Abstract
Δ9-Tetrahydrocannabinol (THC), the psychoactive component of marijuana, and other direct cannabinoid receptor (CB1) agonists produce a number of neurobehavioral effects in mammals that range from the beneficial (analgesia) to the untoward (abuse potential). Why, however, this full spectrum of activities is not observed upon pharmacological inhibition or genetic deletion of either fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), enzymes that regulate the two major endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG), respectively, has remained unclear. Here, we describe a selective and efficacious dual FAAH/MAGL inhibitor, JZL195, and show that this agent exhibits broad activity in the tetrad test for CB1 agonism, causing analgesia, hypomotilty, and catalepsy. Comparison of JZL195 to specific FAAH and MAGL inhibitors identified behavioral processes that were regulated by a single endocannabinoid pathway (e.g., hypomotility by the 2-AG/MAGL pathway) and, interestingly, those where disruption of both FAAH and MAGL produced additive effects that were reversed by a CB1 antagonist. Falling into this latter category was drug discrimination behavior, where dual FAAH/MAGL blockade, but not disruption of either FAAH or MAGL alone, produced THC-like responses that were reversed by a CB1 antagonist. These data indicate that AEA and 2-AG signaling pathways interact to regulate specific behavioral processes in vivo, including those relevant to drug abuse, thus providing a potential mechanistic basis for the distinct pharmacological profiles of direct CB1 agonists and inhibitors of individual endocannabinoid degradative enzymes.
Keywords: hydrolase, inhibitor, metabolism
N-arachidonoyl ethanolamine (anandamide or AEA) (1) and 2-arachidonoylglycerol (2-AG) (2, 3) are lipid transmitters that serve as endogenous ligands for the cannabinoid G-protein-coupled receptors CB1 and CB2. These lipids and receptors, along with the enzymes that biosynthesize and degrade AEA and 2-AG, form the endogenous cannabinoid (endocannabinoid) system, which regulates a diverse number of physiological processes in mammals, including pain, cognition, emotionality, neurodegeneration, feeding, and inflammation (4).
CB1 and CB2 receptors are also activated by Δ9-tetrahydrocannabinol (THC), the psychoactive component of marijuana (4). Most of the neurobehavioral effects of THC and other direct cannabinoid receptor agonists are mediated by the CB1 receptor (5, 6), likely reflecting its widespread and abundant expression in the nervous system (7, 8). CB1 agonism produces medicinally useful activities, such as analgesia, but also a number of undesirable side effects, including locomotor and cognitive impairments, as well as abuse liability. To date, it has proved difficult to uncouple these beneficial and untoward properties, thus limiting the therapeutic utility of direct CB1 agonists.
Inhibitors of AEA and 2-AG degradation offer a potentially attractive alternative strategy to stimulate the endocannabinoid system (9–12). Indeed, despite the structural similarity shared by AEA and 2-AG, distinct enzymes inactivate these lipids and thus serve as key points of control over specific endocannabinoid signaling events in vivo (13). Fatty acid amide hydrolase (FAAH) is the major degradative enzyme for AEA (14–16). In contrast, although several enzymes can hydrolyze 2-AG, this reaction appears to be primarily catalyzed by monoacylglycerol lipase (MAGL) in the nervous system (17). The designation of FAAH and MAGL as principal AEA and 2-AG degradative enzymes, respectively, is supported by a combination of genetic and pharmacological studies. For instance, FAAH(−/−) mice or animals treated with selective FAAH inhibitors possess >10-fold elevations in brain levels of AEA and other N-acyl ethanolamines (18–20) but no alterations in 2-AG (20–22). Conversely, mice treated with the selective MAGL inhibitor JZL184 show 8- to 10-fold increases in brain 2-AG levels without changes in AEA content (23, 24).
Pharmacological studies have revealed that selective FAAH and MAGL inhibitors produce an intriguing subset of the behavioral effects observed with direct CB1 agonists, including analgesia in multiple acute and chronic pain models (23, 25–27). Inhibition of MAGL, but not FAAH, also causes CB1-dependent hypomotility (23). However, neither FAAH nor MAGL inhibitors induce the cataleptic behavioral responses observed with direct CB1 agonists. Moreover, FAAH inhibitors have proved inactive in models of drug abuse, including drug discrimination assays (28). Collectively, these findings indicate that selective blockade of FAAH or MAGL can disassociate some of the beneficial and undesirable effects of CB1 activation.
Although the different pharmacological profiles of CB1 agonists and endocannabinoid hydrolase inhibitors are potentially exciting from a therapeutic perspective, they also present a frustrating mechanistic conundrum. Simply put, why do selective FAAH and MAGL inhibitors differ in their actions, and why do they produce only a subset of the behavioral effects observed with direct CB1 agonists? One possibility is that AEA and 2-AG signaling pathways interact in the nervous system to affect behavioral processes beyond those regulated by either endocannabinoid alone. To test this idea, we describe herein an inhibitor, JZL195, that inactivates both FAAH and MAGL with high efficacy and selectivity in vivo. Using JZL195, we discover behavioral processes in which elevations in AEA and 2-AG combine to give additive effects. These behaviors include catalepsy and THC-like drug discrimination responses, indicating that some of the untoward effects of direct CB1 agonists constitute points of crosstalk between endogenous AEA and 2-AG signaling pathways.
