Abstract
The novel cyclopenta[b]benzofuran, silvestrol, isolated from the fruits and twigs of Aglaia foveolata, has been found to exhibit very potent in vitro cytotoxic activity against several human cancer cell lines. Furthermore, it was active in the in vivo P388 murine leukemia model. In this study, the mechanism of cytotoxicity mediated by silvestrol in the LNCaP (hormone-dependent human prostate cancer) cell line was investigated. Silvestrol induced an apoptotic response, disrupted the mitochondrial trans-membrane potential and caused cytochrome c release into the cytoplasm. Immunoblot analysis indicated that, at the protein level, silvestrol produced an increase of bcl-xl phosphorylation with a concomitant increase of bak. Furthermore, caspase-2, -9 and -10 appeared to be involved in silvestrol-mediated apoptosis. In contrast, the involvement of caspase-3 and caspase-7 was not detected, either by immunoblot or caspase-3/7-like activity analysis, indicating that these pathways do not play a crucial role in silvestrol-induced apoptosis. To investigate the relative contribution of the caspases, inhibition of apoptosis with four different cell-permeable inhibitors was studied (Boc-D-Fmk, Z-VDVAD-FMK, Z-LEHD-FMK, and Z-AEVD-FMK). Only the general caspase inhibitor, Boc-D-Fmk, completely inhibited the formation of apoptotic bodies. In contrast, caspase-2 and caspase-9 selective inhibitors induced about a 40% decreased apoptotic response, whereas the caspase-10 selective inhibitor caused about a 60% reduction in apoptosis compared to silvestrol only treated cells. Taken together, the studies described herein demonstrate the involvement of the apoptosome/mitochondrial pathway and suggest the possibility that silvestrol may also trigger the extrinsic pathway of programmed cell death signaling in tumor cells.
Keywords: Silvestrol, LNCaP cells, apoptosis, apoptosome, caspase-10
Natural products have played an important role in cancer chemotherapy by providing several new drugs and lead structures for further development (1, 2). The cyclopenta[b]benzofuran core, found only in plants of the genus Aglaia (Meliaceae), has afforded interesting lead structures due to its unique carbon skeleton and the potent biological activity of some members of this compound class, known also as rocaglate or rocaglamide derivatives (3, 4). In terms of their potential antitumor propensities, cyclopenta[b]benzofurans have been reported to exhibit potent antiproliferative and cytostatic activity against human cancer cell lines (3, 5). They block protein synthesis and induce cell-cycle arrest at the G2/M transition in certain tumor cell lines (6). Furthermore, these compounds inhibit NF-κB activity by blocking inducible NF-κB DNA binding activity and I-κB degradation as well as expression of NF-κB target genes in T-lymphocytes (7). In regard to their NF-κB inhibitory activity, it was demonstrated that a synthetic derivative of rocaglaol is able to reduce tissue inflammation and neuronal cell death by inhibiting NF-κB and AP-1 signaling, resulting in significant neuroprotection in animal models of neurodegeneration (8). In addition, some rocaglamide derivatives have been suggested as a new source of NF-AT specific inhibitors for the treatment of certain inflammatory diseases (9).
In our recent work, the cyclopenta[b]benzofuran, silvestrol, isolated from the fruits and twigs of Aglaia foveolata, has been found to show very potent in vitro cytotoxic activity against several human cancer cell lines (10). Its potency was comparable to that of the well-known anticancer drug, paclitaxel (Taxol®). Furthermore, silvestrol exhibited potent inhibitory activity in vivo against several human cancer cells, which were cultivated in hollow fibers, and implanted intraperitoneally in mice (10). The natural product was also active in the P388 murine leukemia model (10). Interestingly, silvestrol possesses an unusual dioxanyloxy group at the C-6 position, which is a major structural difference from other cyclopenta[b]benzofurans, and it was suggested that this pendant group is important for its potent cytotoxic activity (10, 11). Synthesis of this dioxanyloxy substituent has been completed and synthesis of the molecule of silvestrol is underway (11). Since silvestrol is worthy of further investigation as an anticancer drug candidate, a better understanding of its cellular mechanism of action is warranted. Therefore, the present work was carried out to study silvestrol-mediated apoptosis in the LNCaP human prostate carcinoma cell line.
Two apoptosis pathways are relatively well understood at the molecular level. In the intrinsic pathway, apoptotic signaling impacts mitochondria to induce the release of mitochondrial cytochrome c into the cytosol, where it binds to the adaptor protein Apaf-1 (apoptotic protease-activating factor 1) and procaspase-9. These lead to the formation of the apoptosome and subsequent activation of executioner caspases, such as caspase-3 or -7 (12). In the extrinsic pathway, the cell surface death receptor Fas (CD95/Apo-1), a member of the tumor necrosis factor receptor family, is activated by binding of its ligand leading to the formation of the death-inducing-signaling-complex (DISC). DISC formation then triggers the sequential activation of the initiator caspases, caspase-8 or -10, and the executioner caspases, caspase-3 or -7, either directly or through a mitochondrial pathway.
