Abstract
Objective
Deficits in sustained attention may represent an endophenotype for bipolar disorder (BD). One heritable measure of sustained attention is intrasubject variability in response time (ISV-RT). We tested the hypothesis that, compared with controls, both youths with BD and those at familial risk for the disorder would have increased ISV-RT.
Method
Subjects were 28 patients with BD, 26 unaffected youths with a first-degree relative with BD, and 24 control youths without an affected relative, all aged 7 to 17 years. Subjects completed the Flanker Continuous Performance Test.
Results
Bipolar disorder and at-risk youths had increased ISV-RT, compared with the controls. Differences were independent of comorbid psychopathology in youths with BD and present in psychiatrically healthy at-risk youths.
Conclusions
Increased ISV-RT may be a risk marker for BD. Further research is needed to investigate the neural and genetic underpinnings of this deficit, as well as the specificity of the finding to BD.
Keywords: bipolar disorder, sustained attention, endophenotype
Although research in schizophrenia has begun to identify endophenotypes, such as deficits in working memory,1–3 the search for endophenotypes in bipolar disorder (BD) is in its infancy.4 Several behavioral markers, such as deficits in executive function, sustained attention, verbal memory, and face emotion labeling, have been implicated as possible BD endophenotypes.4,5 Sustained attention is defined as the capacity to maintain performance on a cognitive task in the presence of distracting stimuli,6 and measures include target sensitivity and intrasubject variability in response time (RT). Intrasubject variability in response time (ISV-RT) reflects variability in the time that a subject takes to respond to stimuli during an attentional task. Adult BD probands and their relatives make more errors than controls on sustained attention tasks.7–9 With regard to ISV-RT specifically, one study found that BD adults had increased ISV-RT on a continuous performance test.10 Although ISV-RT is highly heritable in control populations,11 ISV-RT has not been studied in relatives of patients with BD.
Attention-deficit/hyperactivity disorder (ADHD) is a common comorbidity in pediatric BD, even when the diagnosis is based on symptoms during euthymia.12,13 Intrasubject variability in RT is increased in both individuals with ADHD and their relatives.14,15 Given the high BD-ADHD comorbidity,16 the presence of increased ISV-RT in adult BD patients,10 and the heritability of ISV-RT in both control populations and those with ADHD, we tested whether ISV-RT would be increased in youths with BD and their relatives, compared with the controls. If so, then increased ISV-RT may be a candidate endophenotype for BD.
To be considered an endophenotype, or heritable bio-marker, the construct must show association with illness, state independence, greater prevalence in nonaffected family members than in the general population, heritability, and cosegregation with the illness within families.17 Here, we present evidence that increased ISV-RT meets the first three of these criteria in pediatric patients with BD. The identification of endophenotypes is crucial; discovery of trait-based markers will ultimately aid in earlier identification of BD. Moreover, endophenotypes can lead to early intervention initiatives and ultimately prevention.
We examined ISV-RT in BD probands and their relatives on the Flanker Continuous Performance Test, which assesses attention in the context of interference and has been used to demonstrate increased ISV-RT in patients with ADHD.18,19 We hypothesized that, compared with the controls, both subjects with BD and children at familial risk for BD would have increased ISV-RT. If confirmed, our findings would suggest that increased ISV-RT may be a candidate endophenotype for BD.
METHOD
Subjects
Participants were enrolled in an institutional review board–approved protocol at the National Institute of Mental Health in Bethesda, MD. Minors (aged 7–17 years) and their guardians gave written informed assent and consent, respectively.