Results
Development of JZL195, a Selective Dual Inhibitor of AEA and 2-AG Hydrolysis.
Previous attempts to characterize dual inhibition of FAAH and MAGL have used fluorophosphonate reagents, such as isopropyldodecylfluorophosphonate (IDFP) (29). However, interpreting the pharmacology of IDFP is difficult for several reasons. First, IDFP inhibits many serine hydrolases, including not only FAAH and MAGL but also the alternative 2-AG hydrolase ABHD6, hormone sensitive lipase (HSL), neuropathy target esterase (NTE), and the ether–lipid metabolic enzyme KIAA1363 (29). Second, IDFP displaces CB1 receptor agonist binding with an IC50 of 2 nM and stimulates GTP binding at CB1 with an IC50 of 3 μM (30). Third, mice acutely treated with IDFP die within 48 h after treatment by a non-CB1 mechanism (29). Because of these shortcomings, it has been difficult to evaluate the pharmacological impact of concurrently elevating both 2-AG and AEA in vivo. With this goal in mind, we sought to create a more selective dual FAAH/MAGL inhibitor.
We designed dual FAAH/MAGL inhibitors based on an electrophilic N-carbonyl piperidine/piperazine structural motif common to both the MAGL-selective inhibitor JZL184 (23, 24) and the FAAH-selective inhibitors PF-622 (31) and PF-3845 (19) (Fig. 1A). We then performed iterative cycles of medicinal chemistry around an N-substituted piperazine carbamate scaffold, using the serine hydrolase-directed probe fluorophosphonate rhodamine (FP-Rh) (32) in competitive activity-based protein profiling (ABPP) screens (33) to concurrently optimize inhibitor potency and selectivity in mouse brain proteomes. These competitive ABPP studies identified neuropathy target esterase (NTE) as a common “off-target” for many N-carbonyl piperidine carbamates [Fig. 1B and Supporting Information (SI) Table S1]. Because NTE is required for mouse viability (34) and is responsible for the delayed onset toxicity of nerve agents (35), we sought to minimize the activity of dual FAAH/MAGL inhibitors toward this additional brain enzyme. This effort culminated in the generation of JZL195, an N-(3-phenoxybenzyl) piperazine carbamate (Fig. 1A and Fig. S1), that produced near-complete blockade of FP-Rh labeling of both mouse brain FAAH and MAGL at concentrations as low as 100 nM (IC50 values of 13 and 19 nM, respectively; Fig. 1C and Fig. S2), while showing only modest and incomplete inhibitory activity against NTE (IC50 > 5 μM, ≈50% maximal inhibition; Fig. 1C, Fig. S2). At higher concentrations, JZL195 inhibited ABHD6 (Fig. 1C and Fig. S2), but not any of the other brain serine hydrolases detected in our competitive ABPP assays (Fig. 1C). We furthermore confirmed that JZL195 (100 μM) does not inhibit mouse brain acetylcholinesterase activity (Fig. S3). Substrate hydrolysis assays using recombinant enzymes expressed in COS7 cells or brain membrane preparations confirmed that JZL195 inhibited both FAAH and MAGL with high potency, affording IC50 values of 2 and 4 nM, respectively (Fig. 1 D and E). At the highest concentrations tested, JZL195 blocked ≈95% of the brain 2-AG hydrolysis activity (Fig. 1E), which was a slightly greater effect than has been observed with the MAGL-selective inhibitor JZL184, which showed 85% maximal blockade of brain 2-AG hydrolysis activity (23). This finding is consistent with previous work indicating that ABHD6 (and possibly FAAH) also contributes to total brain 2-AG hydrolysis activity (17). JZL195 did not show appreciable binding to either the CB1 or CB2 receptor (IC50 values >20 μM) and did not increase [35S]GTPγS binding over basal at any concentration tested (Fig. S4). Finally, JZL195 also inhibited rat and human FAAH and MAGL enzymes with IC50 values in the range of ≈10–100 nM based on competitive ABPP assays (Fig. S2).
Fig. 1.
Development of a dual FAAH and MAGL inhibitor, JZL195. (A) Structures of the FAAH-selective inhibitors PF-622 and PF-3845, the MAGL-selective inhibitor JZL184, and dual FAAH-MAGL inhibitors. (B) Serine hydrolase activity profiles of brain membranes after incubation with dual FAAH-MAGL inhibitors (1 μM) as determined by competitive ABPP using the serine hydrolase-directed probe fluorophosphonate-rhodamine (FP-Rh). (C) Concentration-dependent effects of JZL195 on mouse brain membrane serine hydrolases. (D and E) Blockade of AEA (D) and 2-AG (E) hydrolysis activity for MAGL and FAAH, respectively, recombinantly expressed in COS7 cells (black traces), or from mouse brain membranes (blue traces). For B–E, samples were treated with inhibitor for 30 min at 37 °C before addition of FP-Rh (1 μM) (B and C), 100 μM AEA (D), or 100 μM 2-AG (E). For D and E, data are presented as means ± SEM of three independent experiments.
