Summary
Meiosis is a highly specialized cell division that requires a significant reorganization of the canonical cell cycle machinery and the use of meiosis-specific cell cycle regulators. The Anaphase Promoting Complex (APC) and a conserved APC adaptor, Cdc20/Fzy are required for anaphase progression in mitotic cells. The APC has also been implicated in meiosis, though it is not yet understood how it mediates these non-canonical divisions. Cort is a diverged Cdc20 homologue expressed in the female germ-line of Drosophila where it functions with the Cdk1-interacting protein, Cks30A, to drive anaphase in meiosis II. Here we show that Cort functions together with the canonical mitotic APC adaptor, Cdc20/Fzy to target the three mitotic cyclins for destruction in the egg and drive anaphase progression in both meiotic divisions. In addition to controlling cyclin destruction globally in the egg, Cort and Fzy appear to both be required for local destruction of cyclin B on spindles. We find that cyclin B associates with spindle microtubules throughout meiosis I and meiosis II, and dissociates from the meiotic spindle in anaphase II. Fzy and Cort are required for this loss of cyclin B from the meiotic spindle. Our results lead to a model in which the germline specific APCCort cooperates with the more general APCFzy both locally on the meiotic spindle, and globally in the egg cytoplasm to target cyclins for destruction and drive progression through the two meiotic divisions.
Keywords: Fzy, Cort, Cks, APC, Drosophila, cell cycle, meiosis
Introduction
The cell divisions of female meiosis and the ensuing mitotic cycles of early embryogenesis represent two examples of non-canonical cell cycles. Meiosis differs from the typical mitotic cycle in several respects. Most notably, two divisions occur in sequence without an intervening S-phase, resulting in the production of 4 haploid gametes. Also, the first meiotic division involves segregation of homologous chromosomes and occurs without sister chromatid segregation, while the 2nd meiotic division involves the segregation of sister chromatids as in mitosis. The regulation of meiosis requires a significant reorganization of the canonical cell cycle machinery and the use of a number of meiosis specific cell cycle regulators (reviewed in (Marston and Amon, 2004)). One example is in the regulation of anaphase, the coordinated series of events that results in the segregation of chromosomes to produce two daughter nuclei. In mitoticaly dividing cells, anaphase progression depends critically on the inactivation of the mitotic kinase, Cdk1 and the release of sister chromatid cohesion through destruction of cohesin complexes. These events are controlled by an E3 Ubiquitin ligase, the Anaphase Promoting Complex (APC) in association with an adaptor protein Cdc20 or Fzy (reviewed in (Peters, 2002)), that targets mitotic cyclins and securin for destruction. The role of the APC in meiosis appears to be more complex than in mitotic cells. For example the APC only partially inhibits Cdk1 activity between meiotic divisions (Gross et al., 2000) and sister chromatid cohesion persists at centromeres through anaphase I (Katis et al., 2004) (Kitajima et al., 2004). It is not yet clear how the activity of the APC is modified in these specialized cell divisions.
In most eukaryotes, the meiotic cell cycle is followed by another atypical cell cycle, the cleavage divisions of early embryogenesis. In Drosophila, these cleavage cycles occur as a series of synchronized, rapid nuclear divisions and are referred to as syncytial divisions. The female meiotic cell cycle is not only temporally closely linked to the syncytial mitotic cell cycle, but it also occurs within a shared cytoplasm, that of the egg. Therefore these two distinct cell cycles share a common pool of cell cycle regulators and may share common strategies for spatially and temporally regulating cell cycle progression within a syncytium.
One way in which the syncytial cell cycle is modified appears to be in the limited destruction of mitotic cyclins in each cell cycle, apparently by restricting their destruction to the area of the mitotic nuclei. While there is evidence that cyclin destruction is spatially regulated in somatic cells (Kallio et al., 1998; Rieder et al., 1997), this strategy appears to be of particular importance in the syncytial embryo of Drosophila as a means to conserve mitotic cyclins for the duration of the rapid syncytial divisions. Several lines of evidence suggest that at least one cyclin, cyclin B, undergoes limited local destruction on mitotic spindles in the syncytial embryo (Edgar et al., 1994; Huang and Raff, 1999; Raff et al., 2002; Su et al., 1998). It is not yet known what mediates this local cyclin B destruction, and it is also not known if this is unique to the syncytial mitotic cell cycle or if it occurs in the preceding meiotic divisions.
Drosophila represents an excellent model system for understanding how the canonical cell cycle machinery is developmentally modified, and novel cell cycle regulators are utilized to control meiosis and syncytial divisions. Cort encodes a Cdc20/Cdh1 related protein that appears to be required specifically in female meiosis (Chu et al., 2001; Lieberfarb et al., 1996; Page and Orr-Weaver, 1996), and functions with a germ-line specific Cks gene, Cks30A to mediate the destruction of cyclin A (Swan et al., 2005; Swan and Schupbach, 2005). Here we show that the canonical APC adaptor, Fzy, functions together with Cort to target mitotic cyclins for destruction and drive anaphase in both meiosis I and meiosis II. Female meiosis, like the subsequent syncytial mitotic cell cycles, appears to involve local destruction of cyclin B, and we find that both Cort and Fzy are required for this process.
Materials and Methods
Drosophila stocks
The two cort mutants (Schupbach and Wieschaus, 1989) have similar meiotic phenotypes (Page and Orr-Weaver, 1996)and were analyzed as transheterozygotes. CortQW55 has a conserved Y303C change and cortRH65 encodes a truncated protein lacking the 7th WD repeat. Cks30A corresponds to the remnants gene and Cks30AKO was generated by site directed mutagenesis and is a molecular null, lacking a start codon (Swan et al., 2005). Fzy6 and fzy7 (Dawson et al., 1995) are temperature sensitive lethal alleles that are female sterile at 22°C. These were analyzed as transheterozygotes. Double mutant cort, fzy chromosomes were generated by recombining cortQW55 with fzy 6 and recombining cortRH65 with fzy7. All experiments with fzy mutants and cort,fzy double mutants were performed on eggs from females kept at 29°C for 3 to 5 days. UAS-HA-cort was made by PCR amplification of genomic cort (including introns), followed by cloning into pUASp with a 2X Hemaglutin (HA) tag at the N-terminus. Expression of UAS-HA-Cort using the nosGal4-VP16 driver results in rescue of the female sterility of cort mutants (data not shown). UAS-Fzy and UAS-Fzr were obtained from Christian Lehner (Sigrist and Lehner, 1997). UAS-CyclinB-TPM-GFP was obtained from Jordan Raff (Raff et al., 2002). Wing expression of UAS-HA-Cort, UAS-Fzy and UAS-Fzr was driven by ptcGal4 and enGal4. With these drivers, UAS-Fzy and UAS-Fzr expression results in pupal lethality. To observe the wing phenotype in enGal4/UAS-Fzy, flies were raised at 18°C to reduce expression levels and permit survival to adult.
Antibody stainings
To observe early meiotic events in wild-type, mature eggs were activated to undergo meiosis in vitro as described (Page and Orr-Weaver, 1997). For later meiotic events in wild-type, eggs were collected from 0 to 20 minute collections. To detect cyclin B, eggs from 0-2 hour collections or from activated oocytes were fixed in 100% Methanol, rehydrated gradually, blocked in PBST, 1%BSA and incubated with rabbit anti-cyclin B antiserum (from Jordan Raff) at 1/500. Rat anti-α-Tubulin (Cappel) was used at 1/500 and DNA was labeled either with mouse anti-Histones (Chemicon) at 1/1000 or Oligreen (Molecular Probes) at 1/500. Rat anti-Sub antibody (Jang et al., 2005) was used at 1/3000. FISH was performed on 0-2 hour eggs or dissected oocytes, using a probe to a repeated 359 bp repeat sequence unique to the centromeric region of the X-chromosome (Dernburg, 2000). For immunostaining of wing discs, 3rd instar larvae were collected from crosses of UAS-HA-Cort, UAS-Fzr or UAS-Fzy to ptcGal 4. Discs were fixed 30 minutes in 3.7% formaldehyde/PBST, extracted 1 hour in PBST + .3% Triton X-100, and labeled with rabbit anti cyclin B, B3 (from Christian Lehner) at 1/500, or rabbit anti cyclin A (from David Glover) at 1/500. Discs were also labeled with rat anti HA antiserum (Roche) at 1/500 and mouse anti B-gal antiserum (Promega) at 1/500.
