Abstract
ATP released from cells is known to activate plasma membrane P2X (ionotropic) or P2Y (metabotropic) receptors. In skeletal muscle cells, depolarizing stimuli induce both a fast calcium signal associated with contraction and a slow signal that regulates gene expression. Here we show that nucleotides released to the extracellular medium by electrical stimulation are partly involved in the fast component and are largely responsible for the slow signals. In rat skeletal myotubes, a tetanic stimulus (45 Hz, 400 1-ms pulses) rapidly increased extracellular levels of ATP, ADP, and AMP after 15 s to 3 min. Exogenous ATP induced an increase in intracellular free Ca2+ concentration, with an EC50 value of 7.8 ± 3.1 μm. Exogenous ADP, UTP, and UDP also promoted calcium transients. Both fast and slow calcium signals evoked by tetanic stimulation were inhibited by either 100 μm suramin or 2 units/ml apyrase. Apyrase also reduced fast and slow calcium signals evoked by tetanus (45 Hz, 400 0.3-ms pulses) in isolated mouse adult skeletal fibers. A likely candidate for the ATP release pathway is the pannexin-1 hemichannel; its blockers inhibited both calcium transients and ATP release. The dihydropyridine receptor co-precipitated with both the P2Y2 receptor and pannexin-1. As reported previously for electrical stimulation, 500 μm ATP significantly increased mRNA expression for both c-fos and interleukin 6. Our results suggest that nucleotides released during skeletal muscle activity through pannexin-1 hemichannels act through P2X and P2Y receptors to modulate both Ca2+ homeostasis and muscle physiology.
Introduction
Activation of skeletal muscle cells promotes a contractile response through “excitation-contraction” coupling. This is a complex process that allows coupling between membrane depolarization and Ca2+ release from the sarcoplasmic reticulum, inducing a fast increase in intracellular free Ca2+ levels that allows interactions of filaments required for muscle contraction. Two major proteins account for the excitation-contraction coupling process: the dihydropyridine receptor (DHPR),2 located in transverse (T) tubules of the plasma membrane, and the ryanodine receptor (RyR), a Ca2+ channel present in the sarcoplasmic reticulum. In skeletal muscles, DHPR acts both as a voltage sensor and a slow L-type Ca2+ channel (Cav1.1). Action potential propagation through muscle fibers promotes a transient T-tubule membrane depolarization, evoking conformational changes of the DHPR that are transmitted to the RyR, which opens and releases calcium to the cytosol for contraction development (1–4).
In addition to taking part in contraction, intracellular free Ca2+ controls different muscle cells processes, including metabolic pathway activation, differentiation, hypertrophy, and gene expression (5–7). We have previously shown that tetanic electrical stimulation of skeletal myotubes evokes a fast calcium transient during stimulation and a slow calcium transient that peaks 60–100 s later. The fast signal is blocked by ryanodine, and the slow component is dependent on 1,4,5-inositol trisphosphate (IP3) generation and Ca2+ release from the sarcoplasmic reticulum through IP3 receptors (8, 9). We have demonstrated that the slow calcium signal is dependent on DHPR activation, Gβγ complex release, phosphoinositide 3-kinase and phospholipase C activation, and IP3 increases (8–10). The slow calcium transient evoked by membrane depolarization has been associated with nuclear Ca2+ accumulation related to expression of early genes (5) and with activation of transcription pathways and expression of a number of skeletal muscle genes (11–13). Slow calcium signals have been assigned a role in the “excitation-transcription” process, which has possible effects on and implications for cell homeostasis maintenance. This process may shed light on muscle cell plasticity induced by physiological activity.
Mediators between DHPR activation and the Gβγ complex remain unknown. One option is that the Gβγ complex interacts directly with DHPR and could be released after its activation. It has been shown that voltage-activated Ca2+ channels (L-, P/Q-, N-, and R-types) have binding sites for Gβγ (14–16). Activation of these channels through Gβγ has been demonstrated, but the opposite process, i.e. release of Gβγ by the channel, has not been confirmed. Another possibility for DHPR is that it either activates a G protein-coupled receptor (GPCR) directly or fosters the release of a ligand for such receptors that will activate the pathway of heteromeric Gα/Gβγ. This is a likely possibility, because a slow calcium signal takes several seconds to develop, suggesting the activation of signaling pathways instead of direct interactions. It is important to note that in physiological conditions, the skeletal muscle is subjected simultaneously to many stimuli, including membrane depolarization, as well as hormones, metabolites, and extracellular molecules that can activate GPCRs and control cellular processes. It is interesting then to analyze the possible interactions between voltage sensors (such as DHPR) and different GPCRs as a basic mechanism possibly involved in the modulation of muscle plasticity.
Extracellular ATP, a co-transmitter in adrenergic and cholinergic synapses, has several functions in the central and peripheral nervous systems (17, 18). In addition, autocrine/paracrine ATP signaling occurs in many cell types (epithelium, endothelium, fibroblasts, etc.) that release nucleotides (ATP, UTP) to the extracellular medium under resting conditions and after physiopathological events, such as hypoxia, cell swelling, shear stress, or inflammation, through nonlytic mechanisms (19, 20). Depending on the cell type, ATP release by exocytosis or through ABC transporters, stretch- and voltage-activated channels, or connexin hemichannels has been described (19–21). Recently, pannexin (Pnx) hemichannels have also been proposed as relevant ATP conduits (22, 23). The ATP (or UTP) released is quickly metabolized to the di- and monophosphate nucleotides through ectonucleotidases located at the cell surface or the extracellular matrix. Finally, they are converted into nucleosides to be recaptured by cells (24). ATP, UTP, and their metabolites have been implicated in the regulation of physiological processes such as epithelial secretion, vascular tone, platelet aggregation, pain sensitivity, and actions as trophic factors (20, 25–29).
There are two main families of receptors for extracellular nucleotides: the P2X receptors, which are ion channels activated by purine triphosphate nucleotides; and the P2Y receptors, GPCRs activated by both di- and triphosphate purine or pyrimidine nucleotides. Seven P2X receptor subtypes (P2X1–7) and eight P2Y receptor subtypes (P2Y1,2,4,6,11,12,13,14) have been cloned and characterized pharmacologically (25, 29). Particularly in skeletal muscle, both short term (30) and long term (31) responses mediated by activation of nucleotide receptors have been described. The role of ATP as a co-transmitter with acetylcholine at the neuromuscular junction (17, 32) has also been documented. However, a role for ATP in signaling pathways involved in long term events such as proliferation or muscle development has just begun to be explored (33). ATP is released from skeletal muscle cells in response to muscle contraction (34, 35). Moreover, P2Y receptors are able to increase both their sensitivity to agonists and their activity responding to changes in membrane potential (36–38). Several P2Y receptor subtypes regulate the activity of voltage-dependent Ca2+ (N-type) and K+ (M-type) channels and modulate the cystic fibrosis transmembrane conductance regulator Cl− channel (25). Recently, functional nucleotide receptors (P2X4, P2X5, P2X7, P2Y1, and P2Y4) have been described in rat myotubes that evoke calcium transients when stimulated with exogenous nucleotides (39). In differentiated cells derived from human skeletal muscle, the activation of P2Y1 receptors by exogenous agonists promotes calcium transients dependent on the IP3 pathway but independent of RyRs, which leads to ERK1/ERK2 activation (40). Considering all of this background, extracellular ATP and its metabolites could be proposed as muscle plasticity regulators, which opens some very interesting questions about the interaction of nucleotide receptors with proteins such as DHPR, relevant to excitation-contraction and excitation-transcription events.
In this work we have found evidence that ATP is released by the physiological activity of skeletal muscle cells and is metabolized extracellularly, that P2Y/P2X functional receptors are expressed in this system, and that calcium transients can be evoked upon nucleotide exposure. Moreover, calcium transients evoked by tetanic electrical activity can be inhibited by interfering with extracellular nucleotide pathways. We have postulated that extracellular nucleotides can be crucial mediators between electrical stimulation and both the fast and slow calcium transients involved, respectively, in muscle activity and plasticity.
EXPERIMENTAL PROCEDURES
Reagents
ATP, ADP, UTP, UDP, and apyrase grade VII from potato, suramin, MRS2179, cytosine arabinoside, penicillin, streptomycin, and amphotericin B were obtained from Sigma-Aldrich. Dulbecco's modified Eagle's medium-F12, bovine serum, and fetal bovine serum were from Invitrogen. Collagenase type II was from Worthington Biochemical Corp. Pnx1 mimetic blocking peptide 10pnx1 (WRQAAFVDSY) was from Biosynthesis Inc. (Lewisville, TX). Fluo3-acetoxymethylester (fluo3-AM) was obtained from Molecular Probes (Eugene, OR). CompleteTM Mini protease inhibitors were from Roche Applied Science, and protein A/G-agarose was from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-DHPR α-1 antibody was from Affinity Bioreagents (Golden, CO). Rabbit anti-P2Y2 receptor antibody was from Zymed Laboratories Inc. (South San Francisco, CA). Rabbit anti-Pnx1 antibody was generated at the laboratory of Dr. Juan Carlos Sáez, Pontificia Universidad Católica, Santiago, Chile, and its characterization will be published elsewhere. Secondary horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies were from Pierce Biotechnology. Enhanced chemiluminescence (ECL) reagents were from Amersham Biosciences.
Cell Culture
Neonatal rat myotubes were cultured as described previously (41). Briefly, muscle tissue from the hind limbs of 12–24-h postnatal rat pups was dispersed mechanically and then treated with 0.2% (w/v) collagenase for 15 min with mild agitation. The suspension was filtered through a Nytex membrane or lens tissue paper and spun down at low speed. Then 10–15 min of preplating was performed for the enrichment of myoblasts; cells were plated at densities of 3.5 × 105 cells/dish (35 mm) or 9.5 × 105 cells/dish (60 mm). The plating medium was Dulbecco's modified Eagle's medium-Ham's F-12, 10% bovine serum, 25% fetal calf serum, 100 mg/liter penicillin, 50 mg/liter streptomycin, and 2.5 mg/liter amphotericin B. For fluorescence measurements, cells were plated on round coverslips pretreated with a 1% gelatin solution placed in the culture dishes for 30 min. To eliminate remaining fibroblasts, 10 mm cytosine arabinoside was added on the third day of culture. After 36 h in culture, fetal calf serum concentration was reduced to 1.8% (v/v) to induce differentiation. Myotubes in the dish, some spontaneously contracting, with an estimated purity of 90% were visible after the fifth day of culture; these were used for experiments after 5–7 days in culture.
Muscle Fiber Culture
BALB/c mice (5–7 weeks old) were used in this study. Animal care and manipulation were in agreement with protocols approved by the bioethical committee of the University of Chile. Isolated muscle fibers from mouse flexor digitorum brevis (FDB) were obtained by enzymatic digestion of the whole muscle with collagenase for 90 min at 450–500 units/ml followed by mechanic dissociation with fire-polished Pasteur pipettes. Isolated fibers were seeded on Matrigel-coated coverslips in Dulbecco's modified Eagle's medium supplemented with 10% horse serum. Fibers where used at 20 h after seeding.