This initial characterization suggested that JZL195 was a potent and selective inhibitor of MAGL and FAAH in brain proteomes, with additional potential to block ABHD6, an enzyme that has also been shown to hydrolyze 2-AG (17). To test whether these features of JZL195 were maintained in vivo, we treated mice with JZL195 (20 mg·kg−1, i.p., 2 h), killed them, and analyzed their brain proteomes using a shotgun liquid chromatography–mass spectrometry (LC-MS) platform, termed ABPP-MudPIT (36), that displays enhanced resolution and sensitivity compared with gel-based ABPP methods. ABPP-MudPIT confirmed that JZL195 caused near-complete inhibition of MAGL, FAAH, and ABHD6 but did not inhibit any of the other ≈40 serine hydrolases identified in the mouse brain proteome (Fig. S5 and Table S2), including NTE, which was also confirmed by gel-based ABPP (Fig. S6).
We observed time-dependent inhibition of MAGL and FAAH by JZL195, consistent with a covalent mechanism of inactivation, and therefore measured kobs [I]−1 values for JZL195 against endocannabinoid hydrolases in the mouse brain membrane proteome. JZL195 showing slightly higher kobs [I]−1 values for FAAH (8000 ± 500 M−1·s−1) and MAGL (5700 ± 600 M−1·s−1) compared to ABHD6 (3600 ± 500 M−1·s−1), in good agreement with our previously determined IC50 values (Fig. S2). This analysis also revealed that the FAAH and MAGL inhibitory values exhibited by JZL195 were similar to those of the selective inhibitors PF-3845 and JZL184, respectively (Table S3).
JZL195 Inhibits Endocannabinoid Hydrolysis and Elevates 2-AG and AEA Levels in Vivo.
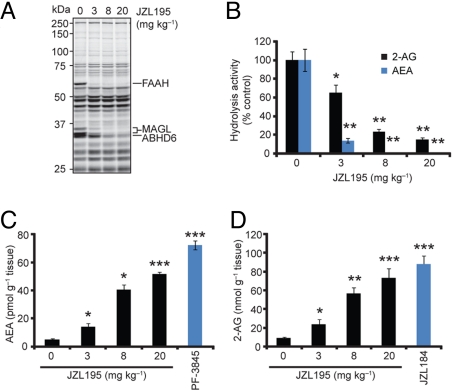
We next evaluated the hydrolysis rates and endogenous levels of AEA and 2-AG in brains from mice treated with varying doses of JZL195. Male C57BL/6 mice administered JZL195 (3–20 mg·kg−1, i.p., 4 h) showed dose-dependent reductions in brain FAAH, MAGL, and ABHD6 activities as judged by gel-based ABPP (Fig. 2A) that correlated with near-complete inhibition of both AEA and 2-AG hydrolysis (Fig. 2B). These effects were accompanied by dramatic elevations in brain levels of AEA (Fig. 2C) and 2-AG (Fig. 2D) that approximated the increases observed with selective FAAH (PF-3845, 10 mg·kg−1, i.p.) or MAGL (JZL184, 40 mg·kg−1, i.p.) inhibitors. We observed a trend of increased endocannabinoid levels at 20 mg·kg−1 compared with 8 mg·kg−1 JZL195, which may reflect a more rapid rate of FAAH and MAGL inhibition at the higher dose of JZL195, providing a longer time period for endocannabinoid accumulation.
Fig. 2.
JZL195 dose-responsively inhibits FAAH and MAGL in vivo and raises brain AEA and 2-AG levels. (A and B) Serine hydrolase activity profiles (A) and 2-AG and AEA hydrolytic activities (B) of brain membranes prepared from mice treated with JZL195 at the indicated doses (3–20 mg·kg−1, i.p.) for 4 h. (C and D) Brain levels of AEA (C) and 2-AG (D) from mice treated with JZL195 at the indicated doses (3–20 mg·kg−1, i.p.) for 4 h. For C and D, data from mice treated with selective MAGL (JZL184, 40 mg·kg−1, i.p.) and FAAH (PF-3845, 10 mg·kg−1, i.p.) inhibitors are also shown, respectively. For B–D, *, P < 0.05; **, P < 0.01; ***, P < 0.001 for inhibitor-treated versus vehicle-treated animals. Data are presented as means ± SEM. n = 3–5 mice per group.