Our results have demonstrated that silvestrol induces apoptosis through the mitochondrial/apoptosome pathway, suggesting that it follows the well-characterized intrinsic pathway. However, silvestrol-mediated apoptosis did not induce the activation of two major executioner caspases, caspases-3 and -7. We also show the contribution of caspase-10, implicating the potential involvement of the extrinsic pathway in silvestrol-induced apoptosis.
Materials and Methods
Cell culture
The human prostate carcinoma cells, hormone dependent LNCaP, were obtained from American Type Culture Collection (Rockville, MD, USA) and cultured in RPMI-1640 cell culture medium supplemented with 10% heat-inactivated fetal bovine serum and 1% PSF (100 units/ml penicillin G, 100 μg/ml streptomycin sulfate, 250 ng/ml amphotericin B), supplemented with 0.1 nM testosterone. The cells were maintained at 37°C and 5% CO2.
Chemicals and antibodies
Silvestrol, {6-O-demethyl-6-[6-(1,2-dihydroxyethyl)-3-methoxy-1,4-dioxan-2-yl]-aglafolin}, was isolated from the bark and twigs of Aglaia foveolata Pannell (Meliaceae), as described previously (10, 11). Four different cell-permeable inhibitors of caspases (Boc-D-Fmk, Z-VDVAD-FMK, Z-LEHD-FMK, and Z-AEVD-FMK), anti-caspase-2, anti-caspase-10, anti-Apaf-1, and anti-cytochrome c antibodies were obtained from Calbiochem (La Jolla, CA, USA). Anti-caspase-3, anti-caspase-7, anti-caspase-9, anti-poly (ADP-ribose) polymerase (PARP), anti-bak, and anti-bcl-xl were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Colony formation assay
The effect of silvestrol on LNCaP colony formation was evaluated as described previously (13). LNCaP cells in log phase growth were plated in a 100-mm tissue culture dish (250 cells/dish). After an incubation period of 24 h, the cells were treated with silvestrol (30 nM or 120 nM). Following additional incubation periods of 24, 48 and 72 h, dishes were washed with PBS and cultured in drug-free medium for 7 days. Colonies were then fixed with methanol, stained with Giemsa stain (Fisher Scientific, Itasca, IL, USA), and counted.
TUNEL Assay for quantification of apoptosis
The APO-DIRECT™ apoptosis kit obtained from Phoenix Flow Systems (San Diego, CA, USA) was used for the quantification of apoptosis. The cells were seeded at a density of 7×104 cells/ml in 100-mm culture dishes and were treated with 30 nM or 120 nM concentrations of silvestrol for 24 h. The cells were trypsinized, washed with PBS, and fixed with 1% (w/v) paraformaldehyde in PBS on ice for 30 min. After centrifugation, the cells were suspended in 70% (v/v) ethanol at −20°C until use. All ethanol was removed from the reaction tubes and cells were washed twice with PBS. The cells were processed for labeling with fluorescein-tagged deoxyuridine triphosphate nucleotide at 22°C – 24°C overnight, washed, and incubated with propidium iodide/RNase A solution in the dark for 30 min at room temperature. The labeled cells were then analyzed by flow cytometry.
Inhibition of apoptosis
To investigate the relative contribution of different caspases in silvestrol-induced apoptosis, 4,6-diamidino-2-phenylindole (DAPI) staining of the nucleus was performed in the presence of four different cell-permeable inhibitors of caspases: 50 μM Boc-D-Fmk as a general caspase inhibitor; 10 μM Z-VDVAD-FMK as a caspase-2 inhibitor (14); 20 μM Z-LEHD-FMK as a caspase-9 inhibitor; and 10 μM Z-AEVD-FMK as a caspase-10 inhibitor. Caspase inhibitors employed for the current studies were based on previously reported data (15, 16). LNCaP cells (6×104/ml) were plated in 6-well plates and incubated for 24 h. The cells were treated with 240 nM silvestrol for 24 h with or without four different cell-permeable inhibitors of caspases 1 h prior to treatment with silvestrol. The cells were harvested, washed, and fixed with methanol-acetic acid (1:1) for 30 min at room temperature. Cells were stained with DAPI (1μg/ml) on glass slides for 15 min at room temperature. DAPI staining of the nucleus was observed under fluorescence microscopy. Three independent experiments were performed and more than 150 cells were counted for each sample. The percentage of apoptotic nuclei was determined.
Analysis of caspases-3 and -7-like activity
The Apo-ONE™ homogeneous caspase-3/7 assay kit (Promega, Madison, WI, USA) was used to measure the activities of caspase-3 and -7. Cells (7×104 cells/ml) were treated with silvestrol (15 nM, 30 nM, 60 nM, 120 nM, and 240 nM) or etoposide for 24 h in a black 96-well plate. Etoposide, which is known to activate caspases-3/7 in LNCaP cells (17), was used as a positive control for this assay. At the end of the treatment, lysis buffer and the substrate (Z-DEVD-rhodamine 110) were mixed and added to the cells. Upon sequential cleavage and removal of the DEVD peptides by caspase-3 and -7 activity and excitation at 499 nm, the rhodamine 110-leaving group became intensely fluorescent. The emission maximum is 521 nm. The amount of fluorescent product generated was proportional to the amount of caspase-3 and -7 cleavage activity present in the sample. The samples were measured in triplicate. Caspase-3 and -7 activity was indicated by net fluorescence.
