Abstract
Previous studies suggest that carotenoids and tocopherols (vitamin E compounds) may be inversely associated with prostate cancer risk, yet little is known about how they affect prostate cancer progression and survival. We investigated whether serum α-tocopherol, β-carotene, and retinol concentrations, or the α-tocopherol and β-carotene trial supplementation, affected survival of men diagnosed with prostate cancer during the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study, a randomized, double-blind, placebo-controlled primary prevention trial testing the effects of β-carotene and α-tocopherol supplements on cancer incidence in adult male smokers in southwestern Finland (n=29,133). Prostate cancer survival was examined using the Kaplan-Meier method with deaths from other causes treated as censoring, and using Cox proportional hazards regression models with hazard ratios (HR) and 95% confidence intervals (95% CI) adjusted for family history of prostate cancer, age at randomization, benign prostatic hyperplasia, age and stage at diagnosis, height, BMI, and serum cholesterol. As of April 2005, 1,891 men were diagnosed with prostate cancer and 395 died of their disease. Higher serum α-tocopherol at baseline was associated with improved prostate cancer survival (HR=0.67, 0.45–1.00), especially among cases who had received the trial’s α-tocopherol intervention and who were in the highest quintile of α-tocopherol at baseline (HR=0.51, 0.20–0.90) or at the 3-year follow-up measurement (HR=0.26, 0.09–0.71). Serum β-carotene, serum retinol, and supplemental β-carotene had no apparent effects on survival. These findings suggest that higher α-tocopherol (and not β-carotene or retinol) status increases overall prostate cancer survival. Further investigations, possibly including randomized studies, are needed to confirm this observation.
Although rates vary throughout the world, prostate cancer is the most common cancer diagnosed in the United States, accounting for an estimated 186,320 new cases and 28,660 deaths annually (1). The few well-established risk factors for prostate cancer incidence include increasing age, race/ethnicity (being African American or Jamaican), and having a positive family history (1, 2). Additional factors such as height, physical activity, body mass index (BMI), hormones, and diet are under investigation (2–4). Environmental factors such as diet are thought to be significant contributors as migrants tend to take on the prostate cancer risk profiles of their new country after arriving. In one study of Japanese men who emigrated to Los Angeles, incidence rates were 4–9 times higher for the first generation immigrants compared to men living in Japan, and by the second generation the rates were approximately the same as the general U.S. population (5). Fortunately, 5 year survival is almost 100%, when diagnosed with localized or regional disease, and there are approximately 2 million prostate cancer survivors in the U.S. (1, 6). Yet, little is known about how diet affects prostate cancer progression and survival.
Most studies have investigated diet as an etiological factor for prostate cancer and a considerable number have focused on carotenoids and tocopherols, but with mixed results. Two large cohort studies, NIH-AARP Diet and Health Study and the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study, reported protective associations of alpha-tocopherol with advanced prostate cancer (7, 8), whereas no associations were seen between overall prostate cancer risk and individual carotenoids, retinol, or tocopherols in the European Prospective Investigation Cohort (EPIC) (9) or the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial (10). Vitamin E was protective in smokers, as were β-carotene supplements in men with low β-carotene intake, in the PLCO trial (10). Currently, several suspected risk factors, such as BMI, soy, fish oil, meat intake, calcium, lycopene, vitamins C and E, and selenium, are also being studied in relation to prostate cancer progression and survival (11–13). However, such research investigating carotenoids and tocopherols is scant. One notable exception came from a cohort of health professionals that examined pre and post- diagnostic diet and found that consumption of tomato sauce and fish were protective against disease progression, although tocopherols and individual carotenoids were not assessed (14). Carotenoids and tocopherols (vitamin E) are thought to affect disease risk, progression, and survival through several mechanisms.
Carotenoids, the naturally-occurring pigments found in many fruits and vegetables, have the antioxidant capacity to quench singlet oxygen and thus, are thought to prevent conditions related to oxidative stress, such as cardiovascular disease and many cancers (15). Vitamin E consists of four tocopherols and four tocotrienols, of which, α-tocopherol is the most biologically active form in humans and is present in plant and seed oils, nuts, margarine, seeds, and cereal grains. Also an antioxidant, α-tocopherol is hypothesized to prevent cancer by inhibiting formation of carcinogens such as nitrosamines, by decreasing cell proliferation, or by increasing antibody production and enhancing cell-mediated immunity (16).
In this report, we prospectively examined whether serum α-tocopherol, β-carotene, and retinol measured at study entry and after 3 years affected survival time in men diagnosed with prostate cancer while enrolled in the ATBC Study from 1985 to 2005.
Methods
Study Population
Data were from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study, which was a randomized, double-blind, placebo-controlled primary prevention trial designed to study the effects of β-carotene and α-tocopherol supplements on cancer incidence in 29,133 Caucasian male smokers aged 50–69 years in southwestern Finland enrolled between 1985 and 1988 (17). Men who had prior cancer or serious illness or who reported current use of dietary supplements with > 20 mg vitamin E, >20,000 IU vitamin A, or >6 mg β-carotene were ineligible. Enrolled men were randomly assigned to one of four groups based on a 2 × 2 factorial design: 1) α-tocopherol (dl-alpha-tocopherol acetate 50 mg/day), 2) β-carotene (20 mg/day), 3) both supplements, or 4) placebo capsules for 5–8 years until death or trial closure. Although the trial ended on April 30, 1993, on-going follow-up continues through the Finnish Cancer Registry. As of April 30, 2005, there were 1,891 men diagnosed with prostate cancer and 395 deaths due to prostate cancer. The study was approved by the institutional review boards of the US National Cancer Institute and the National Public Health Institute of Finland, and written informed consent was obtained from each participant before randomization.
Case Identification
Incident prostate cancer cases (International Classification of Diseases 9, code 185), diagnosed by April 30, 2005 (i.e., up to 20 years of follow-up), were identified through the Finnish Cancer Registry, which provides nearly 100% case ascertainment (18). For cases diagnosed through September 2001, the medical records were reviewed centrally by one or two study oncologist(s) for diagnostic confirmation and staging, and cases with available histopathologic or cytologic specimens were reviewed and confirmed by pathologists. Prostate cancer cases diagnosed after September 2001 had only the Finnish Cancer Registry data for site, histology, and date of diagnosis. Advanced cases (n = 397) were defined as those cases with stage III or IV of the tumor-node-metastasis staging system, as defined by the American Joint Committee on Cancer (19). Stage information was only available for those cases diagnosed through September 2001; all cases without stage information were analyzed as a separate missing category. Finland has not adopted population-based prostate-specific antigen screening programs, and only 1 of 246 cases that occurred during the trial period was detected by prostate-specific antigen screening (20). Based on data on the prostate cancers diagnosed by September 2001, we estimate that about 10% or fewer of the prostate cancer cases were initially detected through an elevated prostate-specific antigen screen. Deaths were identified from the Register of Causes of Death. Specific causes were derived from the official underlying cause of death.
Data Collection
At baseline, study subjects completed general risk factor, smoking, and medical history questionnaires, a food frequency questionnaire of the previous 12 months, and an exam where height and weight were measured by specially trained registered nurses (21). Participants also provided an overnight fasting serum sample, at baseline and 3 years, that was protected from light and stored at −70°C until it was analyzed for α-tocopherol, β-carotene, and retinol using high-performance liquid chromatography (22). Total and HDL-cholesterol concentrations were measured using an enzymatic assay (CHOD-PAP method; Boehringer Mannheim) (23).