The study included three groups of children—pediatric probands with “narrow phenotype” BD,20 youths at risk for BD by virtue of having a first-degree relative with the disorder, and typically developing control children. Children's diagnoses were assigned using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version (K-SADS-PL),21 administered separately to children and parents by clinicians with demonstrated interrater reliability (κ > 0.9). Narrow phenotype BD required a history of at least one manic or hypomanic episode, meeting full DSM-IV-TR duration criteria, with elevated mood and at least three B criterion symptoms. Comorbid diagnoses (e.g., ADHD) were assigned based on symptoms present during euthymia. At-risk children had either a full biological sibling with narrow phenotype BD or a parent with DSM-IV-TR bipolar I or II disorder. At-risk children with anxiety or disruptive behavior disorders were included to avoid recruiting a particularly resilient group; other psychopathology was exclusionary. In particular, at-risk children with mood disorders were excluded because such disorders (e.g., depressive episode) may indicate that the child was already affected with BD. Where multiple siblings from a family were enrolled, data from only the oldest at-risk sibling was included in an attempt to more closely match subjects on age. Parent diagnoses were confirmed by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition22 or the Diagnostic Interview for Genetic Studies.23
Exclusion criteria for all of the participants included IQ lower than 70, pervasive developmental disorders, substance abuse within the past 2 months, or significant medical illness. Children's Depression Rating Scale24 and Young Mania Rating Scale25 ratings were collected for BD and at-risk youths. The Wechsler Abbreviated Scale of Intelligence (WASI)26 was administered to all of the participants.
Behavioral Task
The computerized Flanker Continuous Performance Test (Cognitive Therapeutics)27 assesses attention in the context of interference, by requiring the subjects to press a button in response to a target arrow presented along with distractor stimuli (flankers). The participants were instructed to press the left arrow key when the target arrow key pointed left and the right arrow key when the target pointed right. Participants completed 48 trials of each of three conditions (neutral, congruent, and incongruent), in random order, for a total of 144 trials. In the neutral condition, the flankers were horizontal lines, whereas in the other conditions, the flankers were arrows pointing in the same (congruent) or opposite (incongruent) direction as the target arrow.
Data Analysis
Between-group differences in age and IQ were measured using an analysis of variance, and differences in sex distribution were assessed by χ2. Age differed significantly between groups and thus served as a covariate in subsequent analyses.
Outliers more than two SDs above or below the group means for percent correct responses or SD of RT were removed. Accuracy of responses and RT were recorded separately for congruent, neutral, and incongruent trials. Mean RT and ISV-RT (the SD of each individual's mean RT) were calculated, again separately for each trial condition.
An omnibus multivariate analysis of covariance (MANCOVA), with age included as a covariate, was performed including all variables (i.e., accuracy, mean RT, ISV-RT) for all three trial types (i.e., congruent, neutral, incongruent). This controlled for type I errors. After finding a significant omnibus result, subsequent post hoc analyses included separate ANCOVAs for each variable in each trial type to clarify specific measures contributing to the between-group difference detected with the MANCOVA. Cohen d for ISV-RT measures were computed using the mean and SD, separately for each trial type.
In secondary analyses, data from BD and at-risk subjects with lifetime diagnoses of ADHD were excluded because of reported associations between increased ISV-RT and ADHD.14,28 In addition, to ensure that increased ISV-RT in at-risk youths was not associated with subsyndromal ADHD symptoms, we created a continuous subthreshold ADHD variable using responses to the items in the K-SADS-PL ADHD module. Specifically, we computed the mean value of the scores for all items in the K-SADS-PL ADHD module and then performed Pearson correlations between ADHD symptoms and ISV-RT. We also ran post hoc analyses excluding noneuthymic youths with BD, and BD and at-risk subjects with anxiety diagnoses to control for mood state and comorbid anxiety disorders, respectively. In the at-risk sample, t tests compared ISV-RT between those that were at risk because of a BD parent to those that were at risk because of a sibling with narrow phenotype BD. Finally, we compared medicated versus unmedicated patients with BD, unmedicated patients with BD versus controls, and examined the presence of each category of psychotropic medication on ISV-RT performance.
RESULTS
Seventy-eight children completed the Flanker task: 28 BD, 26 at-risk, and 24 normal volunteer (NV) subjects (Table 1). The at-risk subjects were younger than the BD and NV subjects (F2,75 = 6.87, p < .01), so subsequent analyses controlled for age. There were no between-group differences in sex or IQ. Eighty-nine percent (n = 25) of the BD subjects had a comorbid diagnosis, with a mean of 2.5 (SD 1.8) additional diagnoses. Twenty-seven percent (n = 7) of at-risk subjects carried Axis I diagnoses, specifically anxiety disorders (n = 5) and/or ADHD (n = 3). More than half (52%, n = 14/27 [mood rating data unavailable for 1 patient with BD]) of the youths with BD were euthymic (i.e., Children's Depression Rating Scale score < 40 and Young Mania Rating Scale score ≤ 12). Eighty-six percent (n = 24) of the BD youths were medicated while being tested, with a mean of 2.75 (SD 1.48) medications, including anticonvulsants (71%), atypical antipsychotics (57%), antidepressants (39%), lithium (32%), stimulants (18%), and anxiolytics (14%). All at-risk and control youths were unmedicated and euthymic at the time of testing.