Western analysis
Extracts were prepared in 2X sample buffer from wild-type and mutant eggs collected over a 2-hour period. The wild-type eggs derive from unfertilized females (crossed to XO males). Western blotting was performed by standard techniques. Antibodies were mouse anti Cyclin A and mouse anti cyclin B (both from Developmental Studies Hybridoma Bank), rabbit anti cyclin B3 (Sigrist et al., 1995)and rabbit anti Pim (Stratmann and Lehner, 1996), and rabbit anti PSTAIR (Santa Cruz).
Results
Cort and Fzy are required for the completion of meiosis I and II
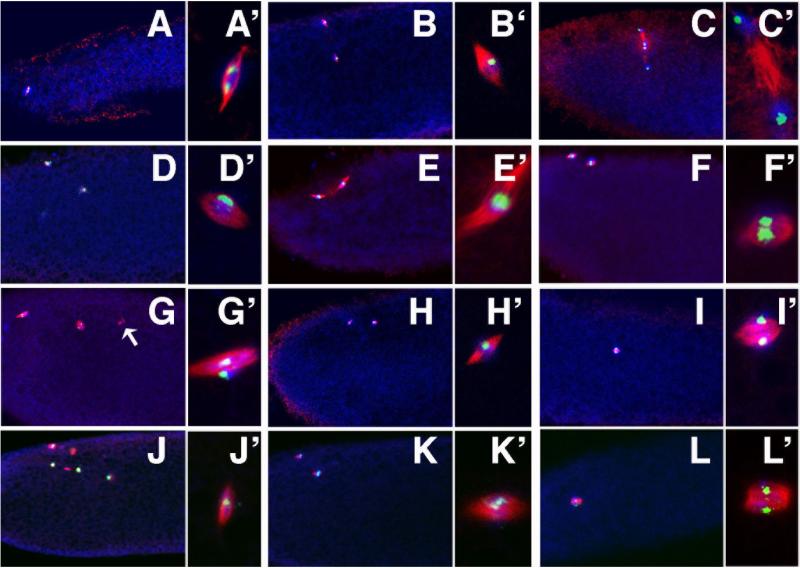
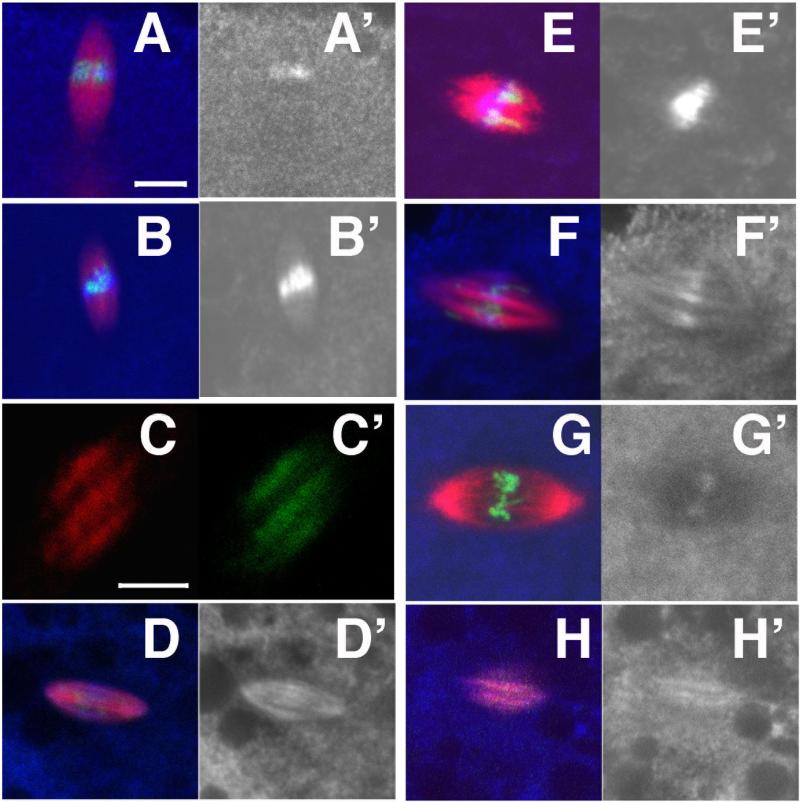
The Drosophila genome contains 4 Cdc20/Cdh1 genes. Fzr2 appears to be exclusively transcribed in the male germline (Jacobs et al., 2002), while Fzr is transcribed in the female germline (Sigrist and Lehner, 1997), but the protein is not detectable in early embryos, either by Western or by in vivo functional assays (Jacobs et al., 2002; Raff et al., 2002). To determine the role of APC complexes in female meiosis, we focused on the canonical Cdc20, fzy and a female specific Cdc20 homologue, Cort, both of which are highly expressed in the female germ-line (Chu et al., 2001; Dawson et al., 1995). We re-examined the meiotic phenotypes of cort and fzy mutants separately and in double mutant combinations by observing spindles and DNA and by following chromosome segregation using FISH against an X-chromosome probe. Fzy temperature sensitive mutants were analyzed at 29°C, and therefore, to control for temperature effects, wild-type and cort mutants were examined at both room temperature and 29°C. Drosophila female meiosis arrests in metaphase of the first meiotic division until ovulation. At this stage the egg contains a single spindle near the anterior cortex and this spindle contains two X-chromosome signals representing the two pairs of sister chromatids (Figure 1A, A’). Upon ovulation, meiosis resumes. In metaphase of meiosis II, two tandemly arranged spindles form around the products of the first meiotic division. Both metaphase spindles contain a single sister chromatid pair (Figure 1B,B’). In anaphase II sister chromatids separate, resulting in 4 meiotic products, each with a single X-chromosome (Figure 1C,C’). Meiosis is completed very rapidly after ovulation and at 22°C only 1% (n=220) of eggs from a 0-2 hour collection are still in meiosis. The remainder of eggs contain arrested meiotic products (polar bodies). Similarly, in eggs from females kept at 29°C, only 4% (n=113) are in meiosis. In addition, 3% of eggs contain aberrant spindles near the cortex, suggesting a low level disruption of meiosis at this temperature. As previously described, eggs from cort mutant females (hereafter referred to as cort eggs) contain two spindles near the anterior cortex of the egg, indicative of an arrest in meiosis II (Chu et al., 2001; Lieberfarb et al., 1996; Page and Orr-Weaver, 1996) (Figure 1D). Similarly, at 29°C, 90% (n=78) of eggs contain 2 meiotic spindles. Both of the spindles contain a single X-chromosome signal (Figure 1D’), indicating an arrest in metaphase, prior to sister chromatid separation.
Figure 1.
Cort and Fzy are required for the completion of meiosis I and meiosis II. In all panels microtubules are red, a 359bp centromeric repeat unique to the X-chromosome is labeled green, and DNA is blue. Panels (A‘ -L’) are higher magnification views of one of the spindles in A-L). Mitotic spindles derived from the male pronucleus (when present) are more internal and are not shown in these images. (A-C) Meiosis in wild-type eggs obtained by in vitro activation (A) or 0-20 minute egg collections (B,C). In metaphase of meiosis I (A) the egg contains a single spindle with 2 discrete X-chromosome signals representing 2 pairs of sister chromatids. In metaphase II (B), there are two tandemly arranged spindles each containing a single X-chromosome signal. In anaphase II (C), sister chromatids separate and both spindles contain two discrete X-chromosome signals. (D-L) 0-2 hour eggs from females of the genotypes described. (D) In cortQW55/cortRH65 (females at 29°C for 3-5 days), eggs contain 2 spindles, with 1 X-chromosome each, indicating arrest in metaphase II. (E,F) Cks30AKO/Cks30AKO eggs with 2 spindles and either 1 X-chromosome (E) or 2 (F), indicating a delay as early as metaphase of meiosis II. (G) In fzy6/fzy7 at 29°C the majority of eggs contain two spindles, both with 2 X-chromosome signals, indicating arrest in anaphase II. Arrow indicates small spindle and associated chromatin.(H,I) In cortQW55,fzy6/cortRH65,fzy7 at 29°C, eggs contain either two spindles with 1 X-chromosome each, indicating a metaphase II arrest, or a single spindle with 2 X-chromosomes, indicating a metaphase I arrest). (J-L) Eggs from nosGal4VP16/UAS-cyclinB-TPM-GFP arrest with multiple spindles as in (J) or with 2 spindles (K), suggesting a meiosis II arrest, or with a single spindle (L), indicative of a meiosis I arrest.
