Abstract
The two most common HIV-associated renal diseases, HIV-associated nephropathy and HIV-immune-complex kidney disease, share the common pathologic finding of hyperplasia within the glomerulus. Podocyte injury is central to the pathogenesis of these diseases; however, the source of the proliferating glomerular epithelial cell remains a topic of debate. Parenchymal injury has been linked to direct infection of renal epithelial cells by HIV-1, although the mechanism of viral entry into this non-lymphoid compartment is unclear. Although transgenic rodent models have provided insight into viral proteins responsible for inducing renal disease, such models have important limitations. Rodent HIV-1 models, for instance, cannot replicate all aspects of immune activation, a process that could have an important role in the pathogenesis
Key points
HIV-associated nephropathy (HIVAN) and HIV immune-complex kidney disease (HIVICK) are the most common renal diseases in patients with HIV-1.
HIVAN seems to be caused directly by HIV-1 infection, possibly via disruption of normal homeostatic functions of mature podocytes caused by HIV proteins Nef, Vpr and Tat.
Contrary to HIVAN, HIVICK is not observed in rodent models of HIV-1, which might mean that virus replication or immune response to viral proteins—not expression of viral genes alone—induce this condition.
The mechanism by which renal parenchymal cells are infected by HIV-1 does not seem to involve the same CD4 chemokine co-receptor mechanism that the virus uses to infect immune cells.
Hyperplastic injury in the glomerulus is a common pathogenic event of the HIV-associated renal diseases, although the source of the proliferative epithelial cell is unclear.
Immune activation, both systemic and within the kidney, is dramatically altered in HIV-1 infection and might have a role in precipitating and exacerbating HIV-associated renal diseases.
Introduction
HIV-associated renal diseases, like most chronic kidney diseases, are caused by a combination of genetic and environmental factors. The two most common HIV-associated renal conditions are a collapsing glomerulopathy known as HIV-associated nephropathy (HIVAN) and a collection of immunoglobulin-related glomerulonephritidies collectively known as HIV immune-complex kidney disease (HIVICK). HIVAN is the most common renal complication among patients with HIV-1 and is predominantly seen in patients of African descent.1,2 The immune-complex disorders are more frequently diagnosed in patients of non-African descent than in those of African descent, although the prevalence of these disorders can vary widely depending on patient demographics.
This Review discusses the pathogenesis of the HIV-associated renal diseases, emphasizing the role of HIV-1 as an environmental pathogen that perturbs the homeostatic processes of the host. Genetically determined host responses to HIV-1 are also important, but little is known about the genes involved in these processes. The genetics of HIV-associated renal diseases, which have been reviewed elsewhere,3 are not discussed in detail here. We will focus on unresolved and controversial issues regarding pathogenesis, including mechanisms of infection within the kidney, interpretation of data from rodent disease models, the contribution of host immune responses, and the source of the proliferating glomerular epithelial cell (Box 1).
Box 1. Unanswered questions relating to the pathogenesis on HIV-associated nephropathy.
What is the route of renal cell infection?
Are mesangial and endothelial renal cells infected by HIV-1?
-
Which viral proteins contribute to disease?
Are intracellular or extracellular effects responsible?
Are (individual or combined) effects of proteins Nef, Vpr, Tat and gp120 responsible for any pathogenic effect?
-
What is the identity of the proliferating cells that form the glomerular pseudocrescents?
Podocytes?
Parietal cells?
Stem-cell-derived cells?
-
What is the contribution of immune activation?
-
Is immune activation initiated by immune cells?
Is activation a response to infection?
Is activation a local response to inflammation?
Is immune activation initiated by renal cells?
-
The current consensus
HIVAN and HIVICK are parenchymal renal diseases that occur secondary to infection of the host by HIV-1. Various animal models, including natural infections by related lentiviruses (simian immunodeficiency virus or recombinant simian-human immunodeficiency virus in primates4–6 and feline immunodeficiency virus in cats7) as well as the many transgenic rodent models (discussed below), support the paradigm that HIV-1 infection and expression of viral gene products cause the HIV-associated renal diseases. Although intravenous drug use is common in the patient populations most susceptible to HIVAN, since the earliest reports, HIVAN and HIVICK have been identified as pathologically distinct from renal diseases linked solely to illicit drug use, such as heroin nephropathy.8,9 In addition, several reports have confirmed that HIVAN can also develop in patients who acquired HIV by routes of transmission other than intravenous drug use, such as perinatal infection.10,11
A strong genetic predisposition exists for the development of HIVAN, with the overwhelming majority of cases occurring in individuals of African descent.3 However, only ~10% of African Americans with HIV-1 develop renal complications, which indicates genetic diversity within the African American population. Similarly, preliminary reports from Africa suggest that the prevalence of HIVAN varies widely with geographical location, an observation that conforms with the high degree of genetic diversity within African populations.12 Genome-wide association studies have identified a candidate gene associated with most of the attributable risk for the development of HIV-associated focal segmental glomerulosclerosis in African Americans.13,14 The function of this gene in renal cells and how its allelic variation might predispose to kidney disease, however, is not yet known.
HIV-1 infection within the kidney
Several groups have shown that HIV-1 nucleic acids are present in both the non-lymphoid and lymphoid renal compartments in kidneys of patients diagnosed with HIVAN (Figure 1).15–18 This observation indicates that the kidney could serve as a reservoir for HIV-1 in this patient population. Of the non-lymphoid compartments, HIV-1 has been detected in renal epithelial cells, including glomerular visceral and parietal epithelial cells and tubular epithelial cells. Whether HIV-1 also infects renal epithelial cells in patients without HIVAN has not been conclusively established, as available reports on very small numbers of patients have provided conflicting results.15,17 If renal epithelial cells harbor HIV-1 in otherwise normal kidneys, then the genetic predilection for HIVAN associated with African ancestry would probably not be related to the mechanisms that preside to viral entry.