TABLE 1.
Demographics and Clinical Characteristics for Patients With BD, Youths With Familial Risk for BD, and Control Subjects
| Group |
||||
|---|---|---|---|---|
| Characteristic | BD (n = 28) | AR (n = 26) | NV (n = 24) | p |
| Age (mean) | 14.0 ± 2.3 | 12.0 ± 3.0 | 14.3 ± 1.8 | .01 |
| Full-scale IQa(mean) |
108.1 ± 13.8 |
114.3 ± 15.3 |
109.8 ± 12.1 |
.26 |
| |
n (%) |
n (%) |
n (%) |
|
| Sex (male) | 14 (50) | 18 (69) | 14 (58) | .35 |
| Any Axis I diagnosis | 28 (100) | 7 (27) | ||
| Bipolar disorder I | 22 (79) | 0 (0) | ||
| Anxiety disorder | 16 (57) | 5 (19) | ||
| ADHD | 19 (68) | 3 (12) | ||
| Oppositional defiant disorder | 9 (32) | 0 (0) | ||
| Unmedicated | 4 (14) | 26 (100) | ||
| Euthymica | 14/27 (52) | 24/24 (100) | ||
| Depresseda | 3/27 (11) | 0 (0) | ||
| Hypomanic, manic, or mixeda | 10/27 (37) | 0 (0) | ||
| CDRS,a mean | 29.96 ± 14.56 | 19.79 ± 4.88 | ||
| YMRS,a mean | 9.63 ± 6.99 | 2.73 ± 3.69 | ||
Note: AR = at risk; BD = bipolar disorder; CDRS = Children's Depression Rating Scale; NV = normal volunteer; YMRS = Young Mania Rating Scale.
IQ scores and mood ratings were unavailable for 1 BD and 2 AR youths; the means and percentages for these groups is based on n = 27 BD and n = 24 AR.
The omnibus MANCOVA demonstrated a significant group effect (F18,132 = 1.87, p = .02). Post hoc ANCOVAs demonstrated significant group effects for each variable included in the omnibus MANCOVA (i.e., ISV-RT, accuracy, and RT; all p's < .04). Post hoc ANCOVAs examining ISV-RT indicated that groups differed on congruent (F2,74 = 9.30, p < .01), neutral (F2,74 = 8.33, p < .01), and incongruent (F2,74 = 6.77, p < .01) trials.
In all analyses, both subjects with BD (all p's < .01; Cohen d for congruent, d = 1.64; neutral, d = 1.34; incongruent, d = 1.18) and those at risk (all p's < .03; congruent, d = 1.21; neutral, d = 1.23; incongruent, d = 1.17) had increased ISV-RT, compared with the controls. The youths with BD and those at risk did not differ from each other on ISV-RT (all p's > .14; congruent, d = 0.18; neutral, d = 0.18; incongruent, d = 0.11; Table 2).
TABLE 2.