Statistical Methods
Follow-up time was calculated from the date of diagnosis to the earliest of death from prostate cancer, death from non-prostate cancer causes, and the censoring date (April 30, 2005). Deaths from non-prostate cancer causes were treated as independent censoring events. All men diagnosed with prostate cancer (n=1,891) were included in analyses of baseline serum biomarkers, but only those diagnosed after 3 years (n=1,557) were included in analyses with serum biomarkers at 3 years. Baseline descriptive characteristics are presented as means (continuous variables) or proportions (categorical variables) for prostate deaths and survivors and compared using student’s t-test or chi-square test. Survival was evaluated using Kaplan-Meier survival plots and Cox proportional hazards regression models for each quintile of serum α-tocopherol, β-carotene, and retinol measured at baseline and 3 years. The quintile cut-points, which were estimated from baseline analyte distributions in survivors, were 9.4, 10.9, 12.2, and 14.9 mg/L for α-tocopherol, 104, 155, 210, and 299 μg/L for β-carotene, and 492, 554, 613, and 691 μg/L for retinol. The quintile cut-points used to classify serum α-tocopherol measured 3 years after accrual were 11.5, 13.5, 16.0, and 19.0 mg/L. The proportional hazards assumption was tested by examining Schoenfeld residuals and was upheld in all analyses. Adjusted hazard ratios and 95% confidence intervals were calculated using Cox proportional hazards regression models, adjusted for age at randomization, BMI, age at diagnosis, advanced stage at diagnosis, number of years smoked, and serum cholesterol in α-tocopherol analyses, as it affects bioavailability (24). To account for missing data, we adjusted stage at diagnosis in three categories: 1) before September, 2001 and stage I or II, 2) before September, 2001 and stage III or IV, and 3) after September, 2001 or missing. Additional covariates that were considered but not included were daily number of cigarettes, age at smoking initiation, weight, height, family history of prostate cancer, benign prostatic hyperplasia, physical activity (no activity or light-to-moderate work activity and ≥ moderate leisure activity), urban residence, education, marital status, and dietary intakes of protein, fat, polyunsaturated fatty acids, vitamin C, and lycopene. Effect modification was evaluated in stratified multivariate analyses and also tested by adding product interaction terms and comparing p values for the likelihood ratio tests (<0.05) for the models with and without interaction terms. We examined whether the association with survival differed by several factors, including treatment arm, BMI, height, smoking status, alcohol use, physical activity, family history of cancer, and self-reported dietary intake of alcohol, fish, red meat, dietary fats, fruits, vegetables, and several micronutrients. All statistical tests were two-sided and p values ≤ 0.05 were considered statistically significant. Data analyses were conducted using Stata (version SE 9, STATACorp, College Station, TX).
Results
Of the 29,133 men enrolled in the ATBC Study, 1,891 were diagnosed with prostate cancer during 1985–2005; 89 of these diagnoses were made within 3 years of accrual. Of the 1,891 with prostate cancer, 395 died from prostate cancer, 527 died from non-prostate cancer causes, and 969 were alive on April 30, 2005. Median follow-up time was 3.4 years (range= 0–15.8) for prostate cancer deaths, compared to 4.1 years (range= 0–17.8) for those who died from other causes or survived through the analysis period (referred to here as “survivors”). Comparisons of baseline characteristics between those with fatal prostate cancer and survivors are presented in Table 1. Overall, survivors were slightly younger upon enrollment (mean 57.9 vs. 59.6 years), older upon diagnosis (mean 70.5 vs. 68.2 years), and had a shorter smoking history (35.7 years vs. 37.6 years). Survivors were also more likely to be physically active (21% vs. 14%), less likely to have advanced prostate cancer at diagnosis (21% vs. 69%), more likely to have missing information on stage at diagnosis (47% vs. 15%), and also more likely to elect radical surgery (23% vs. 4%). In the intervention trial, those with fatal prostate cancer were slightly more likely to have been assigned to the β-carotene only group (31%), but there was no significant difference among the other intervention groups (placebo, α-tocopherol only, or both β-carotene and α-tocopherol). There were also no statistically significant differences in baseline serum nutrient biomarkers between groups.
Table 1.
Selected baseline characteristics (means with standard deviations and proportions) among 1,891 men diagnosed with prostate cancer in the ATBC Study
| Characteristics | Survivors (n=1496) | Deceased (n=395) | p value |
|---|---|---|---|
| Age at randomization (yr) | 57.9 ± 5.1 | 59.6 ± 5.1 | <0.0001 |
| Age at diagnosis (yr) | 70.5 ± 5.4 | 68.2 ± 6.3 | <0.0001 |
| Survival (yr) | 4.1 ± 3.3 | 3.4 ± 2.8 | <0.0001 |
| Body mass index (kg/m2) | 26.4 ± 3.7 | 26.3 ± 3.6 | 0.73 |
| Cigarettes smoked (per day) | 19.8 ± 8.7 | 19.2 ± 8.7 | 0.21 |
| Years of smoking (yr) | 35.7 ± 8.9 | 37.6 ± 8.7 | 0.0002 |
| Benign prostatic hyperplasia (%) | 6 | 4 | 0.20 |
| Prostate cancer family history (%) | 6 | 8 | 0.17 |
| Stage at diagnosis (% stage 3 or 4) | 21 | 69 | <0.0001 |
| Missing stage at diagnosis (%) | 47 | 15 | <0.0001 |
| Elected radical surgery (%) | 23 | 4 | <0.0001 |
| Physical activity (% active) | 21 | 14 | 0.005 |
| Education (% elementary) | 71 | 72 | 0.35 |
| Married (% married) | 81 | 83 | 0.18 |
| Urban residence (% big town) | 46 | 45 | 0.85 |
| Total energy (kcal/day) | 2694 ± 728 | 2698 ± 834 | 0.94 |
| Intervention group | |||
| Placebo (%) | 26 | 26 | 0.80 |
| α-tocopherol only (%) | 23 | 22 | 0.45 |
| β-carotene only (%) | 25 | 31 | 0.02 |
| Both (%) | 26 | 22 | 0.18 |
| Serum biomarkers at baseline | |||
| α-tocopherol (mg/L) | 11.9 ± 3.1 | 11.7 ± 3.1 | 0.29 |
| β-carotene (μg/mL) | 221 ± 185 | 222 ± 185 | 0.88 |
| Retinol (μg/mL) | 594 ± 130 | 594 ± 131 | 0.55 |
| Cholesterol (mmol/L) | 6.2 ± 1.1 | 6.2 ± 1.2 | 0.87 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.80 |
Table 2 describes demographic, dietary, and biomarker characteristics for each quintile of baseline serum α-tocopherol. Men in the higher quintiles of baseline serum α-tocopherol tended to be more physically active, more likely to live in an urban residence, and have more formal education than those in the lower quintiles. Conversely, most of the self-reported dietary intakes examined were significantly associated with the quintile of baseline serum α-tocopherol. Vitamins C, D, and E, selenium, α-tocopherol, β-carotene, polyunsaturated fat, α-linolenic acid, vegetables, fruits, and fish were positively associated with baseline serum α-tocopherol quintiles, whereas saturated fat was inversely associated. Associations were similar for serum α-tocopherol measured at 3 years, although with some attenuation for vitamin C, selenium, and β-carotene (data not shown). Serum baseline concentrations of β-carotene, retinol, and total cholesterol were positively associated with serum baseline concentrations of α-tocopherol, while serum HDL cholesterol was inversely associated. Although missing stage at diagnosis and radical surgery election were more likely among survivors in Table 1, there was no association with either factor and quintile of serum α-tocopherol at baseline or 3 years.
