Abstract
The wound response prohormone jasmonic acid (JA) accumulates rapidly in tissues both proximal and distal to injury sites in plants. Using quantitative liquid chromatography-mass spectrometry after flash freezing of tissues, we found that JA accumulated within 30 s of injury in wounded Arabidopsis leaves (p = 3.5 e−7). JA augmentation distal to wounds was strongest in unwounded leaves with direct vascular connections to wounded leaves wherein JA levels increased significantly within 120 s of wounding (p = 0.00027). This gave conservative and statistically robust temporal boundaries for the average velocity of the long distance signal leading to distal JA accumulation in unwounded leaves of 3.4–4.5 cm min−1. Like JA, transcripts of the JA synthesis gene LIPOXYGENASE2 (LOX2) and the jasmonate response gene JAZ10.3 also accumulated to higher levels in directly interconnected leaves than in indirectly connected leaves. JA accumulation in a lox2-1 mutant plant was initiated rapidly after wounding then slowed progressively compared with the wild type (WT). Despite this, JAZ10.3 expression in the two genotypes was similar. Free cyclopentenone jasmonate levels were similar in both resting WT and lox2-1. In contrast, bound cyclopentenone jasmonates (arabidopsides) were far lower in lox2-1 than in the WT. The major roles of LOX2 are to generate arabidopsides and the large levels of JA that accumulate proximal to the wound. LOX2 is not essential for some of the most rapid events elicited by wounding.
Introduction
Some organisms are more exposed to wounding than others. At one extreme would be many endoparasites and, at the other extreme, most plants because they are constantly exposed to physical aggression by mobile herbivores. For this reason the whole body wound responses of plants have to be rapid enough to counter this danger (1). In response to leaf damage, plant cells both proximal to the wound site as well as those in distal positions change their behavior in a highly coordinated manner to promote survival. It is now known that much of this coordination of defense in response to wounding and herbivory is due to the production of the prohormone jasmonic acid (JA)2 (2, 3). JA is made in a multistep series of reactions involving both chloroplasts and peroxisomes (4) prior to being converted into biologically active ligands such as jasmonoyl-l-isoleucine (JA-Ile) (5, 6) and jasmonoyl-l-tryptophan (7).
From the standpoint of hormone synthesis in general, one of the interesting features of JA synthesis is its rapidity. JA was recently found to accumulate within less than 120 s in wounded Arabidopsis leaves (8). This prompted the application of leaf flash-freezing techniques to JA analysis, whereby tissue is frozen in liquid nitrogen within 3 s of harvest. This technique provided the first evidence consistent with a rapid (∼3 cm min−1) systemic signal displacement from wounded to unwounded leaves leading to distal JA accumulation (8). The finding of fast JA accumulation in tissues distal to the wound is supported by the recent literature. Jasmonate responses such as the expression of some JASMONATE-ZIM domain (JAZ) genes (9, 10) take place within 15 min in unwounded rosette leaves of Arabidopsis plants when only a few leaves are wounded (11, 12). Moreover, an ∼17-fold increase in detectable JA in distal leaves 5 min after wounding (8) fits well with a 8-fold increase in the levels of JA-Ile observed independently at this same time point (12). It is still an open question whether the rapid JA accumulation in response to wounding results from metabolism of free or esterified OPDA and dinor-OPDA pools (12, 13) or takes place as an entirely de novo event beginning with fatty acid oxygenation (4).
The initial reactions of JA synthesis involve the 13-LOX-catalyzed incorporation of molecular oxygen into chloroplastic triunsaturated fatty acids (14). The regulation of this step is still poorly defined, and whether JA synthesis is initiated on free or esterified fatty acids is unknown. Nevertheless, it is clear that a major site of JA production occurs in thylakoids (reviewed in Refs. 4 and 13) where at least part of triunsaturated fatty acid production also occurs (15). In Arabidopsis, four 13-LOXs (LOXs 2, 3, 4, and 6) could potentially contribute to JA synthesis in vegetative and reproductive tissues (16); however, LOX2 is known to contribute the major part (∼75%) of JA measured 4 h after wounding leaves (17). Fully consistent with this, a dominant version of a microRNA (miR319a) that indirectly and negatively regulates LOX2 gene expression was found to suppress 75% of wound-induced JA synthesis in leaves (18). Less is known about control over LOX2 protein and enzyme activity in vivo. The fact that LOX2 is a canonical LOX suggests that it should require divalent cations such as Ca2+ for activity (see Ref. 19 for a review). Indeed, Ca2+ transients are thought to be important for initiating JA synthesis in potato (1, 20), and LOX2 transcripts and LOX enzyme activity are up-regulated in a cation channel mutant known to alter K+ and Ca2+ homeostasis in Arabidopsis (21, 22). In summary, LOX2 is the dominant but not the only LOX enzyme responsible for JA synthesis in wounded leaves. The effects of this major LOX on the timing of both JA synthesis and gene expression after wounding are largely unexplored.
In this work quantitative approaches were used to address the following questions: 1) How quickly does JA accumulate in the wounded leaf? 2) How fast is the mobile signal leading to JA accumulation in leaves distal to wounds? 3) Is long distance signaling dependent on vascular interconnections of leaves? 4) Is jasmonate response gene expression, including that of LOX2, affected by vascular architecture? 5) How does mutation of a major LOX (LOX2) involved in JA synthesis affect the timing of JA accumulation and gene expression? For much of the JA measurement data presented, we worked in the time frame of 180 s, which is defined here as “rapid.”