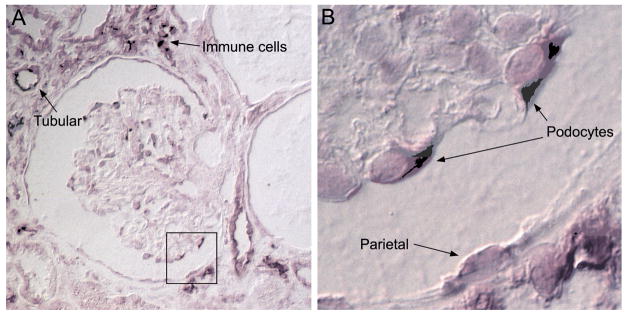
Figure 1.
Renal biopsy sample from a patient with HIVAN. HIV-1 messenger RNA is detected via an in situ hybridization methodology that leads to the formation of a purple stain.15 In infected cells, viral messenger RNAs transcribed from viral DNA are primarily found in the cytoplasm. a Hybridization-positive tubular epithelial cells and immune cells have infiltrated the interstitium (60× magnification). b Viral messenger RNAs are also present in podocytes and parietal epithelial cells (100× magnification). Abbreviation: HIVAN, HIV-associated nephropathy.
Unlike the well-described mechanisms of HIV-1 infection of lymphocytes, macrophages, and dendritic cells, the route by which HIV-1 enters renal epithelial cells is poorly understood. HIV-1 envelope glycoprotein gp120 isolated from renal biopsies of patients with HIVAN were found to be dual-tropic and could infect lymphocytes expressing either CXCR-4 or CCR5 (chemokine co-receptors of T-cell surface glycoprotein CD4), which are most commonly responsible for HIV-1 entry.19,20 Renal epithelial cells, however, do not normally express T-cell surface glycoprotein CD4, CCR5, or CXCR-4.18,21–23 Alternatively, HIV-1 could use other chemokine receptors for infection, such as chemokine C-C motif ligand 3 (CCL3)24 or the Duffy antigen receptor complex (DARC). DARC expression is upregulated in the kidneys of children with HIVAN,25 however no association was found between a DARC polymorphism prevalent among African Americans and HIVAN,26 although homozygosity for this polymorphism does increase susceptibility to lymphocyte infection.27
Infected immune cells are also found in the kidneys of patients with HIV-associated renal diseases and could serve as a proximal cellular source of HIV-1 for renal epithelial cells. One early study using tubular epithelial cells isolated from biopsies or urine of children with HIVAN showed that HIV-1 virions from infected macrophages could be detected in membrane-bound, intracellular vesicles within the tubular cells when the two cell-types were cultured together.28 This observation suggests that trans-infection by phagocytosis of released virus or perhaps during direct cell-to-cell contact with infected leukocytes is a plausible mechanisms of viral entry into renal epithelial cells. Indeed, similarly to infection of dendritic cells via the lectin DC-SIGN,29,30 DEC-205 on renal epithelial cells has been show to support internalization of HIV-1 in vitro; subsequent rescue of virus by co-cultured T-cells suggests that productive infection of renal epithelial cells is possible.31,32 Likewise, exosomes released from infected renal dendritic cells may fuse with or be phagocytosed by adjacent renal epithelial cells.33,34 Any of these and other non-conventional mechanisms of viral entry are avenues for future research to investigate how HIV-1 infects non-lymphoid renal tissue.
Infection of non-epithelial renal cells
Strong in vivo evidence indicates that renal epithelial cells (both glomerular and tubular) harbor HIV-1 nucleic acids, which suggests infection of these cells by HIV-1.15–17,19,35 In these studies, no evidence suggested that glomerular mesangial or endothelial cells harbor HIV-1. However, several in vitro studies have reported on the susceptibility of these other glomerular cells to infection, although their results are conflicting.36–39 Interpretation of these in vitro studies could be complicated by the implementation of suboptimal methods (e.g. use of laboratory-adapted virus, rodent cells, or immortalized cell lines) and the achievement of only marginal levels of viral replication. Evidence exists that mesangial cells express HIV-1 receptor and co-receptors,36 eliminating the issue with renal epithelial cell infection described above; however, the existence of these receptors has not been confirmed by other studies.18,21–23 Results from reports on the infection of mesangial cells in vitro are inconclusive. Two studies found opposite results with regard to infection using the same method (infection of primary human mesangial cells with HIV-1 IIIB),36,39 whereas a third study reported that mesangial cells were resistant to infection by common T-cell-tropic and macrophage-tropic viruses but were susceptible to rare brain-tropic viral isolates.37 Infection of endothelial cell, also a T-cell surface glycoprotein CD4 and co-receptor negative cell type, is unlikely. Extrapolating from extensive work in the area of HIV-related central nervous system complications, endothelial cells cannot fully support the viral lifecycle and are not considered a significant contributor to virus production in vivo.40 Although the hypothesis of direct infection by HIV-1 of mesangial or renal endothelial cells remains inconclusive, these renal cell types could suffer deleterious effects from released viral proteins or cytokines secreted from other infected cells (see discussion below).
Interpreting data from rodent models
Much of our current understanding of the pathogenesis of HIVAN in vivo has relied on data collected from transgenic rodents expressing various HIV-1 genes (reviewed elsewhere41). Mice and rats cannot be infected by HIV-1, so transgenic animals allow only for the study of the post-integration phase of the HIV-1 lifecycle. In addition, almost all rodent HIV-1 disease models use replication-defective proviral DNA, such that no new virus can be produced, and immune response typical of productive viral infections are abrogated. Nevertheless, because viral proteins often hijack the host cellular machinery to support viral replication and altering normal cellular functions,42 this experimental approach has provided mechanistic insight.
Many HIV-1 transgenic models display the clinical and pathological features of HIVAN, but none display those of HIVICK. This observation suggests that expression of viral genes alone, not replicative infection, can induce HIVAN, whereas virus production or immune responses to viral proteins might be required to induce HIVICK. The transgenic models developed initially expressed most of the HIV-1 genome and used the native viral promoter, LTR, to drive gene transcription in many tissues including the kidney.43 These transgenic models were engineered to delete the gag and pol genes (Δgag-pol) but retain env and the six accessory genes nef, tat, rev, vif, vpr and vpu, and produced a renal disease remarkably similar to HIVAN.44 Similarly, expression of this Δgag-pol proviral DNA under the control of a podocyte-restricted promoter also recreated a HIVAN-like pathology, which indicates that podocyte injury has a primary role in disease induction.45 Another model, however, which expressed an intact, full length provirus under the control of the CD4 promoter recreated a renal disease displaying pathology similar to HIVAN, including tubulointerstitial fibrosis, inflammation, microcysts and protein casts.46 Expression under CD4 promoter control is restricted to T-cells, monocytes, macrophages, and dendritic cells, and strong transgene expression was detected in the kidney (glomeruli and interstitium).