Performance on the Flanker Task, by Trial Type, in BD, At-Risk, and Control Youthsa
| BD (n = 28) | AR (n = 26) | NV (n = 24) | F2,74 | Significant Group Effects | |
|---|---|---|---|---|---|
| Proportion correct | |||||
| Congruent | 0.70 ± 0.03 | 0.78 ± 0.03 | 0.87 ± 0.03 | 7.98 | BD < NV p < .01 |
| AR < NV p = .04 | |||||
| Neutral | 0.73 ± 0.03 | 0.78 ± 0.03 | 0.86 ± 0.03 | 5.01 | BD < NV p < .01 |
| Incongruent | 0.60 ± 0.03 | 0.68 ± 0.03 | 0.74 ± 0.03 | 6.34 | BD < NV p < .01 |
| Mean response time | |||||
| Congruent | 514.86 ± 13.36 | 480.24 ± 14.53 | 438.28 ± 14.60 | 7.71 | BD > NV p < .01 |
| AR > NV p = .05 | |||||
| Neutral | 511.68 ± 14.91 | 478.68 ± 16.21 | 440.30 ± 16.28 | 4.81 | BD > NV p < .01 |
| Incongruent | 563.82 ± 17.40 | 525.21 ± 18.92 | 499.48 ± 19.00 | 3.30 | BD > NV p < .01 |
| Intrasubject variability in response time | |||||
| Congruent | 156.38 ± 8.52 | 145.83 ± 9.27 | 104.40 ± 9.31 | 9.30 | BD > NV p < .01 |
| AR > NV p < .01 | |||||
| Neutral | 145.66 ± 8.15 | 133.31 ± 8.86 | 98.03 ± 8.90 | 8.33 | BD > NV p < .01 |
| AR > NV p < .02 | |||||
| Incongruent | 170.98 ± 10.78 | 152.74 ± 11.72 | 113.60 ± 11.77 | 6.77 | BD > NV p < .01 |
| AR > NV p < .03 |
Analysis covaried for age; means and SEs adjusted for age.
Post hoc ANCOVAs examining accuracy and RT also demonstrated between-group differences on all three trial types. Unlike for ISV-RT, some of these interactions were driven by differences between BD and control groups (all p's < .01; Table 2); the at-risk youths had poorer accuracy (p = .04) and longer RTs (p = .05) thanthe controls for congruent trials only. Neither accuracy nor mean RT differed significantly between the BD and at-risk groups.
When the noneuthymic youths with BD were excluded, euthymic subjects with BD had increased ISV-RT compared with the controls for all the three trial types (congruent, p = .02; neutral, p = .03; incongruent, p = .02).
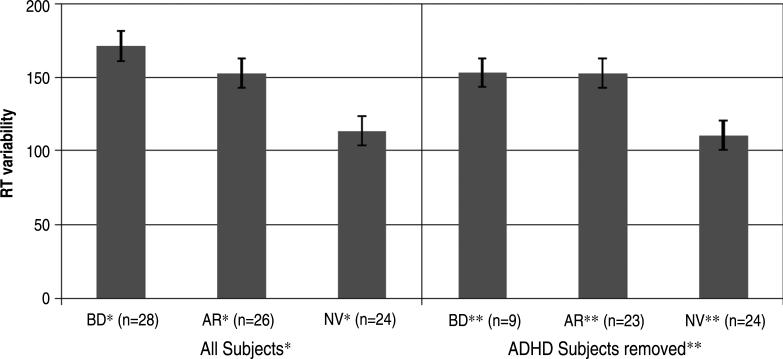
When data from the 19 children with BD (68%) and the 3 at-risk children (12%) with lifetime diagnoses of ADHD were removed from the analysis, differences in ISV-RT between the 23 remaining at-risk and 24 control youths remained significant for all the three task conditions (AR > NV, all p's < .02). With the subjects with ADHD removed, ISV-RT differed significantly between the 9 youths with BD and the 24 controls on congruent and incongruent trials (BD > NV p < .01 and p = .03, respectively), with a trend for increased ISV-RT in the subjects with BD on neutral trial RTs (p = .08; Fig. 1). To determine whether increased ISV-RT in the at-risk sample was related to subsyndromal ADHD symptoms, we performed a Pearson correlation between a continuous variable of ADHD symptoms, based on K-SADS-PL responses, and the three types of ISV-RT. The mean ADHD score was 1.15 (SD 0.33) among the 21 at-risk children for which scores were available. There was no correlation between degree of ADHD symptoms and ISV-RT for congruent (r = –0.09, p = .68), neutral (r = –0.07, p = .75), or incongruent (r = –0.07, p = .76) trials.
Fig. 1.
Mean reaction time variability on Flanker task incongruent trials by group subjects with attention-deficit/hyperactivity disorder are excluded in the right side of the figure. AR = at risk; BD = bipolar disorder; NV = normal volunteer; RT = response time. *F2,74 = 6.77, p < .01, BD > NV (p < .01), AR > NV (p < .03); **F2,52 = 4.43, p < .02, BD > NV (p = .03), AR > NV (p < .02).