Table 2.
Selected characteristics (means and proportions) of men with prostate cancer by baseline serum α-tocopherol quintile
| Serum α-tocopherol quintiles (mg/L) | ||||||
|---|---|---|---|---|---|---|
| Q1 (n=379) | Q2 (n=378) | Q3 (n=389) | Q4 (n=368) | Q5 (n=377) | ||
| Characteristics | (4.2–9.4) | (9.44–10.9) | (10.91–14.7) | (14.73–15.4) | (15.5–17.86) | p value |
| Deceased (n=395) (%) | 21.9 | 24.1 | 19.5 | 19.3 | 19.6 | 0.42 |
| Advanced prostate cancer (%) | 36 | 36 | 34 | 34 | 35 | 0.98 |
| Missing stage at diagnosis (%) | 38.3 | 39.4 | 36.8 | 44.3 | 41.1 | 0.69 |
| Age at randomization (y) | 59.0 | 58.5 | 58.1 | 57.9 | 57.8 | 0.12 |
| Age at diagnosis (y) | 70.4 | 70.2 | 69.7 | 70.1 | 69.7 | 0.40 |
| Body mass index (kg/m2) | 25.6 | 26.2 | 26.3 | 26.5 | 27.3 | 0.42 |
| Cigarettes/d | 20.3 | 20.3 | 19.8 | 18.8 | 19.1 | 0.36 |
| Years of smoking | 37.5 | 36.8 | 35.2 | 35.8 | 35.4 | 0.17 |
| Benign prostatic hyperplasia (%) | 2.9 | 2.7 | 2.8 | 4.6 | 5.0 | 0.57 |
| Prostate cancer family history (%) | 5.0 | 7.3 | 7.3 | 4.8 | 5.8 | 0.58 |
| Physical activity (% active) | 14.0 | 18.5 | 17.2 | 24.2 | 23.3 | 0.001 |
| Education (% elementary) | 79.7 | 74.3 | 70.2 | 71.2 | 63.4 | <0.0001 |
| Married (%) | 80.2 | 78.3 | 81.5 | 85.6 | 85.2 | 0.12 |
| Urban residence (%) | 38.0 | 46.3 | 45.5 | 46.5 | 51.2 | 0.002 |
| Elected radical surgery (%) | 13.1 | 17.9 | 15.4 | 22.4 | 17.9 | 0.54 |
| Dietary Intake (daily) | p for trend | |||||
| Vitamin E (mg) | 10.3 | 11.2 | 12.0 | 12.8 | 14.4 | <0.0001 |
| Zinc (mg) | 15.4 | 15.7 | 15.5 | 15.8 | 16.0 | 0.06 |
| Vitamin D (mcg) | 4.5 | 5.1 | 5.3 | 5.4 | 6.0 | <0.0001 |
| Vitamin C (mg) | 89.9 | 96.7 | 99.0 | 107.5 | 107.4 | <0.0001 |
| Selenium (mcg) | 88.3 | 89.4 | 89.5 | 90.2 | 91.8 | 0.04 |
| Retinol (mg) | 1.5 | 1.6 | 1.5 | 1.6 | 1.6 | 0.07 |
| Total fat (g) | 125.3 | 125.3 | 121.9 | 122.7 | 124.1 | 0.58 |
| Saturated fat (g) | 57.7 | 55.8 | 52.1 | 51.3 | 49.7 | <0.0001 |
| Polyunsaturated fat (g) | 10.1 | 10.9 | 12.0 | 12.9 | 15.2 | <0.0001 |
| α-Linolenic acid (mg) | 1487 | 1591 | 1664.8 | 1782.3 | 1943.3 | <0.0001 |
| Energy (kcal) | 2736 | 2694 | 2666 | 2672 | 2710 | 0.65 |
| Cholesterol (mg) | 588.5 | 598.6 | 579.4 | 573.0 | 566.0 | 0.10 |
| α-tocopherol (mg) | 8.9 | 9.6 | 10.3 | 11.0 | 12.6 | <0.0001 |
| β-carotene (mg) | 1.9 | 2.0 | 2.1 | 2.3 | 2.3 | <0.0001 |
| Alcohol (g) | 19.5 | 17.2 | 16.8 | 14.6 | 15.7 | 0.18 |
| Vegetables (g) | 664.3 | 694.1 | 731.1 | 758.9 | 778.8 | <0.0001 |
| Fruit (g) | 179.4 | 194 | 225.3 | 231.2 | 241.9 | <0.0001 |
| Fish (g) | 32.8 | 39.3 | 39.6 | 38.0 | 41.3 | <0.0001 |
| Serum biomarkers at baseline | ||||||
| β-carotene (μg/mL) | 166.8 | 190.1 | 229.3 | 257.5 | 262.6 | <0.0001 |
| Retinol (μg/mL) | 554.7 | 576.8 | 582.0 | 611.1 | 650.7 | <0.0001 |
| Cholesterol (mmol/L) | 5.2 | 5.9 | 6.2 | 6.6 | 7.2 | <0.0001 |
| HDL cholesterol (mmol/L) | 1.3 | 1.2 | 1.2 | 1.2 | 1.1 | <0.0001 |
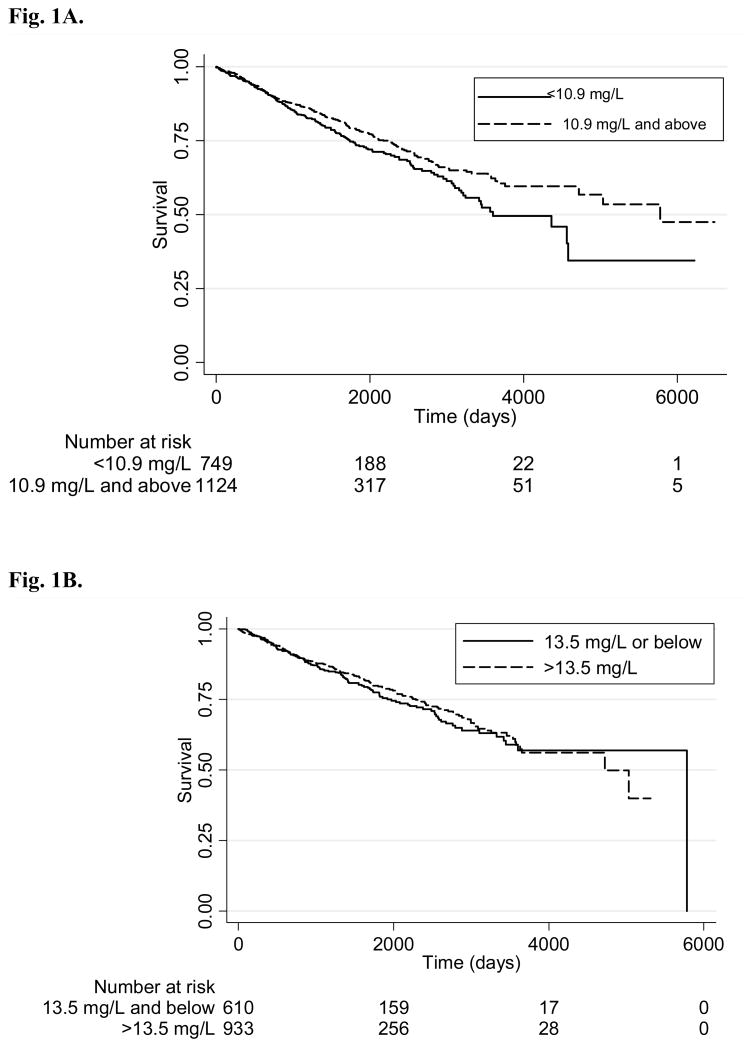
Adjusted hazard ratios for prostate cancer mortality were examined by baseline serum α-tocopherol, β-carotene, and retinol (Table 3). Higher baseline serum α-tocopherol was associated with improved prostate cancer survival (p for trend= 0.03). Neither serum β-carotene nor retinol had a demonstrable effect. Those with α-tocopherol levels above the second quintile at baseline, which corresponds to concentrations ≥ 10.9 mg/L, had a hazard ratio HR= 0.73 (95% CI 0.57 to 0.93), which is suggestive of a possible threshold effect. Figure 1A shows a comparison of the survival curves for those in the top three quintiles versus those in the bottom two quintiles.