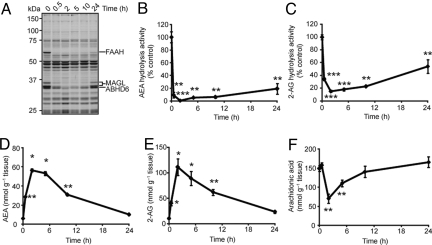
A time course analysis of mice given one administration of JZL195 (20 mg·kg−1, i.p.) revealed that blockade of FAAH and MAGL lasted at least 10 h as judged by gel-based ABPP or AEA and 2-AG hydrolysis assays (Fig. 3 A–C). Brain AEA (Fig. 3D) and 2-AG (Fig. 3E) levels were also sustained over this time course to a degree that corresponded well with extent of FAAH and MAGL inhibition. It has been previously shown that only partial (≈15–30%) recovery of these enzyme activities is required to fully restore brain levels of their endocannabinoid substrates (23, 37). JZL195 treatment also decreased brain arachidonic acid to a level that was, in stoichiometry, equivalent to the elevations in brain 2-AG (Fig. 3F). This result is consistent with previous findings indicating that 2-AG is a physiologic precursor for arachidonic acid in the brain (23). Similar data were obtained after oral administration of JZL195 (Fig. S7), indicating that this compound can be administered by multiple routes to inactivate both FAAH and MAGL in vivo. Importantly, unlike animals treated with the general hydrolase inhibitor IDFP, which die within 2 days after a single administration (29), mice could be chronically treated with JZL195 for several days (once per day for 6 days) without any lethality or overt signs of toxicity. Together, these data indicate that JZL195 is a highly efficacious polypharmacology tool to simultaneously augment brain levels of AEA and 2-AG in vivo.
Fig. 3.
Time course analysis of inhibitory activity of JZL195 in vivo. (A–C) Serine hydrolase activity profiles (A) and AEA (B) and 2-AG (C) hydrolytic activities of brain membranes prepared from mice treated with JZL195 (20 mg·kg−1, i.p.) for the indicated times. (D–F) Brain levels of AEA (D), 2-AG (E), and arachidonic acid (F) from mice treated with JZL195 (20 mg·kg−1, i.p.) for the indicated times. For B–F, *, P < 0.05; **, P < 0.01, ***, P < 0.001 for inhibitor-treated versus vehicle-treated control animals. Data are presented as means ± SEM. n = 3–5 mice per group.
Behavioral Effects of JZL195—Evidence for Endocannabinoid Crosstalk in Vivo.
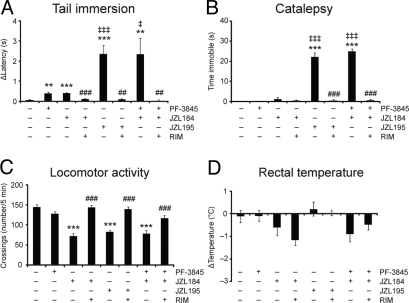
To assess whether concurrent elevations in AEA and 2-AG produced CB1-dependent behavioral effects, we screened JZL195-treated mice in the “tetrad test” for cannabinoid behavior, consisting of assays for antinociception, catalepsy, hypomotility, and hypothermia (38). For these studies, we also treated groups of mice with the specific FAAH and MAGL inhibitors PF-3845 (10 mg·kg−1, i.p.) and JZL184 (40 mg·kg−1, i.p.), respectively. As has been reported previously (20, 23, 26), mice treated with selective FAAH or MAGL inhibitors showed a small, but significant antinociceptive response in the tail-immersion assay of thermal pain sensation (Fig. 4A). Interestingly, JZL195 (20 mg·kg−1, i.p.) produced a much greater antinociceptive response in this assay compared with inhibitors of either FAAH or MAGL alone (Fig. 4A). A similar profile was observed in the acetic acid writhing test of visceral pain sensation, where JZL195 produced a more pronounced antinociceptive effect than either JZL184 or PF-3845 (Fig. S8). In the bar test, JZL195-treated animals displayed marked catalepsy, whereas neither PF-3845 nor JZL184 were active in this assay (Fig. 4B). We also observed hyperreflexia for several of the JZL184- and JZL195-treated animals when assayed on the bar test (Table S4). This behavior, which has been described as “popcorning” and has been observed for direct CB1 agonists (39), is exemplified by a reluctance to grasp the bar and accompanied instead by biting at the bar, urination, and/or extreme jumping (Movie S1). Both JZL195 and JZL184, but not PF-3845, also caused hypomotility in an open field test (Fig. 4C), suggesting that this phenotype, like hyperreflexia, is primarily driven by the 2-AG/MAGL pathway. In contrast, none of the inhibitors produced hypothermic effects (Fig. 4D). Finally, all of the behavioral effects observed with JZL195 were completely blocked by pretreatment with the CB1 antagonist rimonabant (3 mg·kg−1, i.p.) (Fig. 4 A–D).
Fig. 4.