Mitochondrial membrane potential analysis
The mitochondrial membrane potential permeability was measured using 40 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6; Molecular Probes, Eugene, OR, USA). Cells (7×104 cells/ml) were plated in 100-mm culture dishes and incubated with silvestrol. At the end of incubation, cultured cells were incubated with DiOC6 for fifteen min at 37°C. Cells were washed with PBS before resuspension in 500μl PBS containing 40 nM DiOC6 and 30 μg/ml propidium iodide. Fluorescence intensities were analyzed by flow cytometry with excitation and emission settings of 484 and 500 nm, respectively. Histograms showed only viable, propidium iodide-negative cells.
Subcellular fractionation
The ApoAlert® Cell Fractionation kit (Clontech Laboratories, Inc., Palo Alto, CA, USA) was used as per the manufacturer’s protocol to separate mitochondria from the cytosol of LNCaP cells treated with silvestrol. After silvestrol exposure, cells as well as the floating cells were harvested in isotonic buffer including protease inhibitor cocktail and DTT, incubated on ice for 10 mins and homogenized. Samples were transferred to microfuge tubes and spun at 700 ×g for 10 min at 4°C to eliminate nuclei and unbroken cells. The resulting supernatant was centrifuged at 104×g for 25 min at 4°C to obtain supernatant as a cytosolic fraction and the pellet as a mitochondrial fraction. To confirm that the mitochondrial fraction was successfully separated from the cytosolic fraction, each sample was probed with COX4 antibody. These subcellular fractions were tested for the presence of cytochrome c and bak.
Immunoblotting
Silvestrol-treated cells, including the floating cells, were washed three times with ice-cold PBS and lysed in 500 μl of buffer (20 mM Tris base (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF). Cells were scraped from the plate and sonicated. Lysates were incubated at 0–4°C for 30 min and vigorously vortexed before centrifugation at 12,500 ×g for 15 min at 4°C. Total proteins (30 μg) were separated on 8–15% SDS-PAGE, and blotted onto polyvinylidene difluoride membranes using a semi-dry blotting system (Fisher-Scientific, Springfield, NJ, USA). The membrane was blocked with 5% nonfat dry milk in TBST (20 mM Tris-HCl (pH 7.6), 8.2 g/l NaCl, and 0.1% Tween 20) for 1 h at room temperature before incubation with primary antibody diluted with 5% nonfat dry milk in TBST at 4°C overnight. The membranes were washed three times with TBST and incubated with secondary antibody conjugated with horseradish peroxidase at room temperature for 1 h. Proteins were visualized using an enhanced chemiluminescence system (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA) after three washings in TBST.
Results
Silvestrol is cytotoxic towards LNCaP cells
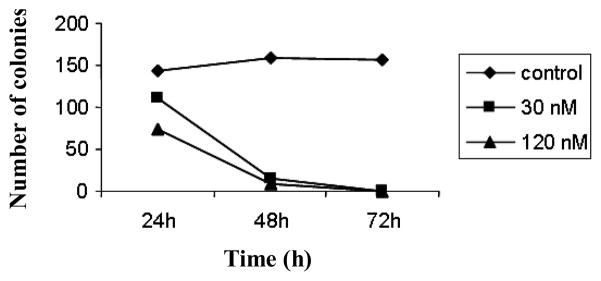
We previously reported the potent activity of silvestrol (Figure 1) in inhibiting the growth of various human tumor cell lines, including the human hormone-dependent prostate carcinoma, LNCaP (10). In order to better understand the possible mode of action of this agent, we assessed the effect of silvestrol on colony formation using LNCaP cells. As shown in Figure 2, the number of colonies was significantly reduced by treatment with silvestrol. More specifically, exposure of tumor cells with the natural product for more than 48 h resulted in complete inhibition of colony formation, suggesting that silvestrol functions by a cytotoxic rather than cytostatic mechanism in the LNCaP cell line.
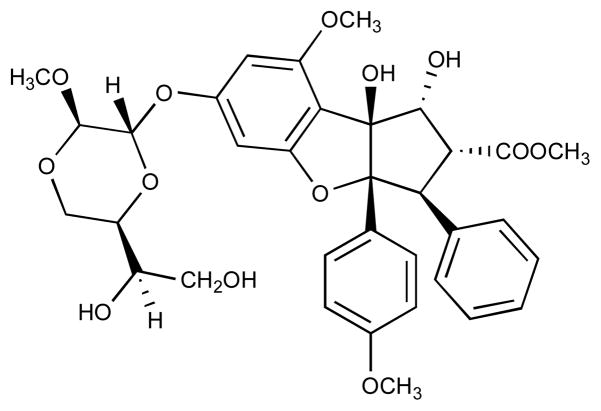
Figure 1.
Structure of silvestrol.
Figure 2.