When data from the 16 children with BD and 5 at-risk children with anxiety disorders were removed, the remaining subjects with BD (n = 12) and those at risk (n = 21) exhibited increased ISV-RT compared with the controls on congruent (all p's < .01) and incongruent (all p's < .01) trials. The at-risk subjects also had increased ISV-RT compared with the controls on neutral trials (p = .02), and the subjects with BD trended toward increased ISV-RT compared with the controls (p = .11).
Post hoc t test revealed no differences between at-risk subjects with affected siblings (n = 16) versus those with affected parents (n = 10; p = .81, p = .67, and p = .38 for congruent, neutral, and incongruent trial types, respectively). Post hoc t tests revealed no differences between medicated (n = 24) and unmedicated (n = 4) patients with BD (p = .31, p = .65, p = .75 for congruent, neutral, and incongruent trial types, respectively). However, the subset of unmedicated (n = 4) patients with BD differed from the controls (p < .01 for all three trial types). Finally, the presence of any particular type of medication, including anticonvulsants (n = 20), atypical antipsychotics (n = 16), antidepressants (n = 11), lithium (n = 9), stimulants (n = 5), or anxiolytics (n = 4) did not influence ISV-RT performance in the patients with BD (all p's > .15).
DISCUSSION
We found that pediatric patients with BD and children at risk for BD had increased ISV-RT on the Flanker CPT. Previous studies have identified increased ISV-RT in adult patients with BD10 and sustained attention deficits in BD and at-risk populations.7–9 Here, we extend that literature by demonstrating that increased ISV-RT is present in predominately euthymic youths with BD and unmedicated relatives, who were youths free of mood disorders.
Our finding of increased ISV-RT in both probands and at-risk subjects was not driven solely by ADHD, as between-group differences remained when data from the youths with BD and at-risk youths with ADHD were excluded. Furthermore, there was no correlation between increased ISV-RT and subthreshold ADHD symptoms in at-risk youths, a negative finding which may reflect the relatively low rate of ADHD symptoms in this group. Taken together, analyses suggest that the between-group differences we identified do not reflect the influence of ADHD. However, these analyses are subject to type II error.
There is a paucity of candidate endophenotypes for BD, as compared with schizophrenia or ADHD. Possible BD endophenotypes include deficits in attention,4 verbal learning and memory,29,30 and face emotion labeling.5 Research has investigated sustained attention deficits in patients with BD. It seems that such deficits are independent of impairments in working memory31 and are present in euthymic adult patients,7,32–34 suggesting that sustained attention is trait rather than state related. After controlling for comorbid ADHD, the youths with BD also show sustained attention deficits.35
Increased ISV-RT, which has been studied extensively in ADHD, seems to reflect deficits in sustained attention and/or arousal.36 It may index efficiency of neural signaling and reflect a failure to adopt a consistent response strategy.37,38 Fast-frequency ISV-RT, as identified by fast Fourier transformation,18 may reflect sustained attention deficits resulting from moment-to-moment variation in top-down control, whereas predominately slow-frequency ISV-RT may represent declining arousal over the course of the task.39 Such techniques should be used to further characterize the deficits that we identified in the youths with BD and their relatives and to possibly differentiate their patterns of variation from those subjects with other psychiatric disorders.
Neuroimaging techniques may elucidate the neurophysiology of ISV-RT. A recent functional magnetic resonance imaging study found that brief lapses in attention, as indexed by trials with long reaction times, were associated with reduced anterior cingulate and prefrontal activation before stimulus onset, increased activity in frontal and parietal regions, and less deactivation of the default mode network.40 The latter is comprised of brain regions that fire spontaneously during rest and become less active during processes requiring attention.41 It includes the precuneus, posterior cingulate, medial prefrontal, and middle temporal gyrus, among other regions.40,42 Consistent with Weissman et al.,40 Kelly et al.19 reported an association between increased ISV-RT and the strength of the negative correlation between activation in the default-mode network and a “task-positive” network. Finally, increased ISV-RT was found in patients with brain lesions in the dorsolateral and superior medial frontal cortices,43 supporting the importance of frontal control in maintaining attention and consistent response strategy.