Comparison of the effects of single versus dual inhibitors of FAAH and MAGL in the tetrad test for cannabinoid behavior. (A) Selective FAAH (PF-3845, 10 mg·kg−1, i.p.) and MAGL (JZL184, 40 mg·kg−1, i.p.) inhibitors produce antinociceptive effects in the tail immersion assay of thermal pain sensation. The dual FAAH/MAGL inhibitor JZL195 (20 mg·kg−1, i.p) or dual treatment with individual inhibitors (JZL184 + PF-3845) produces a much greater antinociceptive effect in this assay. Average baseline latency was 0.56 ± 0.04 s and did not differ among treatment groups. (B) JZL195, or JZL184 + PF-3845, but not PF-3845 or JZL184 alone, produces robust catalepsy in the bar test. (C) JZL195 and JZL184, but not PF-3845, produce hypomotility an open-field test. (D) JZL195, JZL184, and PF-3845 do not cause hypothermia. For A–C, all FAAH/MAGL inhibitor effects were blocked by pretreatment with the CB1 antagonist rimonabant (RIM, 3 mg·kg−1, i.p). **, P < 0.01, ***, P < 0.001 for vehicle–vehicle versus vehicle–JZL195, vehicle–JZL184, or vehicle–PF-3845 treated mice; †, P < 0.05; †††, P < 0.001 for vehicle–JZL195 versus vehicle–JZL184 or vehicle–PF-3845 treated mice; ##,P < 0.01, ###,P < 0.001; for vehicle–JZL195, vehicle–JZL184, or JZL184/PF-3845 versus rimonabant–JZL195, rimonabant–JZL184, or rimonabant–JZL184-PF 3845-treated respectively. Data are presented as means ± SEM. n = 6–14 mice per group.
The marked CB1-dependent antinociception and catalepsy observed in JZL195-treated animals suggested that dual inhibition of FAAH and MAGL coordinately stimulates AEA and 2-AG signaling pathways in vivo to regulate specific behavioral processes. To fortify this premise and test whether inhibition of ABHD6 also contributes to these effects, we evaluated two additional cohorts of mice in the tetrad test: (i) mice treated with both PF-3845 and JZL184, and (ii) FAAH(−/−) mice treated with JZL184. Both of these groups of dual FAAH/MAGL-inhibited animals showed the same pattern of behavioral responses in the tetrad test that was observed for JZL195-treated animals (Fig. 4 A–D and Fig. S9). As neither PF-3845 nor JZL184 show substantial activity against ABHD6 (19, 23), we conclude that selective blockade of both FAAH and MAGL is sufficient to produces additive endocannabinoid activity in pain and catalepsy assays.
Dual Inhibition of MAGL and FAAH Produces THC-Like Effects in Drug Discrimination.
The outcome of our pharmacological studies in the tetrad test suggested that dual inhibition of FAAH and MAGL more closely mimics the effects of direct CB1 agonists compared to selective blockade of either enzyme alone. To directly address whether animals perceive dual FAAH/MAGL inhibition as similar to direct CB1 agonism, we used a drug discrimination paradigm. Drug discrimination serves as an animal model for marijuana intoxication, and drugs that substitute for THC in this assay are predicted to have marijuana-like subjective effects in humans (40). In this model, mice are trained to distinguish between THC (5.6 mg·kg−1, s.c.) and vehicle treatment by administration of the appropriate drug to animals upon inserting their snout for food reward into a THC- or vehicle-associated nose poke aperture. After a training period of 34 weeks (representing 170 trials), mice were given a single dose of vehicle, THC (5.6 mg·kg−1, i.p.), or JZL195 (40 mg·kg−1, i.p.) and were asked to discriminate the drugs as THC- or vehicle-like over a time period of 15 min. Vehicle- and THC-treated animals responded appropriately by inserting their snout into the nose poke associated with vehicle or THC administration, respectively, for food reward (Fig. 5A). Remarkably, mice treated with JZL195 showed THC-appropriate responses >80% of the time, and this behavioral pattern was completely blocked by pretreatment with rimonabant (3 mg·kg−1, i.p.) (Fig. 5A). A similar level of THC-appropriate responding was observed for FAAH(−/−) mice treated with JZL184 (Fig. 5B). In contrast, neither FAAH(−/−) mice nor JZL184-treated FAAH(+/+) mice showed strong THC-appropriate responding, although the latter animals showed partial generalization to THC (Fig. 5B), possibly reflecting a larger contribution of the MAGL/2-AG pathway to this behavior. These data, along with previous studies using selective FAAH inhibitors (28, 41), indicate that blockade of individual endocannabinoid degradation pathways is insufficient to promote full THC-like discriminative stimulus effects. In contrast, dual blockade of both FAAH and MAGL mimicked treatment with a direct CB1 agonist, providing evidence that AEA and 2-AG pathways can crosstalk in vivo to promote the discriminative stimulus effects of an illicit drug.
Fig. 5.