Table 3.
Adjusted* hazard ratios for prostate cancer mortality by baseline serum α-tocopherol, β-carotene, and retinol
| Category | n (deaths) | Hazard Ratio | 95% CI | P | p for trend |
|---|---|---|---|---|---|
| Serum α-tocopherol (range mg/L) | |||||
| Lowest Quintile (<9.4) | 379 (83) | 1 | |||
| 2nd Quintile (9.4– 10.9) | 378 (91) | 0.94 | (0.69, 1.28) | 0.71 | |
| 3rd Quintile (10.9– 12.2) | 389 (76) | 0.68 | (0.48, 0.96) | 0.03 | |
| 4th Quintile (12.3– 14.0) | 368 (71) | 0.75 | (0.52, 1.07) | 0.11 | |
| 5th Quintile (>14.0) | 377 (74) | 0.67 | (0.45, 1.00) | 0.05 | 0.03 |
| Serum α-tocopherol (range mg/L) | |||||
| Lowest & 2nd Quintile (< 10.9) | 757 (174) | 1 | |||
| 3rd– 5th Quintile (≥10.9) | 1134 (221) | 0.73 | (0.57, 0.93) | 0.01 | |
| Serum β-carotene (range mg/L) | |||||
| Lowest Quintile (<105) | 381 (77) | 1 | |||
| 2nd Quintile (105– 155) | 383 (82) | 1.07 | (0.78, 1.46) | 0.68 | |
| 3rd Quintile (156– 210) | 372 (76) | 1.04 | (0.76, 1.43) | 0.80 | |
| 4th Quintile (211– 299) | 378 (74) | 0.80 | (0.58, 1.12) | 0.19 | |
| 5th Quintile (>299) | 377 (86) | 1.01 | (0.74, 1.39) | 0.95 | 0.49 |
| Serum retinol (range mg/L) | |||||
| Lowest Quintile (<493) | 386 (74) | 1 | |||
| 2nd Quintile (493– 554) | 374 (78) | 1.13 | (0.82, 1.56) | 0.45 | |
| 3rd Quintile (555– 613) | 378 (92) | 1.20 | (0.88, 1.64) | 0.25 | |
| 4th Quintile (614– 691) | 376 (74) | 1.10 | (0.79, 1.52) | 0.57 | |
| 5th Quintile (>691) | 377 (77) | 1.17 | (0.84, 1.63) | 0.34 | 0.44 |
Adjusted for age at randomization, BMI, age at diagnosis, advanced stage of prostate cancer at diagnosis, years of smoking, and for α-tocopherol, cholesterol.
Figure 1.
Fig. 1A. Prostate cancer survival by serum α-tocopherol at baseline. Kaplan-Meier plots of the probability of not dying from prostate cancer from the date of diagnosis for those in the 1st and 2nd quintiles of baseline serum α-tocopherol and those in the 3rd – 5th quintiles (≥ 10.9 mg/L). Below each graph is the total of men at risk for the same time points. Log-rank test for equality of survivor functions: Pr>chi2 = 0.03.
Fig. 1B. Prostate cancer survival by serum α-tocopherol at three years. Kaplan-Meier plots of the probability of not dying from prostate cancer from the date of diagnosis for those in the 1st and 2nd quintiles of serum α-tocopherol at three years after entry on trial and those in the 3rd -5th quintiles (> 13.5 mg/L). Only men with a prostate cancer diagnosis after three years from entry on trial are included. Below each graph is the total of men at risk for the same time points. Log-rank test for equality of survivor functions: Pr>chi2 = 0.52.
Although there were no statistically significant interactions, there appeared to be a considerable difference in survival by α-tocopherol supplementation status (Table 4). Longer prostate cancer survival was observed for those who received α-tocopherol supplementation (either α-tocopherol only or α-tocopherol and β-carotene combined) and had higher serum α-tocopherol at baseline (p for trend= 0.04). The strongest relationship was seen for those who received α-tocopherol supplementation and were in the highest serum α-tocopherol quintile (HR= 0.51, 95% CI 0.20, 0.90). Higher baseline serum β-carotene, regardless of supplementation status, had no effect on prostate cancer survival. We also restricted the analyses to those receiving the single nutrient supplement to assess the whether the combined α-tocopherol and β-carotene supplement acted differently. The overall trend was the same; only those who received α-tocopherol supplements had significantly improved survival with the strongest effect seen for the highest serum α-tocopherol quintile (HR= 0.29, 95% CI 0.11, 0.74).
Table 4.
