Abstract
Interleukin-1β (IL-1β) is an important pro-inflammatory cytokine that is secreted by unconventional means in a caspase-1-dependent manner. Using a one-step immunoprecipitation approach to isolate endogenous caspase-1 from the monocytic THP1 cell line, we identified previously undescribed binding partners using mass spectrometry. One of the proteins identified was Rab39a, a member of the Rab GTPase family, a group of proteins that have important roles in protein trafficking and secretion. We confirmed by co-immunoprecipitation that Rab39a binds caspase-1. Knock down of Rab39a with small interfering RNA resulted in diminished levels of secreted IL-1β but had no effect on induction of pro-IL-1β mRNA by lipopolysaccharide. Rab39a contains a highly conserved caspase-1 cleavage site and was cleaved in the presence of recombinant caspase-1 or lipopolysaccharide. Finally, overexpression of Rab39a results in an increase in IL-1β secretion, and furthermore, overexpression of a Rab39a construct lacking the caspase-1 cleavage site leads to an additional increase in IL-1β secretion. Altogether, our findings show that Rab39a interacts with caspase-1 and suggest that Rab39a functions as a trafficking adaptor linking caspase-1 to IL-1β secretion.
Introduction
IL-1β2 is a potent pro-inflammatory cytokine produced by monocytes, macrophages, and dendritic cells. It is first synthesized as biologically inactive pro-IL-1β and is processed by caspase-1 into mature and biologically active IL-1β. It is subsequently released into the extracellular milieu (1). Most proteins that are secreted from the cell contain signal peptides that direct their transport to the plasma membrane through the endoplasmic reticulum-Golgi pathway. However, certain proteins, including IL-1β, do not contain signal peptides and are secreted by unconventional means, the mechanism of which is not entirely understood. Several models for IL-1β secretion have been described (for review, see ref. 2), including the lysosome-dependent pathway (3, 4), microvesicle shedding (5, 6), and exosome release (7).
Caspase-1 is an important regulatory molecule in the innate immune response that is activated in the inflammasome in response to certain pro-inflammatory stimuli. The inflammasome is a multiprotein complex, which, along with caspase-1, also contains a Nod-like receptor (NLR) protein such as NLRP1, NLRP3, or IPAF and the adaptor protein ASC (8). A diverse range of stimuli can activate inflammasomes such as bacteria, viruses, and in the case of the NLRP3 inflammasome danger signals such as uric acid and ATP and aggregated materials such as silica and asbestos (for review, see ref. 9).
Besides its well documented role in IL-1β processing and in the inflammasome, other roles for caspase-1 are emerging. A recent study describes how caspase-1 is important for the secretion of IL-1β as well as many other unconventionally secreted proteins such as fibroblast growth factor-2 and IL-1α. Using iTRAQ proteomics, 77 leaderless proteins with extracellular functions whose secretion is mediated by caspase-1 were identified. Many of these proteins are involved in inflammation, cytoprotection, or tissue repair (10).
In this study we used an immunoprecipitation approach to isolate caspase-1 from THP1 cells and then identified binding partners using mass spectrometry. One of the proteins identified was Rab39a. The human Rab protein family consists of >60 members. They are associated with specific membranes and are required for vesicle movement in pathways such as secretion and endocytosis (for review, see ref. 11). Rab39a is a relatively uncharacterized member of the Rab GTPase family. It was initially described by Stankovic et al. (12) as a novel Rab protein that is widely expressed in a variety of tissues, including spleen and small intestine and in peripheral leukocytes. Rab39a shows 78% identity at the amino acid level to another recently described Rab protein, Rab39b (13).