EXPERIMENTAL PROCEDURES
Sample Preparation and Analysis
Protocols were adapted from (8, 23) to allow quantitative JA measurements. Rapid (∼3 s) freezing of all samples was employed (8). Approximately 300–500 mg of frozen leaf material was weighed accurately (note that there was no JA generation during thawing). A 200-ng/ml solution (50 μl) of 18O-JA internal standard (IS) (24) was added prior to extraction with isopropanol. The extract was prepurified on a C18 solid phase extraction cartridge using MeOH:H2O (60:40, v/v) for elution. After evaporation to dryness, the residue was reconstituted in 100 μl of MeOH:H2O (60:40, v/v). Ultrahigh pressure liquid chromatography/time-of-flight mass spectrometry analyses were performed on a Micromass LCT Premier time-of-flight mass spectrometer from Waters (Milford, MA) with an electrospray interface coupled to an Acquity UPLC system (Waters). Optimized chromatographic conditions were obtained by computer modeling using the software Osiris (Datalys, Grenoble, France). The separation was carried out on a Waters UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm) with the following solvent system: A = 0.1% formic acid-H2O, B = 0.1% formic acid-acetonitrile. A gradient elution was performed at a flow rate of 0.6 ml/min at 60 °C under these conditions: 5–53% B in 4 min, 53–100% B in 2 min, holding at 100% B for 3 min and reconditioning at 5% B for 4 min. Time-of-flight mass spectrometry parameters were optimized in negative ion mode for the electrospray source and the ion guide for maximal sensitivity by infusing a JA standard solution at 1 μg/ml. Pilot experiments as well as results in Glauser et al. (8) were used to estimate the range of expected JA levels in tissues. The time-of-flight detector response function was linear over the range of 0.5–50 pmol of JA injected. All of the values we report for plant tissues fall within the range of linearity. The limit of quantitation (LOQ; 10 times signal-to-noise ratio) was equivalent to 20 pmol JA g−1 fresh mass (FM). We verified that 18O-JA was not converted to 16O-JA (24) during extraction. The calibration curve was performed in the matrix as follows: control plants were extracted as above, with the exception that no IS was added before extraction. After solid phase extraction the dried extract was resuspended in 50 μl of the 200 ng/ml solution of 18O-JA IS and 50 μl of 50, 100, 400, and 1200 ng/ml solutions of 16O-JA. The 16O-JA concentrations of the calibration points were thus 25, 50, 200, and 600 ng/ml. For experiments using time points from 5–90 min after wounding, the following modification was made: a 2 μg/ml solution (50 μl) of 18O-JA IS was employed, and the final sample volume was 1 ml. For the calibration curve, the extract was resuspended in 50 μl of the 2 μg/ml solution of 18O-JA IS and 950 μl of 50, 100, 400, and 1200 ng/ml solutions of 16O-JA. Reconstructed ion chromatograms of 16O-JA and 18O-JA were extracted at m/z 209.1178 ± 0.02 and 213.1254 ± 0.02 Da, respectively. Peak areas for 16O-JA were integrated and normalized to those of the corresponding 18O-JA IS. The concentration in samples was determined from the calibration curve. For semi-quantitative estimation of bound and unbound cyclopentenone jasmonate pools (see Table 1), freezing powder from flash-frozen plants was extracted into boiling isopropanol. MeOH:H2O (85:15, v/v) was used for solid phase extraction elution. Ultrahigh pressure liquid chromatography conditions were modified as follows compared with JA quantification: 5–89% B for 7 min, 89–100% B for 0.1 min, holding at 100% B for 3 min and reconditioning at 5% for 4 min. Compounds analyzed were JA, OPDA, dinor-OPDA, arabidopsides A, B, and C, and a “lyso-arabidopside” (sn2-O-(dinor-oxophytodienoyl)-monogalactosyl monogylceride) (25). The experimental design with respect to wounded and distal leaves was the same as that used in Fig. 1 (B and C).
TABLE 1.
Free and esterified jasmonates in healthy and wounded Arabidopsis leaves
Amounts of free and bound jasmonates were determined semi-quantitatively in unwounded leaves (UNWND), wounded leaves (WOUNDED) 3 min after wounding and in leaves distal to the wound (W DISTAL) at the same time point for WT and the lox2-1 mutant. The abundance of the different jasmonates was obtained from integrated peak areas of extracted ion chromatograms, which are given as ± S.D. (n = 3). ND, not detected. Lyso-arabidop, sn2-O-(dinor-oxophytodienoyl)-monogalactosyl monogylceride (25).
| WT |
lox2-1 |
|||||
|---|---|---|---|---|---|---|
| UNWND | WOUNDED | W DISTAL | UNWND | WOUNDED | W DISTAL | |
| JA | ND | 20.9 ± 10.4 | 11.8 ± 3.5 | ND | 16.0 ± 6.9 | 5.2 ± 2.8 |
| OPDA | 16.5 ± 5.9 | 246 ± 61 | 50.1 ± 13.3 | 18.0 ± 5.8 | 103 ± 43 | 22.1 ± 6.5 |
| dinor-OPDA | ND | 65.6 ± 18.1 | 3.8 ± 0.5 | ND | 12.1 ± 4.4 | ND |
| Arabidopside A | 166 ± 16 | 4482a ± 646 | 393 ± 140 | ND | 29.3 ± 28.1 | ND |
| Arabidopside B | 28.3 ± 2.3 | 2923 ± 610 | 77.3 ± 10.0 | ND | 5.2 ± 6.5 | ND |
| Arabidopside C | 3.2 ± 0.7 | 967 ± 460 | 8.3 ± 3.5 | ND | 3.2 ± 3.0 | ND |
| Lyso-arabidop | 9.6 ± 4.6 | 2310 ± 402 | 25.4 ± 9.9 | 3.2 ± 2.6 | 56.2 ± 28.8 | 5.9 ± 2.2 |
a This value is a minimum because of detector saturation.
FIGURE 1.