Calcium Measurements in Rat Skeletal Myotubes
Cytosolic Ca2+ images were obtained from single, nonspontaneously contracting myotubes preloaded with fluo3-AM using a fluorescence microscope (Olympus TO41, New Hyde Park, NY) equipped with a cooled charge-coupled device camera and image acquisition system (MCD 600, Spectra Source, Westlake Village, CA) or an inverted confocal microscope (LSM 5 Pascal, Carl Zeiss, Jena, Germany). Myotubes were washed twice with Krebs buffer (145 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 5.6 mm glucose, 10 mm HEPES, pH 7.4) and loaded with 5.4 μm fluo3-AM (added from a stock in dimethyl sulfoxide; 20% pluronic acid) for 30 min at 37 °C. After loading, myotubes were washed with Krebs buffer and used within 2 h. For the Ca2+-free condition, the washout and the measurement were performed in Ca2+-free saline (145 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm EGTA, 5.6 mm glucose, 10 mm HEPES, pH 7.4). Cell-containing coverslips were mounted in a 1-ml capacity chamber and placed in the microscope for fluorescence measurements after excitation with a filter system or a 488 nm wavelength argon laser beam. Cells were stimulated using a pair of external platinum electrodes placed within 3 mm of each other and set 1–2 mm over the cells. The electrical field stimulation (45 Hz, 400 1-ms pulses) was performed as described previously (9). Control experiments were performed showing that this stimulus could be repeatedly applied to a myotube with no deleterious effect. Fluorescence images were collected every 6 s (epifluorescence microscopy) or 1.9 ms (line scan, confocal microscopy). Intracellular Ca2+ was expressed as a percentage of fluorescence intensity relative to basal fluorescence. Fluorescence data (F) normalized with respect to basal fluorescence (F0) were expressed as (F − F0)/F0.
Calcium Measurements in Isolated Fibers
Isolated FDB fibers were incubated for 30 min with 5 μm fluo3-AM at room temperature, and electrical stimulation was applied with a couple of platinum electrodes connected through an isolation unit to a stimulator. Fibers were stimulated with a tetanus protocol (45 Hz, 400 0.3-ms pulses). During stimulation experiments, fibers were kept in Krebs buffer. A series of images during stimulation experiments was obtained with a confocal microscope. After excitation with a 488 nm wavelength argon laser, fluorescence images were collected every 1.8 s and analyzed frame by frame. Images from each experiment were processed identically before quantization by outlining cell fluorescence; fluorescence data (F) were normalized with respect to basal fluorescence (F0) and expressed as (F − F0)/F.
mRNA Determinations
Total RNA from rat skeletal myotubes was extracted with TRIzol® reagent (42). The reverse transcription (RT) reaction was performed with 1 μg of total RNA using an oligo(dT) primer. PCR was carried out using forward and reverse primers specific for P2X or P2Y receptor subtypes (43–47), interleukin-6 (IL-6), or c-fos as detailed in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA amplification was used as the internal control. After an initial denaturing for 10 min at 94 °C, amplifications using of the RT products were carried out for 25–30 cycles as follows: denaturing at 94 °C for 30 s, annealing at 54 °C (P2Y receptor subtypes), 56 °C (IL-6, GAPDH), or 58 °C (P2X receptor subtypes, c-fos) for 30 s, and extension at 72 °C for 30 s. After completion of the cycles, a final 10-min extension at 72 °C was carried out. mRNA for all P2X and P2Y receptor subtypes was detected in whole rat brain as a control for method efficacy (not shown). PCR products were analyzed by electrophoresis in 1.5% agarose gels. Amplifications without the RT step were made to exclude possible contamination with genomic DNA.
TABLE 1.
PCR primers used for detection of mRNA in myotubes derived from neonatal rat primary culture and mouse C2C12 cell line
| mRNA | Forward (5′–3′) | Reverse (5′–3′) | PCR product |
|---|---|---|---|
| bp | |||
| Rat samples | |||
| P2Y1 | CCCTAACTATGATGCAGCTT | GCTGCATCTTTATCACCCTG | 151 |
| P2Y2 | GGCCCGAGAGCTCTTTAGC | GCAAAGCGGACCAGTCTCT | 386 |
| P2Y4 | GGGGACAAGTATCGAAACCA | GCCCCTGCAGTTAGTTCCCTT | 208 |
| P2Y6 | GACACCTGTGTTTCGGGGAC | CCTCTACAGGAGGGGCCTT | 259 |
| P2Y11 | ACTGGTGGTTGAGTTCCTGG | TCAGGTGGGAGAAGCTGAGT | 410 |
| P2Y12 | CAGAAATTCCTTGATGAGCA | ATGTGGTGATTTCCTTGGAG | 175 |
| P2Y13 | TGTGCACTTTCTCATCCGTG | TTGCCAGGAAGAGAGTTG | 570 |
| P2Y14 | CGCAATATATTCAGCATTGTGC | TCAGGAAAGCACAGATACTTTG | 317 |
| P2X1 | GTTCAGCATGAAGACAGGCA | GTATAGATGTGTGAGGGGCC | 136 |
| P2X2 | TGTGACTGGGAAACAGAAACC | AGGAGATGGCAGGGAACC | 114 |
| P2X3 | AAGAAGGGCTGCTATTTCTGC | AGGCATGCAAGGGGTAAAG | 126 |
| P2X4 | TCTGGTGTGCTGTTGGCTGG | ACCTGAGAGAGCCTCCTTCC | 152 |
| P2X5 | ACTTAGGGAAGAGCAAACTCC | AGCAAGAGCTGAACTGCACA | 156 |
| P2X6 | AGGCTAGGGTGAAAGCAACA | GCAGGAATATCAGGTTCTTTGG | 201 |
| P2X7 | TAAAGTTTGGATGTGGCTTGG | TCTGTGTGGTGTGTGGTGTG | 156 |
| IL-6 | CCAATTTCCAATGCTCTCCT | ACCACAGTGAGGAATGTCCA | 190 |
| c-fos | AGGCCGACTCCTTCTCCAG | CAGATAGCTGCTCTACTTTGC | 298 |
| GAPDH | CAACTTTGGCATTGTGGAAG | CTGCTTCACCACCTTCTTG | 295 |
| Mice samples | |||
| P2Y1 | TGGCGTGGTGTACCCTCTCAAGTC | CGGGACAGTCTCCTTCTGAATGTA | 558 |
| P2Y2 | CTGGAACCCTGGAATAGCAC | GCTGGTGGTGACGAAGTAGA | 513 |
| P2Y4 | AGCCCAAGTTCTGGAGATGGTG | GGTGGTTCCATTGGCATTGG | 492 |
| P2Y6 | CACCTGTGATTTGGCAACTG | TCTTGGCAAATGGATGTGAA | 336 |
| P2Y11 | ACTGGTGGTTGAGTTCCTGG | TCAGGTGGGAGAAGCTGAGT | 410 |
| P2Y12 | CACCTCAGCCAATACCACCT | AACATGAAGGCCCAGATGAC | 453 |
| P2Y13 | GAAGAGAGGCACATGCAACA | TTACTAATGCCAGGCCAACC | 345 |
| P2Y14 | CAGTGCATGGAGCTCAAAAA | GCAGCCGAGAGTAGCAGAGT | 347 |
| P2X1 | CATTGTGCAGAGAACCCAGAA | ATGTCCTCCGCATACTTGAAC | 776 |
| P2X2 | ACGTTCATGAACAAAAACAAG | TCAAAGTTGGGCCAAACCTTTGG | 360 |
| P2X3 | CTGTATATCAGACTTCTTCACCTACGA | TTATGTCCTTGTCGGTGAGGTTAG | 596 |
| P2X4 | GAGAATGACGCTGGTGTGCC | TTGGTGAGTGTGCGTTGCTC | 356 |
| P2X5 | TCCACCAATCTCTACTGC | CCAGGTCACAGAAGAAAG | 400 |
| P2X6 | CGATTCACTCTCCAGTCCG | GGTCCTCCAGTAGAAACCG | 427 |
| P2X7 | AAGTCTCTGCCTGGTGTC | GGCATATCTGAAGTTGTAGC | 401 |
Obtaining Samples for Extracellular Nucleotide Measurement
Rat skeletal myotubes grown on 35-mm plates were used 5–7 days after seeding. The extracellular medium was replaced by 1.5 ml of Krebs solution 1 h before the nucleotide assay. To obtain a homogeneous electrical stimulation of all the cells in the dish, we built a stimulation device consisting of a row of six platinum wires intercalated 1 cm apart with alternate polarity across a circular plastic holder that fit in the dish. The two terminals were connected to a stimulator unit with controls for the frequency, intensity, and duration of each pulse (9). Cells were stimulated with a tetanus protocol (45 Hz, 400 1-ms pulses), and extracellular aliquots were removed from 0 to 60 min post-stimulus. One dish/point was used to avoid ATP release for changes in extracellular volume. After samples were collected, cells from each plate were lysed, and proteins were quantified by a Coomassie Plus protein assay (Pierce). Data are expressed as pmol of extracellular nucleotide/mg of protein.
Nucleotide Separation by HPLC with Fluorescence Detection
Extracellular nucleotides were derivatized for sensitive quantitation of adenyl purines as fluorescent 1,N6-etheno species. Each extracellular sample (200 μl) was added over 100 μl of ice-cold phosphate-citrate buffer (77 mm Na2HPO4, 61 mm citric acid, pH 4) and maintained on ice until derivatization. After the addition of 10 μl of chloroacetaldehyde, samples were heated for 40 min at 80 °C as described elsewhere (48, 49). The reaction was stopped by being incubated on ice for 5 min. After samples were kept for 24 h at 4 °C, an automated Merck/Hitachi HPLC apparatus equipped with a fluorescence detector (VWR-Hitachi) was used for the identification and quantification of ethenylated species at excitation and emission wavelengths of 230 and 420 nm, respectively. Each sample (20 μl) was injected into a reverse-phase column (Chromolith® Performance RP-18e, 100-3 mm) equilibrated with the mobile phase (200 mm NaH2PO4·H2O, 200 mm Na2HPO4·2H2O, 5 mm tetrabutylammonium, adjusted to pH 6 using H3PO4) at 1.5 ml/min. Data from test samples were compared against known concentrations of derivatized adenosine, AMP, ADP, and ATP analyzed in parallel.
ATP Detection by Luciferin/Luciferase Assay
Fifty μl of extracellular samples was added to 20 μl of CellTiter-Glo® luminescent cell viability assay (Promega, Madison, WI). After a 10-min incubation in the dark, samples were quantified in a luminometer. In parallel, a standard curve from 1 fmol to 100 pmol of ATP was performed using the same kit. The lineal range was obtained from 100 fmol to 10 pmol of ATP. Sample readings were interpolated on a standard curve to detect ATP concentrations under each condition.