Adjusted* hazard ratios for prostate cancer mortality by trial supplementation and baseline serum α-tocopherol and β-carotene
| Category | # cases (#deaths) | Hazard Ratio | 95% CI | P | p for trend |
|---|---|---|---|---|---|
| Serum α-tocopherol (mg/L) | |||||
| No α-tocopherol supplement | |||||
| Lowest Quintile | 202 (44) | 1 | |||
| 2nd Quintile | 200 (56) | 1.05 | (0.70, 1.60) | 0.80 | |
| 3rd Quintile | 214 (38) | 0.71 | (0.44, 1.16) | 0.17 | |
| 4th Quintile | 170 (35) | 0.77 | (0.46, 1.29) | 0.32 | |
| 5th Quintile | 201 (49) | 0.86 | (0.51, 1.47) | 0.58 | 0.39 |
| α-tocopherol supplement | |||||
| Lowest Quintile | 177 (39) | 1 | |||
| 2nd Quintile | 178 (35) | 0.82 | (0.50, 1.29) | 0.37 | |
| 3rd Quintile | 175 (38) | 0.64 | (0.39, 1.04) | 0.07 | |
| 4th Quintile | 198 (36) | 0.74 | (0.44, 1.23) | 0.24 | |
| 5th Quintile | 176 (25) | 0.51 | (0.20, 0.90) | 0.02 | 0.04 |
| Combined | |||||
| No Supp-Lowest Quintile | 202 (44) | 1 | |||
| No Supp-2nd Quintile | 200 (56) | 0.99 | (0.75, 1.30) | 0.92 | |
| No Supp-3rd Quintile | 214 (38) | 0.67 | (0.50, 0.90) | 0.01 | |
| No Supp-4th Quintile | 170 (35) | 0.59 | (0.43, 0.81) | 0.001 | |
| No Supp-5th Quintile | 201 (49) | 0.82 | (0.61, 1.10) | 0.19 | |
| Supp-Lowest Quintile | 177 (39) | 0.76 | (0.58, 1.00) | 0.05 | |
| Supp-2nd Quintile | 178 (35) | 0.69 | (0.52, 0.92) | 0.01 | |
| Supp-3rd Quintile | 175 (38) | 0.56 | (0.41, 0.77) | <0.001 | |
| Supp-4th Quintile | 198 (36) | 0.63 | (0.46, 0.86) | 0.004 | |
| Supp-5th Quintile | 176 (25) | 0.63 | (0.44, 0.88) | 0.01 | 0.28 |
| Serum β-carotene (mg/L) | |||||
| No β-carotene supplement | |||||
| Lowest Quintile | 193 (46) | 1 | |||
| 2nd Quintile | 196 (50) | 1.10 | (0.69, 1.75) | 0.69 | |
| 3rd Quintile | 196 (41) | 1.05 | (0.65, 1.69) | 0.84 | |
| 4th Quintile | 209 (42) | 0.78 | (0.43, 1.16) | 0.17 | |
| 5th Quintile | 193 (43) | 0.99 | (0.62, 1.59) | 0.98 | 0.42 |
| β-carotene supplement | |||||
| Lowest Quintile | 188 (31) | 1 | |||
| 2nd Quintile | 187 (32) | 1.04 | (0.68, 1.61) | 0.85 | |
| 3rd Quintile | 176 (35) | 1.05 | (0.68, 1.63) | 0.82 | |
| 4th Quintile | 169 (32) | 0.91 | (0.58, 1.40) | 0.66 | |
| 5th Quintile | 184 (43) | 1.04 | (0.67, 1.60) | 0.86 | 0.90 |
Adjusted for age at randomization, BMI, age at diagnosis, advanced stage of prostate cancer at diagnosis, years of smoking, and for α-tocopherol, cholesterol.
To examine possible trial effects seen elsewhere (25), survival was also compared by supplementation status and the period of time in which prostate cancer was diagnosed: during the controlled trial (before May 1993), 6-year post-trial follow-up (through April 30, 1999), and through April 30, 2005 (12-year post-trial). The α-tocopherol supplementation death hazard ratios were HR= 0.60 (95% CI 0.29, 1.24) during the trial, HR= 0.71 (95% CI 0.38, 1.30) in the 6-year post-trial period, and HR= 0.98 (95% CI 0.43, 2.25) in the 12-year post-trial period. For β-carotene, the corresponding hazard ratios were HR= 1.02 (95% CI 0.56, 1.84), HR= 0.96 (95% CI 0.58, 1.57), and HR= 1.17 (95% CI 0.57, 2.39).
Prostate cancer survival was examined by quintile of serum α-tocopherol measured three years into the study, following the onset of supplementation (Table 5). The results were similar to those for baseline measurements, with higher on-study α-tocopherol levels being related to improved overall survival (p for trend= 0.12), particularly for cases in the highest quintile who were receiving the α-tocopherol supplementation (HR= 0.26, 95% CI 0.09, 0.71). However, the highest three quintiles did not have significantly improved prostate cancer survival compared to the bottom two quintiles (p=0.52, Figure 1B). Compared to a common referent of no α-tocopherol supplementation/lowest serum quintile, the only statistically significant association was poorer survival for those in the lowest quintile and receiving supplementation (HR= 2.82, 95% CI 1.11, 7.15). Although, cases in most other categories experienced increased survival, the interaction test was not significant.
Table 5.
Adjusted* hazard ratios for prostate cancer mortality by serum α-tocopherol at 3 years and supplementation status
| Category | # cases (#deaths) | Hazard Ratio | 95% CI | p | p for trend |
|---|---|---|---|---|---|
| Serum α-tocopherol (range mg/L) | |||||
| Lowest Quintile (<11.5) | 315 (66) | 1 | |||
| 2nd Quintile (11.5 – 13.4) | 301 (60) | 0.96 | (0.68, 1.36) | 0.83 | |
| 3rd Quintile (13.5 – 16.0) | 341 (78) | 0.93 | (0.67, 1.30) | 0.68 | |
| 4th Quintile (16.1 – 19.0) | 295 (44) | 0.74 | (0.50, 1.09) | 0.13 | |
| 5th Quintile (>19.0) | 305 (55) | 0.80 | (0.54, 1.17) | 0.24 | 0.12 |
| No α-tocopherol supplement | |||||
| Lowest Quintile | 289 (61) | 1 | |||
| 2nd Quintile | 228 (44) | 0.97 | (0.65, 1.45) | 0.90 | |
| 3rd Quintile | 172 (39) | 0.95 | (0.61, 1.49) | 0.83 | |
| 4th Quintile | 74 (13) | 0.85 | (0.45, 1.63) | 0.63 | |
| 5th Quintile | 40 (8) | 0.80 | (0.38, 1.69) | 0.56 | 0.52 |
| α-tocopherol supplement | |||||
| Lowest Quintile | 26 (5) | 1 | |||
| 2nd Quintile | 73 (16) | 0.41 | (0.15, 1.14) | 0.09 | |
| 3rd Quintile | 169 (39) | 0.36 | (0.14, 0.94) | 0.04 | |
| 4th Quintile | 221 (31) | 0.24 | (0.09, 0.64) | 0.01 | |
| 5th Quintile | 265 (47) | 0.26 | (0.09, 0.71) | 0.01 | 0.02 |
| Combined | |||||
| No Supp-Lowest Quintile | 289 (61) | 1 | |||
| No Supp-2nd Quintile | 228 (44) | 0.93 | (0.62, 1.39) | 0.71 | |
| No Supp-3rd Quintile | 172 (39) | 0.87 | (0.57, 1.34) | 0.53 | |
| No Supp-4th Quintile | 74 (13) | 0.95 | (0.50, 1.82) | 0.88 | |
| No Supp-5th Quintile | 40 (8) | 0.67 | (0.31, 1.43) | 0.30 | |
| Supp-Lowest Quintile | 26 (5) | 2.82 | (1.11, 7.15) | 0.03 | |
| Supp-2nd Quintile | 73 (16) | 1.27 | (0.73, 2.21) | 0.40 | |
| Supp-3rd Quintile | 169 (39) | 1.08 | (0.71, 1.62) | 0.72 | |
| Supp-4th Quintile | 221 (31) | 0.80 | (0.51, 1.24) | 0.31 | |
| Supp-5th Quintile | 265 (47) | 0.84 | (0.55, 1.27) | 0.40 | 0.24 |
| Combined quintiles† (range mg/L) | |||||
| No Supp- 1st & 2nd Quintile (≤13.5) | 517 (105) | 1 | |||
| No Supp-3rd– 5th Quintile (>13.5) | 286 (60) | 0.90 | (0.64, 1.27) | 0.56 | |
| Supp- 1st & 2nd Quintile (≤13.5) | 99 (21) | 1.48 | (0.92, 2.39) | 0.11 | |
| Supp-3rd– 5th Quintile (>13.5) | 655 (117) | 0.93 | (0.71, 1.22) | 0.59 | 0.81 |
Adjusted for age at randomization, BMI, age at diagnosis, advanced stage of prostate cancer at diagnosis, years of smoking, and for α-tocopherol, cholesterol
Men in 1st and 2nd quintiles of serum α-tocopherol were compared to those in the 3rd –5th quintiles. The value between 2nd and 3rd quintile of serum α-tocopherol at 3 years was 13.5 mg/L.