Several neuroimaging studies have focused on possible mechanisms mediating increased ISV-RT in patients with ADHD. Using resting state functional magnetic resonance imaging, Castellanos and colleagues44 and Uddin and colleagues45 found that, compared with the controls, the patients with ADHD had decreased network connectivity in the default mode network. Imaging studies have not focused on ISV-RT in patients with BD. However, Strakowski et al.46 found overactivation of the ventral prefrontal and the parietal cortices, as well as the parahippocampus/amygdala, in subjects with BD performing a sustained attention task, compared with the controls. Further research on the mechanisms mediating increased ISV-RT in BD is warranted, with specific exploration of default mode network function in BD.
A few studies have explored genetic influences on ISV-RT. In the control subjects, increased Met loading on the COMT Val158Met genotype was associated with decreased ISV-RT on a continuous performance test,47 whereas a study in subjects with ADHD found associations between increased ISV-RT and a 10-repeat allele of the DAT1.48 DAT1 and COMT have been identified as possible susceptibility genes for BD,49,50 raising the possibility that variations in ISV-RT may be related to dopaminergic dysregulation in BD.
Our study, in combination with the literature, suggests that deficits in sustained attention may be a somewhat nonspecific endophenotypic marker for psychopathology. Indeed, ISV-RT may be an attentional trait that is shared across many groups. Increased ISV-RT has been detected in patients with ADHD,18,37 schizophrenia,51 BD,7–9 and sustained attention deficits have been observed in the first-degree relatives of patients with ADHD14,15,52 and BD.8,53 This lack of specificity is not surprising because deficits in sustained attention are characteristic of each of these illnesses, and indeed, there is evidence for overlapping pathophysiological mechanisms54,55 (e.g., as previously noted, COMT and dopaminergic genes have been implicated in BD, schizophrenia, and ADHD). Furthermore, offspring of adults with BD are at risk for a number of illnesses other than BD, including ADHD.56,57 It is also possible that different psychopathologies could demonstrate a similar deficit, but the deficit may be mediated by different circuitry. Subsequent research may reveal some endophenotypes specific to BD and some that are shared with other illnesses, reflecting both shared and unique risk-related genes.
To explore this possibility, future research should include pathological control groups that do not exhibit deficits in sustained attention to ascertain whether ISV-RT is specific to psychiatric conditions manifesting this symptom, rather than being a more global marker for psychopathology. We are currently testing the hypothesis that the youths with BD and their at-risk relatives have increased ISV-RT relative to subjects with anxiety disorders, as well as to the controls. Future studies are needed to identify clusters of endophenotypes that may assist in determining at-risk youths who will be more or less likely to develop BD.
Additional limitations of our study include small sample sizes and the heterogeneous nature of the at-risk group, which includes both siblings and offspring of BD probands. However, post hoc analyses demonstrate no difference in ISV-RT between these two at-risk subsamples. The “narrow phenotype” criteria used to diagnose BD not only require distinct episodes (as does DSM-IV-TR) but also are more stringent than DSM-IV-TR in requiring an episode of elation. Therefore, our results are not generalizable to youths who receive the diagnosis of BD in the absence of discrete episodes or to those whose episodes are characterized by irritability without euphoria. Most patients with BD were medicated, and some were not euthymic, but our findings were present in both euthymic youths with BD and unmedicated euthymic at-risk youths, suggesting that increased ISV-RT is not due to the confounding effects of mood state or medication. However, analyses only examining particular classes of medications (e.g., stimulants) may have been under-powered because of small sample sizes.
Some of our subjects have comorbid ADHD. Our post hoc analyses excluded subjects with ADHD, and there was a lack of correlation between ISV-RT and ADHD symptoms, suggesting that increased ISV-RT is related independently to BD and familial risk for BD. However, our analyses did not directly control for ADHD symptoms. Finally, the at-risk group (12 years) was significantly younger than the other two groups (14 years). Although our analyses included age as a covariate, it is important to note that, throughout adolescence, response inhibition continues to improve and has a linear association with age.58,59 Therefore, the 2-year age gap between our at-risk subjects and other samples represents a developmentally sensitive time during which changes in brain maturation and behavioral performance are continuing to occur. Future studies should include samples matched on age. However, even if matched on age, it is possible that response inhibition may be delayed in patients with BD. Thus, although the control and BD groups were age-matched, the BD patients may perform worse because of developmental delays. Studies are needed that follow patients with BD and at-risk youths longitudinally.