Myotube Stimulation with Conditioned Buffer
ATP activity released by electrical stimulation over calcium transients was assessed. Skeletal myotubes grown on 35-mm plates were used 5–7 days after seeding. The extracellular medium was replaced by 1 ml of Krebs solution 1 h before the assay. The whole plate was stimulated electrically with tetanus (45 Hz, 400 1-ms pulses); 0.7 ml of the extracellular buffer was removed 15 s later and placed over fluo3-loaded myotubes in 0.3 ml of buffer. Intracellular calcium transients were recorded as detailed previously.
Co-Immunoprecipitation Assay
Skeletal myotubes were solubilized for 1 h in 200 μl of lysis buffer (20 mm Tris-HCl, pH 7.8, 0.1% Nonidet P-40, 5 mm EDTA, 10 mm EGTA, 140 mm NaCl, 10% glycerol, and protease inhibitors). A 20-min, 15,000 × g supernatant fraction was incubated for 30 min with 10 μg of protein A/G-agarose as a preclearing strategy. The beads were spun down by centrifugation and washed three times with 200 μl of washing buffer (25 mm HEPES, pH 7.5, 0.2% Nonidet P-40, 140 mm NaCl, 0.1% bovine serum albumin, 10% glycerol, and protease inhibitors). After the preclearing step, whole cell extracts were incubated for 4 h with mouse anti-DHPR α-1 (1:400), rabbit anti-P2Y2 receptor (0.8 μg/ml), or rabbit anti-Pnx1 (1:200) antibodies and then incubated for 30 min with 50 μg of protein A/G-agarose beads. The beads pellet was washed three times with washing buffer. Proteins were resolved by SDS-PAGE in 7–10% gels, transferred to polyvinylidene difluoride filters, and blotted with the corresponding antibody.
Statistical Analysis
Data of n experiments were expressed as mean ± S.E. The significance of difference among treatments was evaluated using a t test for unpaired data or analysis of variance followed by Dunnett's post test for multiple comparisons. A p value of <0.05 was considered statistically significant.
RESULTS
mRNA for P2X and P2Y Receptor Subtypes Expressed in Skeletal Myotubes
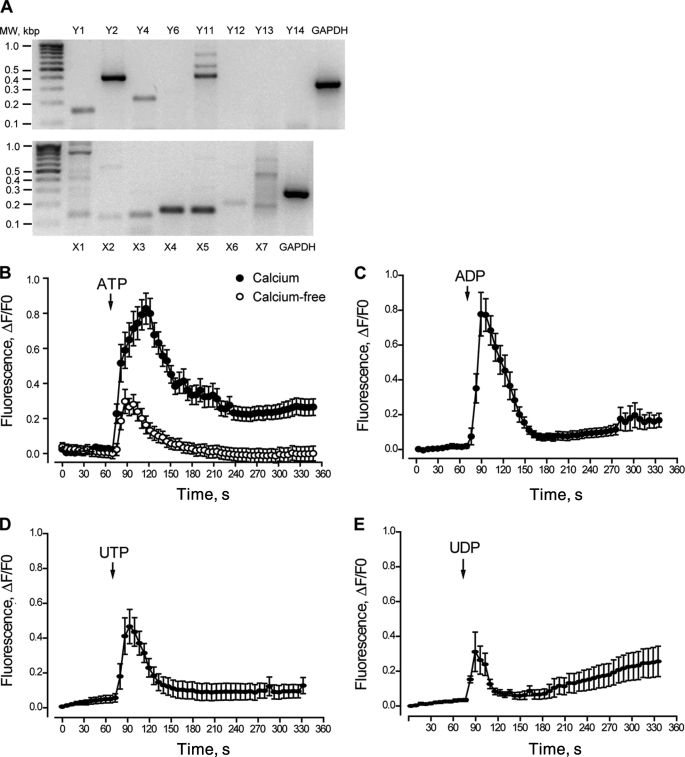
Screening was performed for all P2Y and P2X receptor subtypes. mRNA for several P2Y and for all P2X receptor subtypes was detected by RT-PCR in newborn rat skeletal myotubes (Fig. 1A). Strong bands for P2Y1, P2Y2, P2Y4, and P2Y11 receptors were detected in myotubes derived from newborn rat primary culture (Fig. 1A). Although a pale band was observed for the P2Y6 receptor, no mRNA detection was observed for P2Y12, P2Y13, or P2Y14 in our samples. When P2X mRNA expression was assessed, all subtypes were detected as selective bands of different intensities (Fig. 1A). Predominant bands for P2X4 and P2X5 receptors were observed. The mRNA for each P2X and P2Y receptor subtype was detected in whole rat brain as a control of method efficacy (not shown). As a positive control, GADPH mRNA was detected in all experiments.
FIGURE 1.
Molecular and pharmacological determination of P2Y/P2X receptors in rat skeletal myotubes. A, mRNA for several P2Y and P2X receptor subtypes is expressed in skeletal myotubes derived from newborn rat primary cultures. Total RNA was extracted from differentiated skeletal myotubes, and the mRNA for all the P2X/P2Y receptor subtypes was assessed by RT-PCR and detected in 1.5% agarose gels on the basis of their estimated molecular weight. A representative gel of three different RNA extractions is presented. B–E, natural agonists for P2Y/P2X receptors evoke calcium transients in skeletal myotubes. The effect of ATP, ADP, UTP and UDP (500 μm) over intracellular calcium changes was assessed in skeletal myotubes. A sequence of images was taken with the charge-coupled device camera attached to the epifluorescence microscope side port, which was equipped with the correct filters to capture fluo3-AM fluorescence to monitor the intracellular Ca+2 level. The analyzed regions of interest were from whole myotubes, considering both cytosolic and nuclear components. Stimuli were applied where indicated by an arrow and maintained throughout the recording period. Traces correspond to mean ± S.E. For each condition, 20–50 cells coming from 4–10 independent coverslips were quantified. In B, ATP was assessed either in regular Krebs buffer (solid circles, 1 mm Ca+2) or in calcium-free Krebs buffer (open circles, 0 Ca+2 plus 2 mm EGTA). All other nucleotides were assessed in regular Krebs buffer.
Given that primary cultures can contain not only muscle cells but also fibroblasts, we assessed P2Y and P2X receptor mRNA expression in skeletal myotubes derived from the C2C12 mouse cell line. In these cells, we detected all of the P2Y receptor subtypes, including P2Y12–14, which was absent in the primary cultures. All P2X receptor subtypes were also detected in these samples (not shown).
Exogenous Nucleotides Evoke Calcium Transients in Skeletal Myotubes
We tested exogenous nucleotide applications for the production of calcium transients in skeletal myotubes. 500 μm ATP, ADP, UTP, or UDP evoked intracellular Ca2+ increases in these cells, with different amplitudes and kinetics (Fig. 1, B–E). The relative intensity of the fluorescence peak obtained was ATP > ADP > UTP > UDP. ADP, UTP, or UDP, which activate only selective P2Y receptors, showed an initial Ca2+ increase that clearly returned to basal values in the first 60–100 s. In contrast, Ca2+ transients evoked by ATP, which activated both the P2X and P2Y receptors, showed an initial increase with a slow subsequent reduction that did not reach basal values and lasted at least 4.5 min after the stimulus started. This result suggests that the ATP-evoked Ca2+ transient is composed of two or more kinetic components. To distinguish between ATP effects over P2X or P2Y receptors, we assessed Ca2+ transients evoked by ATP in a calcium-free buffer. This maneuver removed P2X responses and saved P2Y-mediated events. Under this condition, we observed that the Ca2+ signal was smaller and came back to basal values, whereas the signal in the Ca2+-containing medium was larger and appeared to have more than one component (Fig. 1B). Considering the total area under the Ca2+ transients for the initial 100 s, nearly 20% of the ATP signal was still present in the calcium-free medium.
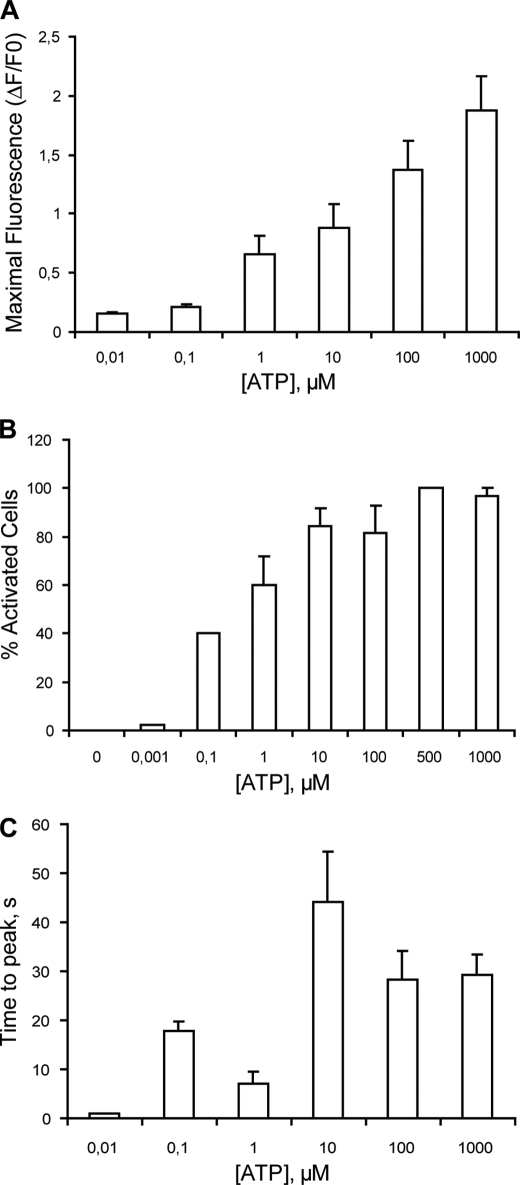
An ATP concentration-response curve was obtained for Ca2+ transients. Both maximal fluorescence and the percentage of activated cells increased in a concentration-dependent manner with ATP (Fig. 2, A and B). An analysis of the time to peak of Ca2+ transients evoked by different ATP concentrations rendered two maximal values, at 0.1 and 10 μm ATP, suggesting the presence of more than one type of ATP receptor (Fig. 2C).
FIGURE 2.
Quantitation and kinetics of calcium transients evoked by ATP in skeletal myotubes. ATP increases intracellular Ca2+ in a concentration-dependent manner in skeletal myotubes. Cells were incubated with 0.001 μm-1 mm ATP, and calcium transients were measured as described in the legend for Fig. 2. From the calcium signals we analyzed the maximal fluorescence reached (A), the percentage of activated cells (B), and the time to peak (C). Values were expressed as mean ± S.E. Each coverslip was used to assay only one ATP concentration. Each bar represents values obtained in 12–25 cells from three independent coverslips.