We have investigated the nature of the interaction between caspase-1 and Rab39a, with particular interest in its role in IL-1β secretion. Our findings suggest that Rab39a functions as a trafficking adaptor linking caspase-1 to IL-1β secretion.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
PCMV-HA was purchased from Clontech. Rab39a and Rab39b (ATCC) were subcloned into PCMV-HA. AU-1-caspase-1 was a gift from Prof. Seamus Martin, Dept. of Genetics, Trinity College, Dublin, Ireland. Antibodies used were: anti-caspase-1 (A19) (Santa Cruz Biotechnology); anti-β-actin (Sigma), and anti-HA (Covance). Human recombinant caspase-1 and YVAD-Cmk were from Calbiochem. Agonists used were 2-kDa macrophage-activating lipopeptide (Malp-2) from Alexis, LPS from Escherichia coli, serotype EH100, from Alexis, Pam3Cys from Calbiochem, and ATP from Sigma.
Cell Culture and Transient Transfection
Human peripheral blood mononuclear cells (PBMCs) were isolated from human blood and maintained in RPMI 1640 medium supplemented with 10% fetal calf serum and 1% penicillin/streptomycin solution (v/v). 293T cells were transfected with Genejuice (Novagen). THP1 cells, PBMCs, and Raw 264.7 cells were transfected with the Amaxa system (Lonza) using Nucleofector solution V and Nucleofector program V-001, Y-001, or D-032, respectively.
Mass Spectrometry
Cell-free extracts were prepared from THP1 cells and incubated at 37 °C for 30 min to assemble inflammasomes as described previously (14). Samples were diluted in immunoprecipitation buffer (20 mm HEPES-KOH (pH 7.5), 50 mm NaCl, 0.3% CHAPS, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, I mm dithiothreitol, 2 μg/ml aprotinin, 1 μg/ml leupeptin, and 250 μm phenylmethylsulfonyl fluoride) and precleared with protein A/G-agarose beads (Santa Cruz Biotechnology) at 4 °C for 1 h. Precleared cell-free extracts were incubated with fresh protein A/G- agarose beads (Santa Cruz Biotechnology) and anti-caspase-1 antibody (Santa Cruz Biotechnology) overnight at 4 °C. Captured complexes were washed three times in immunoprecipitation buffer and eluted into two-dimensional gel sample buffer (8 m urea, 4% CHAPS, 0.05% SDS, 100 mm dithiothreitol, 0.03% bromphenol blue, 0.2% amphylotes). Proteins were separated using two-dimensional gel electrophoresis, silver stained, and in-gel protein digestion and protein identification by MALDI-TOF using a Voyager DE-PRO mass spectrometer were carried out as described previously (14).
Cytokine Analysis
IL-1β and TNF-α were measured by ELISA according to the manufacturer's instructions (R&D Systems). Each experiment was done in triplicate, and data are expressed as picograms/ml (mean ± S.D.) for a representative of at least three independent experiments. For comparison between two groups, Student's t test was used. A p value of < 0.05 was considered significant.
siRNA
Control siRNA and hRab39a siRNA (GGAGCGGUUCAGAUCAAUAtt) were from Ambion. THP1 cells or PBMCs were transfected using Amaxa Nucleofector (Lonza) according to the manufacturer's protocol (Nucleofector solution V, Nucleofector program V-001 or Y-001, respectively) with 1.5 μg of siRNA/106 cells. Cells were incubated at 37 °C for 48 h. Cells were stimulated with 100 ng/ml LPS 24 h prior to harvesting.
Real Time PCR
RNA extractions were carried out with RNeasy minikit (Qiagen). cDNA was created using a First Strand Synthesis Kit (Invitrogen). SYBR Green real time PCR (Invitrogen) was then carried out on the cDNA using primers specific for Rab39a or Rab39b and glyceraldehyde-3-phosphate dehydrogenase as a control: Rab39a forward, TCTACCAGTTCCGCCTCATC; Rab39a reverse, ATTGATCTGAACCGCTCCTG; Rab39b forward, TTTTTCCCCATTCTCTGGAA; Rab39b reverse, AGCGGTCCTCCGTTTTTAAT.