In summary, the finding of increased ISV-RT in children with BD and those at risk supports the idea that increased ISV-RT may be one of several risk markers for BD. The identification of endophenotypes will ultimately aid in early identification, intervention, and possibly prevention of illness. Increased ISV-RT may result from inefficient top-down control, present across a number of psychopathologies characterized by sustained attention deficits. This deficit may reflect overlapping pathophysiological mechanisms among these illnesses or may result from distinct mechanisms in different illnesses. Future comparative imaging studies are needed to explore the underlying pathophysiology of increased ISV-RT in the various disorders.
Acknowledgments
Funding support for this work was provided by the Intramural Research Program of the National Institute of Mental Health. Funding support for Melissa H. Rooney was provided through the Clinical Research Training Program, a public-private partnership supported jointly by the National Institutes of Health (NIH) and Pfizer (via a grant to the Foundation for NIH from Pfizer).
The authors thank the children and families who made this study possible, along with the members of the Section on Bipolar Spectrum Disorders at the National Institute of Mental Health. The authors also thank Robert M. Bilder, Ph.D., ABPP-CN, Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine at University of California at Los Angeles, for statistical support.
Footnotes
Dr. Brotman and Dr. Rooney contributed equally to this article.
This article was reviewed under and accepted by Ad Hoc Editor David R. Rosenberg, M.D.
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuro-psychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glahn DC, Therman S, Manninen M, et al. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- 3.Horan WP, Braff DL, Nuechterlein KH, et al. Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2008;103:218–228. doi: 10.1016/j.schres.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- 5.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 6.Pasini A, Paloscia C, Alessandrelli R, Porfirio MC, Curatolo P. Attention and executive functions profile in drug naïve ADHD subtypes. Brain Dev. 2007;29:400–408. doi: 10.1016/j.braindev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 8.Klimes-Dougan B, Ronsaville D, Wiggs EA, Martinez PE. Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol Psychiatry. 2006;60:957–965. doi: 10.1016/j.biopsych.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Winters KC, Stone AA, Weintraub S, Neale JM. Cognitive and attentional deficits in children vulnerable to psychopathology. J Abnorm Child Psych. 1981;9:435–453. doi: 10.1007/BF00917794. [DOI] [PubMed] [Google Scholar]
- 10.Bora E, Vahip S, Akdeniz F. Sustained attention deficits in manic and euthymic patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1097–1102. doi: 10.1016/j.pnpbp.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Kuntsi J, Rogers H, Swinard G, et al. Reaction time, inhibition, working memory and “delay aversion” performance: genetic influences and their interpretation. Psychol Med. 2006;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh MK, DelBello MP, Kowatch RA, Strakowski SM. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8:710–720. doi: 10.1111/j.1399-5618.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 13.Dickstein DP, Rich BA, Binstock AB, et al. Comorbid anxiety in phenotypes of pediatric bipolar disorder. J Child Adolesc Psychopharmacol. 2005;15:534–548. doi: 10.1089/cap.2005.15.534. [DOI] [PubMed] [Google Scholar]
- 14.Andreou P, Neale BM, Chen W, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychol Med. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle AE, Willcutt EG, Seidman LJ, et al. Attention-deficit/hyperactivity disorder endophenotypes. Biol Psychiatry. 2005;57:1324–1335. doi: 10.1016/j.biopsych.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Galanter CA, Leibenluft E. Frontiers between attention deficit hyperactivity disorder and bipolar disorder. Child Adolesc Psychiatr Clin N Am. 2008;17:325–346. doi: 10.1016/j.chc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 18.Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Leibenluft E, Charney DS, Towbin KT, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York: 2002. [Google Scholar]