Cks30A, like cort is required for proper completion of meiosis II, consistent with a model in which Cks promotes the activation of APCCort (Swan et al., 2005). However, while cort mutants invariably arrest in the 2nd meiosis, in Cks30 most oocytes eventually complete meiosis, though they are delayed in doing so (Swan et al., 2005). In 0-2 hour collections of Cks30AKO eggs, 26% are in meiosis II (n=46). In 58% of these, both spindles have a single X-chromosome signal and are therefore in metaphase of meiosis II (Figure 1E,E’), while 42% have 2 X-chromosomes per spindle and are therefore in anaphase of meiosis II (Figure 1F,F’). Therefore, loss of Cks30A results in a meiotic phenotype similar to but weaker than cort, suggesting that Cks30A activity enhances but is not essential for the function of the APCCort.
In Drosophila, as in most eukaryotes, Cdc20/Fzy is the critical APC adaptor in mitosis, and is essential for anaphase progression in most cell types (Dawson et al., 1993; Dawson et al., 1995; Sigrist et al., 1995). It is not yet known if Fzy is also required for anaphase progression in the meiotic divisions. To address this question we analyzed female meiosis in eggs produced by females mutant for fzy. Fzy, unlike cort, or Cks30A is essential for viability, and germ-line clones of a null allele do not produce eggs (data not shown). However, temperature sensitive allele combinations raised at the permissive temperature are viable and have been used to study the role of fzy in early embryogenesis. Fzy6/fzy7 raised at the permissive temperature of 22°C are female sterile and embryos arrest in the first mitosis (Dawson et al., 1993). Meiosis appears unaffected in these eggs (data not shown). To achieve a stronger phenotype, we shifted fzy6/fzy7 females to the restrictive temperature of 29°C. In addition to the mitotic arrest, eggs from fzy6/fzy7 females kept at 29°C (hereafter referred to as fzy eggs) display defects in meiosis. 74% (n=78) of fzy eggs contain 2 spindles near the cortex (Figure 1G), indicative of a meiosis II delay or arrest. In most cases both spindles contain two X-chromosome signals (Figure 1G’), indicating that sister chromatid separation has occurred and therefore they are in anaphase of meiosis II. Often, as in Figure 1G’, the two X-chromosomes are often not properly aligned along the spindle axis, likely a result of prolonged arrest. In rare cases, we detect more than 2 X-chromosome signals per spindle (data not shown), suggesting that DNA replication can occur during the aberrant meiosis in fzy eggs. We do not see meiotic spindles with only a single X-chromosome indicating that meiosis does not detectably delay or arrest in metaphase of meiosis II. In addition to the two major spindles, eggs often contain one or more smaller spindles with associated chromatin, near the two major ones (arrow, Figure 1G), possibly resulting from chromosome loss at the first meiotic division. 13% of embryos contain one or more spindles at the anterior cortex in addition to a polar body, suggesting a partial completion of meiosis, while 6% of embryos contain only polar bodies at the anterior cortex, and therefore appear to complete meiosis.
8% of fzy eggs contain only a single spindle near the cortex, possibly indicative of a meiosis I arrest. The same percentage of eggs from cort mutants raised at 29°C also arrest with a single meiotic spindle, (in agreement with previous findings (Page and Orr-Weaver, 1996)), suggesting the possibility that cort and fzy play partially redundant roles in meiosis I. To test this possibility, we analyzed the phenotype of a fzy, cort double mutant raised at 29°C. 74% (n=57) of fzy, cort double mutant eggs contain two spindles, each with a single X-chromosome signal (Figure 1H,H’), indicating that they arrest in metaphase of the 2nd meiotic division. 26% of eggs contain only a single spindle containing two X-chromosome signals (Figure 1I,I’), indicating an arrest in meiosis I. We conclude that the two APC adaptors, Cort and Fzy are necessary for anaphase progression in both meiotic divisions, performing partially redundant roles in meiosis I and non-redundant roles in meiosis II.
In addition to its role in anaphase, Cks30A is required earlier in meiosis, for assembly or maintenance of the first meiotic spindle (Pearson et al., 2005; Swan et al., 2005). To determine if spindle assembly or metaphase I arrest is affected in cort or fzy mutants, we analyzed chromosome alignment in unactivated oocytes using the X-chromosome FISH probe. In wild-type, in metaphase I, the autosomes are aligned at the spindle equator while the X-chromosomes are typically precociously segregated to either pole. We find that chromosomes are properly aligned in both cort and fzy mutants, and in cort,fzy double mutants (see Supplemental Fig. 1). Therefore, with the caveat that we are not able to study null alleles of cort and fzy, we conclude that the first requirement for cort and fzy in meiosis is in anaphase of meiosis I.
Cyclin destruction is necessary for completion of meiosis in Drosophila
In mitotic cells of most eukaryotes, the APCFzy promotes anaphase by targeting cyclins and other mitotic regulators for destruction. The importance of cyclin destruction in the two meiotic divisions is less clear. To determine if cyclin destruction is necessary for female meiosis in Drosophila, we examined meiotic progression in eggs from females expressing a destruction box mutated form of cyclin B, Cyclin B-TPM-GFP (Raff et al., 2002). When expressed in the female germ-line, cyclin B-TPM-GFP results in mitotic arrest at a variable stage of the syncytial mitotic cycle in the majority of embryos, indicating that cyclin B destruction is necessary for anaphase progression in these cell cycles (Raff et al., 2002). To determine if failure to destroy cyclin B also disrupts meiosis we expressed Cyclin BCBTM with the strong germ-line driver nosGal4VP16 at 29°C (to induce higher expression). Under these conditions, almost all embryos arrest in the first mitotic division (data not shown). In addition to this mitotic arrest, only 38% (n=51) appear to complete female meiosis as judged by the presence of polar bodies and the absence of spindles at the dorsal anterior of the egg. 50% of eggs contain multiple small spindles in the dorsal anterior, possibly a result of meiotic spindle breakdown and/or chromosome mis-segregation (Figure 1J,J’). The remaining 14% of eggs appear to arrest in meiosis. 4% of eggs have two spindles with either 1 or 2 X-chromosomes, indicative of arrest in metaphase or anaphase of meiosis II (Figure 1K,K’). 10% of eggs contain a single spindle at the dorsal anterior, typically with two X-chromosome signals, indicative of a meiosis I arrest (Figure 1L,L’). Therefore, cyclin B destruction is necessary for the proper completion of female meiosis in Drosophila.