Disruption of the Extracellular ATP Pathway Strongly Inhibits Calcium Transients Evoked by Electrical Stimulation in Both Neonatal Rat Myotubes and Adult Muscle Fibers
As reported previously (9), a tetanic electrical stimulation (45 Hz, 400 1-ms pulses) elicits two distinguishable Ca2+ transients, a fast one related to excitation-contraction coupling and a slow one related to gene expression (Fig. 3, A–C).
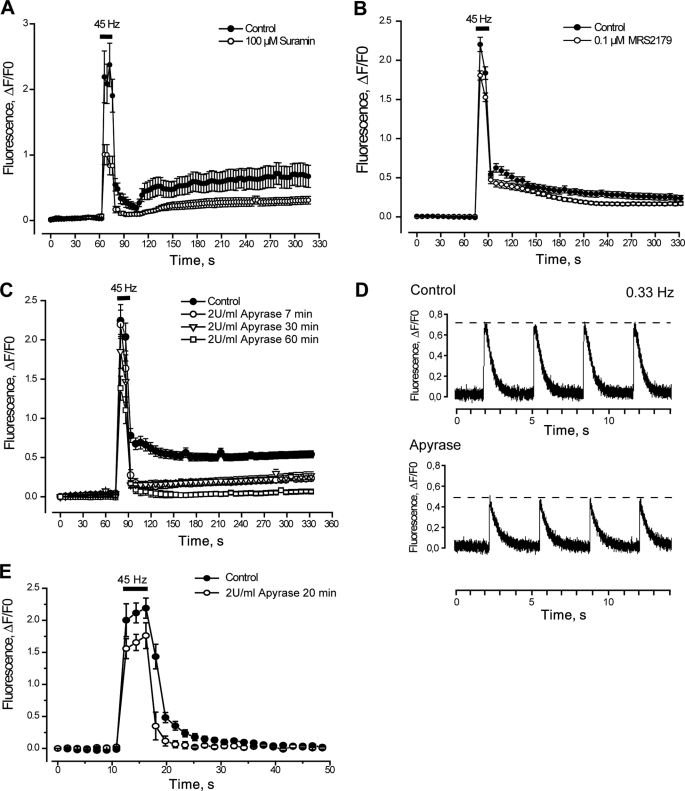
FIGURE 3.
Blockade of the P2Y/P2X receptor signaling reduces calcium transients evoked by tetanic electrical stimulation. A, fast and slow calcium transients evoked by tetanus (45 Hz, 400 1-ms pulses) are strongly reduced after general P2Y/P2X blockade using 100 μm suramin for 20 min prior to and during the protocol. A sequence of images shows fluo3 fluorescence to monitor the intracellular Ca+2 level (representative of n = 33–43 cells, five coverslips, three different cultures). B, a small reduction in calcium transients evoked by tetanus is observed when a specific P2Y1 receptor antagonist (MRS2179) was added for 20 min prior to and during the protocol (n = 107–149 cells, six coverslips, three different cultures). C, apyrase reduced in a time-dependent manner the calcium transients evoked by tetanus (n = 13–47 cells, four coverslips, three different cultures). A–C, experiments were performed using the epifluorescence microscope. For each coverslip, calcium transients evoked by tetanus before and after the inhibitor were tested. Values are expressed as mean ± S.E. D, to accurately detect changes in the fast calcium signal, we used a single pulse protocol (0.33 Hz, 1-ms pulses) and acquired fluorescence data every 1.9 ms by line scan confocal microscopy. As shown in the representative tracing, there was an evident decrease in the fast signal amplitude after 20 min of incubation with apyrase. E, isolated fibers from mouse FDB muscle loaded with fluo3-AM were stimulated with a couple of platinum electrodes with a tetanic protocol (45 Hz, 400 0.3-ms pulses). Fluorescence images were acquired in a confocal microscope every 1.8 s and analyzed frame by frame. Calcium transients evoked by tetanus were assessed before and after incubation with 2 units/ml apyrase for 20 min. Apyrase treatment evoked a significant reduction in both fast and slow calcium transients promoted by tetanus (n = 3 fibers, two different cultures). Values are expressed as mean ± S.E.
Blockade of Purinergic Receptors
We assessed the Ca2+ transients evoked by tetanic electrical stimulation in the presence of 100 μm suramin, a nonselective antagonist for all P2X and P2Y receptor subtypes, or 0.1 μm MRS2179, a selective blocker of the P2Y1 receptor subtype. Cells were incubated with blockers 20 min before and during the protocol. Suramin reduced by 50–60% both the fast and slow Ca2+ signals evoked by tetanic electrical stimulation (Fig. 3A and Table 2). MRS2179 reduced the fast Ca2+ signal by only 15% and the slow calcium signal evoked by tetanus by about 30% (Fig. 3B and Table 2). These results suggest that a combination of several P2X/P2Y receptor subtypes may mediate Ca2+ responses induced by electrical stimulation of skeletal muscle cells.
TABLE 2.
Effect of several blockers over maximal fast and slow calcium signal evoked by tetanic stimulation (45 Hz, 400 pulses, 1 ms each)
Data were analyzed using Dunnett's t test for one-tail comparison between several treatments and control. n, number of cells analyzed. Controls are derived from 17 different cultures. For treatments, cells correspond to 3–5 different cultures. NS, not significant.
| Blocker | Fast calcium signal | p value | Slow calcium signal | p value | n |
|---|---|---|---|---|---|
| % maximal value | % maximal value | ||||
| Control | 100.2 ± 3.4 | 100.1 ± 5.5 | 282 | ||
| 2 units/ml apyrase, 7 min | 98.7 ± 8.1 | NS | 21.8 ± 7.2 | <0.05 | 13 |
| 2 units/ml apyrase, 30 min | 83.1 ± 9.7 | NS | 22.1 ± 5.7 | <0.01 | 16 |
| 2 units/ml apyrase, 60 min | 62.2 ± 6.9 | <0.05 | 15.3 ± 6.5 | <0.01 | 20 |
| 100 μm suramin, 20 min | 47.5 ± 7.2 | <0.01 | 36.3 ± 8.1 | <0.01 | 43 |
| 0.1 μm MRS2179, 20 min | 82.8 ± 2.7 | <0.01 | 68.4 ± 7.2 | <0.01 | 139 |
| 100 μm oleamide, 20 min | 61.6 ± 10.2 | NS | 61.0 ± 25.9 | NS | 15 |
| 100 μm oleamide, 30 min | 35.8 ± 6.0 | <0.05 | 8.8 ± 3.2 | <0.05 | 17 |
| 100 μm anti-Pnx1, 20 min | 49.4 ± 7.7 | <0.01 | 53.8 ± 9.6 | <0.05 | 25 |
| 100 μm anti-Pnx1, 30 min | 42.0 ± 7.0 | <0.01 | 27.9 ± 7.0 | <0.01 | 24 |
Extracellular ATP Metabolization
Incubation of cells with apyrase, an enzyme that metabolizes extracellular ATP to AMP, strongly reduced Ca2+ transients evoked by electrical stimulation in a time-dependent manner. 7 min of incubation with apyrase reduced the initial component of the slow Ca2+ signal evoked by tetanus by 80%, with no significant changes over the fast Ca2+ signal (Fig. 3C and Table 2). Although the slow Ca2+ signal was completely abolished after 1 h of incubation with apyrase, the tetanus-evoked fast Ca2+signal was reduced only 40% (Fig. 3C and Table 2).
To better analyze the effects of apyrase over the fast Ca2+ component, assays were performed in skeletal myotubes using a line scan acquisition in a confocal microscope. This protocol allowed us to obtain images every 1.9 ms so as to resolve the fast Ca2+ signal (Fig. 3D). We used a stimulation frequency of 0.33 Hz, described as a promoter of only the fast but not the slow calcium signals, in our system (9). Under these conditions, a 20-min treatment with apyrase decreased the amplitude of electrical stimulation-evoked calcium signals from 0.63 ± 0.02 to 0.52 ± 0.03 arbitrary units (p < 0.01, n = 99–106 cells). Moreover, when the decaying phase of calcium transients was fit by a first-order exponential function, the exponential time constants were modified from 462.18 ± 16.08 to 377.32 ± 14.98 ms after apyrase treatment (p < 0.001, n = 99–106 cells). The rise time of the calcium signal was not altered after apyrase incubation. Representative traces of these protocols are shown (Fig. 3D). Then, without extracellular ATP and ADP, the tetanus-evoked calcium signals, which were smaller in magnitude, returned faster to basal levels. These results suggest that extracellular ATP has a role in promoting both fast and slow calcium signals evoked by tetanic electrical stimulation.
To assess the role of extracellular nucleotides in an adult skeletal model, we isolated skeletal fibers from mouse FDB and tested the effect of apyrase on calcium transients evoked by tetanus (45 Hz, 400 0.3-ms pulses). In this model, tetanus-evoked calcium signals are somehow different than those found in newborn-derived skeletal myotubes. We observed the corresponding fast calcium transient during tetanus stimulation followed by a slow return to basal values lasting 15–20 s (Fig. 3E, closed circles). This “foot-like” component has been related to the slow calcium transient of myotubes.3 Extracellular ATP degradation by apyrase treatment reduced by 20% the fast calcium signal evoked by tetanus in skeletal fibers (Fig. 3E, open circles). The slow calcium signal evoked by tetanus in this system was also much reduced after 20 min of apyrase incubation, reaching basal values less than 5 s after tetanus stimulation ended (Fig. 3E, open circles). Thus, as in the primary cultured myotubes, extracellular ATP is an important mediator for calcium transients evoked by electrical stimulation in adult skeletal fibers.