Silvestrol is cytotoxic to LNCaP cells. LNCaP cells were plated in a 100 mm tissue culture dish (250 cells/dish), and treated with 30 nM and 120 nM silvestrol for 24, 48, or 72 h. Dishes were then washed and cultured in drug-free medium for an additional 7 days. Colonies were then fixed, stained with Giemsa stain, and scored. Results are expressed as the means of triplicate determinations.
Silvestrol induces apoptosis, which can be prevented by Boc-D-Fmk
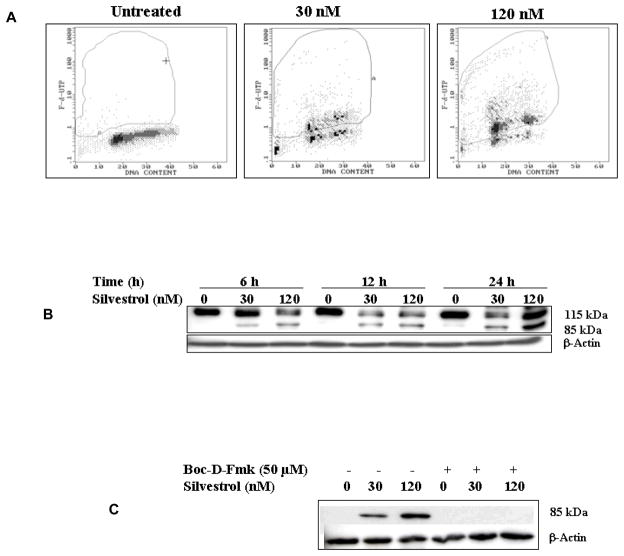
As an explanation for its cytotoxic effect, the capability of silvestrol to induce apoptosis in the LNCaP cell line was investigated. Apoptosis was measured by the TUNEL assay. As shown in Figure 3A, silvestrol treatment of LNCaP cells resulted in a 53% and 77% increase in TUNEL-positive cells when exposed to 30 nM or 120 nM silvestrol, respectively. A dose- and time-dependent increase of PARP cleavage was also observed after treatment with silvestrol, confirming that this compound induced apoptosis in LNCaP cells (Figure 3B).
Figure 3.
Silvestrol induces apoptosis in LNCaP cells and the general caspase inhibitor, Boc-D-Fmk, inhibits silvestrol-induced apoptosis. (A) The TUNEL assay was used for quantification of silvestrol-induced apoptosis. LNCaP cells were treated with 30 nM or 120 nM silvestrol for 24 h, harvested, fixed, stained with FITC-dUTP and analyzed by flow cytometry as detailed in “Material and Methods.” (B) Immunoblot of PARP cleavage with treatment of silvestrol (30 nM or 120 nM). (C) LNCaP cells were treated with 30 nM or 120 nM silvestrol for 24 h in the absence or presence of 50 μM Boc-D-Fmk. An antibody specific for cleaved PARP was used.
Inhibition of silvestrol-mediated apoptosis by a general caspase inhibitor, Boc-D-Fmk, was observed in the immunoblotting for PARP cleavage, suggesting the involvement of certain caspases. The cleaved PARP (85 kDa) was not observed in cells pretreated with the general caspase inhibitor, Boc-D-Fmk (Figure 3C).
Silvestrol induces apoptosis through the mitochondrial pathway
It has been suggested that a dissipation of the mitochondrial transmembrane potential (ΔΨm) precedes apoptosis and may represent an early signaling event (18). It is known to be part of the death pathway of cells exposed to chemotherapeutic drugs and several proteins located between the inner and outer mitochondrial membranes can affect depolarization. Several studies suggest that phosphorylation of the anti-apoptotic proteins, bcl-2 or bcl-xl, make them inactive, thus promoting apoptosis, possibly by freeing bax or bak from (bcl-2 or bcl-xl)/(bak or bax) dimers and by releasing cytochrome c from the mitochondria to the cytoplasm.
Using the DiOC6 fluorescent probe, we analyzed the ΔΨm of LNCaP cells treated with silvestrol. Silvestrol treatment of 30 nM or 120 nM showed a slight ΔΨm reduction that was not reduced further by extending the incubation to time 24 h (Figure 4A). However, silvestrol treatment did induce cytochrome c release from mitochondria to the cytoplasm (Figure 4B). In addition, proteins located in the mitochondrial membrane were tested by immunoblotting and it was found that phosphorylated bcl-xl and bak were associated with silvestrol-induced apoptosis, indicating the involvement of mitochondria in this phenomenon (Figure 4B).
Figure 4.
Silvestrol induces apoptosis through the mitochondrial pathway. (A) LNCaP cells were treated with silvestrol as indicated in the figure. The cells were then sequentially stained with DiOC6 and propidium iodide followed by flow cytometric analysis. Note the leftward shift in the peaks silvestrol exposed cells relative to controls, which is indicative of mitochondrial membrane depolarization. (B) Immunoblot of the effect of silvestrol on cytochrome c, bak, and phosphorylated bcl-xl.