Co-immunoprecipitation and Immunoblotting
293T cells were transfected using Genejuice (Novagen) with the indicated plasmids where the total amount of DNA was kept constant. 36 h later, cells were lysed in immunoprecipitation lysis buffer (150 mm NaCl, 50 mm Tris-HCl (pH 8), 1% Nonidet P-40, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 1 μg/ml aprotinin). An aliquot of lysate was removed for whole cell lysate analysis. Lysates were precleared twice with protein A/G beads (Santa Cruz Biotechnology). Precleared cell-free extracts were incubated with fresh protein A/G-agarose beads and anti-caspase-1 antibody (Santa Cruz Biotechnology) for 4 h at 4 °C. The immune complexes were washed three times in immunoprecipitation buffer and eluted by the addition of sample buffer followed by SDS-PAGE and immunoblotting using the indicated antibodies.
Rab39a Cleavage Assay
293T cells were transfected as indicated, and lysates were used as substrates in a Rab39a cleavage assay, as described for IL-1β (1) and Mal (15) using recombinant caspase-1. Alternatively, Raw 264.7 cells were transfected as indicated and treated with 100 ng/ml LPS for 3 h followed by 5 mm ATP for 1 h.
Site-directed Mutagenesis of Rab39a
Point mutations were introduced into the Rab39a gene sequence using the QuikChangeTM site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions.
RESULTS
Rab39a Is a Previously Undescribed Binding Partner of Caspase-1
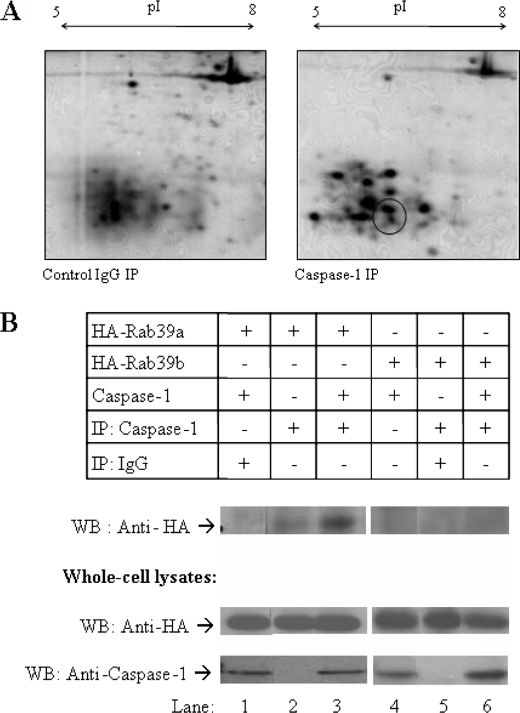
At the outset we wished to uncover components of the inflammasome to understand its functions and its regulation further. To do this, we used an immunoprecipitation approach to isolate caspase-1 from cell-free extracts of THP1 cells. Immunoprecipitates were washed extensively and eluted into sample buffer and resolved on two-dimensional polyacrylamide gels. Silver staining was used to visualize gels. Protein spots were compared with those from an IgG control immunoprecipitation. Protein spots that were differentially immunoprecipitated with caspase-1 were excised from the gels and were analyzed by MALDI-TOF mass spectrometry. Of these proteins, the Ras-related protein Rab39a (Swiss Protein Database accession no. Q14964) was detected in caspase-1 immunoprecipitates in the area indicated (Fig. 1A) with 25% sequence coverage.
FIGURE 1.
Rab39a is a novel binding partner of caspase-1. A, caspase-1 complexes were isolated from THP1 cell-free extracts. Proteins co-precipitating (IP) with caspase-1 were compared with proteins co-precipitating from an IgG control and were analyzed by two-dimensional gel electrophoresis (pI 5–8 first dimension and 12% SDS-PAGE second dimension). Protein spots of interest were identified by mass spectrometry. Rab39a was identified in the indicated area. B, 293T cells were transiently transfected with plasmids encoding caspase-1 and HA-Rab39a or HA-Rab39b. Caspase-1 was immunoprecipitated, and samples were probed with HA to show interaction. Whole cell lysates were immunoblotted (WB) with anti-HA anti-caspase-1. Results shown are each representative of two to four experiments.