Cort and Fzy are required for the destruction of mitotic cyclins in the egg
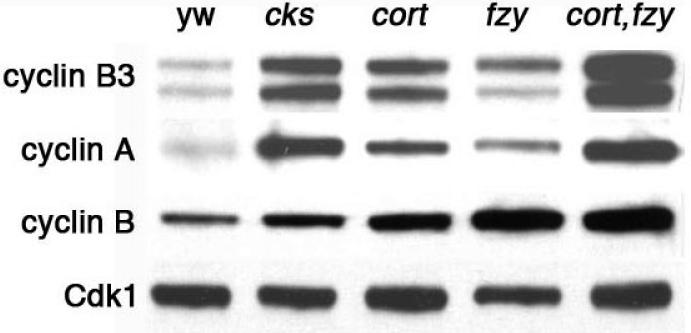
The above results suggest the possibility that the meiotic arrest in cort and fzy eggs could be caused by a failure to destroy mitotic cyclins. In Drosophila it is not known if the APCFzy has any role in cyclin destruction during meiosis. On the other hand the APCCort has been implicated with Cks30A in cyclin A destruction in the female germ-line (Swan et al., 2005). To determine the respective roles of cort and fzy in cyclin destruction in female meiosis, we compared cyclin levels in egg extracts from cort, fzy, cort,fzy double mutants and from Cks30A. All of these mutants arrest at or before entry into the first mitotic cell cycle, and therefore, for control extracts we used unfertilized, and therefore non-cycling, wild-type eggs. As previously reported, Cks30A and cort eggs contain high levels of cyclin A protein (Swan et al., 2005) (Figure 2). Cyclin A levels are not elevated in egg extracts from fzy6/fzy7 mutants raised at 22°C (data not shown). However, eggs from fzy6/fzy7 females kept at 29°C show a clear elevation in cyclin A levels, and cort, fzy double mutants have an even greater elevation in cyclin A levels (Figure 2). Therefore, fzy and cort are both required for cyclin A destruction in the Drosophila egg. Cyclin B and B3 levels are also elevated in fzy and cort single mutants, and more so in fzy, cort double mutant (Figure 2), indicating that Cort and Fzy cooperate in the destruction of all three mitotic cyclins. Comparing the relative effects of cort mutants and fzy on the different cyclins suggests that Cort is more important for cyclin A and cyclin B3 destruction, while Fzy is more important for cyclin B destruction. Therefore, the two APC adaptors may have different target preferences
Figure 2.
Cks30A, Cort and Fzy regulate overall cyclin levels in the egg. Western blots of eggs aged 0-2 hours from unfertilized (females crossed to X0 males) wild-type (yw), Cks30AKO/Cks30AKO, cortQW55,fzy6/cortRH65,fzy7 at 29°C, and cortQW55, fzy6 / cortRH65, fzy7 (at 29°C) probed for cyclin B3, cyclin A, and cyclin B. Cdk1, a stable cell cycle regulator, serves as a loading control. Cyclin B is slightly elevated in Cks30A, more so in fzy and cort and highly elevated in fzy/cort double mutants. Cyclin A and cyclin B3 are elevated in cort, and fzy and more so in cks30A and fzy, cort double mutants.
In Xenopus and mice, Cks1 is necessary for the activation of the APCFzy complex by associating with Cdk1 and promoting its phosphorylation of APC subunits Cdc27 and Cdc16 (Patra and Dunphy, 1998; Spruck et al., 2003). Drosophila Cks30A interacts with Cdk1 in the Drosophila germ-line, and is required for cyclin A destruction (Swan et al., 2005). Cks30A eggs also have elevated cyclin B3 levels, and both cyclin A and B3 are at levels higher than in cort or fzy single mutants, and approaching that of the fzy, cort double mutant (Figure 2). This could be explained if Cks30A activity is required for the function of both APCFzy and APCCort complexes. Cyclin B, in contrast, is not strongly affected in Cks30A (Figure 2) arguing that Cks30A plays a lesser role in promoting the activity of APCFzy and APCCort in cyclin B destruction.
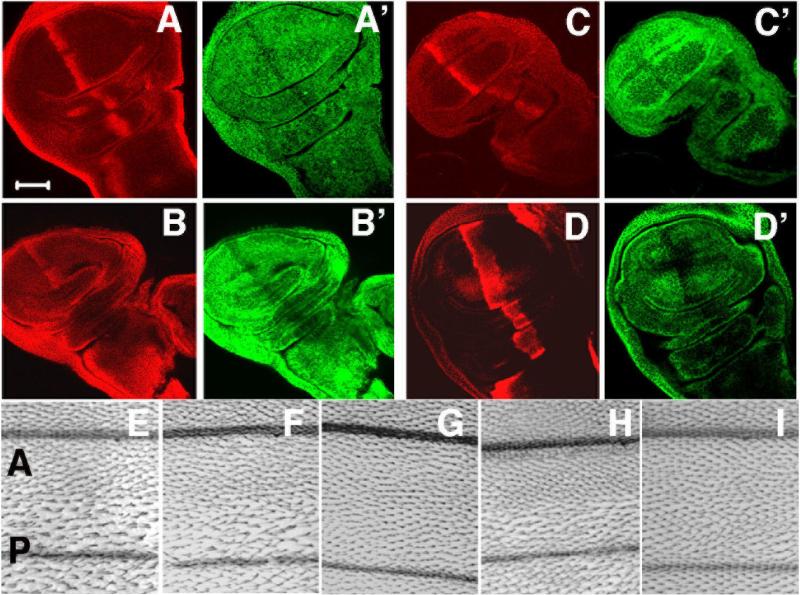
The above results indicate that Cort, like other Cdc20/Cdh1 family proteins functions in the targeting of mitotic cyclins for destruction. To further test the ability of Cort to target cyclins for destruction, we expressed HA-tagged Cort in a stripe of cells in the wing imaginal disc using the Gal4/UAS system and then looked at cyclin levels by immunolocalization. The expression of HA-Cort results in a corresponding decrease in cyclin A, B and B3 (Figure 3A,B,C), consistent with these cyclins being targeted for destruction by Cort. A similar effect is seen upon the over-expression of Fzy or Fzr (Figure 3D and data not shown). Therefore, Cort is able to target all of the mitotic cyclins for destruction, consistent with a proposed role as an APC adaptor.
Figure 3.
Cort can mediate destruction of mitotic regulators and its activity depends on Cks30A. (A-C) UAS-HA-Cort expressed under ptcGal4 and detected with anti-HA antibodies. Cyclins A (A’), B (B’) and B3 (C’) are reduced in the stripe corresponding to Cort expression. (D,D’) UAS-Fzy expressed under ptcGal4 (detected with anti β-gal antibodies (D) results in a corresponding reduction in cyclin A levels (D’). (E-H) Magnified area of an adult wing from Drosophila expressing HA-Cort (E,G,H) or UAS-Fzy (F) in the posterior half of the wing (P) using enGal4. The anterior half of the wing (A) serves as a control. Expression of UAS-HA-Cort (E) or UAS-Fzy (F) in the posterior wing results in wider spacing of wing hairs. (G) Wing hair spacing phenotype is suppressed when HA-Cort is expressed in a Cks30AKO/ Cks30AKO background. (H) Co-expression of UAS-Flag-Cks30A with HA-Cort in a Cks30AKO/ Cks30AKO background restores the wing hair spacing phenotype. (I) Expression of Flag-Cks30A alone does not affect bristle spacing.
The reduction of cyclin levels would be expected to inhibit mitosis in the wing imaginal disc. Each cell in the wing secretes a single bristle and mitotic failure results in fewer, but larger cells and consequently fewer wing hairs (Weigmann et al., 1997). Indeed, the expression of Fzy or HA-Cort in the posterior compartment of the wing disc, using the enGal4 driver, leads to fewer but larger cells as judged by an increase in the spacing between the wing hairs (Figure 3E,F). To test the possibility that Cks30A is required for the activation of the APCCort, we used enGal4 to express HA-Cort in Drosophila that are also lacking zygotic expression of Cks30A. In the Cks30A background, the wing hair spacing phenotype is suppressed (Figure 3G). It is largely restored if Flag-Cks30A is co-expressed with HA-Cort in the Cks30A mutant background (Figure 3H), while expression of Flag-Cks30A alone has no effect (Figure 3I). Therefore, Cks30A is required for Cort activity.