To further investigate the mechanisms of pathogenesis associated with the expression of specific HIV-1 gene products in various renal compartments, additional transgenic models were created with fewer HIV-1 genes. In these studies, viral protein Nef has received considerable attention, but the results obtained exemplify the challenges and pitfalls of transgenic modeling. Protein Nef evolved to help HIV-1 escape host immunity by downregulating key cell surface receptors on lymphocytes, including T-cell surface glycoproteins CD3, CD4, and CD28, and major histocompatibility complex class I,47 and most protein Nef functions appear to be fully supported in murine cells.48 A series of transgenic models based on the CD4 promoter system described above were created that systematically deleted individual HIV-1 genes.49 This study found that none of the Δnef models developed the disease, but when nef was expressed, even when it was the only gene expressed,50 the kidneys developed glomerosclerosis—which is indicative of late-stage podocyte injury in HIVAN—and marked tubulointerstitial nephritis. However, in other studies Δnef transgenic models displayed kidney pathology and a high level of proteinuria.51,52 When protein Nef is expressed only in podocytes, results are inconsistent as well. One group found no renal pathology or proteinuria associated with this transgenic model but detected loss of cell-cycle quiescence and differentiation anomalies in glomeruli.53 Another group observed instead glomerulosclerosis—without appreciable glomerular epithelial cell proliferation—which was dependent on the level of nef expression54.
Although much attention has focused on nef, other viral genes such as vpr and tat could be involved in the pathogenesis of kidney disease. Like protein Nef, protein Vpr seems to have a role in HIVAN pathogenesis and in supporting key aspects of the viral lifecycle, specifically nuclear import and integration.55 Transgenic mice expressing a Δgag-pol/vpr transgene did not develop renal disease or proteinuria, despite intact nef expression.51 Studies on the expression of vpr (from either a podocyte or macrophage-restricted promoter) showed that protein Vpr can induce kidney disease and that its concomitant expression with protein Nef exacerbates renal injury.51,54 Protein Tat, a potent transcriptional co-activator, is difficult to study in context of the native provirus because it controls the expression of the viral LTR. Thus, protein Tat can only be studied when its expression is controlled by another promoter. Exogenously added protein Tat glomerular collapse.58
These discrepancies could have a number of explanations. For example, transgene expression levels have been associated with disease occurrence or severity in different founders with the same transgene.43,54 Accurate comparisons of expression levels among the various models would be impossible to achieve from the published data and would require a direct head-to-head analysis. Alternatively, forced expression of viral products might have toxic effects and cause renal injury unrelated to the true disease process in natural infections by the virus, as was elegantly demonstrated in a focal segmental glomerulosclerosis rat model obtained through podocyte expression of a diphtheria toxin receptor transgene.59 Moreover, by using renal-cell or tissue-specific promoter systems to express viral products, central and peripheral immunologic tolerance to these products can be bypassed, confounding studies aimed at determining how viral products hijack host molecular pathways.33,60 In addition, the cooperative pathogenic effect of HIV-1 in non-lymphoid and lymphoid tissues could be required to precipitate or accelerate renal disease. Indeed, disruptions in immunity and insurgence of inflammation are the predominant features of HIV-1 infection in humans. This combination of effects has yet to be fully modeled in rodents, although perturbations in immune cell populations have been observed in HIV-1 transgenic mice and rats through downregulation cell surface receptors on T-cells by protein Nef.49,61–65 Finally, HIV-1 proteins such as Tat, Nef, and Vpr can be released from cells that express them and enter or interact with adjacent or distal cells that do not.66–70 Thus, compartmentally-expressed viral products might induce pathology in another compartment within the kidney through the bystander effects of extracellular viral products. This hypothesis is supported by several in vitro studies evaluating the effects of exogenous viral proteins on renal cell behavior. The direct application of purified viral proteins such as the precursor envelope polyprotein gp160 and its major surface fragment gp12071–74 or Tat56,57 to renal cell cultures can induce a variety of responses relevant to HIVAN cell phenotypes. Such responses include activation of intracellular signaling cascades, loss of key differentiation proteins, proliferation, and apoptosis.
Role of host immune response
In a case report75 that examined renal biopsy samples from a patient with HIVAN before and after antiretroviral treatment, expression of viral mRNAs within infected renal epithelial cells was unchanged despite dramatic improvements in renal function and pathology. Since none of the antiretroviral drugs block viral gene transcription, this case suggested that renal function and pathology can improve despite ongoing HIV-1 expression within the non-lymphoid renal compartment. What did decrease with antiretroviral therapy in this patient was the systemic production of HIV-1, marked by an improvement in the patient’s immune and inflammatory status.
Acute HIV-1 infection induces a robust immune response to the virus, whereas progressive HIV-1 disease causes a persistent state of immune activation before the patient’s immune system collapses in AIDS.76 Both the acute and chronic stages of HIV-1 infection induce innate and adaptive immune responses, with marked increases in T-cell activation and turnover, and high levels of circulating pro-inflammatory cytokines such as the tumor necrosis factor. Since interstitial inflammation is a prominent feature of HIV-associated renal diseases, these states of immune activation can have a negative impact on the kidney, regardless of whether or not pro-inflammatory cytokines are produced within the organ. In support of this hypothesis, case reports and retrospective studies on the use of anti-inflammatory corticosteroids to treat HIVAN have consistently found improvements in renal function.77,78 What remains unknown in HIVAN is which immune and inflammatory effectors are most responsible for mediating injury to the renal parenchyma.