Potential associations between prostate cancer survival and treatment status, BMI, height, smoking status, alcohol use, physical activity, and dietary intake of vitamins C, D, and E, fish, fruits, vegetables, calcium, selenium, lycopene, total carotenoids, α-linolenic acid, and polyunsaturated fat were also examined (data not shown). Overall, none of these associations were statistically significant, but there were trends toward greater prostate cancer survival for men who were taller than 178cm, with a HR= 0.77 (95% CI 0.56, 1.02), and trends toward poorer survival for men in the highest quartile of pack-years smoked, with HR= 1.30 (95% CI 0.99, 1.72).
Discussion
Higher serum and supplemental α-tocopherol appeared to improve overall prostate cancer survival in this investigation. Overall, estimates were similar for the baseline and 3 year serum α-tocopherol determinations, suggesting both dietary and supplemental vitamin E may contribute to improved prostate cancer survival. Baseline serum values reflect dietary intake as men who reported taking supplements above minimum limits upon enrollment were not eligible to participate in the trial, whereas 3 year serum concentrations resulted from dietary intake and the intervention supplementation. The strongest survival associations were seen for men who received α-tocopherol supplementation and had high serum α-tocopherol concentrations at baseline or 3 years, as well as during the first six years following the trial supplementation. Neither serum nor supplemental β-carotene or serum retinol had apparent effects on survival. These findings build upon previous research showing a 41% reduction of prostate cancer mortality in response to α-tocopherol supplementation in the controlled trial component of the ATBC Study from 1985–1993 (20). The data presented here included an additional 12 years of follow-up. Our findings stand in contrast to those recently reported for two large, randomized controlled studies, the Physicians’ Health Study (PHS) II and the Selenium and Vitamin E Cancer Prevention Trial (SELECT). PHS II tested vitamins C and E and found no effect of vitamin E supplementation on prostate cancer mortality (HR=1.01, 95% CI 0.64, 1.58) or incidence (HR=0.97, 95% CI 0.85, 1.09) following up to 10 years of intervention (26). Similarly, SELECT tested selenium and vitamin E in over 35,000 healthy men and resulted in a non-significant increased rate of prostate cancer among the men receiving the supplemental vitamin E (HR=1.13, 99% CI 0.95, 1.35) compared to those receiving the vitamin E placebo (27). The dose of vitamin E used in the PHS II was 400 IU synthetic α-tocopherol every other day, half the effective daily dosage of 400 IU daily all rac-α-tocopheryl acetate used in SELECT. Several factors could account for these differing results. First, our analysis was based on measured serum alpha-tocopherol, whereas PHS II and SELECT tested and reported on their supplementation groups. The intervention dose in the ATBC Study was 50 IU dl-α-tocopherol acetate/day, 4 to 8 times lower than the other trials and also had a longer period of follow-up (up to 20 years). PHS II and SELECT evaluated much higher dosages for shorter periods and have not yet reported on prospectively measured vitamin E blood levels which could reveal a different association compared to the high-dose supplementation. Second, PHS II and SELECT were conducted in the U.S. where PSA testing is common and those enrolled in SELECT were required to undergo prostate cancer screening prior to study entry in contrast to the ATBC Study. If vitamin E affects advanced prostate cancer in particular, as has been observed (8), then an unscreened population would likely show a stronger beneficial relationship. Third, both recently reported trials were comprised of more diverse study populations with relatively few smokers compared to the ATBC Study participants. It is possible that the survival benefit of vitamin E is only experienced by smokers.
There are few other studies investigating vitamin E and prostate cancer survival. Two studies included daily vitamin E supplements within their interventions; one found improved survival in combination with ω-3 polyunsaturated fatty acids (27, 28) and another concluded that a vegan diet with soy, fish oil, and several micronutrient supplements delayed disease progression (29). Although each study demonstrated sizeable effects, it is not possible to distinguish the contribution of supplemental vitamin E from the other dietary intervention components. However, dietary agents have been shown to impact prostate cancer survival. An investigation within the Health Professionals Follow-up Study examined pre- and post- diagnostic diet and found high intake of tomato sauce (HR=0.56, 95% CI 0.38, 0.82) and fish (HR=0.73, 95% CI 0.52, 1.02) were protective against disease progression (14). As prostate cancer is a disease with relatively good survival, there is interest in other causes of death in men diagnosed with prostate cancer. We found a trend toward protection for all-cause mortality (including prostate cancer) (HR=0.67, 95% CI 0.52, 0.87 for highest versus lowest quintile; Ptrend = 0.01) which was slightly attenuated when those who died of prostate cancer were excluded (HR=0.70, 95% CI 0.46, 0.98 for highest versus lowest quintile; Ptrend = 0.11), suggesting a possible effect for α-tocopherol on other causes of death in men with prostate cancer. Although meta-analyses suggest vitamin E has no effect on all-cause mortality (30), lower mortality rates were observed for men with higher serum vitamin E at baseline in another analysis within the ATBC data (31).
There is considerably more evidence available for the relationship between vitamin E and prostate cancer risk than for survival. Several, but not all, observational and intervention studies have shown a strong inverse association between α-tocopherol and the risk of developing prostate cancer, particularly for advanced cancers or among smokers (32). For example, analysis from the PLCO trial found no association with risk in the general population, but vitamin E was strongly protective in smokers (RR=0.29, 95% CI 0.12, 0.68) (10). Similar work using the ATBC Study with 19 years of follow-up found that serum α-tocopherol was associated with reduced risk of prostate cancer (RR=0.80, 95% CI 0.66, 0.96 for highest versus lowest quintile), especially advanced disease (RR=0.56, 95% CI 0.36, 0.85) (8), whereas the original effect seen in the trial period of the study was a 32% reduction in prostate cancer risk with α-tocopherol supplementation (20). New observational studies are needed to tease apart the relationship between prostate cancer risk and α-tocopherol from supplementation versus serum levels as well as the possible interactions with smoking. Similar investigations are needed to determine the role of nutritional factors in prostate cancer survival.
The mechanisms through which vitamin E might affect the development and progression of prostate cancer are not fully understood; however, a number of biologically plausible pathways have been suggested. α-Tocopherol is thought to affect carcinogenesis by detoxifying oxidizing radicals via its antioxidant properties, inducing cell cycle arrest in prostate cancer cells (33), decreasing cell growth by down-regulating the phosphoinositide 3-kinase pathway (34), enhancing cell-mediated immunity (16), targeting transcription factors, such as NF-κB, that contribute to malignant transformation and cell growth (35), and decreasing serum androgen concentrations (36). Many of these biological actions are likely to be involved in prostate cancer survival.