Cyclin B associates dynamically with the meiotic spindle
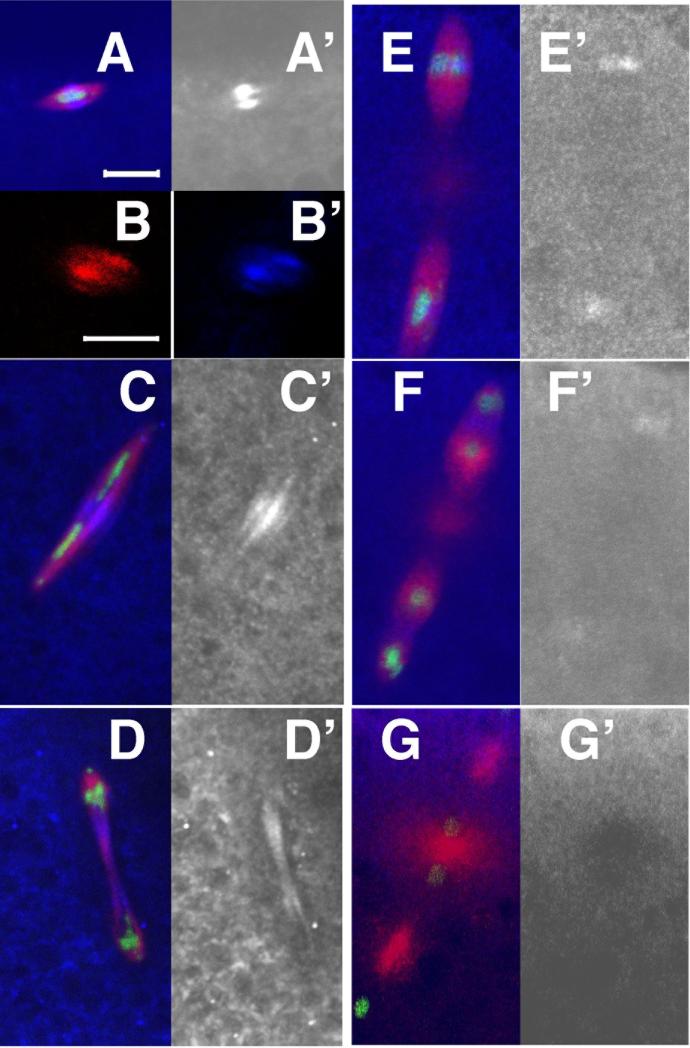
Cyclin B undergoes incomplete destruction in the syncytial mitotic cycles, apparently as a result of localized destruction restricted to spindles (Edgar et al., 1994; Huang and Raff, 1999; Raff et al., 2002; Su et al., 1998). It is not known how this local destruction is mediated and it is also not known if localized cyclin B destruction is unique to the syncytial mitotic cycles or if it also occurs in the preceding meiotic divisions. To determine if cyclin B is subject to localized destruction in female meiosis, we first determined the localization of cyclin B in wild-type meiosis. In Drosophila females, meiosis is arrested in metaphase of the first meiotic division until ovulation., cyclin B accumulates at high levels on the metaphase I spindle. (Figure 4A) (Pearson et al., 2005). This cyclin B accumulation is non-uniform and appears to be focused at the meiotic spindle mid-zone, the region of the meiotic spindle where non-kinetochore microtubules from either pole overlap. The meiotic spindle mid-zone (or meiotic metaphase central spindle) appears to play a specialized role in establishing spindle bipolarity and recruiting chromosomal passenger proteins to the meiotic spindle (Jang et al., 2005). To confirm that cyclin B associates with the spindle mid-zone, we double labeled oocytes for cyclin B and the spindle mid-zone component, Subito (Sub) (Jang et al., 2005). Cyclin B and Sub appear to precisely colocalize (Figure 4B), confirming that cyclin B specifically associates with the spindle mid-zone in metaphase of meiosis I. In anaphase of meiosis I the spindle mid-zone extends as the spindle elongates and chromosomes segregate to either pole (Jang et al., 2005). Cyclin B persists on the spindle mid-zone throughout anaphase I (Figure 4C,D). Upon assembly of the 2nd meiotic spindle, cyclin B appears to redistribute to the spindle mid-zone of the newly formed meiosis II spindles (Figure 4E). Cyclin B persists at the spindle mid-zone after the onset of anaphase (Figure 4F), but is no longer detected later in anaphase (Figure 4G). Therefore, cyclin B is associated with the meiotic spindle mid-zone throughout meiosis, and dissociates from the spindle late in anaphase II. This pattern of accumulation suggests that cyclin B, presumably in complex with Cdk1 plays a unique role at the meiotic mid-zone in meiosis I and meiosis II, and that it is targeted for destruction at this site in anaphase II.
Figure 4.
Cyclin B associates with the spindle in female meiosis. (A-D) In vitro activated oocytes, and (E-F) 0-20 minute egg collections from wild-type (yw) females. In all panels except (B,B’) microtubules are red, DNA is green and cyclin B is blue. Cyclin B channel is shown by itself in grayscale in right hand panels (labeled with (‘). (A) In metaphase I high levels of cyclin B accumulate on the spindle mid-zone. (B,B’) Metaphase I spindle labeled with antibodies to Sub (B) and cyclin B (B’) reveals colocalization at the spindle mid-zone. (C,D) In anaphase I, cyclin B persists at the spindle mid-zone. (E) In metaphase II, cyclin B accumulates at the mid-zone on both spindles. (F) Cyclin B persists at lower levels at the mid-zone in anaphase II. (G) Late in anaphase II cyclin B is no longer detected on the meiotic spindle. Scale bar in A = 5um and applies for all images except (B,B’). Scale bar in B = 2um.
Cort, Fzy and Cks30A are required for the local destruction of cyclin B
To determine if the dissociation of cyclin B from meiotic and mitotic spindles in anaphase reflects local destruction by the APCCort or APCFzy, we compared cyclin B distribution in wild-type (Figure 5A), and in eggs from cort and fzy females at 29°C. In cort, cyclin B accumulates on the arrested meiotic spindles (Figure 5B) (only one of the two meiosis II spindles is shown). This accumulation is significantly higher than that detected in wild-type metaphase II, suggesting that cyclin B is stabilized on the arrested spindle. As in wild-type meiosis, cyclin B associates specifically with the overlapping microtubules of the spindle mid-zone, co-localizing with the mid-zone component Sub (Figure 5C). Fzy eggs also arrest with elevated levels of cyclin B on the meiosis II spindles (Figure 5D). However, rather than exclusively accumulating at the spindle mid-zone, cyclin B is at lower levels more uniformly along the spindle. The finding that mutations in cort and fzy, result in a stable association of cyclin B with the meiotic spindle strongly supports a model in which cyclin B loss from the meiotic spindle in anaphase is a result of localized destruction by APCCort and APCFzy complexes.
Figure 5.
Cyclin B is stabilized on the meiotic spindle in cort, fzy and Cks30A. In all panels except (C, C’), Tubulin is red, DNA is green and cyclin B is blue. Cyclin B is shown by itself in grayscale in right hand panels (labeled with ‘). (A) In wild-type, cyclin B accumulates on the spindle mid-zone in metaphase of meiosis II. (B) Arrested meiosis II spindle from cortQW55/cortRH65 at 29°C with high levels of cyclin B associated with the mid-zone of the spindle. (C,C’) Co-labeling of a metaphase II spindle from cortQW55/cortRH65 with antibodies to Sub (C) and cyclin B (C’) reveals colocalization at the spindle mid-zone. (D) Meiosis II spindle from fzy6/fzy7 with accumulation of cyclin B along the length of the spindle. (E) Metaphase arrested meiotic spindle from cortQW55,fzy6/cortRH65,fzy7 with high level of cyclin B at the spindle mid-zone. (F) Meiotic spindle from Cks30AKO/Cks30AKO with elevated levels of cyclin B on the spindle mid-zone and along the spindle. (G, H) Mitotic spindles in yw (G) and fzy6/fzy7 (H). In wild-type, cyclin B associates with the spindle mid-zone late in metaphase, before disappearing from the spindle in anaphase. In fzy, cyclin B associates along the length of the arrested mitotic spindle. Scale bars in (A) and (F) equal 5um. Scale bar in (A)= 5um applies to all panels except C, C’. Scale bar in C = 2um.