The pathogenic role of immune activation is clearer for HIVICK than HIVAN, because of the extensive research on immunoglobulin-mediated glomerulonephritidies. HIVICK is typically associated with concurrent infections, such as hepatitis C, and is characterized by immune complex deposition that includes complement, HIV-1 antigens and reactive antibodies.79 Patients with HIV-1 often have high levels of circulating antigens and robust polyclonal antibody responses which could predispose to immune-complex deposition and subsequent complement activation in the kidney. This lupus-like scenario initiates a secondary inflammatory response involving immune cell recruitment and cytokine secretion.80 Of course, as HIV-1 infection progresses to AIDS, patients with HIVAN or HIVICK become more susceptible to new infections or reactivation of latent infections, which would contribute to immune dysregulation and potentially exacerbate renal injury.81
All immune and inflammatory responses are mediated by NF-κB, a ubiquitous protein complex that acts as a pleiotropic transcription factor that participates in T and B-cell activation, cytokine gene expression, regulation of cell survival and proliferation, and is itself a key activator of the HIV-1 promoter. In HIVAN, podocytes exhibit an elevated and persistent activation of NF-κB.82 This intrinsic activation has been associated with podocyte proliferation and apoptosis in a mouse model of HIVAN.82,83 In addition, renal tubular epithelial cells infected in vitro express a large array of immune mediators, many of which are regulated by NF-κB.84 Secretion of pro-inflammatory cytokines tumor necrosis factor and interleukin-6 from renal tubular was shown to enhance viral replication in infected macrophages.85 Blockade of NF-κB activation in a transgenic AIDS mouse model resulted in marked improvements in renal function and pathology including a reduction in immune cell infiltrates.86 Together, the interconnected pathways that rely on NF-κB activation in leukocytes and the renal parenchyma could create a kidney microenvironment where inflammatory injury and fibrosis are intensified.
The debate on podocyte proliferation
The unifying pathological feature of HIVAN and HIVICK is hyperplastic injury within the glomerulus. Glomerular crescents are commonly found in many immunoglobulin-related glomerular diseases, including HIVICK. Glomerular pseudocrescents are pathognomonic lesions in adults with collapsing glomerulopathies such as HIVAN.87 In pediatric HIVAN, pseudocresentic injury is not typical, whereas mesangial proliferation is prominent.11 Abnormal proliferation is also commonly observed in tubular epithelial cells in HIVAN, with microcysts forming along the length of the nephron.88
Crescents were originally believed to be composed of proliferating parietal epithelial cells, whereas pseudocrescents were considered different because they were originally believed to be composed of proliferating visceral epithelial cells (podocytes). However, recent studies using more advanced cell lineage methods have challenged this cell type distinction (described below). Until the observation of pseudocrescents in HIVAN, adult podocytes had long been believed to be terminally differentiated and unable to proliferate. In other forms of glomerulosclerosis such as diabetic nephropathy, injured podocytes that die are not known to regenerate. Early immunohistochemical studies of HIVAN biopsies and transgenic mouse models of HIVAN suggested that diseased podocytes have increased levels of markers for cell-cycle engagement and progression, and mitosis. .89–91 However, these cells did not express many of the markers typical of differentiated podocytes.89 This observation instigated a debate over whether conclusively identifying diseased podocytes from other cell-types that may be present in pseudocrescents is possible. Several reports have since suggested that pseudocrescents in HIVAN and other collapsing glomerulopathies are not of podocyte origin because their marker composition was more consistent with either parietal glomerular epithelial cells92–94 or macrophages95 than with podocytes. Furthermore, two groups have provided evidence that podocytes could be regenerated from glomerular cells other than visceral glomerular epithelial cells, specifically parietal glomerular epithelial cells96 or renal glomerular stem cells.97 These studies, however, do not exclude a pathogenic contribution of podocyte proliferation. Indeed, proliferating podocytes can initiate the formation of and populate true glomerular crescents.95,98 Overall, evidence does support the hypothesis that pseudocrescents can be populated by cells that do not originate from mature, differentiated podocytes.
If mature podocytes have the capacity to reenter the cell cycle, then podocyte differentiation would not be an irreversible process of post-mitotic permanence, but one of reversible quiescence. This scenario could have implications for future therapies, which could be directed at the regeneration of podocytes following injury. Alternatively, if the proliferating cells in HIVAN pseudocrescents are not derived from mature podocytes, then HIVAN would be like other forms of glomerulosclerosis where mature podocytes do not proliferate, but die or are shed into the urine. In the latter case, determining whether pseudocrescents are populated by podocyte or -non-podocyte precursors would become the primary research objective for regenerative therapies. Despite the unclear origin of the proliferating glomerular cell in HIVAN, what remains at issue is uncontrolled hyperplasia, the major pathologic anomaly in HIVAN glomeruli.
The role of podocyte dedifferentiation
During normal cell development, transcriptional reprogramming occurs to shift global patterns of gene expression away from genes associated with proliferation to those associated with differentiation.99 This inverse relationship between a cell’s proliferative capacity and its differentiation state is necessary in mature organs to achieve and preserve normal tissue architecture and cell function.
