.1128/JVI.00622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Toole TE, et al. Affinity modulation of the αIIbβ3 integrin (platelet GPIIb-IIIa) is an intrinsic property of the receptor. Cell Regul. 1990;1:883–893. doi: 10.1091/mbc.1.12.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinacker A, et al. Role of the integrin αvβ6 in cell attachment to fibronectin. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- 45.Omerovic J, Lev L, Longnecker R. The amino terminus of Epstein–Barr virus glycoprotein gH is important for fusion with B cells and epithelial cells. J Virol. 2005;79:12408–12415. doi: 10.1128/JVI.79.19.12408-12415.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gline SE, Cambier S, Govaerts C, Nishimura SL. A 50-Α separation of the integrin αvβ3 extracellular domain C termini reveals an intermediate activation state. J Biol Chem. 2004;279:54567–54572. doi: 10.1074/jbc.M406582200. [DOI] [PubMed] [Google Scholar]
- 47.Li QX, Turk SM, Hutt-Fletcher LM. The Epstein–Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oba DE, Hutt-Fletcher LM. Induction of antibodies to the Epstein–Barr virus glycoprotein gp85 with a synthetic peptide corresponding to a sequence in the BXLF2 open reading frame. J Virol. 1988;62:1108–1114. doi: 10.1128/jvi.62.4.1108-1114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulford DJ, Lowrey P, Morgan AJ. Co-expression of the Epstein–Barr virus BXLF2 and BKRF2 genes with a recombinant baculovirus produces gp85 on the cell surface with antigenic similarity to the native protein. J Gen Virol. 1995;76:3145–3152. doi: 10.1099/0022-1317-76-12-3145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.