Prostate cancer survival was greater for men with baseline serum α-tocopherol ≥ 11 mg/L, regardless of supplementation status, but there was slightly better survival for those receiving the vitamin E supplementation (HR= 0.51, 95% CI 0.27, 0.96). Similar results were seen after 3 years among those in the highest α-tocopherol quintiles who received supplementation had considerably better survival (HR= 0.29, 95% CI 0.10, 0.82). Vitamin E supplementation has been associated with decreased prostate cancer risk in several cohort studies, which reflects self-selected supplement use rather than randomized assignment in an intervention trial. Long-term (10 years average) supplement use of vitamin E (≥ 400 IU) was associated with the reduced risk of advanced prostate cancers (HR= 0.43, 95% CI 0.19, 1.00) in the VITamins And Lifestyle (VITAL) cohort study (37). A similar non-significant trend was seen for advanced cases in nonsmokers, but not current or former smokers in the NIH-AARP Diet and Health Study, a large cohort study of men 50 to 71 years; however, supplemental vitamin E intake was unrelated to prostate cancer risk in the total population (7). Other recent analyses suggest possible harm from such supplements (38, 39). Our analyses suggest that both dietary vitamin E, as evidenced by baseline levels, and supplemental vitamin E as reflected in on-study serum concentrations at 3 years, impacted survival favorably.
When all categories of serum α-tocopherol at 3 years and supplementation were compared to men in the lowest quintile without supplementation, there appeared to be a non-significant trend with better survival in the highest quintiles. Unexpectedly, however, the poorest survival was seen for men in the lowest quintile of serum α-tocopherol who had received supplementation, and not all men who received supplements were in the higher α-tocopherol quintiles at 3 years even though supplemental vitamin E contributes much more to biochemical status than do dietary sources (40). It is important to note that there were very few participants (n=27, including 5 cases) in this category, however, and the finding could be due to chance. It is also foreseeable that less healthy men may be less likely to take supplements, yet compliance, as measured by capsule adherence and number of visits, was no different for these 27 participants than the general study population. Finally, it is possible that these men may be more likely to have a genetic polymorphism that is related to both survival and vitamin E metabolism. Vitamin E levels have been shown to modify prostate cancer risk for polymorphisms in magnanese-superoxide dismutase (MnSOD) and XRCC1, for example, with stronger associations seen for aggressive cancers (41–43). At present, genotyping data to test this hypothesis are not available for all cases.
Our findings that serum β-carotene and retinol were unrelated to prostate cancer survival mirror other observational and intervention studies that generally found no association with either nutrient and prostate cancer risk (9, 44). Results for β-carotene and prostate cancer risk have been inconsistent. There was no association detected in the Physicians Health Study (RR= 1.0, 95% CI 0.9, 1.1) (45), a randomized trial of aspirin and 50 mg β-carotene on alternate days, but β-carotene was associated with an increased risk of aggressive prostate cancer in a nested case-control study using data from the PLCO Trial (RR= 1.67, 95% CI 1.03, 2.72) (46).
There are several notable strengths of this study. The availability of a large number of prostate cancer cases with considerable post-diagnosis follow-up provided substantial power to detect potential associations with survival that may have been obscured in smaller studies. The use of serum nutrient biomarkers reduces the measurement error associated with self-reported data for dietary and supplement intake. Participants were exceptionally adherent during the trial period, with 96% of supplement capsules taken as scheduled and there was no off-trial supplement use reported. Finally, because PSA screening was not widely employed at the time of the ATBC trial, the potential for lead-time bias caused by overdiagnosis of cancers that are not clinically relevant was low.
Limitations of the study include its being conducted in a relatively homogeneous cohort of male Finnish smokers, which limits the generalizability of our findings to nonsmokers and other races and ethnicities. We did not have comprehensive data concerning prostate cancer treatment; however, surgery was distributed equally across categories of serum α-tocopherol (baseline and 3 years) and supplementation status among those who died of prostate cancer. Another limitation was lack of detailed data on stage and grade of prostate cancer in those diagnosed after September 2001. However, cross-tabulations of α-tocopherol levels by stage in those diagnosed at or before September 2001 failed to reveal a correlation of α-tocopherol levels with stage. Thus, stage is unlikely to confound our results. Moreover we adjusted stage and period of diagnosis in three categories (1) before September, 2001 and stage I or II, 2) before September, 2001 and stage III or IV, and 3) after September, 2001 or missing). Gleason score, a strong predictor of survival, was available only for cases diagnosed before May 1, 1993 (<10% of cases) and thus, not included in these analyses. An examination of available data showed that Gleason score was strongly associated with survival as expected, but was not associated with serum α-tocopherol or β-carotene at either baseline or 3 years reducing concerns about potential confounding. We did not have information on serum levels or diet after 3 years on-study or post-trial supplement use, thus changes in diet or supplement use could have resulted in misclassification that would have biased our results to toward the null. This was an observational study of prostate cancer cases, even though it was conducted within the ATBC cohort for a randomized trial. Thus, we cannot exclude the possibility of residual confounding by unmeasured or unknown characteristics, especially clinico-pathological factors that may be related to survival, even though we adjusted for a number of potential confounders.
In summary, our results suggest dietary and supplemental α-tocopherol may improve prostate cancer survival. Data from similar studies in other populations would be useful. Considering the large number of men diagnosed with prostate cancer, such nutritional modifications could have a significant impact if subsequent randomized trials in men with newly diagnosed prostate cancer prove a benefit.
Acknowledgments
Funding
This research was supported in part by the Intramural Research Program of the NIH and the National Cancer Institute. Additionally, this research was supported by U.S. Public Health Service contracts N01-CN-45165, N01-RC-45035, and N01-RC-37004 from the National Cancer Institute, Department of Health and Human Services.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Signorello LB, Adami H-O. Prostate Cancer. In: Adami H-O, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. Oxford: Oxford University Press; 2002. [Google Scholar]
- 3.Sequoia JSP, Wright ME, McCarron P, et al. A Prospective Investigation of Height and Prostate Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2174–2178. doi: 10.1158/1055-9965.EPI-06-0467. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RB, Goldman B, Tangen CM, et al. Association of Body Mass Index With Response and Survival in Men With Metastatic Prostate Cancer: Southwest Oncology Group Trials 8894 and 9916. J Urol. 2007;178:1946–1951. doi: 10.1016/j.juro.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu H, Ross R, Bernstein L, et al. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. 2007 [Google Scholar]
- 7.Wright ME, Weinstein SJ, Lawson KA, et al. Supplemental and Dietary Vitamin E Intakes and Risk of Prostate Cancer in a Large Prospective Study. Cancer Epidemiol Biomarkers Prev. 2007;16:1128–1135. doi: 10.1158/1055-9965.EPI-06-1071. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein SJ, Wright ME, Lawson KA, et al. Serum and Dietary Vitamin E in Relation to Prostate Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1253–1259. doi: 10.1158/1055-9965.EPI-06-1084. [DOI] [PubMed] [Google Scholar]