The difference in site of cyclin B accumulation on the meiotic spindle between cort and fzy could be a result of Cort and Fzy having distinct sites of activity. In this model, Cort mediates cyclin B destruction at the spindle mid-zone while Fzy targets cyclin B along the length of the spindle. One consequence of this model is that fzy, cort double mutants might have a cyclin B accumulation that is the sum of that of the two single mutants. Alternatively, Cort and Fzy may mediate cyclin B destruction at different stages of meiosis. In this model, Cort mediates cyclin B destruction in metaphase when cyclin B is primarily at the mid-zone, and Fzy functions in anaphase along the entire spindle. This model fits with the time of arrest of cort and fzy in metaphase and anaphase respectively (Figure 1), and it predicts that fzy,cort double mutants would arrest in metaphase with cyclin B at the mid-zone. We find that indeed fzy,cort double mutants accumulate cyclin B largely at the spindle mid-zone and not along the length of the spindle (Figure 5E), and therefore are identical to cort single mutants. Therefore, the different site of accumulation of cyclin B in cort and fzy may reflect different temporal requirements for the APCCort and APCFzy in meiosis.
By Western analysis, Cks30A has little effect on overall cyclin B levels (Figure 3). However immunostaining of eggs from Cks30A reveals cyclin B enriched on meiotic spindles (Figure 5F). Therefore, Cks30A is also required for the destruction of cyclin B on spindles in female meiosis, consistent with a role in activation of APCCort and APCFzy complexes.
In the syncytial embryonic cell cycles Cyclin B associates with the mitotic spindle at metaphase (Huang and Raff, 1999) (Figure 5G), and its destruction on spindles may play a role in anaphase progression. Given that APCCort and APCFzy are both required for the destruction of cyclin B on the meiotic spindle, it seems likely that either or both APC complexes would also be involved in local cyclin B destruction on mitotic spindles. Cort mutants arrest prior to assembly of a mitotic spindle and therefore the role of Cort in localized cyclin B destruction in mitosis could not be determined. Fzy and Cks30A, however, enter into and arrest in the first mitosis. In both mutants the mitotic arrest is associated with a failure to locally destroy cyclin B (Figure 5H and data not shown), arguing that Cks30A and Fzy are necessary for the local destruction of cyclin B in syncytial mitosis in addition to meiosis.
Discussion
In most cell types, in Drosophila and in other metazoans, the APCFzy drives anaphase progression by targeting mitotic cyclins and other mitotic proteins for destruction. The female germ-line is an exception in that the APCFzy is not sufficient. A germ-line specific APC adaptor, Cort, cooperates with Fzy to mediate cyclin destruction in meiosis.
Cort is a functional Cdc20 homologue
The cort gene encodes a diverged member of the Cdc20/Cdh1 family (Chu et al., 2001). Cdc20/Cdh1 homologues interact with the APC and with specific sequences (D-box, Ken box or A-box) found on cyclins and other APC targets. As such, Cdc20/Cdh1 proteins act as specificity factors to target proteins for Ubiquitination and eventual destruction. Cort protein, like all Cdc20/Cdh1 family proteins contains 7 WD domains in the C-terminal half of the protein, implicated in substrate recognition (Pfleger et al., 2001). We also find that Cort has a N-terminal C-box (amino acids 482, 483) and a C-terminal IR tail (amino acids 54-60), both implicated in binding to the APC (Passmore et al., 2003; Schwab et al., 2001; Vodermaier et al., 2003). In addition to containing these conserved functional domains, Cort displays a conserved ability to mediate cyclin destruction. Cort mutations result in over accumulation of cyclins A, B and B3 in the egg (Swan et al., 2005) (and Figure 2), while the ectopic expression of Cort in the wing disc leads to a reduction in levels of these mitotic cyclins (Figure 3). Taken together, these results argue that Cortex encodes a functional member of the Cdc20/Cdh1 family.
Fzy and Cort Co-operate to promote cyclin destruction and meiotic progression
While the Drosophila genome has 4 genes that encode Cdc20/Cdh1 proteins, only two of these proteins, Fzy and Cort are expressed at detectable levels in the female germ-line (Raff et al., 2002) (Jacobs et al., 2002) (Chu et al., 2001). We have studied the role of these two APC adaptors both individually and in double mutants and found that they function together to promote anaphase in both the first and 2nd meiotic divisions of female meiosis. In most cell types in Drosophila and other eukaryotes, a single APC complex, the APCFzy is responsible for cyclin destruction and anaphase progression. It is therefore surprising that in the Drosophila female germ-line, two APC adaptors are necessary for meiotic progression. In the case of meiosis I, Cort and Fzy appear to play largely redundant roles, since only removing both genes results in a significant block in meiosis I. The two APC complexes may also be functionally redundant with respect to global cyclin levels. Mutations in either fzy or cort result in an increase in levels of cyclins A, B and B3, while mutation in both genes results in even further increases in cyclin levels.
While Cort and Fzy have overlapping roles in promoting anaphase I, both are essential for meiosis II. This could simply reflect a greater quantitative requirement for APC activity in meiosis II. Alternatively, the two APC complexes could have distinct roles in the 2nd meiotic division. Consistent with this latter possibility, mutations in cort and fzy result in arrest at different stages of meiosis II: cort mutants arrest with sister chromatids associated, and therefore in metaphase, while fzy mutants almost invariably arrest with separated sister chromatids, and therefore in anaphase. Cort and fzy also result in different patterns of cyclin B stabilization on the arrested spindles, suggesting roles in metaphase and anaphase respectively. Therefore, Cort may function to initiate sister chromatid separation at the onset of anaphase II and Fzy may function primarily later anaphase II. Alternatively, the later arrest seen in fzy could simply reflect the fact that the fzy alleles that we have used are not nulls, and it is possible that a complete loss of Fzy activity would also result in a metaphase arrest as seen in cort. However, comparing the meiosis II phenotypes of fzy and Cks30A null mutants suggests that the later arrest in fzy is not simply due to residual activity. Cks30A null mutants have a weaker meiotic arrest than fzy, since they complete meiosis at high frequency (Swan et al., 2005), but they display a higher frequency of metaphase arrest or delay. The fact that fzy does not similarly cause a delay in metaphase of meiosis II suggests that it is only required at anaphase. Therefore, it is possible that Fzy is critical at anaphase, while Cort is necessary for the metaphase to anaphase transition.
The different temporal requirements for Cort and Fzy prior to and after sister chromatid separation respectively, could be related to their apparent differences in substrate specificity. Western analysis (Figure 2) reveals that Cort is more important for cyclin A and B3 destruction, while Fzy appears to play a greater role in cyclin B destruction in the egg. In mitotic cells, cyclin destruction occurs sequentially, with cyclin A destruction first in prometaphase being a prerequisiste for sister chromatid separation. Cyclin B destruction occurs at anaphase onset and is necessary for later anaphase events, subsequent to sister chromatid separation (Sigrist et al., 1995). Therefore, it is possible that Cort promotes the early stages of meiotic anaphase by targeting cyclin A for destruction, while Fzy is more critical later through its targeting of cyclin B for destruction.
Role of the APC in meiosis
The meiotic cell cycle differs in many respects from the standard mitotic cycle. While APC-mediated destruction of mitotic regulators appears to be required for anaphase progression in most or all mitotic cells, the role of the APC and cyclin destruction in meiosis is not as well understood. Our analysis of the two APC adaptors, Cort and Fzy has permitted an evaluation of the role of the APC complex in Drosophila female meiosis. We find that the APC is required for anaphase progression in both meiotic divisions. Correlating with its requirement for completion of meiosis, the APC is required for the destruction of mitotic cyclins. At least one of these cyclins, cyclin B is a critical substrate in meiosis, since the expression of a stabilized form of cyclin B disrupts meiosis (Figure 1). Therefore, APC activity and cyclin destruction is required for anaphase progression in both meiotic divisions in addition to mitosis. APC activity has been implicated in both meiotic divisions in C. elegans (Furuta et al., 2000; Golden et al., 2000) and in the mouse (Salah and Nasmyth, 2000; Terret et al., 2003) and in the 2nd meiotic division but not the first meiotic division in Xenopus (Peter et al., 2001; Taieb et al., 2001). In yeast, two APC complexes, the mitotic APCFzy and a meiosis specific complex (APCAma1 in S. cerevisiae and APCMfr1 in S. pombe) function together to mediate protein destruction in meiosis (Asakawa et al., 2001; Blanco et al., 2001; Izawa et al., 2005; Salah and Nasmyth, 2000). It now appears that Drosophila also uses two APC complexes in female meiosis, and this may turn out to be a common strategy in other eukaryotes.