To confirm that Rab39a can interact with caspase-1, co-immunoprecipitation experiments were carried out as shown in Fig. 1B. Because of the unavailability of an antibody to Rab39a, a HA-tagged version of Rab39a was constructed. HA-Rab39a and caspase-1 were overexpressed in 293T cells. Caspase-1 was immunoprecipitated from cells and probed with HA. Rab39a successfully co-immunoprecipitated with caspase-1 (top blot, lane 3) confirming the endogenous interaction identified by mass spectrometry. No interaction was seen in the IgG control (top blot, lane 1), confirming that the interaction was specific. Rab39b, which is 78% similar to Rab39a, was unable to co-immunoprecipitate with caspase-1 (top blot, lane 6).
Rab39a Is Required for IL-1β Secretion
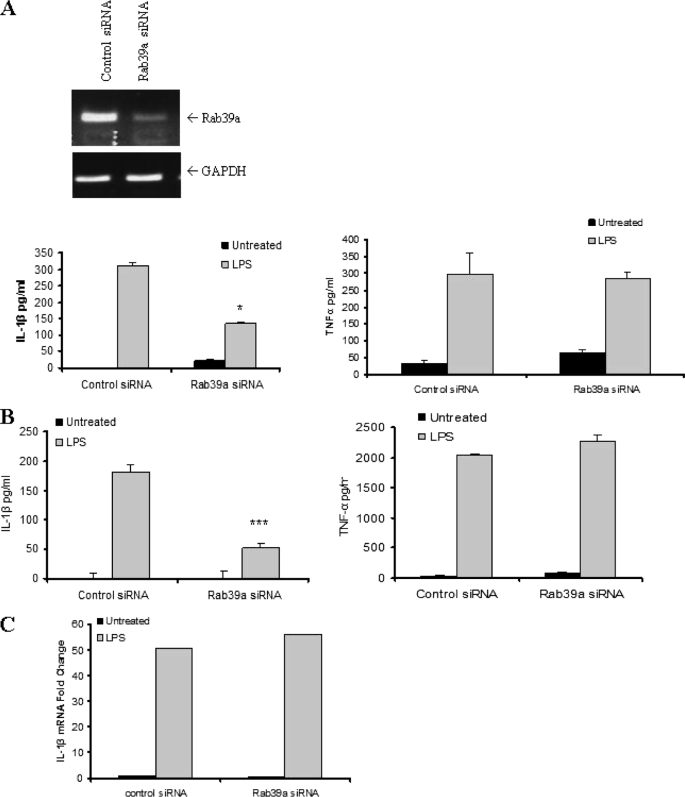
We hypothesized that Rab39a may play a role in the secretion of IL-1β because many Rab family members are involved in trafficking and secretion of proteins (11). To determine whether Rab39a was necessary for IL-1β secretion, siRNA nucleotides targeting Rab39a were transfected into THP1 cells, and secreted IL-1β was measured by ELISA. As shown in Fig. 2A (upper panel), the siRNA knocked down the expression of Rab39a. Compared with control siRNA, knock down of Rab39a resulted in a decrease in secreted IL-1β (left graph). Levels of TNF-α, which is externalized by the classical secretory pathway, were also measured. TNF-α levels remained constant despite Rab39a knock down, indicating that it is specific for IL-1β secretion (right graph).
FIGURE 2.
Rab39a is required for IL-1β secretion. A, knock down of Rab39a is shown by PCR (upper panel). THP1 cells were transfected with siRNA to Rab39a for 48 h. Cells were stimulated with 100 ng/ml LPS overnight before harvesting. IL-1β and TNF-α levels were measured by ELISA. B, PBMCs were transfected with siRNA to Rab39a for 48 h. Cells were stimulated with 100 ng/ml LPS overnight before harvesting. IL-1β and TNF-α levels were measured by ELISA. A and B, results are expressed as mean ± S.D. for triplicate determinations. ***, p < 0.005; *, p < 0.05. All results are representative of three separate experiments. C, RNA from THP1 cells from siRNA experiments was extracted, and mRNA levels of IL-1β were measured by real time PCR. Expression of IL-1β mRNA was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Data were calculated as fold change and are presented relative to that of untreated controls. Results shown are each representative of at least three independent experiments.