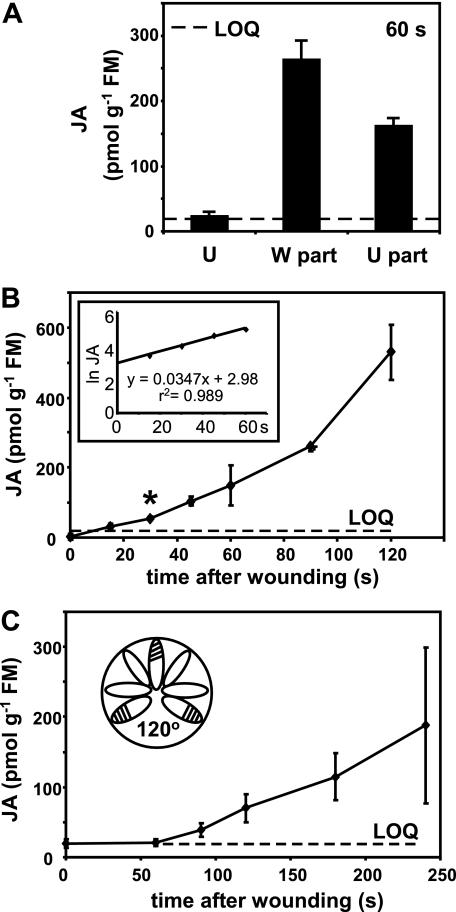
JA accumulation kinetics in wounded and unwounded Arabidopsis leaves. A, JA levels in the wounded part (W part) of leaves compared with the unwounded part (U part) of the same leaves. Levels were measured 60 s after wounding ∼40% of the apical part of fully expanded leaves. U indicates controls from unwounded plants. The data are from four replicates ± S.D. B, time course of JA accumulation in wounded leaves. In this experiment whole leaves including the wounded region were harvested. The data are from four replicates ± S.D. The asterisk at 30 s indicates a statistically significant value (p = 3.5 e−7) that was used in calculations of signal velocity. The inset shows the same data plotted as the natural logarithm of JA level versus time. C, JA accumulation in unwounded distal leaves on rosettes on which three leaves ∼120° apart were wounded. Unwounded interspersed leaves (indicated in the inset) were harvested for JA measurement. The data are from four replicates ± S.D.
Plants
Arabidopsis thaliana accession Col-0 was grown as described by Glauser et al. (8). True leaves were numbered from youngest to oldest. For wounding, 40% of the apical parts of leaves were crushed with metal forceps. The silenced LOX2 line (S-12) and a cognate control line (S-12C) were from Bell et al. (17) and are available as SALK lines (CS3748 and CS3749, respectively). The allene oxide synthase (aos) mutant we used was from Park et al. (26) as described in Mène-Saffrané et al. (27). Tilling (28) was used to isolate a mutant allele of LOX2. The region predicted to encode the catalytic site (bases 2527–3825) was targeted because of a high probability of finding nonsense alleles. One such allele was found and predicted to convert amino acid 630 (a tryptophan) of the WT protein into a stop codon, i.e. converting … GKLWRF to … GKL*. For genotyping, a 1516-bp fragment containing this region of LOX2 was amplified by PCR using the following primers: GGATTATCATGATTTGCTTCTACC and TCAAATAGAAATACTATAAGGAACAC. The WT 1516-bp band cannot be digested with BfmI (SfeI), but the lox2-1 mutation creates a restriction site (restriction at 6–15 h at 37 °C) producing two bands of 864 and 652 bp, respectively. These plants were backcrossed to WT twice prior to experiments. Biocontrol nematodes (Andermatt Biocontrol AG) were watered onto plants at 1700 nematodes/ml of water once each 2 weeks when sciarid flies were seen in growth rooms.
Insect Feeding Trials
Eleven pots of 4.5-week-old plants (two plants/pot) were placed in plexiglass boxes in a growth cabinet at 22 °C, 100 μE s−1 m−2, 70% humidity. Three newly hatched Spodoptera littoralis larvae were placed on each pot. After 9 days of feeding, the larvae were removed and weighed to the nearest 0.01 mg using a Mettler-Toledo MT5 balance (Mettler-Toledo, Greifensee, Switzerland). Caterpillar weights at egg hatch were assumed to be equal; thus, only the final weights were measured. The experiment was repeated three times.
Gene Expression
Total RNA was extracted, and 1 μg was copied into cDNA using the SuperScript II first strand synthesis system (Invitrogen) and oligo(dT) primers according to the manufacturer's instructions. For experiments in Fig. 3, real time quantitative PCR analysis was performed on 100 ng of cDNA in a final volume of 20 μl according to the instructions in the FullVelocity SYBR Green instruction manual (Stratagene, La Jolla, CA). PCR and data analysis were performed, respectively, in an Mx3005P spectrofluorometric thermal cycler (Stratagene) utilizing its software. The data were standardized in relation to At2g28390 (SAND family) (29) (Fig. 3A) or the combined SAND and the ubiquitin-conjugating enzyme At5g25760 transcripts (Fig. 3B), for which levels were found to be stable under the conditions used. For experiments in Fig. 4C we used Power SYBR mix from Applied Biosystems (Foster City, CA) in a final volume of 10 μl. Real time quantitative PCR was performed on 50 ng of cDNA in an Applied Biosystems 7900HT cycler with the following conditions: 50 °C for 2 min, initial denaturation at 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The absence of secondary amplicons in JAZ10.3 amplifications was confirmed by gel electrophoresis. Transcript levels in Fig. 4C were standardized to ubiquitin-conjugating enzyme. At least three biological replicates were used in each experiment: PCR primers LOX2 (At3G45140), 5′-CTATGGAATCTTGCTAAGACTCATG and 5′-CGGCTGAACTTAGCTCTAATGCATA; ubiquitin-conjugating enzyme (AT5G25760), 5′-CAGTCTGTGTGTAGAGCTATCATAGCAT and 5′-AGAAGATTCCCTGAGTCGCAGTT; JAZ10.3 (At5G13220.3), 5′-AAGGAGAGGTAATGATTCTTCAACAAT and 5′-AGTAGGTAACGTAATCTCC; and SAND (At2g28390), 5′-AACTCTATGCAGCATTTGATCCACT and 5′-TGATTGCATATCTTTATCGCCATC.
FIGURE 3.