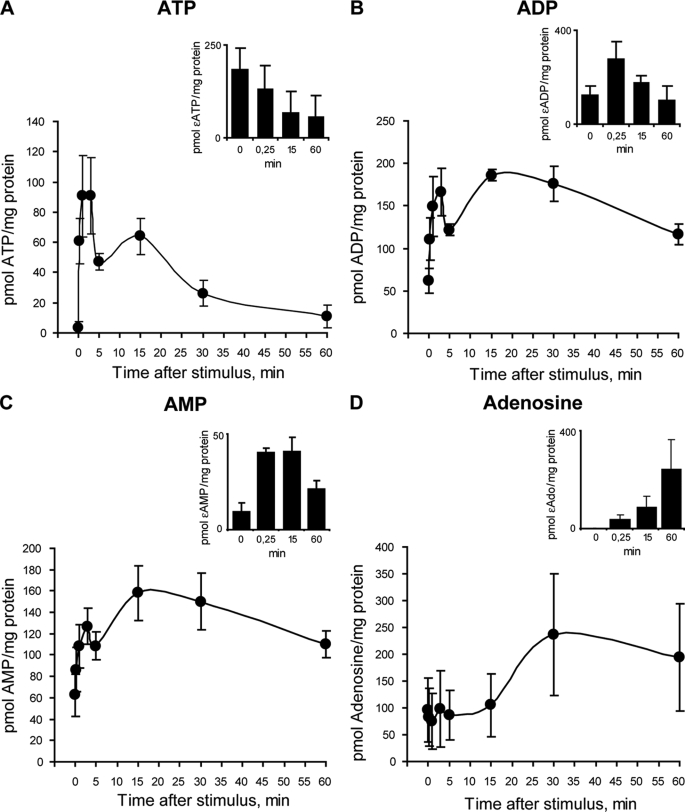
ATP and Its Metabolites Increased in the Extracellular Medium after Tetanic Stimulation or K+ Depolarization of Skeletal Myotubes
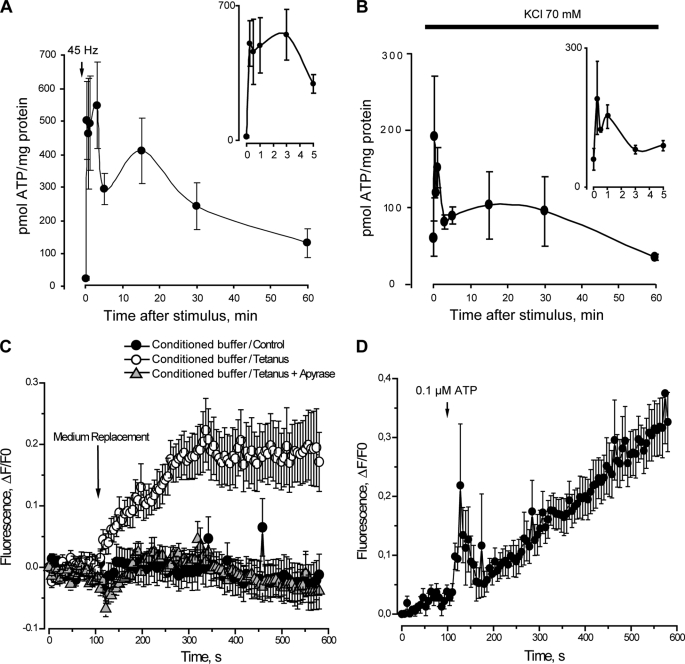
The measurement of ATP release after electrical stimulation using the luciferin/luciferase assay showed two peaks. At 15 s after the stimulus there was a 25-fold increase in extracellular ATP (Fig. 4A). A second peak of extracellular ATP was observed 15 min after the tetanus, which sequentially tended to return to basal levels between 30 and 60 min after the stimulus (Fig. 4A). Measuring both the extracellular ATP and the intracellular ATP content, we detected that in the resting condition extracellular ATP constitutes 0.48 ± 0.05% of the total ATP and increases to 9.2 ± 2.4% 15 s after tetanic electrical stimulation.
FIGURE 4.
ATP release from skeletal myotubes after titanic electrical stimulation or K+-evoked depolarization. A, skeletal myotubes were depolarized by electrical stimulation (45 Hz, 400 1-ms pulses). Aliquots of the extracellular medium were removed at the indicated times after the stimulus. ATP at the samples was measured by luciferin/luciferase assay and quantitated using a calibration standard curve. B, skeletal myotubes were depolarized by 70 mm KCl, and extracellular ATP changes were evaluated as in A. Values are expressed as mean ± S.E. In A and B, enlargements of the x axis during the first 5 min are shown in the insets. C, conditioned saline from electrically stimulated myotubes evokes calcium transients in fluo3-loaded myotubes. Extracellular medium derived from nonstimulated myotubes (control) or from myotubes stimulated electrically (45 Hz, 400 1-ms pulses) in the presence and absence of apyrase (2 units/ml) was added to fluo3-loaded myotubes as described under “Experimental Procedures.” Changes in fluorescence were continually recorded by epifluorescence microscopy as described under “Experimental Procedures.” D, calcium transient elicited by the addition of 0.1 μm ATP is shown for comparison.
To assess a different depolarization stimulus, we measured ATP released under high potassium conditions (70 mm KCl). Extracellular ATP quickly increased at 15 s after the KCl stimulus, with a second peak at 15 min such as occurred with electrical depolarization (Fig. 4B). When analyzing the first 5 min of ATP release induced by electrical stimulation or K+ depolarization, a very fast increase was evidenced, with maximal increments at 15 s (Fig. 4, A and B, insets).
We assessed the role of ATP released after electrical stimulation over calcium transients in skeletal myotubes. We stimulated fluo3-loaded myotubes with a conditioned buffer derived from the extracellular medium of tetanus-activated myotubes. In this condition, intracellular calcium increased significantly (Fig. 4C). No changes in intracellular calcium were observed when conditioned buffer derived from nonstimulated cells or cells stimulated with tetanus in the presence of apyrase were used (Fig. 4C). Considering previous measurements of ATP release taken 15 s after tetanus stimulation, the final ATP concentration in the fluo3-loaded cells was 0.10 ± 0.02 μm (n = 8), which is at the lower limit of concentrations able to elicit calcium signals showing both fast and slow kinetics (Fig. 4D). This result reinforces the role of ATP and its metabolites as extracellular signaling molecules during muscle activity.
To quantify in parallel the ATP and metabolites (ADP, AMP, and adenosine) in the extracellular medium after electrical stimulation, we used HPLC separation coupled with fluorescence detection. Just 30 s after the stimuli, extracellular ATP was increased significantly, reaching a 30-fold maximal increment 1 min later. Extracellular ATP returned to basal levels 60 min after tetanus stimulation (Fig. 5A). Extracellular ADP and AMP reached 2.7- and 2-fold increases at 3 min after electrical stimulation (Fig. 5, B and C). Extracellular adenosine remained at basal levels until 15 min after tetanus stimulation, increasing 2.5-fold at 30–60 min (Fig. 5D). These results are congruent with ATP release evoked by electrical stimulation followed by extracellular metabolization to render sequentially ADP, AMP, and adenosine. It is very interesting to note that ATP, ADP, and AMP showed two peaks of extracellular increments over time, the first at 1–3 min and the second at 15 min after tetanic electrical stimulation (Fig. 5, A–C).
FIGURE 5.
Nucleotides release and metabolization after tetanic stimulation. A–D, to measure ATP and its metabolites at the extracellular medium, skeletal myotubes were stimulated electrically with a tetanus protocol (45 Hz, 400 1-ms pulses), and aliquots of the medium were removed at the indicated times thereafter. Nucleotide samples (ATP, ADP, AMP, and adenosine) were derivatized as described under “Experimental Procedures,” resolved and detected using an HPLC coupled to fluorescence detection, and quantitated using a calibration standard curve. Values are the mean ± S.E. (n = 6–8 series from six independent primary cultures). To assess the ectonucleotidases activities of skeletal myotubes, a standard of ϵ-ATP was applied to the extracellular medium. Insets, at different times, extracellular aliquots were removed to quantify ϵ-ATP (A), ϵ-ADP (B), ϵ-AMP (C), and ϵ-adenosine (D) by HPLC as an indicator of the ATP metabolism ability of these cells. Values are the mean ± S.E. (n = 3–5 series from three independent primary cultures).
To study the ectonucleotidase activities in our system, we assessed the ability of skeletal myotubes to metabolize an etheno-ATP standard (ϵ-ATP) applied in the extracellular buffer solution. The use of an ϵ-ATP standard makes the measurement independent of endogenously released nucleotides. At different times, aliquots were removed and processed to measure ϵ-ATP or its derivatives (ϵ-ADP, ϵ-AMP, and ϵ-adenosine) by HPLC. A significant reduction in ϵ-ATP was detected from 15 s to 60 min, with sequential increases in ϵ-ADP, ϵ-AMP, and ϵ-adenosine levels (Fig. 5, insets).
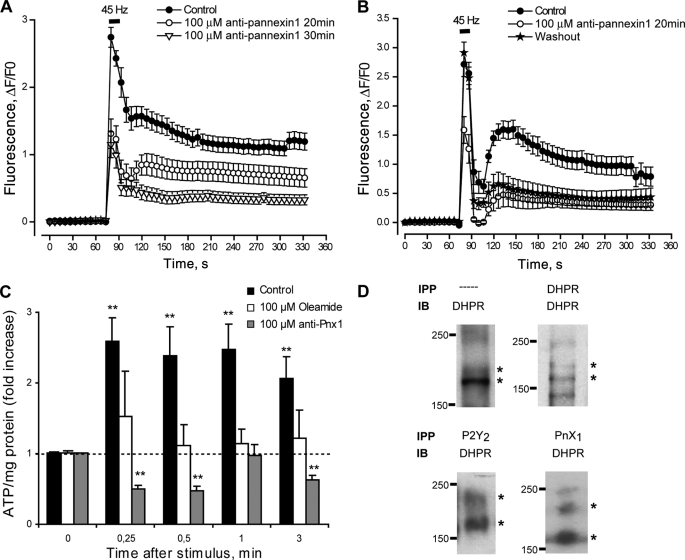
Blockade of ATP Release through Pannexin Hemichannels Reduces Calcium Transients Evoked by Electrical Stimulation
ATP release to the extracellular medium through connexin or pannexin hemichannels has been established in different systems (22, 23, 50, 51). The nonselective blockade of hemichannels using 100 μm oleamide applied 30 min before and during the protocol reduced by 65 and 90%, respectively, the fast and slow calcium signals evoked by tetanus (Table 2). Considering that adult skeletal muscles do not form gap junctions and express connexin subunits poorly (10, 52–55), we addressed the possibility that pannexin hemichannels were the route for ATP release in our system. Incubation with the anti-pannexin-1 peptide 10panx1 (100 μm) 20–30 min before and during the protocol strongly reduced both the fast and slow calcium signals evoked by tetanus (Fig. 6A and Table 2). Interestingly, washout of the peptide for up to 60 min totally restored the fast but not the slow calcium signals evoked by tetanus (Fig. 6B). Incubation with either oleamide or 10panx1 totally blocked the ATP release after tetanic stimulation (Fig. 6C).
FIGURE 6.