Cks30A role in activating the APC
Cks30A belongs to a highly conserved family of proteins that binds to and stimulates the activity of the mitotic kinase, Cdk1. In Xenopus, the Cks30A homologue, Xep9 stimulates the Cdk-dependent phosphorylation of APC subunits, and thereby promotes the activation of the APCFzy complex (Patra and Dunphy, 1998). Our results suggest that Cks30A may have a similar role in stimulating both the APCFzy and APCCort in Drosophila female meiosis. First, Cks30A, like cort and fzy is required for the completion of meiosis II, and like fzy, is required for the completion of the first mitotic division of embryogenesis (this work, Figure 1 and (Lieberfarb et al., 1996; Page and Orr-Weaver, 1996; Swan et al., 2005). Second, Cks30A, like Cort and Fzy is necessary for global cyclin destruction in the Drosophila egg and local cyclin B destruction on the meiotic spindle (Figures 2 and 5). Global levels of cyclins A and B3 are elevated more in Cks30A mutants than in single mutants for cort or fzy, consistent with Cks30A activating both Cort and Fzy. Third, we have shown that Cks30A is necessary for the activity of ectopically expressed Cort in the adult wing (Figure 3). Cks30A may also play a role in activating APCFzy in mitotic cells. We have found that the temperature sensitive fzy6 allele is lethal at all temperatures in a Cks30A null background (AS and TS, unpublished) suggesting that Cks30A dependent activation of APCFzy becomes essential when Fzy activity is compromised.
Though Cks30A appears to promote the activity of the APCCort and APCFzy, these complexes appear to retain some activity in the absence of Cks30A. While cort and fzy cause an arrest in meiosis II, Cks30A null mutants typically are only delayed in meiosis II (Swan et al., 2005). Also, while cyclin A and B3 levels are elevated more in Cks30A eggs than in either fzy or cort, their levels are still not as high as in cort,fzy double mutants, arguing that Fzy and Cort can destroy cyclins A and B3 to some degree in the absence of Cks30A. Cyclin B destruction is even less dependent on Cks30A, since cyclin B levels are affected less in Cks30A mutants than in either cort or fzy single mutants. Therefore, Cks30A may be more critical for the activity of APCCort and APCFzy complexes on cyclins A and B3 than on cyclin B. The relatively weaker meiotic arrest in Cks30A mutants compared to cort, fzy double mutants may also indicate that the APC has other meiotic targets that can be destroyed in the absence of Cks30A.
Localized cyclin destruction in Drosophila meiosis
Cyclin B undergoes local oscillations in its association with mitotic spindles in syncytial embryos, appearing transiently along the full length of the mitotic spindle in early metaphase, and gradually disappearing from the spindle starting at the centrosomes and ending at the kinetochores (Huang and Raff, 1999). The timing of this loss of cyclin B from the spindle, at the onset of anaphase, corresponds with the timing of cyclin B destruction in other cell types, suggesting the possibility that cyclin B is locally destroyed on the spindle in anaphase. We now show that cyclin B is subject to similar local oscillations in the female meiotic cycles (Figure 4), and that cyclin B destruction is necessary for the completion of female meiosis (Figure 1J-L). Importantly, we demonstrate that the local loss of cyclin B from the spindle in meiosis is dependent on the APC adaptors, Cort and Fzy, and the local loss of cyclin B from the spindle in mitosis depends on Fzy (Figure 5),. These results argue strongly that the local loss of cyclin B from the spindle in anaphase of meiosis II and anaphase of mitosis is actually due to local destruction.
The pattern of cyclin B accumulation and loss from the spindle in meiosis differs in some respects compared to the syncytial mitotic cycles. First, in metaphase of mitosis, cyclin B initially accumulates throughout the spindle microtubules (Huang and Raff, 1999), while in metaphase of the meiotic divisions cyclin B first appears exclusively at the spindle mid-zone. This difference may reflect the fact that the meiotic spindle does not contain centrosomes and therefore, cyclin B may not load onto spindles from centrosomes and progress along the spindles to the kinetochores as has been proposed for mitosis (Huang and Raff, 1999).
Second, the timing of cyclin B destruction appears to be different between the meiotic cycles and the mitotic cycles. Most strikingly, there is no loss of cyclin B from the spindle following meiosis I, implying that local cyclin B destruction may not be necessary for anaphase of meiosis I. In addition, cyclin B loss from the spindle following meiosis II only occurs late in anaphase rather than at the onset of anaphase as in the syncytial mitotic cycles. We do not yet know how cyclin B destruction is prevented in anaphase I and early in anaphase of meiosis II. One possibility is that the spindle assembly checkpoint is locally active during these stages. This checkpoint is required for the proper completion of female meiosis in Drosophila (Fischer et al., 2004; Gilliland et al., 2005) and it will be interesting to see if this requirement reflects a role in inhibiting either APCFzy or APCCort activity.
The specific accumulation of cyclin B at the spindle mid-zone in meiosis may reflect the unique properties of the meiotic spindle. The mid-zone microtubules or central spindle microtubules are a subset of spindle microtubules that do not end in kinetochores but instead overlap at the mid-zone with microtubules from the other pole. In dividing cells the central spindle is critical for cytokinesis, but in female meiosis it appears to have a role in spindle assembly (Jang et al., 2005). Along with cyclin B, chromosomal passenger proteins, AuroraB and INCENP are recruited to the spindle mid-zone. It will be necessary to understand what these proteins do at the mid-zone and how cyclin B destruction at this site may be important for anaphase in meiosis. It will also be important to determine how the APCCort targets cyclin B at the spindle midzone. We have not been able to detect any specific localization of GFP or HA-tagged Cortex in meiosis or in the syncytial embryo (AS and TS, unpublished), but it is possible that its activity is spatially regulated.
In conclusion, our results support a model in which two APC complexes, APCFzy and APCCort cooperate to mediate the destruction of meiotic cyclins and progression through female meiosis
Supplementary Material
Acknowledgements
We are grateful to Christian Lehner and Jordan Raff for fly stocks and Antibodies. We also thank Ian Dawson for fly stocks, and Kim McKim for Antibodies. Thanks to Gordon Gray for fly media. We thank Girish Deshpande for critical reading of the manuscript and members of the Schupbach lab for helpful discussions. This work was supported by the Howard Hughes Medical Institute and Public Health Service Grant PO1 CA41086.