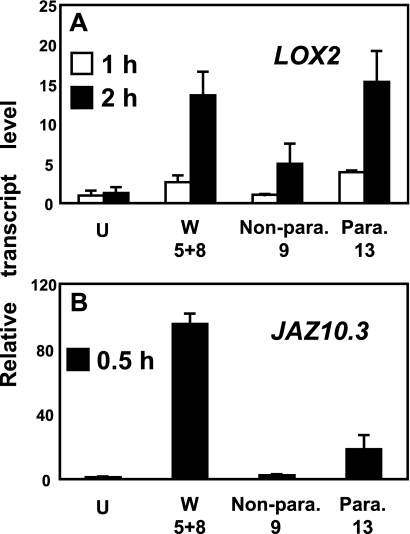
JA marker gene expression in distal leaves. A, levels of LOX2 transcript in unwounded leaves (U), wounded leaves 5 and 8 (W), nonparastichous leaf 9 (Non-para.), and parastichous leaf 13 from the wounded plant (Para.). The data are from three replicates ± S.D. B, similar experiment for the jasmonate response transcript JAZ10.3. The data are from four replicates ± S.D.
FIGURE 4.
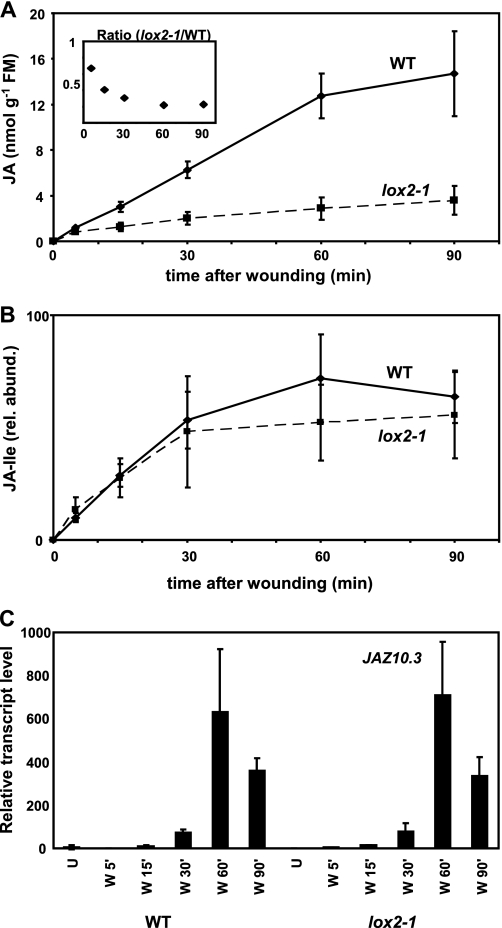
JA, JA-Ile, and JAZ10.3 transcript levels in wounded WT and lox2-1. A, increases in JA levels in wounded leaves of WT and lox2-1. The data are from four replicates ± S.D. The inset shows the decrease in ratio of JA in lox2-1/WT versus time. B, relative abundance (rel. abund.) of JA-Ile in the wounded leaves of WT and lox2-1 plants. C, effect of lox2-1 on JAZ10.3 expression. Leaves were wounded, and transcript levels were measured at the times indicated. The data are the means of three independent replicates ± S.D.
RESULTS
Prior to conducting experiments on wound-induced JA accumulation and temporal jasmonate responses, quantitative methods were employed to establish the range of basal levels of JA in resting leaves. The levels of JA detected in unharmed leaves ranged from the limit of detection (∼7–8 pmol g−1 FM) to 33 pmol g−1 FM, which is above the LOQ (20 pmol g−1 FM; see “Experimental Procedures”) for all experiments. The variability of basal JA levels may correlate to treatments of plants with biocontrol nematodes to prevent contamination of growth rooms with sciarid flies. Untreated plants usually had JA levels below the LOQ. An initial experiment (Fig. 1A) compared wound-induced JA levels in the damaged and undamaged parts of a wounded leaf at 60 s after wound infliction. JA levels were ∼250- and 150 pmol g−1 FM, respectively. A time course of JA accumulation in wounded leaves (Fig. 1B) showed a nonlinear trajectory with JA accumulation starting no later than 30 s post-wounding. When data from the first 60 s were plotted as the natural logarithm (ln) of JA amount versus time, a straight line was obtained (Fig. 1B, inset).
Then, to examine JA build-up distal to injury, three rosette leaves at roughly 120° angles were wounded, and only unwounded interspersed leaves were harvested. JA accumulation in these distal unwounded leaves (Fig. 1C) followed a somewhat similar profile to JA accumulation in the wounded leaf (Fig. 1B), although longer times were needed for JA build-up. In these data large error bars were noted at later time points (180 and 240 s in Fig. 1C). This was taken as a possible indication of heterogeneity in JA build-up in different leaves and prompted an investigation of the role of interleaf vascular architecture in long distance wound responses.
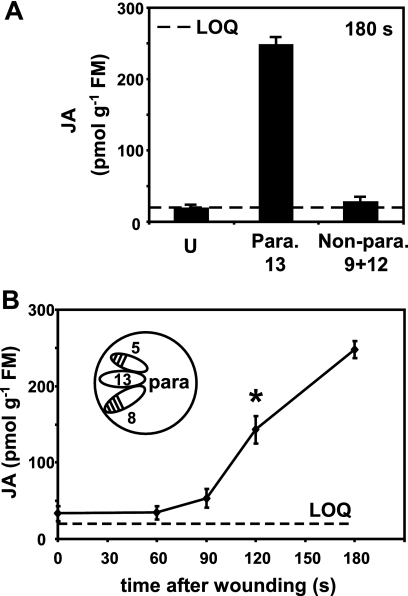
Direct vascular connections between leaves are defined for Arabidopsis, and for this we used the terminology and descriptions of Dengler (30). The nature of interleaf connections (parastichies) is developmentally determined. In much of the adult phase parastichies are n + 5 and n + 8 (for example, leaf 8 is directly connected through the vasculature to leaves 13 and 16). In the juvenile phase the parastichies are somewhat different but still include the n + 5 leaf connections (30). Additionally, the physiological status and in particular the source-sink relationship between leaves affects interleaf signaling (31). Using this knowledge we tested for the differential accumulation of JA in leaves distal to wounds. Leaves 5 and 8 were wounded, and JA was then measured 3 min later in parastichous leaf 13 and nonparastichous leaves 9 and 12 (Fig. 2A). A clear difference was found with JA levels being 9-fold higher (∼250 pmol g−1 FM) in the parastichous leaf than in the nonconnected leaf (∼28 pmol g−1 FM) at this time point. This first observation led us to conduct a time course experiment where leaves 5 and 8 were wounded, and JA levels in leaf 13 were quantitated. JA accumulation in leaf 13 was statistically significant at 120 s post-wounding (Fig. 2B, asterisk). In summary, JA levels increased in wounded leaves by 30 s and in the unwounded parastichous leaf 13 by 120 s post-wounding.