Caspase-2, -9, and -10 are involved in silvestrol-induced apoptosis but caspase-3 and -7 are not
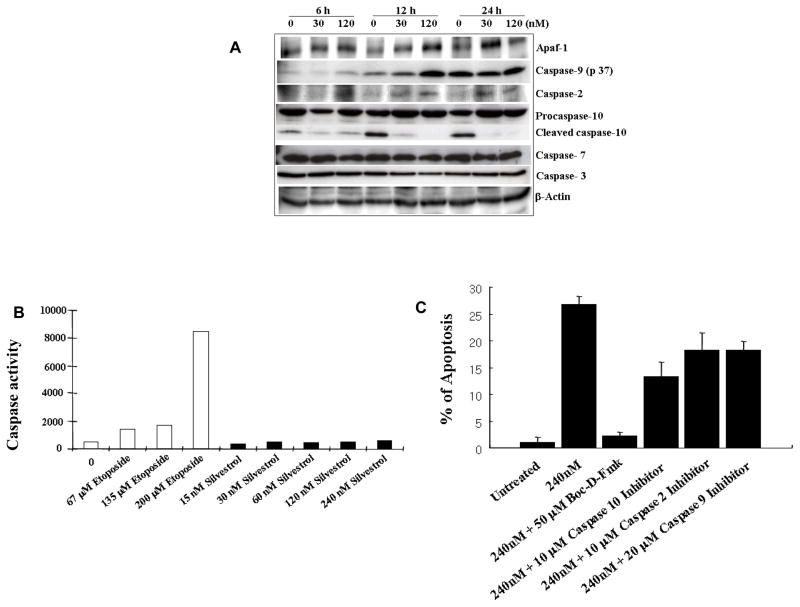
To further define the caspases involved, the processing of caspase-1, -2, -3, -4, -5, -6, -7, -8, -9, and caspase-10 was monitored by immunoblot. This revealed that silvestrol increased the level of cleaved caspase-9 (p37) and caspase-2, as well as an increase of Apaf-1 (Figure 5A).
Figure 5.
Apaf-1, Caspase-2, caspase-9, and caspase-10 are involved in silvestrol-induced apoptosis but caspase-3 and 7 are not. (A) Immunoblot analysis showing the involvement of Apaf-1 and caspase-2, -9, and -10. LNCaP cells were treated with 30 nM and 120 nM silvestrol for 6 h, 12 h, and 24 h. (B) Caspase-3/-7 like activity determined after silvestrol treatment of the LNCaP cells for 24 h using the DEVD-R110 cleavage assay. Etoposide was incubated with LNCaP cells for 24 h as a positive control for the assay. Results are the means of experiments performed in triplicate. (C) Induction of apoptosis induced by 240 nM silvestrol for 24 h in LNCaP cells pretreated with 50 μM Boc-D-Fmk (general caspase inhibitor), 10 μM Z-VDVAD-FMK (caspase-2 inhibitor), 20 μM Z-LEHD-FMK (caspase-9 inhibitor), or 10 μM Z-AEVD-FMK (caspase-10 inhibitor). Apoptosis was quantified by counting nuclei stained with DAPI. Results are the means of three experiments performed in triplicate.
Several studies suggest that Apaf-1 and procaspase-9 assemble into a heptameric wheel-like caspase-activating complex termed the apoptosome. Procapase-9, which is thought to be activated by autoprocessing upon recruitment to the apoptosome, subsequently activates procaspase-3 and -7. However, in this study, immunoblot analysis did not reveal cleavage of procaspase-3 or -7. To confirm the results from immunoblot analysis of caspase-3 and -7, caspase-3 -7-like activity was monitored using the Apo-ONE™ Homogeneous Caspase-3 -7 assay kit. Etoposide served as positive control since it known to induce caspase-3 and -7 activities in LNCaP cells (17). Etoposide concentrations used were based on previous studies (17). As expected from immunoblot analysis, there were no differences between untreated-cells and silvestrol-treated cells at the highest dose of 240 nM. In contrast to silvestrol-treated cells, etoposide-treated cells exhibited significant caspase-3 and -7 like activity (Figure 5B), confirming that neither caspase-3 nor -7 is responsible for silvestrol-induced apoptosis in LNCaP cells.
In addition to the effects on caspase-2 and -9, it was also found that caspase-10 is involved in silvestrol-induced apoptosis (Figure 5A). However, immunoblotting revealed that silvestrol did not induce the cleavage of procaspase-10 for its activation; rather it induced up-regulation of procaspase-10 expression. Furthermore, we did not detect any changes of procaspase-1, -4, -6, or -8 (data not shown).
To confirm the involvement of caspase-2, -9 and -10, and to investigate the relative contribution of these caspases in silvestrol-induced apoptosis, we observed the formation of apoptotic bodies with DAPI staining with four different caspase inhibitors, namely, for general caspase, caspase-2, caspase-10, and caspase-9 inhibition. As illustrated in Figure 5C, all three selective caspase inhibitors demonstrated some reduction in silvestrol-induced apoptosis, although none of them completely inhibited the formation of apoptotic bodies. In contrast, the general caspase inhibitor, Boc-D-Fmk, effectively reduced apoptosis to the level of the untreated cells. Interestingly, the caspase-10 inhibitor was the most effective of the selective inhibitors for the reduction of silvestrol-induced apoptosis while the caspase-2 and caspase-9 inhibitors showed similar but less pronounced effects.