Pannexin-1 hemichannels are involved in ATP release during tetanic stimulation of skeletal myotubes. A, fast and slow calcium components evoked by tetanic electrical stimulation (45 Hz, 400 1-ms pulses) are strongly reduced after pannexin-1 hemichannel blockade using 100 μm of the 10pnx1 peptide 20–30 min prior to and during the protocol (n = 25–79 cells, five coverslips, five different cultures). For each coverslip, calcium transients evoked by tetanus before and after incubation with the peptide were tested. B, the same protocol as described in A was achieved, but after 10pnx1 peptide incubation cells were washed for 60 min, we reassessed the effect of the tetanus over calcium transients. Values are expressed as mean ± S.E. (n = 19–23 cells, two coverslips, two different cultures). C, ATP release evoked by tetanic electrical stimulation was abolished by 100 μm oleamide (a nonselective blocker for connexin and pannexin hemichannels) or 100 μm 10pnx1 peptide. Skeletal myotubes were incubated 20 min before and during the assay with the indicated blocker. Aliquots of the extracellular medium were removed at the indicated times after electrical stimulation, and ATP was measured by a luciferin/luciferase assay as described under “Experimental Procedures.” Values were normalized to 0 control for each treatment and expressed as the mean ± S.E. (n = 4 series from four independent primary cultures). **, p < 0.01, Dunnett's t test, one-tail comparison between each time and their own 0 control. D, DHPR co-precipitates with P2Y2 receptor and pannexin-1 in skeletal myotubes. DHPR was resolved and detected by immunoblot (IB) in samples derived from whole lysates (—) or immunoprecipitated (IPP) previously with anti-DHPR, anti-P2Y2, or anti-Pnx1 as described under “Experimental Procedures.”
DHPR Co-immunoprecipitates with Pannexin-1 and P2Y2 Nucleotide Receptor
Taking into account the results and considering the possibility of a multiprotein complex in skeletal myotubes, we assessed the co-immunoprecipitation among DHPR (voltage sensor), pannexin-1 (ATP-releasing hemichannel), and the P2Y2 metabotropic receptor, chosen because of its strong detection by RT-PCR in our system. By immunoblot of total cell extracts we detected the two reactive bands described previously for DHPR (56–58) between 150 and 220 kDa (Fig. 6D). A weaker, additional band at 250 kDa also was detected frequently. We also detected DHPR by immunoblot in samples derived from DHPR, pannexin-1, and P2Y2 receptor immunoprecipitation (Fig. 6D). This result raises the possibility of a putative protein complex among these three components in the membrane of skeletal myotubes to integrate depolarization with associated fast and slow calcium responses.
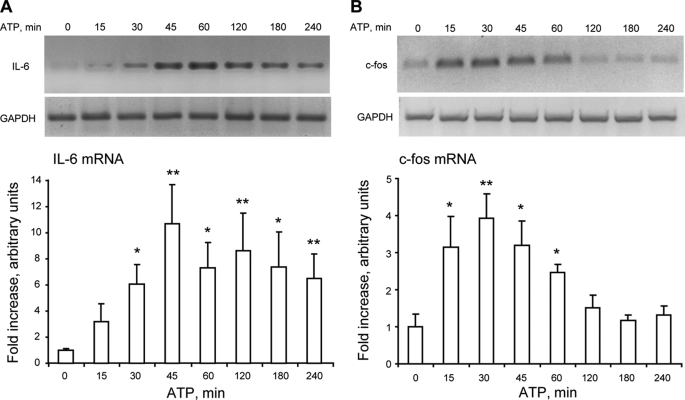
Exogenous ATP Promotes Gene Expression in Skeletal Myotubes
The stimulation of myotubes with 500 μm ATP significantly increased the mRNA for IL-6 (30–240 min, Fig. 7A) and c-fos (15–60 min; Fig. 7B) as detected by semiquantitative RT-PCR. Although there was some variability in the fold over basal increase in these results, in all experiments there was a clear mRNA induction after the addition of ATP.
FIGURE 7.
Exogenous ATP increases both IL-6 and c-fos mRNA in skeletal myotubes. Myotubes were incubated with 500 μm ATP for the times indicated. Total RNA was isolated, and IL-6 (A) or c-fos (B) mRNA levels were analyzed by semiquantitative RT-PCR. The top panels are representative agarose gels for RT-PCR products from IL-6 (A) and c-fos (B) mRNA amplifications with their corresponding GAPDH controls. The bottom panels correspond to results normalized to GAPDH expression and presented as -fold increase of untreated control cells (mean ± S.E. n = 3–8). *, p < 0.05; **, p < 0.01; analyzed by analysis of variance followed by Dunnett's multiple comparison test.
DISCUSSION
In the present work we have demonstrated that nucleotides released to the extracellular medium are the essential mediators in promoting slow calcium signals and can significantly affect fast calcium transients evoked by tetanic stimulation in skeletal muscle cells. As slow calcium signals have been related to the regulation of gene expression (5, 11, 12), our data place extracellular nucleotides as potential modulators of physiological processes such as skeletal muscle activity and plasticity.
Several reports have described the short and long term effects of exogenous nucleotides in skeletal muscles derived from different species (human, rat, mouse, chicken, and frog); calcium mobilization, ERK activation, and gene expression regulation have been observed after skeletal cells incubation with P2X/P2Y receptor agonists (39, 40, 59–64). On the other hand, ATP is released from skeletal muscle cells in response to muscle contraction (34, 35). Previous data from our laboratory indicate that tetanic electrical stimulation evokes a fast calcium signal linked to excitation-contraction coupling and a slow calcium signal related to gene expression control (5, 8, 9). In the present work we focused on the study of extracellular nucleotides released after tetanic stimulation and their putative role as promoters of calcium signals normally related to membrane depolarization.
We detected mRNA expression for several P2Y and all P2X receptor subtypes in skeletal myotubes derived from rat primary cultures. Although the protein expression was established only for the P2Y2 receptor, the functional data and pharmacology are consistent with the presence of multiple receptors for extracellular nucleotides and suggest that these receptors should be relevant for muscle physiology. Future work should unveil the role of particular nucleotide receptor subtypes in both skeletal myotubes and adult muscle fibers.
The exogenous addition of natural P2Y/P2X receptor agonists evokes calcium transients in skeletal myotubes. The ATP-evoked calcium transient is composed of two or more kinetic components; this is in accordance with reported data showing that activation of ionotropic P2X4, P2X5, and P2X7 and metabotropic P2Y1 and P2Y4 purinoreceptors participates in the ATP-evoked calcium transients of multinucleated mice myotubes (39). The biphasic kinetics of the calcium signal evoked by ATP has been described also in human and mice cultured myotubes (65). In those models, ATP-evoked calcium transients have an early, fast component followed by a second, more gradual increase in [Ca2+] (65). After removal of extracellular calcium, the ATP-evoked calcium signal is reduced in amplitude; the slope of the fast initial component decreases and comes back to basal values, suggesting a strong role for P2X receptors on these transients. The remaining 20% calcium response in the absence of extracellular calcium could be attributed to P2Y receptors.
Treatments focused on disrupting the purinergic pathway, which reduced the fast calcium signal by less than 60% while almost completely abolishing the slow calcium component. A complete blockade of the fast signal was not expected, considering that mechanisms for depolarization-evoked fast calcium signals in myotubes are well known; they depend on RyRs and are related to excitation-contraction coupling (1, 3, 4). In this context, nucleotides could only contribute toward modulation of this pathway. It is worth speculating that ATP extrusion after tetanic stimulation could contribute to supplementing the calcium needed to maintain contraction. Excitation-coupled calcium entry is a well known process in myotubes, and it has been linked to DHPR activity (66). Although important in myotubes, the contribution of purinergic receptors to the fast calcium transient appears to be less prominent in adult fibers. Only a 20% reduction of this transient was evident in these cells after a 20-min apyrase treatment (Fig. 3E). In contrast, the extracellular nucleotide pathway appears to be essential for the development of slow calcium signals generated by electrical depolarization in skeletal myotubes and adult fibers. MRS2179, a selective blocker of P2Y1 receptor subtype, slightly but significantly reduced the two calcium components evoked by tetanus. The effect was smaller than that observed with suramin, suggesting an orchestrated effect of several nucleotide receptor subtypes in depolarization-evoked calcium transients. The lack of selective antagonists makes the identification of P2X/P2Y receptor subtypes involved in this process difficult. The enzymatic degradation of endogenous extracellular nucleotides using apyrase confirms the requirement for these mediators in the maintenance of a significant fraction of the fast calcium signal and their fundamental role in the slow calcium component evoked by tetanic electrical stimulation. The accurate analysis of the effect of apyrase over the fast calcium transient evoked by tetanus using line scan confocal microscopy shows that in the absence of extracellular ATP/ADP, this signal has a 17% reduction in amplitude and decays 20% faster than in control conditions. Nevertheless, a relatively long incubation time is needed with apyrase to reduce the fast calcium transient, which may mean that its effect at this level is more complex.
Changes in skeletal muscle sensitivity to ATP have been described in rat, mouse, and chick during development. Both ATP-evoked calcium transients and muscle contraction decrease with postnatal development and are completely absent in adult skeletal muscle fibers (30, 65, 67, 68). To validate our model in an adult preparation, we assessed the apyrase effect over calcium transients evoked by tetanic stimulation in rat adult fibers. Apyrase reduced by 20% the depolarization-evoked fast calcium transient associated with excitation-contraction coupling, whereas it totally abolished the slow transient. Although a reduction in ATP sensitivity has been described for adult skeletal models (30, 65, 67, 68), which led the authors to propose a role for ATP in muscle development, it does not preclude a role for extracellular nucleotides in adult muscle function. It will be important to study the role of P2X, P2Y, and adenosine P1 receptors during development.
ATP release has been demonstrated in vivo in contracting rat and cat skeletal muscles by collecting samples from microdialysis probes inserted in the muscle (34, 35) or ex vivo after a 10-Hz stimulation of mouse hemidiaphragm (69). Enzyme pathways for extracellular ATP metabolization have been also demonstrated in skeletal muscles (70–72). In this work, we determined that ATP is quickly released from skeletal myotubes after tetanic electrical stimulation or K+ depolarization. In all previous works ATP has been measured minutes after the electrical stimulation (32, 34, 35, 69); we detected an initial peak of ATP release between 15 s and 3 min after tetanus stimulation. The size and shape of slow calcium transients elicited by the conditioned media of electrically stimulated myotubes is compatible with that of ATP-elicited transients in a bulk concentration range of 0.1 to 1 mm. We have to consider that the local ATP concentration near the membrane during release must be much higher than that measured in the bulk medium (19).