Knock down of Rab39a in human PBMCs also resulted in a dramatic decrease in IL-1β secretion (Fig. 2B, left graph) but not TNF-α (right graph). Importantly, mRNA levels of IL-1β, induced by LPS, remained constant when Rab39a was knocked down (Fig. 2C), indicating that the decrease in IL-1β might be due to a decrease in secretion and not in induction of the mature form of IL-1β.
Rab39a Can Be Cleaved by Recombinant Caspase-1
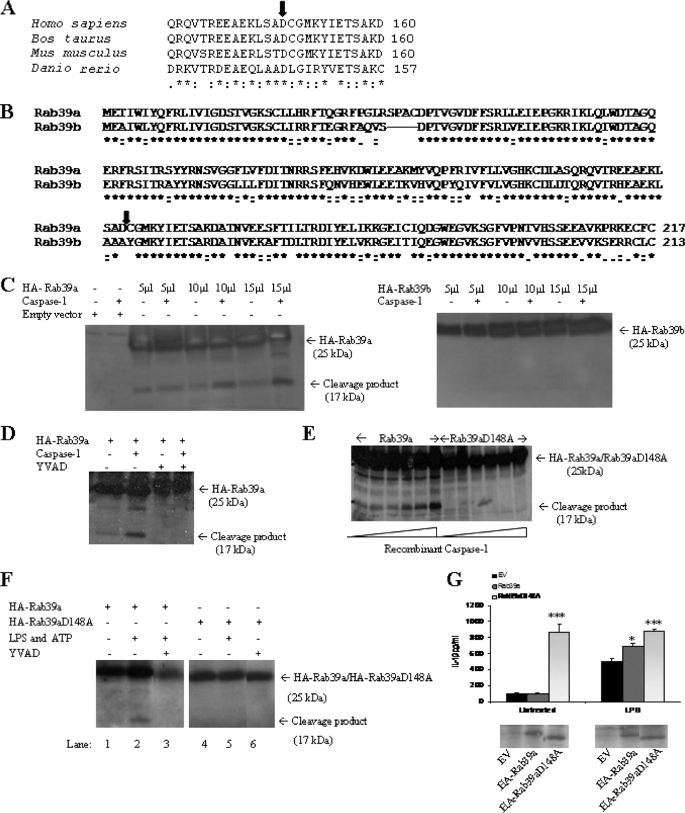
Rab39a contains a putative caspase-1 cleavage site at amino acid 148. This site in Rab39a is highly conserved and is found across many different species (Fig. 3A). Interestingly, Rab39b, which was not found to associate with caspase-1, does not contain this site (Fig. 3B).
FIGURE 3.
Rab39a contains a putative caspase-1 cleavage site and can be cleaved by recombinant caspase-1. A, caspase-1 cleavage is highly conserved. The arrow indicates the conserved putative caspase-1 cleavage site (see expasy peptide cutter site on the web). B, ClustalW alignment of the amino acid sequence of Rab39a and Rab39b is shown. The predicted cleavage site is indicated by the arrow (see expasy peptide cutter site on the web). C, 293T cells were transfected with plasmids encoding HA-Rab39a or HA-Rab39b. Cells were lysed, and increasing volumes of lysate were incubated with recombinant caspase-1 at 30 °C for 90 min. D, 293T cells were transfected with HA-Rab39a in the presence or absence of 100 μm caspase-1 inhibitor (YVAD-Cmk) and then incubated with recombinant caspase-1 at 30 °C for 90 min. E, 293T cells were transfected with HA-Rab39a or HA-Rab39a D148A. Lysates were incubated at 30 °C for 90 min with increasing concentrations of recombinant caspase-1. F, Raw 264.7 cells were transfected with plasmids encoding HA-Rab39a or HA-Rab39aD148A and treated with 100 ng/ml LPS and 5 mm ATP in the presence or absence of 100 μm caspase-1 inhibitor (YVAD-Cmk). Rab39a was detected by immunoblotting for anti-HA. G, HA-Rab39a or HA-Rab39aD148 was transfected into THP1 cells for 48 h. Cells were stimulated with 100 ng/ml LPS overnight before harvesting. Results are expressed as mean ± S.D. for triplicate determinations. ***, p < 0.005; *, p < 0.05. Results are representative of two or three independent experiments. EV, empty vector.