FIGURE 2.
The importance of leaf vascular connections in rapid JA accumulation. A, leaves 5 and 8 were wounded at time 0, and leaves 9 and 12 (no direct vascular connections to leaves 5 and 8 (Non-para.)) and 13 (direct vascular connections to leaves 5 and 8 (Para.)) were harvested for JA measurement 180 s later. Leaves from unwounded plants (U) served as controls. The data are from four replicates ± S.D. B, this experiment used a similar experimental design (inset) in a time course to measure JA accumulation in leaf 13. The asterisk at 120 s indicates a statistically significant value (p = 0.00027) that was used in calculations of signal velocity. The data are from four replicates ± S.D.
Having established that JA production after wounding leaves 5 and 8 was greater in parastichous leaf 13 than in nonparastichous leaves 9 and 12 at the 3-min time point, we asked how this related to gene expression. To do this, relative transcript levels for the JA biosynthesis gene LOX2 (17) and for the jasmonate response transcript JAZ10.3 (formerly JAS1.3) (32) were measured in a similar experimental design. Both transcripts showed a higher induction in connected leaf 13 than in nonconnected leaf 9 after wounding (Fig. 3). An additional experiment with a different primer set designed to amplify all of the predicted JAZ10 transcripts gave similar results (not shown).
After the investigation of JA accumulation in WT plants, we asked the question of whether mutation of LOX2, the gene encoding the major JA-producing LOX in leaves (17), affected JA accumulation kinetics as well as the timing of gene expression. In pilot experiments the transgenic lines generated by Bell et al. (17) were used to investigate the kinetics of JA accumulation after wounding. The possibility that nontarget effects of gene silencing of LOX2 in the transgenic S-12 line of Bell et al. (17) could affect other LOXs was considered. We therefore isolated the nonsense mutant lox2-1. JA accumulation in wounded leaves, gene expression, and biological function after wounding were all investigated in this mutant with several parallel experiments done with the transgenic lines of Bell et al. (17). For example, we found an almost identical reduction of JA levels 90 min after damage of lox2-1 and S12 (the LOX2-silenced line) to ∼25% of those in the WT. However, in the first 1 min after wounding, the levels of JA were statistically similar in lox2-1 (96 ± 13 (S.D., n = 4) pmol g−1 FM), in the WT (124 ± 10 pmol g−1 FM), and also in the LOX2-silenced line S-12 (103 ± 8 pmol g−1 FM) and its empty vector control line S-12C (100 ± 6 pmol g−1 FM). An extended time course experiment comparing JA accumulation in WT and lox2-1 showed that, over time, the relative level of JA in lox2-1 diminished as the wound response progressed out to 90 min (Fig. 4A). Ratios of JA in lox2-1/WT changed most abruptly in the first 15 min. Then after 30 min, these ratios ceased to change greatly over time (Fig. 4A, inset). Contrary to JA levels, the relative levels of JA-Ile were found to be similar in WT and lox2-1 leaves after wounding (Fig. 4B).
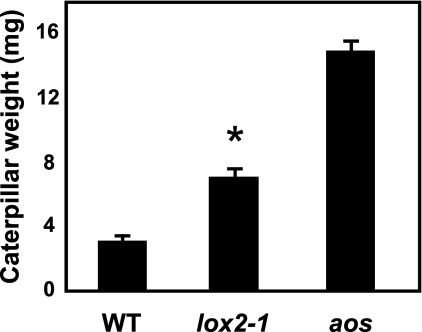
Over the same time course used to measure JA accumulation (i.e. from 5 to 90 min) JAZ10.3 transcript levels were measured and found to be similar in the two plants (Fig. 4C). To rule out the possibility that the JAZ10.3 transcript behaved in an atypical manner compared with other JAZ10 transcripts, this experiment was repeated with a PCR primer set designed to amplify all known JAZ10 isoforms, and this gave similar results (not shown). The larvae of the insect S. littoralis were found to grow more quickly on lox2-1 than on the WT, although they grew still much more quickly on the fully jasmonate-deficient aos line (Fig. 5).
FIGURE 5.
lox2-1 shows enhanced susceptibility to a chewing herbivore. The growth of S. littoralis larvae was tested on WT, lox2-1, and aos mutant plants. Three freshly hatched larvae were placed simultaneously on each genotype (2 plants/pot). Larval weight (means ± S.E.) was measured after 9 days of feeding. The following numbers of insects were recovered: on WT, 81; on lox2-1, 88; and on aos, 88. The asterisk indicates a statistically valid difference (p = 2.1e−11).
Having quantitated JA levels in resting and wounded tissues, we carried out a comparative, semi-quantitative analysis of free and bound jasmonate (arabidopside) levels (Table 1). At the 3-min time point after wounding, JA was inducible in both the wounded and distal leaves of the WT and the lox2-1 mutant. In the wounded leaves of WT plants, the free OPDA levels increased ∼15-fold, and arabidopside levels increased up to 300-fold within 3 min. At the same time, in leaves distal to the wound, free OPDA, dinor-OPDA, and arabidopside levels all increased 2–4-fold compared with levels in unwounded plants. In resting lox2-1 the levels of free OPDA were similar to those in the WT, whereas the levels of the arabidopsides examined were far lower (below the limit of detection) than those in the WT. Upon wounding, free OPDA levels increased proximal to the wound but were essentially stable distal to wounds. Arabidopsides accumulated weakly in wounded lox2-1 leaves but were not detectable distal to the wound at the 3-min time point. In parallel assays S-12 plants gave results similar to those from lox2-1.