References
- Asakawa H, Kitamura K, Shimoda C. A novel Cdc20-related WD-repeat protein, Fzr1, is required for spore formation in Schizosaccharomyces pombe. Mol Genet Genomics. 2001;265:424–35. doi: 10.1007/s004380000429. [DOI] [PubMed] [Google Scholar]
- Blanco MA, Pelloquin L, Moreno S. Fission yeast mfr1 activates APC and coordinates meiotic nuclear division with sporulation. J Cell Sci. 2001;114:2135–43. doi: 10.1242/jcs.114.11.2135. [DOI] [PubMed] [Google Scholar]
- Chu T, Henrion G, Haegeli V, Strickland S. Cortex, a Drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/fizzy protein family. Genesis. 2001;29:141–52. doi: 10.1002/gene.1017. [DOI] [PubMed] [Google Scholar]
- Dawson IA, Roth S, Akam M, Artavanis-Tsakonas S. Mutations of the fizzy locus cause metaphase arrest in Drosophila melanogaster embryos. Development. 1993;117:359–76. doi: 10.1242/dev.117.1.359. [DOI] [PubMed] [Google Scholar]
- Dawson IA, Roth S, Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–37. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A. In Situ Hybridization to Somatic Chromosomes. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbour Laboratory Press; Cold Spring Harbour: 2000. pp. 25–55. [Google Scholar]
- Edgar BA, Sprenger F, Duronio RJ, Leopold P, O'Farrell PH. Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 1994;8:440–52. doi: 10.1101/gad.8.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MG, Heeger S, Hacker U, Lehner CF. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr Biol. 2004;14:2019–24. doi: 10.1016/j.cub.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Furuta T, Tuck S, Kirchner J, Koch B, Auty R, Kitagawa R, Rose AM, Greenstein D. EMB-30: an APC4 homologue required for metaphase-to-anaphase transitions during meiosis and mitosis in Caenorhabditis elegans. Mol Biol Cell. 2000;11:1401–19. doi: 10.1091/mbc.11.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland WD, Wayson SM, Hawley RS. The meiotic defects of mutants in the Drosophila mps1 gene reveal a critical role of Mps1 in the segregation of achiasmate homologs. Curr Biol. 2005;15:672–7. doi: 10.1016/j.cub.2005.02.062. [DOI] [PubMed] [Google Scholar]
- Golden A, Sadler PL, Wallenfang MR, Schumacher JM, Hamill DR, Bates G, Bowerman B, Seydoux G, Shakes DC. Metaphase to anaphase (mat) transition-defective mutants in Caenorhabditis elegans. J Cell Biol. 2000;151:1469–82. doi: 10.1083/jcb.151.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr Biol. 2000;10:430–8. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- Huang J, Raff JW. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. Embo J. 1999;18:2184–95. doi: 10.1093/emboj/18.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa D, Goto M, Yamashita A, Yamano H, Yamamoto M. Fission yeast Mes1p ensures the onset of meiosis II by blocking degradation of cyclin Cdc13p. Nature. 2005;434:529–33. doi: 10.1038/nature03406. [DOI] [PubMed] [Google Scholar]
- Jacobs H, Richter D, Venkatesh T, Lehner C. Completion of mitosis requires neither fzr/rap nor fzr2, a male germline-specific Drosophila Cdh1 homolog. Curr Biol. 2002;12:1435–41. doi: 10.1016/s0960-9822(02)01074-6. [DOI] [PubMed] [Google Scholar]
- Jang JK, Rahman T, McKim KS. The kinesinlike protein Subito contributes to central spindle assembly and organization of the meiotic spindle in Drosophila oocytes. Mol Biol Cell. 2005;16:4684–94. doi: 10.1091/mbc.E04-11-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J Cell Biol. 1998;141:1393–406. doi: 10.1083/jcb.141.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katis VL, Galova M, Rabitsch KP, Gregan J, Nasmyth K. Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr Biol. 2004;14:560–72. doi: 10.1016/j.cub.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427:510–7. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- Lieberfarb ME, Chu T, Wreden C, Theurkauf W, Gergen JP, Strickland S. Mutations that perturb poly(A)-dependent maternal mRNA activation block the initiation of development. Development. 1996;122:579–88. doi: 10.1242/dev.122.2.579. [DOI] [PubMed] [Google Scholar]
- Marston AL, Amon A. Meiosis: cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol. 2004;5:983–97. doi: 10.1038/nrm1526. [DOI] [PubMed] [Google Scholar]
- Page AW, Orr-Weaver TL. The Drosophila genes grauzone and cortex are necessary for proper female meiosis. J Cell Sci. 1996;109(Pt 7):1707–15. doi: 10.1242/jcs.109.7.1707. [DOI] [PubMed] [Google Scholar]
- Page AW, Orr-Weaver TL. Activation of the meiotic divisions in Drosophila oocytes. Dev Biol. 1997;183:195–207. doi: 10.1006/dbio.1997.8506. [DOI] [PubMed] [Google Scholar]
- Passmore LA, McCormack EA, Au SW, Paul A, Willison KR, Harper JW, Barford D. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. Embo J. 2003;22:786–96. doi: 10.1093/emboj/cdg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D, Dunphy WG. Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase- promoting complex at mitosis. Genes Dev. 1998;12:2549–59. doi: 10.1101/gad.12.16.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson NJ, Cullen CF, Dzhindzhev NS, Ohkura H. A preanaphase role for a Cks/Suc1 in acentrosomal spindle formation of Drosophila female meiosis. EMBO Rep. 2005;6:1058–63. doi: 10.1038/sj.embor.7400529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Castro A, Lorca T, Le Peuch C, Magnaghi-Jaulin L, Doree M, Labbe JC. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat Cell Biol. 2001;3:83–7. doi: 10.1038/35050607. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–43. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Pfleger CM, Lee E, Kirschner MW. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 2001;15:2396–407. doi: 10.1101/gad.918201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff JW, Jeffers K, Huang JY. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J Cell Biol. 2002;157:1139–49. doi: 10.1083/jcb.200203035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Khodjakov A, Paliulis LV, Fortier TM, Cole RW, Sluder G. Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage. Proc Natl Acad Sci U S A. 1997;94:5107–12. doi: 10.1073/pnas.94.10.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah SM, Nasmyth K. Destruction of the securin Pds1p occurs at the onset of anaphase during both meiotic divisions in yeast. Chromosoma. 2000;109:27–34. doi: 10.1007/s004120050409. [DOI] [PubMed] [Google Scholar]
- Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989;121:101–17. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Neutzner M, Mocker D, Seufert W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. Embo J. 2001;20:5165–75. doi: 10.1093/emboj/20.18.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist S, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. Embo J. 1995;14:4827–38. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–81. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Spruck CH, de Miguel MP, Smith AP, Ryan A, Stein P, Schultz RM, Lincoln AJ, Donovan PJ, Reed SI. Requirement of Cks2 for the first metaphase/anaphase transition of mammalian meiosis. Science. 2003;300:647–50. doi: 10.1126/science.1084149. [DOI] [PubMed] [Google Scholar]
- Stratmann R, Lehner CF. Separation of sister chromatids in mitosis requires the Drosophila pimples product, a protein degraded after the metaphase/anaphase transition. Cell. 1996;84:25–35. doi: 10.1016/s0092-8674(00)80990-3. [DOI] [PubMed] [Google Scholar]
- Su TT, Sprenger F, DiGregorio PJ, Campbell SD, O'Farrell PH. Exit from mitosis in Drosophila syncytial embryos requires proteolysis and cyclin degradation, and is associated with localized dephosphorylation. Genes Dev. 1998;12:1495–503. doi: 10.1101/gad.12.10.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A, Barcelo G, Schupbach T. Drosophila Cks30A interacts with Cdk1 to target Cyclin A for destruction in the female germline. Development. 2005;132:3669–78. doi: 10.1242/dev.01940. [DOI] [PubMed] [Google Scholar]
- Swan A, Schupbach T. Drosophila Female Meiosis and Embryonic Syncytial Mitosis Use Specialized Cks and CDC20 Proteins for Cyclin Destruction. Cell Cycle. 2005;4:1332–1334. doi: 10.4161/cc.4.10.2088. [DOI] [PubMed] [Google Scholar]
- Taieb FE, Gross SD, Lewellyn AL, Maller JL. Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from Meiosis I to II in Xenopus oocytes. Curr Biol. 2001;11:508–13. doi: 10.1016/s0960-9822(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Terret ME, Wassmann K, Waizenegger I, Maro B, Peters JM, Verlhac MH. The meiosis I-to-meiosis II transition in mouse oocytes requires separase activity. Curr Biol. 2003;13:1797–802. doi: 10.1016/j.cub.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr Biol. 2003;13:1459–68. doi: 10.1016/s0960-9822(03)00581-5. [DOI] [PubMed] [Google Scholar]
- Weigmann K, Cohen SM, Lehner CF. Cell cycle progression, growth and patterning in imaginal discs despite inhibition of cell division after inactivation of Drosophila Cdc2 kinase. Development. 1997;124:3555–63. doi: 10.1242/dev.124.18.3555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.