Thus, the question of whether Rab39a is a substrate of caspase-1 was next addressed. HA-Rab39a was overexpressed in 293T cells, and total cell lysates were incubated with recombinant caspase-1. Western blot analysis revealed the appearance of an additional form of Rab39a at 17 kDa, consistent with cleavage of Rab39a by caspase-1 at the proposed caspase-1 cleavage site. Rab39b, which lacks the cleavage site, showed no additional forms when incubated with recombinant caspase-1 (Fig. 3C). Rab39a could not be cleaved in the presence of YVAD-Cmk, a caspase-1 inhibitor (Fig. 3D).
To confirm the cleavage site in Rab39a, a mutated form of Rab39a was generated by site-directed mutagenesis in which the putative cleavage site Asp148 was replaced by Ala. No cleavage occurred, confirming the cleavage site as Asp148 (Fig. 3E).
Rab39a processing can also take place in macrophages treated with LPS and ATP. Raw 264.7 cells were transfected with HA-Rab39a, and cells were treated with 100 ng/ml LPS for 3 h and 5 mm ATP for 1 h. Western blot analysis revealed the appearance of the 17-kDa form of Rab39a, consistent with cleavage of Rab39a by caspase-1 at the proposed caspase-1 cleavage site. (Fig. 3F, lane 2). This effect was blocked by YVAD-Cmk (lane 3). Significantly; no cleavage took place with HA-Rab39aD148A (lane 5).
Overexpression of HA-Rab39a in THP1 cells resulted in an increase in IL-1β when cells were treated with LPS (Fig. 3G). Interestingly, when HA-Rab39aD148 (which lacks the caspase-1 cleavage site) was overexpressed this strongly increased IL-1β secretion on its own. This was not further enhanced by LPS treatment. The caspase-1-resistant form of Rab39a therefore appears to be able to drive IL-1β production.
Rab39a Can Be Induced by Pro-inflammatory Stimuli
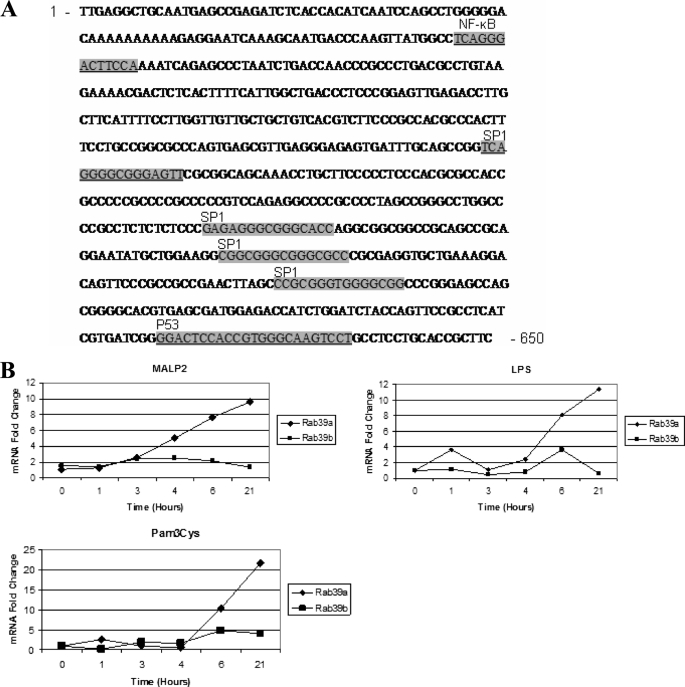
To understand further the role that Rab39a was playing in caspase-1-dependent IL-1β secretion, we examined whether Rab39a, like IL-1β, was inducible by pro-inflammatory stimuli. We analyzed the Rab39a promoter and found that it contains many putative transcription factor-binding sites, including NF-κB sites, p53 sites, and SP1 sites (Fig. 4A).