For a polarized epithelial cell to divide, cytokinesis requires the cell to disengage from cell-cell and cell-matrix contacts and morphologically and functionally dedifferentiate. Mechanistically, whether podocyte dedifferentiation in HIVAN is nothing more than a default response in the context of cell cycle engagement and progression is unclear. In principle, adult podocytes undergoing cell division should lose the slit diaphragm and rearrange foot process cytoskeletal networks, thereby entering a reversible state of effective loss-of-function and dedifferentiation until mitosis is complete. This state is different from the absolute loss-of-function associated with podocyte shedding and death. However, evidence exists that podocytes in models of HIVAN can lose markers of differentiation without evidence of appreciable proliferation;54 whether these altered podocytes also contribute to loss of glomerular function in HIVAN is unknown. Indeed, determining if the primary injury induced by HIV-1 is the loss of podocyte cell-cycle control is of primary importance, as reinstating this control would provide a focus for therapy development. This strategy has been explored in experimental therapeutics for HIVAN. Small molecule inhibitors of cell-cycle cyclin-dependent kinases100–102 can both reduce proliferation and increase podocyte differentiation in the Δgag-pol transgenic mouse model of HIVAN. All-trans retinoic acid was also reported to promote podocyte differentiation by inhibiting mitogenic signaling;103 however, this compound was also shown to promote the morphologic differentiation of diseased podocytes through pathways independent of proliferation.104,105
Conclusions
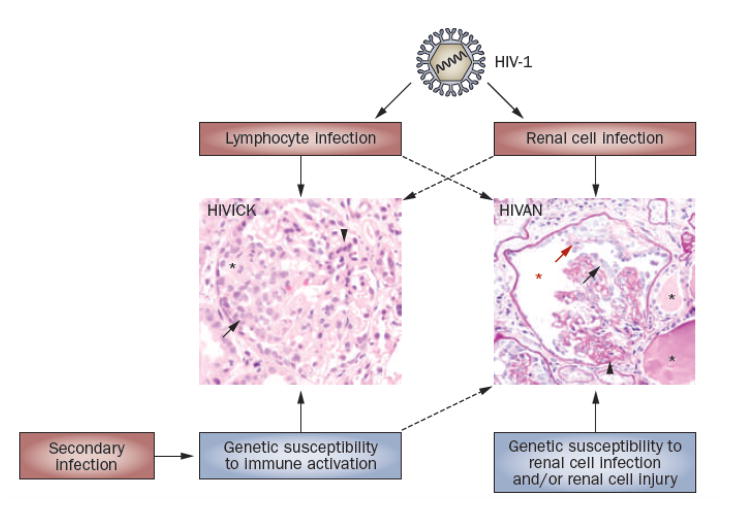
The pathogenesis of HIV-associated renal diseases results from a complicated interplay of viral infection, genetic predisposition, and immune activation (Figure 2). Although evidence supports the idea that direct infection of renal parenchymal cells is pathogenic for HIVAN, systemic and local immune responses might precipitate or exacerbate renal disease. Given the role of viral accessory proteins not only in kidney pathogenesis but also in the response of the host to HIV-1 infection, development of therapeutics directed at inhibiting the function of these accessory proteins seems a logical direction for research. In the immediate future, however, suppressing immune activation or controlling cell proliferation are therapeutic strategies to consider.
Figure 2.
The interplay of environmental (red) and genetic (blue) factors in HIV-associated renal diseases. Of central importance is HIV-1 infection, which occurs in both renal epithelial cells and immunocytes resident and infiltrating the kidney. Although their effect is not fully understood, genetic factors also contribute to pathogenesis, possibly by determining susceptibility to renal cell infection or cell injury response. In HIVICK, and perhaps also HIVAN, immune and inflammatory pathways have a role in disease onset, progression, and severity. Top panel shows typical HIVAN pathology consisting of collapse of the glomerular tuft with segmental areas of sclerosis (thickened basement membranes) and proliferation of epithelial cells adherent to both the tuft and Bowman’s capsule. Microcysts with proteinacious casts can be seen in adjacent tubules. Bottom panel is typical pathology of HIVICK with global sclerosis, mesangial expansion, and hypercellularity encompassing the tuft and Bowman’s capsule. For more detailed information on the pathology of these HIV-associated renal disease, please see an accompanying article in the series. Hematoxylin and eosin stain, both images 60X magnification. Abbreviations: HIVAN, HIV-associated nephropathy; HIVICK, HIV-immune-complex kidney disease..
Acknowledgments
Dr. Bruggeman is supported by NIH grants DK061395 and DK077668 and is a member of the Case Center for AIDS Research supported by NIH grant AI36219. Dr. Nelson is supported by NIH grants DK065498, DK079498, and DK083375.
Footnotes
Review criteria
Information for this article was obtained by searching PubMed with the following search queries: HIV-1 and kidney or glomerulosclerosis or podocyte; Nef and kidney or podocyte not HEK293; Vpr and kidney or podocyte not HEK293, Tat and kidney or podocyte not HEK293; HIVAN; HIV-1 and immune complex and kidney (no constraints were placed on language or publication year) and information presented at the recent meetings of the American Society of Nephrology and International Podocyte Biology Symposium.
References
- 1.Szczech LA, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt CM, et al. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2009;75:428–434. doi: 10.1038/ki.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiryluk K, Martino J, Gharavi AG. Genetic susceptibility, HIV infection, and the kidney. Clin J Am Soc Nephrol. 2007;2 (Suppl 1):S25–S35. doi: 10.2215/CJN.00320107. [DOI] [PubMed] [Google Scholar]
- 4.Alpers CE, et al. Focal segmental glomerulosclerosis in primates infected with a simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1997;13:413–424. doi: 10.1089/aid.1997.13.413. [DOI] [PubMed] [Google Scholar]
- 5.Stephens EB, Tian C, Li Z, Narayan O, Gattone VH. Rhesus macaques infected with macrophage-tropic simian immunodeficiency virus (SIVmacR71/17E) exhibit extensive focal segmental and global glomerulosclerosis. J Virol. 1998;72:8820–8832. doi: 10.1128/jvi.72.11.8820-8832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens EB, Tian C, Dalton SB, Gattone VH. Simian-human immunodeficiency virus-associated nephropathy in macaques. AIDS Res Hum Retroviruses. 2000;16:1295–1306. doi: 10.1089/08892220050117050. [DOI] [PubMed] [Google Scholar]
- 7.Poli A, et al. Renal involvement in feline immunodeficiency virus infection: a clinicopathological study. Nephron. 1993;64:282–288. doi: 10.1159/000187327. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AH, Nast CC. HIV-associated nephropathy. A unique combined glomerular, tubular, and interstitial lesion. Mod Pathol. 1988;1:87–97. [PubMed] [Google Scholar]