DISCUSSION
JA is the source of multiple regulators including JA-Ile (5) and jasmonoyl-l-tryptophan (7). We focused on its build-up in response to wounding. Recent work showed that JA accumulation triggered by the detachment of Arabidopsis leaves took place in less than 120 s, and JA accumulation was also observed in leaves distal to wounds within 2–5 min (8). From this a minimum signal velocity from wounded to unwounded leaves of ∼3 cm min−1 was proposed (8). However, the fact that JA levels in healthy resting leaves are very low (less than 50 pmol g−1 fresh mass) but accumulate in such short time frames upon wounding places demands on analytical methods. The technical aspects considered most critical for the quantification of low levels of JA were as follows. 1) Flash freezing of plant samples in 3 s or less (8). Once frozen, we found that samples did not accumulate JA upon thawing, even if the thawed tissue was further wounded (data not shown). 2) The use of a calibration curve performed in the matrix with an 18O-JA internal standard with an identical retention time to JA. This compensates for the influence of variable matrix effects, in particular ion suppression. 3) Improved sensitivity. For example, we reported previously that 2 min after wound infliction, JA levels in leaves distal to wounds were 4-fold the signal-to-noise ratio. We now report values that equate to 40-fold the signal-to-noise ratio at this time point in these leaves (see Fig. 1C and “Experimental Procedures”). This strong increase in sensitivity was possible on the same instrument used in Glauser et al. (8), mainly by optimization of mass spectrometry tuning to JA and of the chromatographic separation to minimize co-elution leading to ion suppression; 4) a LOQ (20 pmol g−1 FM) based on 10 times the signal-to-noise ratio and a conservative threshold for significant JA accumulation, based on statistically supported values (p < 0.001). We noted that basal levels of JA in resting leaves varied from the limit of detection to slightly above the LOQ. We then measured JA levels in wounded leaves and in leaves distal to the wounds. Fig. 1B shows that JA accumulation in the wounded leaf was significant at 30 s (p = 3.5 e−7) when based on the LOQ value of 20 pmol g−1 FM. A plot of ln JA concentration versus time showed that JA levels doubled approximately each 20 s in the first minute after injury in the wounded leaf. Interestingly, back extrapolation of the curve to the ordinate intercepted at 2.98, whereas the value of e is close to 2.72. Therefore, in these plants, the extrapolated JA level in resting leaves was likely to be 19.7 pmol g−1 FM. This suggests that there are always small JA pools, even in healthy leaves.
The signal velocity rate leading to distal JA accumulation following wounding was then determined. JA accumulation was estimated to be significant at 120 s in leaf 13 when leaves 5 and 8 were wounded (Fig. 2B, p = 0.00027, based on a concentration of 33 pmol g−1 FM measured in unwounded plants). Taking interleaf connections into account gave smaller error bars than the experiment in Fig. 1C, where parastichies were ignored. A time frame for the average long distance signal velocity was estimated based on the shortest distance from the center of the receiver leaf (leaf 13) to the nearest wounded tissue in wounded leaves 5 and 8. Leaf 5 was smaller than leaf 8 at the growth stage we used (5 weeks). The average distance from the wound edge in leaf 5 to the center of leaf 13 was 6.73 ± 0.31 cm (S.D., n = 12). The minimum (slowest) signal velocity from the wound to the receiver leaf was thus 6.73 cm/2 min = 3.4 cm min−1. The maximum (fastest) signal velocity from the wounds to the receiver leaf was based on the estimation (above) that 30 s are required for significant JA accumulation after wounding (Fig. 1B) and that it took 2 min for robust JA accumulation to occur in leaves distal to wounds (Fig. 2B). The maximum signal displacement is thus estimated conservatively as 120–30 = 90 s over an average of 6.73 cm leading to a value of 4.5 cm min−1. The velocity values given are regarded as averages because it is not yet known whether signal generation and displacement speed through the plant are homogenous. To our knowledge our estimates are the first constrained (i.e. maximum and minimum) values for signal velocity leading to a jasmonate response in any plant. The results underscore the importance of considering interleaf vasculature in fully quantitative studies on temporal aspects of long distance signal transfer.
The work of Koo et al. (12) and the results reported herein both support the notion (8) that the mobile signal trafficking rapidly (i.e. in 2 min or less) from wounded to unwounded leaves is unlikely to be a jasmonate. The nature of long distance signals leading to jasmonate responses is unclear and, in Arabidapsis, this signal is not derived from OPDA (12). Evidence suggests the involvement of transmembrane fluxes of ions (reviewed in Refs. 1, 33, and 34). Thus, by inference, water fluxes may also be important. Miller et al. (35) showed that reactive oxygen species mediate fast (8.4 cm min−1) long distance signal propagation along wounded Arabidopsis inflorescences that leads to the expression of a reactive oxygen species reporter gene. The velocity reported was somewhat faster than the interleaf signal velocity we report in the rosette.
As for the accumulation of JA, a dependence on leaf connections was seen for the wound response of JA-regulated transcripts for LOX2 and for JAZ10.3. These results are consistent with reports that both distal wound responses (36) and JA-related gene expression (31), as well as JA accumulation itself (37), are at least to some extent dependent on leaf vascular patterns, leaf structure, and assimilate partitioning (36). Also, in terms of time, JAZ gene expression in distal leaves in response to wounding can take place in the 5–15 min range (11), and this fits well with the reported rates of distal JA (8) and JA-Ile (12) accumulation. The time frame of JA build-up we describe here allowed experimental interventions aimed at deregulating the course of JA accumulation.