Discussion
The results of the present study suggest that silvestrol, a plant-derived cyclopenta[b]benzofuran with an unusual dioxanyloxy ring functionality, induces apoptosis in LNCaP cells. While some members of this compound class are cytostatic against various human cancer cell lines (6, 13), in the present study silvestrol (Figure 1) exhibited a cytotoxic rather than cytostatic effect in a colony formation assay with LNCaP cells (Figure 2). This result is consistent with the observations by others that silvestrol is cytotoxic rather than cytostatic in THP-1 and A549 cells (19). A major difference of silvestrol with other cyclopenta[b]benzofurans is the dioxanyloxy group at the C-6 position, suggesting that the novel dioxanyloxy substituent is important for mediating cytotoxicity.
In the current study, we observed that silvestrol induced cell death through an apoptosis pathway that was inhibited by the general caspase inhibitor, Boc-D-Fmk (Figure 3). Inhibition of apoptosis with pretreatment of Boc-D-Fmk indicated that caspases were functionally and actively involved in silvestrol-induced apoptosis. Recently, induction of apoptosis triggered by certain cyclopenta[b]benzofurans (flavaglines) was reported in colorectal cancer cells (20). Silvestrol induced apoptosis associated with depolarization of the mitochondrial membrane, down-regulation of bcl-xl, and activation of p38 (20). However, the caspases involved in cyclopenta[b]benzofuran-induced apoptosis were not demonstrated. In the present study, the involvement of caspase-2, -9, and -10 were detected (Figure 5A). Although silvestrol treatment did not induce a dramatic change in the mitochondrial membrane potential (Figure 4A), it did induce cytochrome c release, up-regulation of the proapoptotic protein bak and phosphorylation of the antiapoptotic protein bcl-xl, suggesting mitochondrial involvement in silvestrol-induced apoptosis (Figure 4B). In addition, involvement of the apoptosome was suggested by up-regulation of Apaf-1 (Figure 5A), which, along with cytochrome c and caspase-9, is a component of the apoptosome.
It is well known that caspase-2 and -9 are engaged in the intrinsic pathway by responding to changes in mitochondrial potential (21), whereas caspases-8 and -10 are engaged in the extrinsic pathway by detecting activation of the death receptor (22). Activation of these initiator caspases leads to processing of the downstream effector caspases, caspase-3 and -7. Therefore, it would be logical to propose the involvement of caspase-3 or -7, regardless of which pathway is involved. However, in the case of silvestrol-induced apoptosis, we did not detect cleavage of either procaspase-3 or -7 by immunoblot (Figure 5A). This result is surprising since PARP cleavage was observed in silvestrol-treated cells and PARP is a classical substrate of caspase-3 and -7 (23). Furthermore, caspase-7 is known as the predominant caspase in LNCaP cells (24, 25). To confirm the lack of involvement of caspase-3 and -7 in silvestrol-induced apoptosis, caspase-3 and -7-like activities were analyzed and the results were consistent with immunoblot analysis, indicating that caspase-3 and caspase-7 did not play a crucial role in silvestrol-induced apoptosis (Figure 5B). In addition to classic apoptotic mechanisms involving caspases, alternative programmed cell death signaling programs are beginning to emerge and several proteins have been discovered to lead to apoptosis by themselves, in the absence of caspases (26). Based on our results, some of these proteins may be involved and responsible for typical apoptotic cell morphology instead of the effector caspase-3 and -7. Clearly, further investigations in this area are warranted.
The involvement of caspase-10 was also detected in silvestrol-induced apoptosis (Figure 5A), as were caspase-2 and caspase-9. However, immunoblotting revealed an increase of procaspase-10 expression instead of the typical procaspase cleavage for its activation. Most studies on apoptosis are based on the assumption that caspase precursors are activated by cleavage, which is a common mechanism for many zymogens. Although this appears to be true for the executioner caspases, recent research indicates that proximity-induced dimerization without cleavage may be responsible for the activation of caspase-8 and caspase-10 in the DISC (22, 27, 28). Furthermore, it was found that caspase-10 is frequently down-regulated in several tumor cell lines (29) and that caspase-8 is deleted or silenced preferentially in childhood neuroblastoma (30) suggesting that down-regulation of caspase -8 and -10 may contribute to oncogenesis (27). Therefore, it is conceivable that one potential mechanism of silvestrol-induced apoptosis may be via up-regulation of caspase-10 expression, resulting in an increase of procaspase-10, proximity-induced dimerization as well as subsequent activation of the DISC mediated apoptotic pathway.
The ability of four specific caspase inhibitors to block silvestrol-induced apoptosis was studied to confirm caspase involvement and to assess their relative contributions to cell death. Although a general caspase inhibitor totally abrogated this process, inhibitors of caspase-2, -9, or -10 only partially inhibited silvestrol-induced apoptosis. Interestingly, both the caspase-2 and caspase-9 inhibitors exhibited about a 40% reduced apoptotic response, whereas the caspase-10 inhibitor showed about a 60% decrease in apoptosis compared to silvestrol-only treated cells. Since it is known that caspase-2 and -9 are initiator caspases in mitochondrion-dependent apoptosis and caspase-10 is the initiator caspase in death receptor-mediated apoptosis, it seems likely that the contribution of the extrinsic pathway with caspase-10 is greater than the intrinsic pathway involving caspase-2 and -9 in silvestrol-induced apoptosis.