Comparison of the behavioral effects of single versus dual inhibitors of FAAH and MAGL in a THC-appropriate drug discrimination assay. (A) JZL195 (40 mg·kg−1, i.p.) produces THC-appropriate nose pokes at a magnitude similar to THC (5.6 mg·kg−1, s.c.), which is reversed by rimonabant (3 mg·kg−1, i.p.). (B) JZL184 (40 mg·kg−1, i.p.) also shows full substitution for THC in FAAH(−/−) mice, but only incomplete (partial) substitution in FAAH(+/+) mice. For both A and B, an 80% cut-off was used to assign THC-appropriate responses. *, P < 0.05; ***, P < 0.001 for vehicle–vehicle versus vehicle–JZL195 or vehicle–JZL184 treated mice; #,P < 0.05; ###,P < 0.001; for vehicle–JZL195 or vehicle–JZL184 versus rimonabant–JZL195 or rimonabant–JZL184 treated mice, respectively. Data are presented as means ± SEM. n = 6–14 mice per group.
Discussion
Neurotransmitter systems commonly show diversity in their receptor composition (42). For instance, acetylcholine, glutamate, GABA, and monoamines each activate multiple GPCRs and ligand-gated ion channels. Beyond obvious differences in signaling mechanism (e.g., metabotropic versus ionotropic), these receptors also show distinct cellular and subcellular distributions throughout the brain that contribute to their unique roles in neurophysiology and behavior. Pharmacological dissection of the specific functions of such neurotransmitter systems has been achieved in large part through the development of subtype selective receptor agonists and antagonists. The endocannabinoid system presents a strikingly different case, in which multiple ligands (AEA, 2-AG) interact with a single, abundantly expressed receptor (CB1) throughout the nervous system. As such, the physiologic regulation and functions of specific endocannabinoid pathways in the brain is dictated in large part by the respective enzymes that control AEA and 2-AG metabolism.
The recent advent of selective and efficacious inhibitors for each of the two major endocannabinoid degradative enzymes, FAAH and MAGL, has facilitated the functional investigation of AEA and 2-AG signaling pathways, respectively. Despite these advances, our understanding of the unique, and possibly overlapping activities performed by AEA and 2-AG in vivo remains quite limited. Here, we have introduced a complementary “polypharmacology” probe, the dual FAAH/MAGL inhibitor JZL195, for studying simultaneous elevations in both AEA and 2-AG signaling in vivo. Using functional proteomic methods, we show that JZL195 displays high selectivity for FAAH and MAGL in the nervous system, inhibiting only a single additional serine hydrolase target, the alternative 2-AG hydrolase ABHD6. Mice treated with JZL195 show dramatic and sustained inhibition of brain FAAH and MAGL that correlated with ≈10-fold elevations in endogenous AEA and 2-AG levels. These effects on the AEA/FAAH and 2-AG/MAGL systems were similar in magnitude and duration to those observed with selective inhibitors of each individual endocannabinoid pathway (PF-3845 and JZL184, respectively). We could thus directly compare the behavioral effects of single versus dual augmentation of AEA and 2-AG signaling pathways.
Our in vivo pharmacology studies have provided compelling insights into the roles that each endocannabinoid pathway plays in specific behavioral processes. Certain behaviors, such as hypomotility and hyperreflexia, were similarly affected by JZL184 and JZL195 and were not affected by PF-3845, indicating that they are exclusively modulated by the 2-AG/MAGL pathway. Conversely, antinociception was observed in mice treated with either JZL184 or PF-3845, and this effect was dramatically enhanced in mice treated with JZL195 (or mice treated with both JZL184 and PF-3845). This finding is consistent with previous literature indicating that both AEA and 2-AG can independently regulate pain sensation (19, 20, 23, 26), and suggests further that their individual activities are profoundly augmented upon simultaneous blockade of their respective degradative enzymes. An even more striking effect of dual FAAH/MAGL inhibition was observed in the catalepsy test, in which neither JZL184 nor PF-3845 had an effect, but JZL195 (and JZL184 + PF-3845) showed robust activity. The cataleptic effect produced by dual blockade of FAAH and MAGL was intriguing in that it differed qualitatively from the catalepsy caused by direct CB1 agonists. Unlike mice treated with direct CB1 agonists, which typically show a flattened posture, awkward gait, and profound immobility in their home cage environment (Movie S2), dual FAAH/MAGL-disrupted mice exhibited a normal, hunched posture and periodic bouts of movement (without noticeable alterations in gait) in their home cages (Movie S3). However, once placed on the bar apparatus, these mice adopted a CB1 agonist-like cataleptic response, as judged by complete immobility on the bar that was occasionally accompanied by one raised hind limb (Movie S3). These data suggest that dual FAAH/MAGL inhibition produces a “context-dependent” cataleptic response that results in treated animals failing to remove themselves after placement in an awkward position. More generally, the heightened responses of dual FAAH/MAGL-inhibited animals in both pain and catalepsy assays point to specific behavioral processes that are regulated by endocannabinoid crosstalk in vivo.