Extracellular nucleotides reach up to millimolar concentration after cell damage or under nonlytic stimuli conditions such as hypoxia, inflammation, or cell swelling (19, 73–80). In excitable cells such as neurons, chromaffin cells, and platelets, ATP is stored in vesicles and released by calcium-dependent exocytosis (81–83). In non-excitable cells, several mechanisms for ATP release have been proposed: 1) through ABC transporters (84, 85); 2) through stretch- or voltage-activated channels (86–92); 3) through connexin or pannexin hemichannels (19–23). Mechanisms for ATP release from skeletal muscle cells have not been studied. Considering that pannexin hemichannels have emerged as conduits for ATP release in several cellular systems and that they are activated by membrane depolarization, mechanical stress, or intracellular calcium (22, 93), we analyzed their possible role in our system. The expression of pannexin-1 mRNA in adult skeletal muscle has been reported, and pannexin-1 hemichannels have been located in T-tubules of adult skeletal muscle.4 Here, we have gathered evidence suggesting that pannexin-1 hemichannels are essential for both ATP release and tetanus-evoked calcium transients in skeletal myotubes. Recently, functional units by physical association between P2 receptors and hemichannel proteins have been proposed (94). Using transcriptomic strategies, several associations have been suggested; co-immunoprecipitation assays have validated an interaction among P2X7 receptors, pannexin-1, connexin-43, actin, and vinculin in whole brain and cultured astrocytes (94). A supramolecular complex including P2Y1 receptors, connexin-43, actin, and vinculin in cultured astrocytes has also been postulated (94). In agreement with these data, in our model we have shown the co-immunoprecipitation of either pannexin-1 or P2Y2 receptor with DHPR, opening the possibility that such supramolecular complexes could be modulating calcium changes evoked by muscle activity. We first analyzed the interaction with P2Y2 receptors because of their strong mRNA expression in our system; however, other P2X/P2Y receptor subtypes could also be tested as putative components of this complex under resting or stimulated conditions.
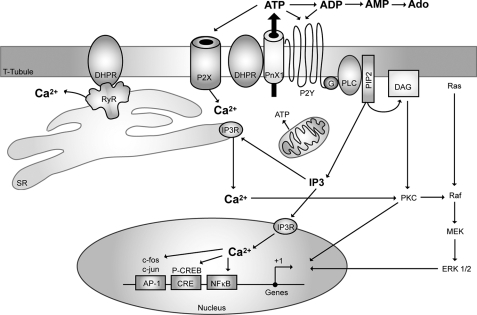
As demonstrated previously for membrane depolarization (5, 11–13), the addition of ATP was able to stimulate mRNA expression for an immediate early gene such as c-fos and for an exercise-induced gene such as IL-6; this reinforces the idea of ATP being the intermediate step between electrical stimulation and intracellular pathways leading to gene expression. The precise time course of events that account for depolarization sensing, ATP release, activation of nucleotide receptors, and calcium movements in skeletal muscle cells still remains to be established, and further work is needed to unveil the mechanisms and characterize the molecular complex that is beginning to emerge and appears to be involved in activity-modulated muscle plasticity. A hypothetical model is depicted in Fig. 8. In this model, membrane depolarization is proposed to activate Cav1.1 dihydropyridine receptors, and its conformational change will in turn activate pannexin-1 channels to release ATP.
FIGURE 8.
Hypothetical schematic model of the proposed role of purinergic receptors in both excitation-contraction and excitation-transcription coupling in skeletal muscle cells. A multimeric protein complex is suggested to exist in the T-tubule membrane, including the dihydropyridine receptor (Cav1.1, DHPR), the purinergic metabotropic P2Y2 receptor, and the pannexin-1 molecule (PnX1). Membrane depolarization will induce the opening of an ATP pathway via PnX1 after a conformational change of the adjacent DHPR. A heterotrimeric G protein will be attached to the P2Y2 receptor, and upon ATP binding, the βγ subunit will sequentially activate phosphoinositide 3-kinase (not shown) and phospholipase C (PLC) to produce IP3 and activate calcium release for the slow calcium transient. Other proteins such as RyR, known to interact with DHPR and P2X receptors (ionotropic, calcium-permeating channel), participate in the fast calcium transient, but whether they are part of the same complex has not yet been evidenced.
Although a direct role for extracellular ATP has not yet been demonstrated in skeletal muscle pathologies, a mild degenerative muscle disease, sarcoglycanopathy, has been associated with the loss of an ecto-ATPase on the surface of the skeletal muscle fibers (70, 95). Furthermore, the altered expression of a human P2X receptor gene in certain degenerative or hyperproliferative muscle disorders has been suggested (96). An increased sensitivity to ATP in dystrophic myotubes has been also established (97). Considering that our data place extracellular nucleotides and their surface receptors as essential mediators between muscle activity and plasticity, it is possible that deregulation of any of the components of this pathway may account for a muscle pathology.
Acknowledgment
We thank Mónica Silva for cell cultures.
This work was supported by Grants from the Fondo de Investigación Avanzada en Areas Prioritarias (Chile) 15010006, and the Fondo Nacional de Desarrollo Cientifico y Tecnológico (Chile) 1080120, FONDAP 13980001, and FONDECYT 3080016.
M. Casas, R. Figueroa, G. Jorquera, M. Escobar, J. Molgó, and E. Jaimovich, manuscript in preparation.
M. A. Riquelme, L. Cea, M. V. L. Bennett, and J. C. Sáez, unpublished observation.
- DHPR
- dihydropyridine receptor
- ϵ-ATP
- etheno-ATP
- ERK
- extracellular signal-regulated kinase
- FDB
- flexor digitorum brevis
- fluo3-AM
- fluo3-acetoxymethylester
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GPCR
- G protein-coupled receptor
- HPLC
- high pressure liquid chromatography
- IL
- interleukin
- IP3
- 1,4,5-inositol trisphosphate
- RT
- reverse transcription
- RyR
- ryanodine receptor
- T
- transverse
- Pnx
- pannexin.
REFERENCES
- 1.Grabner M., Dirksen R. T., Suda N., Beam K. G. (1999) J. Biol. Chem. 274, 21913–21919 [DOI] [PubMed] [Google Scholar]
- 2.Lamb G. D. (2002) Front. Biosci. 7, d834–d842 [DOI] [PubMed] [Google Scholar]
- 3.Protasi F., Paolini C., Nakai J., Beam K. G., Franzini-Armstrong C., Allen P. D. (2002) Biophys. J. 83, 3230–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanabe T., Beam K. G., Adams B. A., Niidome T., Numa S. (1990) Nature 346, 567–569 [DOI] [PubMed] [Google Scholar]
- 5.Carrasco M. A., Riveros N., Ríos J., Müller M., Torres F., Pineda J., Lantadilla S., Jaimovich E. (2003) Am. J. Physiol. Cell Physiol. 284, C1438–C1447 [DOI] [PubMed] [Google Scholar]
- 6.Jaimovich E., Carrasco M. A. (2002) Biol. Res. 35, 195–202 [DOI] [PubMed] [Google Scholar]
- 7.Semsarian C., Wu M. J., Ju Y. K., Marciniec T., Yeoh T., Allen D. G., Harvey R. P., Graham R. M. (1999) Nature 400, 576–581 [DOI] [PubMed] [Google Scholar]
- 8.Eltit J. M., García A. A., Hidalgo J., Liberona J. L., Chiong M., Lavandero S., Maldonado E., Jaimovich E. (2006) J. Biol. Chem. 281, 12143–12154 [DOI] [PubMed] [Google Scholar]
- 9.Eltit J. M., Hidalgo J., Liberona J. L., Jaimovich E. (2004) Biophys. J. 86, 3042–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araya R., Eckardt D., Riquelme M. A., Willecke K., Sáez J. C. (2003) Cell Commun. Adhes. 10, 451–456 [DOI] [PubMed] [Google Scholar]
- 11.Juretic N., García-Huidobro P., Iturrieta J. A., Jaimovich E., Riveros N. (2006) Am. J. Physiol. Cell Physiol. 290, C1428–C1436 [DOI] [PubMed] [Google Scholar]
- 12.Juretic N., Urzúa U., Munroe D. J., Jaimovich E., Riveros N. (2007) J. Cell. Physiol. 210, 819–830 [DOI] [PubMed] [Google Scholar]
- 13.Valdés J. A., Hidalgo J., Galaz J. L., Puentes N., Silva M., Jaimovich E., Carrasco M. A. (2007) Am. J. Physiol. Cell Physiol. 292, C1960–C1970 [DOI] [PubMed] [Google Scholar]
- 14.De Waard M., Hering J., Weiss N., Feltz A. (2005) Trends Pharmacol. Sci. 26, 427–436 [DOI] [PubMed] [Google Scholar]
- 15.Herlitze S., Garcia D. E., Mackie K., Hille B., Scheuer T., Catterall W. A. (1996) Nature 380, 258–262 [DOI] [PubMed] [Google Scholar]
- 16.Ikeda S. R. (1996) Nature 380, 255–258 [DOI] [PubMed] [Google Scholar]
- 17.Silinsky E. M. (1975) J. Physiol. 247, 145–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Kügelgen I., Starke K. (1985) J. Physiol. 367, 435–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarowski E. R., Boucher R. C., Harden T. K. (2003) Mol. Pharmacol. 64, 785–795 [DOI] [PubMed] [Google Scholar]
- 20.Schwiebert E. M., Zsembery A. (2003) Biochim. Biophys. Acta 1615, 7–32 [DOI] [PubMed] [Google Scholar]
- 21.Leybaert L., Braet K., Vandamme W., Cabooter L., Martin P. E., Evans W. H. (2003) Cell Commun. Adhes. 10, 251–257 [DOI] [PubMed] [Google Scholar]
- 22.Bao L., Locovei S., Dahl G. (2004) FEBS Lett. 572, 65–68 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y. J., Maruyama Y., Dvoryanchikov G., Pereira E., Chaudhari N., Roper S. D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6436–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann H. (1999) Trends Pharmacol. Sci. 20, 231–236 [DOI] [PubMed] [Google Scholar]
- 25.Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Knight G. E., Fumagalli M., Gachet C., Jacobson K. A., Weisman G. A. (2006) Pharmacol. Rev. 58, 281–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burnstock G. (2006) Pharmacol. Ther. 110, 433–454 [DOI] [PubMed] [Google Scholar]