FIGURE 4.
Rab39a can be induced by pro-inflammatory stimuli. A, Rab39a promoter contains numerous putative transcription factor binding sites as indicated (adapted from the Genomatix website). B, THP1 cells were stimulated with 100 ng/ml LPS or 5 nm Malp-2 or 1 μg/ml Pam3Cys for the indicated times. mRNA levels were measured by real time PCR with primers specific for Rab39a and Rab39b; expression is normalized to that of glyceraldehyde-3-phosphate dehydrogenase and is presented relative to that of untreated controls. Results shown are representative of three independent experiments.
We analyzed the expression patterns of Rab39a mRNA by quantitative real time PCR in THP1 cells and tested whether expression was altered by pro-inflammatory stimuli. Our results show that Rab39a mRNA was significantly increased in response to LPS, Malp-2, and Pam3Cys (Fig. 4B).
DISCUSSION
IL-1β is a multifunctional cytokine and plays a major role in host defense (16). Unlike other pro-inflammatory cytokines, IL-1β lacks a signal peptide and is secreted via unconventional methods. The process by which it is secreted from the cell has always been contentious. The role of caspase-1 in inflammasome functions and IL-1β processing has been widely described (17). However, a role for caspase-1 in unconventional protein secretion is emerging. A recent paper describes how IL-1β secretion is mediated by caspase-1. This in turn may be mediated by factors that are substrates of caspase-1, the cleavage products of which might have a direct role in IL-1β secretion (10).
Our results unveil further insight into caspase-1-dependent IL-1β secretion. We have shown that the small GTPase protein Rab39a is a novel binding partner of caspase-1 as identified by mass spectrometry and confirmed by co-immunoprecipitation. We provide evidence that Rab39a is necessary for the secretion of IL-1β but not induction of pro-IL-1β because siRNA targeted to Rab39a results in diminished secretion of IL-1β but does not affect mRNA levels of IL-1β.
Bioinformatic analyses indicated that Rab39a contained a conserved caspase-1 cleavage site, and our results demonstrated that Rab39a was cleaved using an in vitro assay. We were also able to show that Rab39a processing could take place in macrophages treated with LPS and ATP. However, this should be interpreted with caution because cleavage of endogenous Rab39a should be shown. We are currently raising an anti-Rab39a antibody to test this. Interestingly, several other Rab proteins (notably Rab4 and Rab14, which are involved in secretion) contain the putative caspase-1 cleavage site. Cleavage of Rab proteins may represent a general mechanism by which caspase-1 modulates protein trafficking within the cell.
Other recent results have also shown that Rab proteins are important for signaling in the innate immune system. For example, Rab7b can negatively regulate both TLR4 and TLR9 signaling by promoting their translocation to the lysosome for degradation (18, 19). The results presented in this paper further add to this realization of the importance of Rab proteins during innate immunity.
We also found that Rab39a was inducible by pro-inflammatory stimuli, implying that its expression may be coordinated with that of IL-1β to promote secretion.
The precise mechanism of IL-1β secretion remains elusive because many models exist as to how this event may occur. Three of the best described models include the lysosome-dependent pathway (3, 4), microvesicle shedding (5, 6), and exosome release (7). In the lysosome-dependent pathway, pro-IL-1β is translocated into secretory lysosomes together with caspase-1. The mature form of IL-1β is produced within the lysosome by caspase-1 cleavage, after which the lysosomes fuse with the plasma membrane and the contents are released into the extracellular space. In microvesicle shedding, caspase-1 activates IL-1β in the cytoplasm and is exported along with the mature cytokine into the extracellular space. The third mechanism, exosome release, is where caspase-1 and IL-1β are contained in the cytoplasm within endosomal internal vesicles that are released as exosomes.
These models involve many unknown trafficking requirements, and interestingly the last two models require the formation of specific vesicles that exit from the cell. Rab proteins have roles in trafficking and secretion, and Rab39a, although largely uncharacterized is likely to function in this manner.