To study the effects of deregulating wound-induced JA accumulation, we used plants in which LOX2 gene expression was silenced (17) as well as a new point mutant (lox2-1). These plants behaved very similarly in our assays. The measured levels of JA in WT and lox2-1 wounded leaves did not differ greatly in the first minutes following wounding, but thereafter a progressively bigger difference in the two genotypes was observed. Data from a pilot experiment illustrated this; the difference in JA content between WT and lox2-1 at 1 min after wounding had a relatively low significance (p = 0.05, not shown), whereas 5 min after wounding, the difference was highly significant (p = 0.0006; Fig. 4A, inset). We noted that between 30 and 60 min post-wounding, the ratio of JA in the two genotypes essentially stabilized over time.
The initially unexpected finding that, following wounding, the levels of JAZ10.3 transcripts in lox2-1 were comparable with those in the WT led us to re-examine the liquid chromatography-mass spectrometry data pool for relative levels of JA-Ile. Consistent with these data, JA-Ile levels in WT and lox2-1 were found to be similar at all time points after wounding. Therefore LOX2 down-regulation does not sufficiently impede the accumulation of JA (and JA-Ile) after wounding to lead to aberrant JAZ10.3 expression. Moreover, the levels of JA that accumulate proximal and distal to wounds in WT plants differ strongly. Induced JA levels in leaves distal to wounds are not reported to reach the nmol g−1 FM values that are reached in the wounded leaf itself (where values can exceed 10 nmol g−1 FM). Despite this, transcript profiles in wounded and distal leaves under our plant culture conditions were very similar (38). Consequently, the levels of JA that accumulate in WT wounded leaves exceed those necessary for the correct temporal expression of JAZ10.3. Moreover, the fact that defenses against the generalist herbivore S. littoralis appear to be compromised in lox2-1 relative to the WT is clearly not a consequence of JAZ10.3 expression.
Potential sources of nascent JA are triunsaturated fatty acids, JA precursors, and all reversibly bound cyclopentenone jasmonates. Arabidopsides (esterified forms of OPDA and dinor-OPDA) have been suggested as potential sources of precursors for JA synthesis (reviewed in Refs. 13 and 39). Unbound pools of OPDA could be the major precursors for rapid JA synthesis (13, 34). However, assessing the relative levels of bound and unbound OPDA and dinor-OPDA is potentially difficult because of the release of these compounds by lipase action in solvents (24). We extracted tissues in boiling isopropanol and found a strong remodeling of the oxylipin signature in damaged WT leaves within 3 min of wounding (Table 1). Arabidopside C levels, for example, increased up to 300-fold compared with resting leaves. Remodeling was less spectacular in distal leaves where the levels of all compounds monitored increased ∼3-fold. Our data agree with the literature where wounding causes strong increases in arabidopside levels (25, 39–41). We also observed an increase in free OPDA in both wounded WT leaves and in leaves distal to wounds. Koo et al. (12) observed increased OPDA levels in the wounded leaf but decreased OPDA levels in leaves distal to wounds. The reason for this is not clear but might be due to different extraction procedures.
Although we cannot unequivocally identify the source of the first JA made in response to wounding, the comparison of WT and lox2-1 plants was informative. Unlike the WT, the unwounded mutant lacked detectable levels of arabidopsides A, B, and C. However, the levels of free OPDA in resting leaves were similar to those in the WT. The fact that JA-Ile, free OPDA in unwounded leaves, and JAZ10.3 expression were similar in the WT and lox2-1 suggests that free OPDA could be a precursor for the JA-Ile pool necessary for correct gene expression. This would be consistent with Koo et al. (12, 34), who proposed that free OPDA is the source of nascent JA-Ile in leaves distal to wounds. It is also possible, however, that one or more of LOXs 3, 4, and 6 contribute directly to rapid OPDA and JA synthesis upon wounding. This will need investigation.
The present results shed new light on LOX2 function. This enzyme is phylogenetically distinct from other 13-LOXs in Arabidopsis and may thus have a role different from that of these other enzymes (16). Our data suggest that the major roles of LOX2 in the leaf are to synthesize arabidopsides and to produce high levels of JA proximal to the wound. Low levels of arabidopsides are produced in wounded lox2-1 leaves (Table 1), and this indicates that other 13-LOXs can also contribute to the synthesis of these compounds. Further work will be needed on lox2-1 (and S-12) plants to fully understand how LOX2 down-regulation might impinge on defense. In our view, and consistent with a proposed role for arabidopsides in chemical defense (39), the lower-than-WT pools of esterified cyclopentenones in lox2-1 may help to reduce the defense capacity of the plants.
Finally, the speed of long distance signals and distribution pattern must be sufficient to prepare tissues for the arrival of small, mobile herbivores as they move over their host plants. The establishment of time constraints for the speed of the long distance wound-initiated signal leading to JA accumulation is an important step in understanding the chronobiology of antiherbivore defense.
Acknowledgments
We thank A. Chételat for technical help, P. Reymond for insect larvae, and D. Caldelari for help with transgenic LOX lines and for valuable discussion. We thank the European Arabidopsis Stock Center for the seeds of the LOX2-silenced line and its vector control.
This work was supported in part by Swiss National Science Foundation Grants 205320-124667/1 (to J.-L. W., S. R., and E. E. F.), 3100A0–122441 (to E. E. F.) and SystemsX.ch (to E. E. F.).
- JA
- jasmonic acid
- JA-Ile
- jasmonoyl-isoleucine
- WT
- wild type
- LOX
- lipoxygenase
- IS
- internal standard
- LOQ
- limit of quantitation
- FM
- fresh mass
- OPDA
- oxo-phytodienoic acid
- UPLC
- ultrahigh-performance liquid chromatography.