- 27.Buvinic S., Briones R., Huidobro-Toro J. P. (2002) Br. J. Pharmacol. 136, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buvinic S., Poblete M. I., Donoso M. V., Delpiano A. M., Briones R., Miranda R., Huidobro-Toro J. P. (2006) J. Physiol. 573, 427–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North R. A. (2002) Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 30.Wells D. G., Zawisa M. J., Hume R. I. (1995) Dev. Biol. 172, 585–590 [DOI] [PubMed] [Google Scholar]
- 31.Ryten M., Dunn P. M., Neary J. T., Burnstock G. (2002) J. Cell Biol. 158, 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunha R. A., Sebastião A. M. (1993) Pflugers Arch. 424, 503–510 [DOI] [PubMed] [Google Scholar]
- 33.Araya R., Riquelme M. A., Brandan E., Sáez J. C. (2004) Brain Res. Brain Res. Rev. 47, 174–188 [DOI] [PubMed] [Google Scholar]
- 34.Li J., King N. C., Sinoway L. I. (2003) J. Appl. Physiol. 95, 577–583 [DOI] [PubMed] [Google Scholar]
- 35.Li J., King N. C., Sinoway L. I. (2005) Circulation 111, 2748–2751 [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Pinna J., Gurung I. S., Vial C., Leon C., Gachet C., Evans R. J., Mahaut-Smith M. P. (2005) J. Biol. Chem. 280, 1490–1498 [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Pinna J., Tolhurst G., Gurung I. S., Vandenberg J. I., Mahaut-Smith M. P. (2004) J. Physiol. 555, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanfield P. (2006) Nat. Cell Biol. 8, 1323–1325 [DOI] [PubMed] [Google Scholar]
- 39.Deli T., Szappanos H., Szigeti G. P., Cseri J., Kovács L., Csernoch L. (2007) Pflugers Arch. 453, 519–529 [DOI] [PubMed] [Google Scholar]
- 40.May C., Weigl L., Karel A., Hohenegger M. (2006) Biochem. Pharmacol. 71, 1497–1509 [DOI] [PubMed] [Google Scholar]
- 41.Jaimovich E., Reyes R., Liberona J. L., Powell J. A. (2000) Am. J. Physiol. Cell Physiol. 278, C998–C1010 [DOI] [PubMed] [Google Scholar]
- 42.Chomczynski P., Sacchi N. (1987) Anal. Biochem. 162, 156–159 [DOI] [PubMed] [Google Scholar]
- 43.Communi D., Gonzalez N. S., Detheux M., Brézillon S., Lannoy V., Parmentier M., Boeynaems J. M. (2001) J. Biol. Chem. 276, 41479–41485 [DOI] [PubMed] [Google Scholar]
- 44.Rodrigues R. J., Almeida T., Richardson P. J., Oliveira C. R., Cunha R. A. (2005) J. Neurosci. 25, 6286–6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scrivens M., Dickenson J. M. (2006) Eur. J. Pharmacol. 543, 166–173 [DOI] [PubMed] [Google Scholar]
- 46.da Silva R. L., Resende R. R., Ulrich H. (2007) Exp. Physiol. 92, 139–145 [DOI] [PubMed] [Google Scholar]
- 47.Hayato R., Ohtubo Y., Yoshii K. (2007) J. Physiol. 584, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarowski E. R., Tarran R., Grubb B. R., van Heusden C. A., Okada S., Boucher R. C. (2004) J. Biol. Chem. 279, 36855–36864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todorov L. D., Mihaylova-Todorova S., Craviso G. L., Bjur R. A., Westfall D. P. (1996) J. Physiol. 496, 731–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reigada D., Lu W., Zhang M., Mitchell C. H. (2008) Neuroscience 157, 396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schenk U., Westendorf A. M., Radaelli E., Casati A., Ferro M., Fumagalli M., Verderio C., Buer J., Scanziani E., Grassi F. (2008) Sci. Signal. 1, ra6. [DOI] [PubMed] [Google Scholar]
- 52.Araya R., Eckardt D., Maxeiner S., Krüger O., Theis M., Willecke K., Sáez J. C. (2005) J. Cell Sci. 118, 27–37 [DOI] [PubMed] [Google Scholar]
- 53.Balogh S., Naus C. C., Merrifield P. A. (1993) Dev. Biol. 155, 351–360 [DOI] [PubMed] [Google Scholar]
- 54.Belluardo N., Trovato-Salinaro A., Mudò G., Condorelli D. F. (2005) Cell Tissue Res. 320, 299–310 [DOI] [PubMed] [Google Scholar]
- 55.von Maltzahn J., Wulf V., Willecke K. (2006) Cell Commun. Adhes. 13, 55–60 [DOI] [PubMed] [Google Scholar]
- 56.De Jongh K. S., Merrick D. K., Catterall W. A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 8585–8589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Jongh K. S., Warner C., Colvin A. A., Catterall W. A. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 10778–10782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai Y., Seagar M. J., Takahashi M., Catterall W. A. (1990) J. Biol. Chem. 265, 20839–20848 [PubMed] [Google Scholar]
- 59.Choi R. C., Man M. L., Ling K. K., Ip N. Y., Simon J., Barnard E. A., Tsim K. W. (2001) J. Neurosci. 21, 9224–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi R. C., Siow N. L., Cheng A. W., Ling K. K., Tung E. K., Simon J., Barnard E. A., Tsim K. W. (2003) J. Neurosci. 23, 4445–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deli T., Tóth B. I., Czifra G., Szappanos H., Bíró T., Csernoch L. (2006) J. Muscle Res. Cell Motil. 27, 617–630 [DOI] [PubMed] [Google Scholar]
- 62.Sandonà D., Danieli-Betto D., Germinario E., Biral D., Martinello T., Lioy A., Tarricone E., Gastaldello S., Betto R. (2005) FASEB J. 19, 1184–1186 [DOI] [PubMed] [Google Scholar]
- 63.Szigeti G. P., Szappanos H., Deli T., Cseri J., Kovács L., Csernoch L. (2007) Pflugers Arch. 453, 509–518 [DOI] [PubMed] [Google Scholar]
- 64.Tsim K. W., Barnard E. A. (2002) Neurosignals 11, 58–64 [DOI] [PubMed] [Google Scholar]
- 65.Cseri J., Szappanos H., Szigeti G. P., Csernátony Z., Kovács L., Csernoch L. (2002) Pflugers Arch. 443, 731–738 [DOI] [PubMed] [Google Scholar]
- 66.Bannister R. A., Pessah I. N., Beam K. G. (2009) J. Gen. Physiol. 133, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collet C., Strube C., Csernoch L., Mallouk N., Ojeda C., Allard B., Jacquemond V. (2002) Pflugers Arch. 443, 771–778 [DOI] [PubMed] [Google Scholar]
- 68.Ryten M., Hoebertz A., Burnstock G. (2001) Dev. Dyn. 221, 331–341 [DOI] [PubMed] [Google Scholar]
- 69.Vizi E. S., Nitahara K., Sato K., Sperlágh B. (2000) J. Auton. Nerv. Syst. 81, 278–284 [DOI] [PubMed] [Google Scholar]
- 70.Delgado J., Moro G., Saborido A., Megías A. (1997) Biochem. J. 327, 899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirley T. L. (1997) J. Biol. Chem. 272, 1076–1081 [DOI] [PubMed] [Google Scholar]
- 72.Megías A., Martínez-Senac M. M., Delgado J., Saborido A. (2001) Biochem. J. 353, 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eltzschig H. K., Eckle T., Mager A., Küper N., Karcher C., Weissmüller T., Boengler K., Schulz R., Robson S. C., Colgan S. P. (2006) Circ. Res. 99, 1100–1108 [DOI] [PubMed] [Google Scholar]
- 74.Filtz T. M., Li Q., Boyer J. L., Nicholas R. A., Harden T. K. (1994) Mol. Pharmacol. 46, 8–14 [PubMed] [Google Scholar]
- 75.Grierson J. P., Meldolesi J. (1995) J. Biol. Chem. 270, 4451–4456 [DOI] [PubMed] [Google Scholar]
- 76.Grygorczyk R., Hanrahan J. W. (1997) Am. J. Physiol. Cell Physiol. 272, C1058–C1066 [DOI] [PubMed] [Google Scholar]
- 77.Lazarowski E. R., Homolya L., Boucher R. C., Harden T. K. (1997) J. Biol. Chem. 272, 24348–24354 [DOI] [PubMed] [Google Scholar]
- 78.Parr C. E., Sullivan D. M., Paradiso A. M., Lazarowski E. R., Burch L. H., Olsen J. C., Erb L., Weisman G. A., Boucher R. C., Turner J. T. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 3275–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watt W. C., Lazarowski E. R., Boucher R. C. (1998) J. Biol. Chem. 273, 14053–14058 [DOI] [PubMed] [Google Scholar]
- 80.Yegutkin G., Bodin P., Burnstock G. (2000) Br. J. Pharmacol. 129, 921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burnstock G. (1997) Neuropharmacology 36, 1127–1139 [DOI] [PubMed] [Google Scholar]
- 82.Evans R. J., Derkach V., Surprenant A. (1992) Nature 357, 503–505 [DOI] [PubMed] [Google Scholar]
- 83.Sorensen C. E., Novak I. (2001) J. Biol. Chem. 276, 32925–32932 [DOI] [PubMed] [Google Scholar]
- 84.Braunstein G. M., Zsembery A., Tucker T. A., Schwiebert E. M. (2004) J. Cyst. Fibros. 3, 99–117 [DOI] [PubMed] [Google Scholar]
- 85.Hazama A., Fan H. T., Abdullaev I., Maeno E., Tanaka S., Ando-Akatsuka Y., Okada Y. (2000) J. Physiol. 523, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benz R., Wojtczak L., Bosch W., Brdiczka D. (1988) FEBS Lett. 231, 75–80 [DOI] [PubMed] [Google Scholar]
- 87.Braunstein G. M., Roman R. M., Clancy J. P., Kudlow B. A., Taylor A. L., Shylonsky V. G., Jovov B., Peter K., Jilling T., Ismailov, Benos D. J., Schwiebert L. M., Fitz J. G., Schwiebert E. M. (2001) J. Biol. Chem. 276, 6621–6630 [DOI] [PubMed] [Google Scholar]
- 88.Caldwell R. A., Clemo H. F., Baumgarten C. M. (1998) Am. J. Physiol. Cell Physiol. 275, C619–C621 [DOI] [PubMed] [Google Scholar]
- 89.Roman R. M., Feranchak A. P., Davison A. K., Schwiebert E. M., Fitz J. G. (1999) Am. J. Physiol. Gastrointest. Liver Physiol. 277, G1222–G1230 [DOI] [PubMed] [Google Scholar]
- 90.Sackin H. (1995) Kidney Int. 48, 1134–1147 [DOI] [PubMed] [Google Scholar]
- 91.Sugita M., Yue Y., Foskett J. K. (1998) EMBO J. 17, 898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor A. L., Kudlow B. A., Marrs K. L., Gruenert D. C., Guggino W. B., Schwiebert E. M. (1998) Am. J. Physiol. Cell Physiol. 275, C1391–C1406 [DOI] [PubMed] [Google Scholar]
- 93.Shestopalov V. I., Panchin Y. (2008) Cell. Mol. Life Sci. 65, 376–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iacobas D. A., Suadicani S. O., Iacobas S., Chrisman C., Cohen M. A., Spray D. C., Scemes E. (2007) J. Membr. Biol. 217, 83–91 [DOI] [PubMed] [Google Scholar]
- 95.Betto R., Senter L., Ceoldo S., Tarricone E., Biral D., Salviati G. (1999) J. Biol. Chem. 274, 7907–7912 [DOI] [PubMed] [Google Scholar]
- 96.Urano T., Nishimori H., Han H., Furuhata T., Kimura Y., Nakamura Y., Tokino T. (1997) Cancer Res. 57, 3281–3287 [PubMed] [Google Scholar]
- 97.Yeung D., Zablocki K., Lien C. F., Jiang T., Arkle S., Brutkowski W., Brown J., Lochmuller H., Simon J., Barnard E. A., Górecki D. C. (2006) FASEB J. 20, 610–620 [DOI] [PubMed] [Google Scholar]