- 9.D’Agati V, Suh JI, Carbone L, Cheng JT, Appel G. Pathology of HIV-associated nephropathy: a detailed morphologic and comparative study. Kidney Int. 1989;35:1358–1370. doi: 10.1038/ki.1989.135. [DOI] [PubMed] [Google Scholar]
- 10.Strauss J, et al. Renal disease in children with the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:625–630. doi: 10.1056/NEJM198909073211001. [DOI] [PubMed] [Google Scholar]
- 11.Ray PE, Xu L, Rakusan T, Liu XH. A 20-year history of childhood HIV-associated nephropathy. Pediatr Nephrol. 2004;19:1075–1092. doi: 10.1007/s00467-004-1558-1. [DOI] [PubMed] [Google Scholar]
- **12.Wools-Kaloustian KK, Gupta SK. Will there be an epidemic of HIV-related chronic kidney disease in sub-Saharan Africa? Too soon to tell. Kidney Int. 2008;74:845–847. doi: 10.1038/ki.2008.326. [DOI] [PubMed] [Google Scholar]
- 13.Kao WH, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp JB, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruggeman LA, et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2079–2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AH, Sun NC, Shapshak P, Imagawa DT. Demonstration of human immunodeficiency virus in renal epithelium in HIV-associated nephropathy. Modern Pathology. 1989;2:125–128. [PubMed] [Google Scholar]
- 17.Kimmel PL, Ferreira-Centeno A, Farkas-Szallasi T, Abraham AA, Garrett CT. Viral DNA in microdissected renal biopsy tissue from HIV infected patients with nephrotic syndrome. Kidney International. 1993;43:1347–1352. doi: 10.1038/ki.1993.189. [DOI] [PubMed] [Google Scholar]
- 18.Eitner F, et al. Chemokine receptor CCR5 and CXCR4 expression in HIV-associated kidney disease. J Am Soc Nephrol. 2000;11:856–867. doi: 10.1681/ASN.V115856. [DOI] [PubMed] [Google Scholar]
- 19.Marras D, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8:522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- 20.Zerhouni-Layachi B, et al. Dual tropism of HIV-1 envelopes derived from renal tubular epithelial cells of patients with HIV-associated nephropathy. AIDS. 2006;20:621–624. doi: 10.1097/01.aids.0000210618.68083.8e. [DOI] [PubMed] [Google Scholar]
- 21.Eitner F, et al. Chemokine receptor (CCR5) expression in human kidneys and in the HIV infected macaque. Kidney Int. 1998;54:1945–1954. doi: 10.1046/j.1523-1755.1998.00211.x. [DOI] [PubMed] [Google Scholar]
- 22.Grone HJ, et al. Spatial and temporally restricted expression of chemokines and chemokine receptors in the developing human kidney. J Am Soc Nephrol. 2002;13:957–967. doi: 10.1681/ASN.V134957. [DOI] [PubMed] [Google Scholar]
- 23.Segerer S, Mack M, Regele H, Kerjaschki D, Schlondorff D. Expression of the C-C chemokine receptor 5 in human kidney diseases. Kidney Int. 1999;56:52–64. doi: 10.1046/j.1523-1755.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez E, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 25.Liu XH, Hadley TJ, Xu L, Peiper SC, Ray PE. Up-regulation of Duffy antigen receptor expression in children with renal disease. Kidney Int. 1999;55:1491–1500. doi: 10.1046/j.1523-1755.1999.00385.x. [DOI] [PubMed] [Google Scholar]
- 26.Woolley IJ, et al. HIV nephropathy and the Duffy antigen/receptor for Chemokines in African Americans. J Nephrol. 2001;14:384–387. [PubMed] [Google Scholar]
- 27.He W, et al. Duffy antigen receptor for chemokines mediates trans-infection of HIV-1 from red blood cells to target cells and affects HIV-AIDS susceptibility. Cell Host Microbe. 2008;4:52–62. doi: 10.1016/j.chom.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray PE, et al. Infection of human primary renal epithelial cells with HIV-1 from children with HIV-associated nephropathy. Kidney Int. 1998;53:1217–1229. doi: 10.1046/j.1523-1755.1998.00900.x. [DOI] [PubMed] [Google Scholar]
- 29.Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 30.Baribaud F, Pohlmann S, Doms RW. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology. 2001;286:1–6. doi: 10.1006/viro.2001.0975. [DOI] [PubMed] [Google Scholar]
- 31.Hatsukari I, et al. DEC-205-mediated internalization of HIV-1 results in the establishment of silent infection in renal tubular cells. J Am Soc Nephrol. 2007;18:780–787. doi: 10.1681/ASN.2006121307. [DOI] [PubMed] [Google Scholar]
- 32.Mikulak J, Teichberg S, Faust T, Schmidtmayerova H, Singhal PC. HIV-1 harboring renal tubular epithelial cell interaction with T cells results in T cell trans-infection. Virology. 2009;385:105–114. doi: 10.1016/j.virol.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 33.John R, Nelson PJ. Dendritic cells in the kidney. J Am Soc Nephrol. 2007;18:2628–2635. doi: 10.1681/ASN.2007030273. [DOI] [PubMed] [Google Scholar]
- 34.Wu L, Kewalramani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanji N, et al. Detection and localization of HIV-1 DNA in renal tissues by in situ polymerase chain reaction. Histol Histopathol. 2006;21:393–401. doi: 10.14670/HH-21.393. [DOI] [PubMed] [Google Scholar]
- **36.Conaldi PG, et al. HIV-persistent infection and cytokine induction in mesangial cells: a potential mechanism for HIV-associated glomerulosclerosis. AIDS. 2000;14:2045–2047. doi: 10.1097/00002030-200009080-00021. [DOI] [PubMed] [Google Scholar]
- 37.Tokizawa S, et al. Infection of mesangial cells with HIV and SIV: identification of GPR1 as a coreceptor. Kidney Int. 2000;58:607–617. doi: 10.1046/j.1523-1755.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 38.Alpers CE, McClure J, Bursten SL. Human mesangial cells are resistant to productive infection by multiple strains of human immunodeficiency virus types 1 and 2. Am J Kidney Dis. 1992;19:126–130. doi: 10.1016/s0272-6386(12)70120-8. [DOI] [PubMed] [Google Scholar]
- 39.Green DF, Resnick L, Bourgoignie JJ. HIV infects glomerular endothelial and mesangial but not epithelial cells in vitro. Kidney Int. 1992;41:956–960. doi: 10.1038/ki.1992.146. [DOI] [PubMed] [Google Scholar]