Having uncovered evidence of CB1-dependent interactions between AEA and 2-AG pathways in multiple components of the tetrad test, we wondered whether such endocannabinoid crosstalk might also influence processes related to drug abuse. We examined this possibility using a drug discrimination paradigm (43), where mice were first trained to distinguish between the CB1 agonist THC and vehicle and then administered individual or dual inhibitors of FAAH and MAGL to determine whether these inhibitors produce “marijuana-like” effects. Dual FAAH/MAGL blockade, but not selective FAAH or MAGL inhibition, was found to cause profound THC-like discriminative stimulus effects that were reversed by the CB1 antagonist rimonabant. Thus, as was observed for pain and catalepsy, AEA and 2-AG pathways interact in a drug discrimination paradigm to produce effects more similar to THC than elevation of either endocannabinoid alone. Future work is required to determine whether endocannabinoid crosstalk will also influence drug discrimination in higher mammals (e.g., primates); however, it seems appropriate to briefly speculate here on some of the potential basic and translational research implications of our findings. First, our findings suggest that endocannabinoid crosstalk may play a role in drug dependence and addiction, which points to the possible clinical liabilities of dual inhibitors of FAAH and MAGL. Conversely, these data provide a mechanistic basis for understanding the lack of abuse potential observed to date with selective FAAH inhibitors. Unlike direct CB1 agonists, FAAH inhibitors have not exhibited activity in models of drug abuse (28) and, on the contrary, have even shown beneficial effects in reducing THC-precipitated withdrawal (44) and the reinforcing effects of nicotine in rats (45), although FAAH blockade has been shown to worsen nicotine withdrawal in mice (46). We hypothesize that the limited abuse potential of selective FAAH inhibitors compared to direct CB1 agonists may reflect a requirement for dual stimulation of AEA and 2-AG pathways to produce the subjective effects of marijuana. Finally, we should also mention that endocannabinoids can interact with protein targets outside of the classical cannabinoid system, for example, TRPV1 (41), and these interactions may also be affected by selective or dual inhibitors of MAGL and FAAH.
In summary, we have shown that the dual FAAH/MAGL inhibitor JZL195 serves as a powerful pharmacological probe to evaluate the behavioral impact of simultaneous elevations in the two principal endocannabinoids AEA and 2-AG. That several instances of enhanced activity were found for JZL195 compared to selective FAAH or MAGL inhibitors argues that AEA and 2-AG signaling pathways engage in extensive interactions in the mammalian nervous system. Whether these interactions occur intrasynaptically (i.e., co-signaling at the same CB1 receptors), intersynaptically (i.e., via crosstalk between distinct AEA- and 2-AG-regulated neuronal circuits), or by a combination of both mechanisms, remains unknown. Regardless of the precise mode of endocannabinoid crosstalk, our data illuminate a provocative and unusual feature of this neurotransmitter system, where ligand (rather than receptor) diversification is exploited to regulate specific mammalian behaviors.
Materials and Methods
Chemical Synthesis of JZL195.
See SI Methods for details on the synthesis of JZL195 and related compounds.
Competitive ABPP Experiments.
Inhibitor selectivity using mouse or rat membrane proteomes was examined using a competitive ABPP method as described previously (33). See SI Methods for details.
Enzyme Activity Assays.
MAGL and FAAH substrate hydrolysis assays were performed using previously described LC-MS assays (17). See SI Methods for details.
In Vitro studies with Inhibitors.
Standard assays were performed by preincubating protein samples with JZL195 for 30 min at 37 °C before the addition of substrate or ABPP probe. Concentration-dependent inhibition curves were obtained from substrate assays or integrated gel band intensities (ImageJ) and were fit using Prism software (GraphPad) to obtain effector concentration for half-maximal response values (IC50). kobs [I]−1 values were obtained using a previously described procedure (23). See SI Methods for details.
In Vivo Studies with Inhibitors.
JZL184, JZL195, PF-3845, or rimonabant were administered to mice as described previously (24). See SI Methods for details.
Measurement of Brain Lipids.
Brain lipid measurements were determined using a previously described procedure (24). See SI Methods for details.
ABPP-MudPIT Analysis of Serine Hydrolases Targeted by JZL195 in Vivo.
ABPP-MudPIT studies were performed following previously described methods (36). See SI Methods for details.
Behavioral Studies.
Mice were evaluated in the tetrad test for cannabinoid effects and in drug discrimination assays as detailed in the SI Methods. Animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committees of the Scripps Research Institute and Virginia Commonwealth University.
Supplementary Material
Acknowledgments.
We thank Tianyang Ji for assistance with the transfection of enzyme cDNAs and the Cravatt laboratory for helpful discussion and critical reading of the manuscript. This work was supported by the US National Institutes of Health (DA017259, DA009789, DA025285, DA03672, DA005274, and T23DA07027, DA014277), the Helen L. Dorris Institute Child and Adolescent Neuro-Psychiatric Disorder Institute, and the Skaggs Institute for Chemical Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909411106/DCSupplemental.