- 23.Nurnberger JI, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 24.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the Children's Depression Rating Scale. J Am Acad Child Adolesc Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- 27.Krabbendam L, Isusi P, Galdos P, et al. Associations between COMTVal158Met polymorphism and cognition: direct or indirect effects? Eur Psychiatry. 2006;21:338–342. doi: 10.1016/j.eurpsy.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 30.Balanzá-Martínez V, Rubio C, Selva-Vera G, et al. Neurocognitive endophenotypes (Endophenocognitypes) from studies of relatives of bipolar disorder subjects: a systematic review. Neurosci Biobehav Rev. 2008;32:1426–1438. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Harmer CJ, Clark L, Grayson L, Goodwin GM. Sustained attention deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia. 2002;40:1586–1590. doi: 10.1016/s0028-3932(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 32.Kolur US, Reddy YC, John JP, Kandavel T, Jain S. Sustained attention and executive functions in euthymic young people with bipolar disorder. Br J Psychiatry. 2006;189:453–458. doi: 10.1192/bjp.bp.106.022921. [DOI] [PubMed] [Google Scholar]
- 33.Najt P, Glahn D, Bearden CE, et al. Attention deficits in bipolar disorder: a comparison based on the Continuous Performance Test. Neurosci Lett. 2005;379:122–126. doi: 10.1016/j.neulet.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 34.Wilder-Willis KE, Sax KW, Rosenberg HL, Fleck DE, Shear PK, Strakowski SM. Persistent attentional dysfunction in remitted bipolar disorder. Bipolar Disord. 2001;3:58–62. doi: 10.1034/j.1399-5618.2001.030202.x. [DOI] [PubMed] [Google Scholar]
- 35.Doyle AE, Wilens TE, Kwon A, et al. Neuropsychological functioning in youth with bipolar disorder. Biol Psychiatry. 2005;58:540–548. doi: 10.1016/j.biopsych.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Di Martino A, Ghaffari M, Curchack J, et al. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;64:607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- 38.Russell VA, Oades RD, Tannock R, et al. Response variability in attention-deficit/hyperactivity disorder: a neuronal and glial energetics hypothesis. Behav Brain Funct. 2006;2:30. doi: 10.1186/1744-9081-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson KA, Robertson IH, Kelly SP, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neurospychologia. 2007;45:2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 41.Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2007:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raichle ME, MacLeod A, Snyder AZ, Powers WJ, Gusnard DS, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- 44.Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uddin LQ, Clare Kelly AM, Biswal BB, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 46.Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP. A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 47.Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Stefanis CN. Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: tuning rather than improving performance. Am J Psychiatry. 2005;162:1752–1754. doi: 10.1176/appi.ajp.162.9.1752. [DOI] [PubMed] [Google Scholar]
- 48.Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Craddock N, Forty L. Genetics of affective (mood) disorders. Eur J Hum Genet. 2006;14:660–668. doi: 10.1038/sj.ejhg.5201549. [DOI] [PubMed] [Google Scholar]
- 50.Keikhaee MR, Fadai F, Sargolzaee MR, Javanbakht A, Najmabadi H, Ohadi M. Association analysis of the dopamine transporter (DAT1)-67A/T polymorphism in bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;135b:47–49. doi: 10.1002/ajmg.b.30174. [DOI] [PubMed] [Google Scholar]
- 51.Kaiser S, Roth A, Rentrop M, Friederich HC, Bender S, Weisbrod M. Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn. 2008;66:73–82. doi: 10.1016/j.bandc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Rommelse NNJ, Altink ME, Martin NC, et al. Relationship between endophenotype and phenotype in ADHD. Behav Brain Functions. 2008;4:4. doi: 10.1186/1744-9081-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Berrettini WH. Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry. 2000;47:245–251. doi: 10.1016/s0006-3223(99)00226-7. [DOI] [PubMed] [Google Scholar]
- 55.Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- 56.DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001;3:325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- 57.Chang KD, Steiner H, Ketter T. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry. 2000;39:453–460. doi: 10.1097/00004583-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- 59.Luna B, Sweeney JA. Studies of brain and cognitive maturation through childhood and adolescence: a strategy for testing neurodevelopmental hypotheses. Schizophr Bull. 2001;27:443–455. doi: 10.1093/oxfordjournals.schbul.a006886. [DOI] [PubMed] [Google Scholar]