- 40.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Rosenstiel P, Gharavi A, D’Agati VD, Klotman P. Transgenic and infectious animal models of HIV-associated nephropathy. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2008121230. in press. [DOI] [PubMed] [Google Scholar]
- 42.Bruggeman LA, et al. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest. 1997;100:84–92. doi: 10.1172/JCI119525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickie P, et al. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991;185:109–119. doi: 10.1016/0042-6822(91)90759-5. [DOI] [PubMed] [Google Scholar]
- 44.Kopp JB, et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci U S A. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong J, et al. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int. 2005;68:1048–1060. doi: 10.1111/j.1523-1755.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 46.Hanna Z, et al. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J Virol. 1998;72:121–132. doi: 10.1128/jvi.72.1.121-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **47.Balog K, Minarovits J. Nef: a pleiotropic modulator of primate lentivirus infectivity and pathogenesis. Acta Microbiol Immunol Hung. 2006;53:51–75. doi: 10.1556/AMicr.53.2006.1.4. [DOI] [PubMed] [Google Scholar]
- 48.Keppler OT, et al. Rodent cells support key functions of the human immunodeficiency virus type 1 pathogenicity factor Nef. J Virol. 2005;79:1655–1665. doi: 10.1128/JVI.79.3.1655-1665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanna Z, et al. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 50.Priceputu E, et al. Primary human immunodeficiency virus type 1 nef alleles show major differences in pathogenicity in transgenic mice. J Virol. 2007;81:4677–4693. doi: 10.1128/JVI.02691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dickie P, et al. Focal glomerulosclerosis in proviral and c-fms transgenic mice links Vpr expression to HIV-associated nephropathy. Virology. 2004;322:69–81. doi: 10.1016/j.virol.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 52.Kajiyama W, Kopp JB, Marinos NJ, Klotman PE, Dickie P. Glomerulosclerosis and viral gene expression in HIV-transgenic mice: role of nef. Kidney Int. 2000;58:1148–1159. doi: 10.1046/j.1523-1755.2000.00271.x. [DOI] [PubMed] [Google Scholar]
- 53.Husain M, D’Agati VD, He JC, Klotman ME, Klotman PE. HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS. 2005;19:1975–1980. doi: 10.1097/01.aids.0000191918.42110.27. [DOI] [PubMed] [Google Scholar]
- 54.Zuo Y, et al. HIV-1 genes vpr and nef synergistically damage podocytes, leading to glomerulosclerosis. J Am Soc Nephrol. 2006;17:2832–2843. doi: 10.1681/ASN.2005080878. [DOI] [PubMed] [Google Scholar]
- 55.Le RE, Benichou S. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology. 2005;2:11. doi: 10.1186/1742-4690-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conaldi PG, et al. Human immunodeficiency virus-1 tat induces hyperproliferation and dysregulation of renal glomerular epithelial cells. Am J Pathol. 2002;161:53–61. doi: 10.1016/S0002-9440(10)64156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doublier S, et al. HIV-1 Tat reduces nephrin in human podocytes: a potential mechanism for enhanced glomerular permeability in HIV-associated nephropathy. AIDS. 2007;21:423–432. doi: 10.1097/QAD.0b013e328012c522. [DOI] [PubMed] [Google Scholar]
- 58.Fung E, et al. Dissecting the Role of HIV-Tat in HIVAN. J Am Soc Nephrol. 2006;17:64A. [Google Scholar]
- 59.Wharram BL, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 60.Faulhaber JR, Nelson PJ. Virus-induced cellular immune mechanisms of injury to the kidney. Clin J Am Soc Nephrol. 2007;2 (Suppl 1):S2–S5. doi: 10.2215/CJN.00020107. [DOI] [PubMed] [Google Scholar]
- 61.Lindemann D, et al. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J Exp Med. 1994;179:797–807. doi: 10.1084/jem.179.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reid W, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reid W, et al. HIV-1 transgenic rats develop T cell abnormalities. Virology. 2004;321:111–119. doi: 10.1016/j.virol.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Santoro TJ, et al. Growth failure and AIDS-like cachexia syndrome in HIV-1 transgenic mice. Virology. 1994;201:147–51. doi: 10.1006/viro.1994.1276. [DOI] [PubMed] [Google Scholar]
- 65.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varin A, et al. Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J Biol Chem. 2005;280:42557–42567. doi: 10.1074/jbc.M502211200. [DOI] [PubMed] [Google Scholar]
- 67.Olivetta E, et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-kappa B activation. J Immunol. 2003;170:1716–1727. doi: 10.4049/jimmunol.170.4.1716. [DOI] [PubMed] [Google Scholar]
- 68.Ensoli B, et al. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 70.Tobiume M, et al. Extracellular Nef protein activates signal transduction pathway from Ras to mitogen-activated protein kinase cascades that leads to activation of human immunodeficiency virus from latency. AIDS Res Hum Retroviruses. 2002;18:461–467. doi: 10.1089/088922202753614227. [DOI] [PubMed] [Google Scholar]
- 71.Kapasi AA, Patel G, Franki N, Singhal PC. HIV-1 gp120-induced tubular epithelial cell apoptosis is mediated through p38-MAPK phosphorylation. Mol Med. 2002;8:676–685. [PMC free article] [PubMed] [Google Scholar]
- 72.Singhal PC, Reddy K, Franki N, Ding G. HIV-1 gp120 envelope protein modulates proliferation of human glomerular epithelial cells. J Cell Biochem. 1999;76:61–70. doi: 10.1002/(sici)1097-4644(20000101)76:1<61::aid-jcb7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 73.Singhal PC, et al. HIV-1 gp160 envelope protein modulates proliferation and apoptosis in mesangial cells. Nephron. 1997;76:284–295. doi: 10.1159/000190193. [DOI] [PubMed] [Google Scholar]
- 74.Singhal PC, Sharma P, Garg P. HIV-1 gp160 protein-macrophage interactions modulate mesangial cell proliferation and matrix synthesis. Am J Pathol. 1995;147:1780–1789. [PMC free article] [PubMed] [Google Scholar]