- 9.Key TJ, Appleby PN, Allen NE, et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr. 2007;86:672–681. doi: 10.1093/ajcn/86.3.672. [DOI] [PubMed] [Google Scholar]
- 10.Kirsh VA, Hayes RB, Mayne ST, et al. Supplemental and Dietary Vitamin E, beta-Carotene, and Vitamin C Intakes and Prostate Cancer Risk. J Natl Cancer Inst. 2006;98:245–254. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 11.Kenfield S, Chang S, Chan J. Diet and Lifestyle Interventions in Active Surveillance Patients with Favorable-Risk Prostate Cancer. Curr Treat Options Oncol. 2007;8:173–196. doi: 10.1007/s11864-007-0034-0. [DOI] [PubMed] [Google Scholar]
- 12.Demark-Wahnefried W, Moyad MA. Dietary intervention in the management of prostate cancer. Curr Opin Urol. 2007;17:168–174. doi: 10.1097/MOU.0b013e3280eb10fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkow SE, Barnard ND, Saxe GA, Ankerberg-Nobis T. Diet and Survival After Prostate Cancer Diagnosis. Nutr Rev. 2007;65:391–403. doi: 10.1111/j.1753-4887.2007.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 14.Chan J, Holick C, Leitzmann M, et al. Diet After Diagnosis and the Risk of Prostate Cancer Progression, Recurrence, and Death (United States) Cancer Causes Control. 2006;17:199–208. doi: 10.1007/s10552-005-0413-4. [DOI] [PubMed] [Google Scholar]
- 15.National Academy of Sciences (NAS) IoM. Dietary References Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and on Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on Scientific Evaluation of Dietary Reference Intakes. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 16.Omenn G. Micronutrients (vitamins and minerals) as cancer-preventive agents. IARC Sci Publ. 1996;139:33–45. [PubMed] [Google Scholar]
- 17.The Alpha-Tocophero Beta Carotene Cancer Prevention Study Group. The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 18.Korhonen P, Malila N, Pukkala E, et al. The Finnish Cancer Registry as follow-up source of a large trial cohort-accuracy and delay. Acta Oncol. 2002;41:381–8. doi: 10.1080/028418602760169442. [DOI] [PubMed] [Google Scholar]
- 19.American Joint Committee on Cancer. Manual for staging of cancer. Philadelphia: J.B. Lippincott Company; 1992. [Google Scholar]
- 20.Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with alpha-tocopherol and beta- carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 21.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 22.Milne DB, Botnen J. Retinol, alpha-tocopherol, lycopene, and alpha- and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin Chem. 1986;32:874–876. [PubMed] [Google Scholar]
- 23.Kattermann R, Jaworek D, Möller G, et al. Multicentre study of a new enzymatic method of cholesterol determination. J Clin Chem Clin Biochem. 1984;22:245–51. doi: 10.1515/cclm.1984.22.3.245. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC. Nutritional epidemiology. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 25.The ATBC Study Group. Incidence of Cancer and Mortality Following {alpha}-Tocopherol and {beta}-Carotene Supplementation: A Postintervention Follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 26.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the Prevention of Prostate and Total Cancer in Men: The Physicians’ Health Study II Randomized Controlled Trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippman SM, Klein EA, Goodman PJ, et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gogos CA, Ginopoulos P, Salsa B, et al. Dietary omega-3 polyunsaturated fatty acids plus vitamin E restore immunodeficiency and prolong survival for severely ill patients with generalized malignancy. Cancer. 1998;82:395–402. doi: 10.1002/(sici)1097-0142(19980115)82:2<403::aid-cncr21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Ornish D, Weidner G, Fair WR, et al. Intensive Lifestyle Changes May Affect The Progression Of Prostate Cancer. J Urol. 2005;174:1065–1070. doi: 10.1097/01.ju.0000169487.49018.73. [DOI] [PubMed] [Google Scholar]
- 30.Bjelakovic G, Nikolova D, Gluud L, Simonetti R, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2008;16:CD007176. doi: 10.1002/14651858.CD007176. [DOI] [PubMed] [Google Scholar]
- 31.Wright ME, Lawson KA, Weinstein SJ, et al. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2006;84:1200–1207. doi: 10.1093/ajcn/84.5.1200. [DOI] [PubMed] [Google Scholar]
- 32.Chan JM, Gann PH, Giovannucci EL. Role of Diet in Prostate Cancer Development and Progression. J Clin Oncol. 2005;23:8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 33.Venkateswaran V, Fleshner N, Klotz L. Modulation of cell proliferation and cell cycle regulators by vitamin E in human prostate carcinoma cell lines. J Urol. 2002;168:1578–82. doi: 10.1016/S0022-5347(05)64524-7. [DOI] [PubMed] [Google Scholar]
- 34.Ni J, Wen X, Yao J, et al. Tocopherol-Associated Protein Suppresses Prostate Cancer Cell Growth by Inhibition of the Phosphoinositide 3-Kinase Pathway. Cancer Res. 2005;65:9807–9816. doi: 10.1158/0008-5472.CAN-05-1334. [DOI] [PubMed] [Google Scholar]
- 35.Crispen PL, Uzzo RG, Golovine K, et al. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: implications for chemoprevention. Prostate. 2007;67:582–590. doi: 10.1002/pros.20468. [DOI] [PubMed] [Google Scholar]
- 36.Hartman TJ, Dorgan JF, Woodson K, et al. Effects of long-term alpha-tocopherol supplementation on serum hormones in older men. Prostate. 2001;46:33–8. doi: 10.1002/1097-0045(200101)46:1<33::aid-pros1005>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Peters U, Littman AJ, Kristal AR, et al. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and lifestyle (VITAL) study cohort. Cancer Causes Control. 2008;19:75–87. doi: 10.1007/s10552-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 38.Lonn E, Bosch J, Yusuf S, et al. Effects of Long-term Vitamin E Supplementation on Cardiovascular Events and Cancer: A Randomized Controlled Trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 39.Lawson KA, Wright ME, Subar A, et al. Multivitamin Use and Risk of Prostate Cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–764. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 40.Satia-Abouta J, Patterson RE, King IB, et al. Reliability and Validity of Self-Report of Vitamin and Mineral Supplement Use in the Vitamins and Lifestyle Study. Am J Epidemiology. 2003;157:944–954. doi: 10.1093/aje/kwg039. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Kantoff PW, Giovannucci E, et al. Manganese Superoxide Dismutase Polymorphism, Prediagnostic Antioxidant Status, and Risk of Clinical Significant Prostate Cancer. Cancer Res. 2005;65:2498–2504. doi: 10.1158/0008-5472.CAN-04-3535. [DOI] [PubMed] [Google Scholar]
- 42.van Gils CH, Bostick RM, Stern MC, Taylor JA. Differences in Base Excision Repair Capacity May Modulate the Effect of Dietary Antioxidant Intake on Prostate Cancer Risk: An Example of Polymorphisms in the XRCC1 Gene. Cancer Epidemiol Biomarkers Prev. 2002;11:1279–1284. [PubMed] [Google Scholar]
- 43.Woodson K, Tangrea JA, Lehman TA, et al. Manganese superoxide dismutase (MnSOD) polymorphism, α-tocopherol supplementation and prostate cancer risk in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (Finland) Cancer Causes Control. 2003;14:513–518. doi: 10.1023/a:1024840823328. [DOI] [PubMed] [Google Scholar]
- 44.Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The Association between Lung and Prostate Cancer Risk, and Serum Micronutrients: Results and Lessons Learned from {beta}-Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2003;12:518–526. [PubMed] [Google Scholar]
- 45.Cook N, Le I, Manson J, Buring J, Hennekens C. Effects of beta-carotene supplementation on cancer incidence by baseline characteristics in the Physicians’ Health Study (United States) Cancer Causes Control. 2000;11:617–26. doi: 10.1023/a:1008995430664. [DOI] [PubMed] [Google Scholar]
- 46.Peters U, Leitzmann MF, Chatterjee N, et al. Serum Lycopene, Other Carotenoids, and Prostate Cancer Risk: a Nested Case-Control Study in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2007;16:962–968. doi: 10.1158/1055-9965.EPI-06-0861. [DOI] [PubMed] [Google Scholar]