References
- 1.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 3.Sugiura T, et al. 2-Arachidonylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 4.Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids and related compounds: Walking back and forth between plant natural products and animal physiology. Chem Biol. 2007;14:741–756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledent C, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 7.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 8.Egertova M, Giang DK, Cravatt BF, Elphick MR. A new perspective on cannabinoid signalling: Complimentary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc R Soc Lond B. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravatt BF, Lichtman AH. Fatty acid amide hydrolase: An emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol. 2003;7:469–475. doi: 10.1016/s1367-5931(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 10.Piomelli D. The endocannabinoid system: A drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- 11.Jonsson KO, Holt S, Fowler CJ. The endocannabinoid system: Current pharmacological research and therapeutic opportunities. Basic Clin Pharmacol Toxicol. 2006;98:124–134. doi: 10.1111/j.1742-7843.2006.pto_376.x. [DOI] [PubMed] [Google Scholar]
- 12.Koutek B, et al. Inhibitors of arachidonyl ethanolamide hydrolysis. J Biol Chem. 1994;269:22937–22940. [PubMed] [Google Scholar]
- 13.Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch DG, Ueda N, Yamamoto S. The fatty acid amide hydrolase (FAAH) Prostaglandins Leuko Essent Fatty Acids. 2002;66:201–210. doi: 10.1054/plef.2001.0358. [DOI] [PubMed] [Google Scholar]
- 16.McKinney MK, Cravatt BF. Structure and function of Fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–432. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 17.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cravatt BF, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn K, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 21.Saghatelian A, et al. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry. 2004;43:14332–14339. doi: 10.1021/bi0480335. [DOI] [PubMed] [Google Scholar]
- 22.Osei-Hyiaman D, et al. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81:273–282. doi: 10.1159/000087925. [DOI] [PubMed] [Google Scholar]
- 23.Long JZ, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo R, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- 26.Lichtman AH, et al. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: Evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- 27.Kinsey SG, et al. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solinas M, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- 29.Nomura DK, et al. Activation of the endocannabinoid system by organophosphorus nerve agents. Nat Chem Biol. 2008;4:373–378. doi: 10.1038/nchembio.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura DK, et al. Monoacylglycerol lipase regulates 2-arachidonoylglycerol action and arachidonic acid levels. Bioorg Med Chem Lett. 2008;18:5875–5878. doi: 10.1016/j.bmcl.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn K, et al. Novel mechanistic class of fatty acid amide hydrolase inhibitors with remarkable selectivity. Biochemistry. 2007;46:13019–13030. doi: 10.1021/bi701378g. [DOI] [PubMed] [Google Scholar]
- 32.Patricelli MP, Giang DK, Stamp LM, Burbaum JJ. Direct visualization of serine hydrolase activities in complex proteome using fluorescent active site-directed probes. Proteomics. 2001;1:1067–1071. doi: 10.1002/1615-9861(200109)1:9<1067::AID-PROT1067>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Leung D, Hardouin C, Boger DL, Cravatt BF. Discovering potent and selective inhibitors of enzymes in complex proteomes. Nat Biotechnol. 2003;21:687–691. doi: 10.1038/nbt826. [DOI] [PubMed] [Google Scholar]
- 34.Winrow CJ, et al. Loss of neuropathy target esterase in mice links organophosphate exposure to hyperactivity. Nat Genet. 2003;33:477–485. doi: 10.1038/ng1131. [DOI] [PubMed] [Google Scholar]
- 35.Johnson MK, Glynn P. Neuropathy target esterase (NTE) and organophosphorus-induced delayed polyneuropathy (OPIDP): Recent advances. Toxicol Lett. 1995;82:459–463. doi: 10.1016/0378-4274(95)03495-1. [DOI] [PubMed] [Google Scholar]
- 36.Jessani N, et al. A streamlined platform for high-content functional proteomics of primary human specimens. Nat Methods. 2005;2:691–697. doi: 10.1038/nmeth778. [DOI] [PubMed] [Google Scholar]
- 37.Fegley D, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 38.Smith PB, et al. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- 39.Patel S, Hillard CJ. Cannabinoid CB(1) receptor agonists produce cerebellar dysfunction in mice. J Pharmacol Exp Ther. 2001;297:629–637. [PubMed] [Google Scholar]
- 40.Balster RL, Prescott WR. Delta 9-tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev. 1992;16:55–62. doi: 10.1016/s0149-7634(05)80051-x. [DOI] [PubMed] [Google Scholar]
- 41.Gobbi G, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel GJ, Agranoff BW, Albers RW, Molinoff PB. Basic Neurochemistry. New York: Raven Press; 1994. [Google Scholar]
- 43.Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1:1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- 44.Schlosburg JE, et al. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009;11:342–352. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melis M, et al. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 2008;28:13985–13994. doi: 10.1523/JNEUROSCI.3221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.