Interestingly, several studies have indicated that LNCaP cells are resistant to Fas-mediated apoptosis, despite expressing both Fas receptor and Fas ligand (31), and are also resistant to TRAIL-induced apoptosis (32, 33). Therefore, it is possible that part of silvestrol’s mechanism of action may be by overcoming resistance to Fas-mediated apoptosis, which could explain the involvement of the extrinsic pathway as seen in our studies. Likewise, studies have demonstrated that anti-Fas treatment of both resistant and sensitive cell lines induced rapid tyrosine phosphorylation ordephosphorylation of multiple proteins in human prostate carcinoma cell lines (31). Specifically, treatment with a protein synthesis inhibitor, cycloheximide, converted the phenotype from Fas-resistant to Fas-sensitive, suggesting that resistance may be regulated by an apoptosis suppressor factor or factors such as a FLICE-inhibitory protein (34, 35). This raises the possibility that silvestrol may inhibit these apoptosis suppressor factors to restore sensitivity to Fas-mediated apoptosis in LNCaP cells.
The studies described herein have demonstrated the involvement of the apoptosome/mitochondrial as well as extrinsic pathway in silvestrol-induced programmed cell death. Furthermore, results from the current study suggest that, in addition to the classic apoptotic mechanisms, alternative programmed cell death processes may also be engaged. For example, additional factors may be involved in silvestrol-induced programmed cell death pathway and identification of these factors could be the focus of future studies. These data should be useful for understanding the apoptotic cellular mechanism mediated by cyclopenta[b]benzofurans and should be helpful in the further characterization of silvestrol as a potential cancer drug candidate.
Acknowledgments
This work was supported by grant U19 CA52956 funded by the National Cancer Institute, USA. We thank Dr. Karen Hagen of the Research Resource Center (University of Illinois at Chicago) for analysis of samples by flow cytometry.
Abbreviations
- Apaf-1
apoptotic protease-activating factor 1
- DAPI
4,6-diamidino-2-phenylindole
- DISC
the death-inducing-signaling-complex
- PARP
poly (ADP-ribose) polymerase
References
- 1.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 2.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 3.Bohnenstengel FI, Steube KG, Meyer C, Nugroho BW, Hung PD, Kiet LC, Proksch P. Structure activity relationships of antiproliferative rocaglamide derivatives from Aglaia species (Meliaceae) Z Naturforsch [C] 1999;54:55–60. [PubMed] [Google Scholar]
- 4.Proksch P, Edrada R, Ebel R, Bohnenstengel FI, Nugroho BW. Chemistry and biological activity of rocaglamide derivatives and related compounds in Aglaia species (Meliaceae) Curr Org Chem. 2001;5:923–938. [Google Scholar]
- 5.King ML, Chiang CC, Ling HC, Fujita E, Ochiai M, McPhail AT. X-ray crystal structure of rocaglamide, a novel antileukemic 1H-cyclopenta[b]benzofuran from Aglaia elliptifolia. J Chem Soc, Chem Comm. 1982:1150–1151. [Google Scholar]
- 6.Bohnenstengel FI, Steube KG, Meyer C, Quentmeier H, Nugroho BW, Proksch P. 1H-cyclopenta[b]benzofuran lignans from Aglaia species inhibit cell proliferation and alter cell cycle distribution in human monocytic leukemia cell lines. Z Naturforsch [C] 1999;54:1075–1083. doi: 10.1515/znc-1999-1212. [DOI] [PubMed] [Google Scholar]
- 7.Baumann B, Bohnenstengel F, Siegmund D, Wajant H, Weber C, Herr I, Debatin KM, Proksch P, Wirth T. Rocaglamide derivatives are potent inhibitors of NF-kappa B activation in T-cells. J Biol Chem. 2002;277:44791–44800. doi: 10.1074/jbc.M208003200. [DOI] [PubMed] [Google Scholar]
- 8.Fahrig T, Gerlach I, Horvath E. A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson’s disease and traumatic brain injury. Mol Pharmacol. 2005;67:1544–1555. doi: 10.1124/mol.104.008177. [DOI] [PubMed] [Google Scholar]
- 9.Proksch P, Giaisi M, Treiber MK, Palfi K, Merling A, Spring H, Krammer PH, Li-Weber M. Rocaglamide derivatives are immunosuppressive phytochemicals that target NF-AT activity in T cells. J Immunol. 2005;174:7075–7084. doi: 10.4049/jimmunol.174.11.7075. [DOI] [PubMed] [Google Scholar]
- 10.Hwang BY, Su BN, Chai H, Mi Q, Kardono LB, Afriastini JJ, Riswan S, Santarsiero BD, Mesecar AD, Wild R, Fairchild CR, Vite GD, Rose WC, Farnsworth NR, Cordell GA, Pezzuto JM, Swanson SM, Kinghorn AD. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J Org Chem. 2004;69:3350–3358. doi: 10.1021/jo040120f. [DOI] [PubMed] [Google Scholar]