- 75.Winston JA, et al. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 76.Douek DC, Roederer M, Koup RA. Emerging Concepts in the Immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eustace JA, et al. Cohort study of the treatment of severe HIV-associated nephropathy with corticosteroids. Kidney Int. 2000;58:1253–1260. doi: 10.1046/j.1523-1755.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 78.Laradi A, Mallet A, Beaufils H, Allouache M, Martinez F. HIV-associated nephropathy: outcome and prognosis factors. Groupe d’ Etudes Nephrologiques d’Ile de France. J Am Soc Nephrol. 1998;9:2327–2335. doi: 10.1681/ASN.V9122327. [DOI] [PubMed] [Google Scholar]
- 79.Cohen SD, Kimmel PL. Immune complex renal disease and human immunodeficiency virus infection. Semin Nephrol. 2008;28:535–544. doi: 10.1016/j.semnephrol.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Vielhauer V, Anders HJ, Schlondorff D. Chemokines and chemokine receptors as therapeutic targets in lupus nephritis. Semin Nephrol. 2007;27:81–97. doi: 10.1016/j.semnephrol.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Waldman M, Marshall V, Whitby D, Kopp JB. Viruses and kidney disease: beyond HIV. Semin Nephrol. 2008;28:595–607. doi: 10.1016/j.semnephrol.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinka S, Bruggeman LA. Persistent NF-{kappa}B activation in renal epithelial cells in mouse modelof HIV-associated nephropathy. Am J Physiol Renal Physiol. 2005;290:F657–F665. doi: 10.1152/ajprenal.00208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ross MJ, Martinka S, D’Agati VD, Bruggeman LA. NF-κB regulates Fas-mediated apoptosis in HIV-associated nephropathy. J Am Soc Nephrol. 2005;16:2403–2411. doi: 10.1681/ASN.2004121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross MJ, et al. HIV-1 infection initiates an inflammatory cascade in human renal tubular epithelial cells. J Acquir Immune Defic Syndr. 2006;42:1–11. doi: 10.1097/01.qai.0000218353.60099.4f. [DOI] [PubMed] [Google Scholar]
- 85.O’Donnell MP, et al. Renal cell cytokine production stimulates HIV-1 expression in chronically HIV-1-infected monocytes. Kidney Int. 1998;53:593–597. doi: 10.1046/j.1523-1755.1998.00789.x. [DOI] [PubMed] [Google Scholar]
- 86.Heckmann A, et al. IKK2 inhibitor alleviates kidney and wasting diseases in a murine model of human AIDS. Am J Pathol. 2004;164:1253–1262. doi: 10.1016/S0002-9440(10)63213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Albaqumi M, Soos TJ, Barisoni L, Nelson PJ. Collapsing glomerulopathy. J Am Soc Nephrol. 2006;17:2854–2863. doi: 10.1681/ASN.2006030225. [DOI] [PubMed] [Google Scholar]
- 88.Ross MJ, Bruggeman LA, Wilson PD, Klotman PE. Microcyst formation and HIV-1 gene expression occur in multiple nephron segments in HIV-associated nephropathy. J Am Soc Nephrol. 2001;12:2645–2651. doi: 10.1681/ASN.V12122645. [DOI] [PubMed] [Google Scholar]
- 89.Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 90.Barisoni L, Bruggeman LA, Mundel P, D’Agati VD, Klotman PE. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 2000;58:173–181. doi: 10.1046/j.1523-1755.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- 91.Nelson PJ, Sunamoto M, Husain M, Gelman IH. HIV-1 expression induces cyclin D1 expression and pRb phosphorylation in infected podocytes: cell-cycle mechanisms contributing to the proliferative phenotype in HIV-associated nephropathy. BMC Microbiol. 2002;2:26. doi: 10.1186/1471-2180-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dijkman H, Smeets B, van der LJ, Steenbergen E, Wetzels J. The parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. Kidney Int. 2005;68:1562–1572. doi: 10.1111/j.1523-1755.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 93.Dijkman HB, et al. Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int. 2006;70:338–344. doi: 10.1038/sj.ki.5001574. [DOI] [PubMed] [Google Scholar]
- 94.Nagata M, et al. Origin and phenotypic features of hyperplastic epithelial cells in collapsing glomerulopathy. Am J Kidney Dis. 1998;32:962–969. doi: 10.1016/s0272-6386(98)70070-8. [DOI] [PubMed] [Google Scholar]
- 95.Barisoni L, Nelson PJ. Collapsing glomerulopathy: an inflammatory podocytopathy? Curr Opin Nephrol Hypertens. 2007;16:192–195. doi: 10.1097/MNH.0b013e32805b726b. [DOI] [PubMed] [Google Scholar]
- 96.Appel D, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ronconi E, et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thorner PS, Ho M, Eremina V, Sado Y, Quaggin S. Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol. 2008;19:495–502. doi: 10.1681/ASN.2006101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spektor TM, Rice JC. Ring around the genes. Nat Cell Biol. 2007;9:1343–1344. doi: 10.1038/ncb1207-1343. [DOI] [PubMed] [Google Scholar]
- 100.Gherardi D, et al. Reversal of collapsing glomerulopathy in mice with the cyclin-dependent kinase inhibitor CYC202. J Am Soc Nephrol. 2004;15:1212–1222. doi: 10.1097/01.asn.0000124672.41036.f4. [DOI] [PubMed] [Google Scholar]
- 101.Nelson PJ, Gelman IH, Klotman PE. Suppression of HIV-1 expression by inhibitors of cyclin-dependent kinases promotes differentiation of infected podocytes. J Am Soc Nephrol. 2001;12:2827–2831. doi: 10.1681/ASN.V12122827. [DOI] [PubMed] [Google Scholar]
- 102.Nelson PJ, D’Agati VD, Gries JM, Suarez JR, Gelman IH. Amelioration of nephropathy in mice expressing HIV-1 genes by the cyclin-dependent kinase inhibitor flavopiridol. J Antimicrob Chemother. 2003;51:921–929. doi: 10.1093/jac/dkg175. [DOI] [PubMed] [Google Scholar]
- 103.He JC, et al. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol. 2007;18:93–102. doi: 10.1681/ASN.2006070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takano Y, et al. Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol. 2007;292:F1573–F1582. doi: 10.1152/ajprenal.00423.2006. [DOI] [PubMed] [Google Scholar]
- 105.Vaughan MR, et al. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int. 2005;68:133–144. doi: 10.1111/j.1523-1755.2005.00387.x. [DOI] [PubMed] [Google Scholar]