REFERENCES
- 1.Maffei M. E., Mithöfer A., Boland W. (2007) Trends Plant Sci. 12, 310–316 [DOI] [PubMed] [Google Scholar]
- 2.Farmer E. E. (2007) Nature 448, 659–660 [DOI] [PubMed] [Google Scholar]
- 3.Howe G. A., Jander G. (2008) Annu. Rev. Plant Biol. 59, 41–66 [DOI] [PubMed] [Google Scholar]
- 4.Wasternack C. (2007) Ann. Bot. 100, 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staswick P. E., Tiryaki I. (2004) Plant Cell 16, 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca S., Chini A., Hamberg M., Adie B., Porzel A., Kramell R., Miersch O., Wasternack C., Solano R. (2009) Nat. Chem. Biol. 5, 344–350 [DOI] [PubMed] [Google Scholar]
- 7.Staswick P. E. (2009) Plant Physiol. 150, 1310–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glauser G., Grata E., Dubugnon L., Rudaz S., Farmer E. E., Wolfender J. L. (2008) J. Biol. Chem. 283, 16400–16407 [DOI] [PubMed] [Google Scholar]
- 9.Browse J. (2009) Annu. Rev. Plant Biol. 60, 183–205 [DOI] [PubMed] [Google Scholar]
- 10.Chico J. M., Chini A., Fonseca S., Solano R. (2008) Curr. Opin. Plant Biol. 11, 486–494 [DOI] [PubMed] [Google Scholar]
- 11.Chung H. S., Koo A. J., Gao X., Jayanty S., Thines B., Jones A. D., Howe G. A. (2008) Plant Physiol. 146, 952–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo A. J., Gao X., Jones A. D., Howe G. A. (2009) Plant J. 59, 974–986 [DOI] [PubMed] [Google Scholar]
- 13.Schaller A., Stintzi A. (2008) in Induced Plant Resistance to Herbivory ( Schaller A. ed) pp. 7–29, Springer, New York [Google Scholar]
- 14.Feussner I., Wasternack C. (2002) Annu. Rev. Plant Biol. 53, 275–297 [DOI] [PubMed] [Google Scholar]
- 15.Andreu V., Collados R., Testillano P. S., Risueño M. C., Picorel R., Alfonso M. (2007) Plant Physiol. 145, 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannenberg G., Martínez M., Hamberg M., Castresana C. (2009) Lipids 44, 85–95 [DOI] [PubMed] [Google Scholar]
- 17.Bell E., Creelman R. A., Mullet J. E. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8675–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schommer C., Palatnik J. F., Aggarwal P., Chetelat A., Cubas P., Farmer E. E., Nath U., Weigel D. (2008) PloS Biol. 6, e230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider C., Pratt D. A., Porter N. A., Brash A. R. (2007) Chem. Biol. 14, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisahn J., Herde O., Willmitzer L., Peña-Cortés H. (2004) Plant Cell Physiol. 45, 456–459 [DOI] [PubMed] [Google Scholar]
- 21.Bonaventure G., Gfeller A., Proebsting W. M., Hörtensteiner S., Chételat A., Martinoia E., Farmer E. E. (2007) Plant J. 49, 889–898 [DOI] [PubMed] [Google Scholar]
- 22.Beyhl D., Hörtensteiner S., Martinoia E., Farmer E. E., Fromm J., Marten I., Hedrich R. (2009) Plant J. 58, 715–723 [DOI] [PubMed] [Google Scholar]
- 23.Glauser G., Guillarme D., Grata E., Boccard J., Thiocone A., Carrupt P. A., Veuthey J. L., Rudaz S., Wolfender J. L. (2008) J. Chromatogr. A 1180, 90–98 [DOI] [PubMed] [Google Scholar]
- 24.Mueller M. J., Mène-Saffrané L., Grun C., Karg K., Farmer E. E. (2006) Plant J. 45, 472–489 [DOI] [PubMed] [Google Scholar]
- 25.Glauser G., Grata E., Rudaz S., Wolfender J. L. (2008) Rapid Commun. Mass Spectrom. 22, 3154–3160 [DOI] [PubMed] [Google Scholar]
- 26.Park J. H., Halitschke R., Kim H. B., Baldwin I. T., Feldmann K. A., Feyereisen R. (2002) Plant J. 31, 1–12 [DOI] [PubMed] [Google Scholar]
- 27.Mène-Saffrané L., Davoine C., Stolz S., Majcherczyk P., Farmer E. E. (2007) J. Biol. Chem. 282, 35749–35756 [DOI] [PubMed] [Google Scholar]
- 28.Henikoff S., Till B. J., Comai L. (2004) Plant Physiol. 135, 630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czechowski T., Stitt M., Altmann T., Udvardi M. K., Scheible W. R. (2005) Plant Physiol. 139, 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dengler N. G. (2006) Can. J. Botany 84, 1660–1671 [Google Scholar]
- 31.Roberts K., Love A. J., Laval V., Laird J., Tomos A. D., Hooks M. A., Milner J. J. (2007) New Phytol. 175, 707–717 [DOI] [PubMed] [Google Scholar]
- 32.Yan Y., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E. E. (2007) Plant Cell 19, 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howe G. A., Schaller A. (2008) in Induced Plant Resistance to Herbivory ( Schaller A. ed) pp. 349–366, Springer, New York [Google Scholar]
- 34.Koo A. J., Howe G. A. (2009) Phytochemistry, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller G., Schlauch K., Tam R., Cortes D., Torres M. A., Shulaev V., Dangl J. L., Mittler R. (2009) Sci. Signal. 2, ra45. [DOI] [PubMed] [Google Scholar]
- 36.Davis J. M., Gordon M. P., Smit B. A. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 2393–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stork W., Diezel C., Halitschke R., Gális I., Baldwin I. T. (2009) PLoS ONE 4, e4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reymond P., Bodenhausen N., Van Poecke R. M., Krishnamurthy V., Dicke M., Farmer E. E. (2004) Plant Cell 16, 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kourtchenko O., Andersson M. X., Hamberg M., Brunnström A., Göbel C., McPhail K. L., Gerwick W. H., Feussner I., Ellerström M. (2007) Plant Physiol. 145, 1658–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buseman C. M., Tamura P., Sparks A. A., Baughman E. J., Maatta S., Zhao J., Roth M. R., Esch S. W., Shah J., Williams T. D., Welti R. (2006) Plant Physiol. 142, 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Böttcher C., Weiler E. W. (2007) Planta 226, 629–637 [DOI] [PubMed] [Google Scholar]