- 11.El Sous M, Rizzacasa MA. Biomimetic synthesis of the novel 1,4-dioxanyloxy fragment of silvestrol and episilvestrol. Tetrahedron Letters. 2005;46:293–295. [Google Scholar]
- 12.Nicholson DW, Thornberry NA. Apoptosis. Life and death decisions. Science. 2003;299:214–215. doi: 10.1126/science.1081274. [DOI] [PubMed] [Google Scholar]
- 13.Lee SK, Cui B, Mehta RR, Kinghorn AD, Pezzuto JM. Cytostatic mechanism and antitumor potential of novel 1H-cyclopenta[b]benzofuran lignans isolated from Aglaia elliptica. Chem Biol Interact. 1998;115:215–228. doi: 10.1016/s0009-2797(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 14.Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 15.Choi C, Kutsch O, Park J, Zhou T, Seol DW, Benveniste EN. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol. 2002;22:724–736. doi: 10.1128/MCB.22.3.724-736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozoren N, Kim K, Burns TF, Dicker DT, Moscioni AD, El-Deiry WS. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2000;60:6259–6265. [PubMed] [Google Scholar]
- 17.Coffey RN, Watson RW, O’Neill AJ, Mc Eleny K, Fitzpatrick JM. Androgen-mediated resistance to apoptosis. Prostate. 2002;53:300–309. doi: 10.1002/pros.10159. [DOI] [PubMed] [Google Scholar]
- 18.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meurer-Grimes BM, Vairo GL, Yu J. Therapeutic compounds and methods. US Patent No. 2003 6710075.
- 20.Hausott B, Greger H, Marian B. Flavaglines: a group of efficient growth inhibitors block cell cycle progression and induce apoptosis in colorectal cancer cells. Int J Cancer. 2004;109:933–940. doi: 10.1002/ijc.20033. [DOI] [PubMed] [Google Scholar]
- 21.Troy CM, Shelanski ML. Caspase-2 redux. Cell Death Differ. 2003;10:101–107. doi: 10.1038/sj.cdd.4401175. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA. 2001;98:13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol. 2002;3:275–283. doi: 10.2174/1389201023378265. [DOI] [PubMed] [Google Scholar]
- 24.Marcelli M, Cunningham GR, Walkup M, He Z, Sturgis L, Kagan C, Mannucci R, Nicoletti I, Teng B, Denner L. Signaling pathway activated during apoptosis of the prostate cancer cell line LNCaP: overexpression of caspase-7 as a new gene therapy strategy for prostate cancer. Cancer Res. 1999;59:382–390. [PubMed] [Google Scholar]
- 25.Marcelli M, Cunningham GR, Haidacher SJ, Padayatty SJ, Sturgis L, Kagan C, Denner L. Caspase-7 is activated during lovastatin-induced apoptosis of the prostate cancer cell line LNCaP. Cancer Res. 1998;58:76–83. [PubMed] [Google Scholar]
- 26.Mathiasen IS, Jaattela M. Triggering caspase-independent cell death to combat cancer. Trends Mol Med. 2002;8:212–220. doi: 10.1016/s1471-4914(02)02328-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002;7:313–319. doi: 10.1023/a:1016167228059. [DOI] [PubMed] [Google Scholar]
- 28.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, Gazdar A, Blenis J, Arnott D, Ashkenazi A. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 30.Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 31.Rokhlin OW, Bishop GA, Hostager BS, Waldschmidt TJ, Sidorenko SP, Pavloff N, Kiefer MC, Umansky SR, Glover RA, Cohen MB. Fas-mediated apoptosis in human prostatic carcinoma cell lines. Cancer Res. 1997;57:1758–1768. [PubMed] [Google Scholar]
- 32.Liang Y, Eid MA, Lewis RW, Kumar MV. Mitochondria from TRAIL-resistant prostate cancer cells are capable of responding to apoptotic stimuli. Cell Signal. 2005;17:243–251. doi: 10.1016/j.cellsig.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Liang H, Salinas RA, Leal BZ, Kosakowska-Cholody T, Michejda CJ, Waters SJ, Herman TS, Woynarowski JM, Woynarowska BA. Caspase-mediated apoptosis and caspase-independent cell death induced by irofulven in prostate cancer cells. Mol Cancer Ther. 2004;3:1385–1396. [PubMed] [Google Scholar]
- 34.Rokhlin OW, Bishop GA, Hostager BS, Waldschmidt TJ, Sidorenko SP, Pavloff N, Kiefer MC, Umansky SR, Glover RA, Cohen MB. Fas-mediated apoptosis in human prostatic carcinoma cell lines. Cancer Res. 1997;57:1758–1768. [PubMed] [Google Scholar]
- 35.Rokhlin OW, Hostager BS, Bishop GA, Sidorenko SP, Glover RA, Gudkov AV, Cohen MB. Dominant nature of the resistance to Fas- and tumor necrosis factor-alpha-mediated apoptosis in human prostatic carcinoma cell lines. Cancer Res. 1997;57:3941–3943. [PubMed] [Google Scholar]