If Rab39a proves to be a physiological substrate, why it would require such cleavage requires further investigation, but it may be necessary for vesicle formation and secretion. We propose the following mechanism of action in which Rab39a associates with caspase-1 and together they are required for efficient IL-1β secretion. During an inflammatory response, Rab39a is induced and binds to caspase-1. As IL-1β is secreted in a caspase-1-dependent manner, we propose that Rab39a is needed to help traffic IL-1β from the cell in a vesicle-dependent manner. Indeed, caspase-1 itself and other components of the inflammasome that also lack signal peptides are also released from activated macrophages (8, 20–21), and Rab39a may be necessary in helping to secrete the entire inflammasome complex from the cell.
Interestingly, overexpression of a Rab39a construct lacking the caspase-1 cleavage site results in a boosting of IL-1β secretion. This suggests that cleavage of Rab39a by caspase-1 would serve as a mechanism of inactivating Rab39a and therefore act as a mechanism to control levels of IL-1β secretion. Hence, overexpression of a construct that cannot be cleaved results in an excess of IL-1β secretion. We are currently exploring the mechanism here.
The association between Rab39a and caspase-1 is highly specific. Rab39a is 78% similar to another Rab protein, Rab39b, but they have very different profiles with regard to caspase-1. Unlike Rab39a, Rab39b was not found to associate with caspase-1 in our screen, and it does not co-immunoprecipitate with caspase-1. It lacks a caspase-1 cleavage site and cannot be cleaved in vitro, and it is not induced by pro-inflammatory stimuli to the same extent as Rab39a. The role of Rab39a in IL-1β secretion is therefore specific.
We have therefore identified Rab39a as a caspase-1-associated protein required for IL-1β secretion. Future studies will aim to understand further precisely how Rab39a participates in this process.
This work was supported by grants from the Health Research Board Science Foundation Ireland and the Wellcome Trust.
- IL-1
- interleukin-1
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- ELISA
- enzyme-linked immunosorbent assay
- HA
- hemagglutinin
- LPS
- lipopolysaccharide
- MALDI-TOF
- matrix-assisted laser desorption ionization time-of-flight
- NF-κB
- nuclear factor κB
- NLR
- Nod-like receptor
- PBMC
- peripheral blood mononuclear cell
- siRNA
- small interfering RNA
- TNF-α
- tumor necrosis factor-α.
REFERENCES
- 1.Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J., Elliston K., Ayala J., Casano F., Chin J., Ding G., Egger L., Gaffney E., Limjuco W., Palyha O., Raju S., Rolando A., Salley J., Yamin T., Lee T., Shively J., MacCross M., Mumford R., Schmidt J., Tocci M. (1992) Nature 356, 768–774 [DOI] [PubMed] [Google Scholar]
- 2.Eder C. (2009) Immunobiology 214, 543–553 [DOI] [PubMed] [Google Scholar]
- 3.Andrei C., Dazzi C., Lotti L., Torrisi M. R., Chimini G., Rubartelli A. (1999) Mol. Biol. Cell 10, 1463–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrei C., Margiocco P., Poggi A., Lotti L. V., Torrisi M. R., Rubartelli A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9745–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKenzie A., Wilson H. L., Kiss-Toth E., Dower S. K., North R. A., Surprenant A. (2001) Immunity 15, 825–835 [DOI] [PubMed] [Google Scholar]
- 6.Pelegrin P., Barroso-Gutierrez C., Surprenant A. (2008) J. Immunol. 180, 7147–7157 [DOI] [PubMed] [Google Scholar]
- 7.Qu Y., Franchi L., Nunez G., Dubyak G. R. (2007) J. Immunol. 179, 1913–1925 [DOI] [PubMed] [Google Scholar]
- 8.Martinon F., Burns K., Tschopp J. (2002) Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
- 9.Martinon F., Mayor A., Tschopp J. (2009) Annu. Rev. Immunol. 27, 229–265 [DOI] [PubMed] [Google Scholar]
- 10.Keller M., Rüegg A., Werner S., Beer H. D. (2008) Cell 132, 818–831 [DOI] [PubMed] [Google Scholar]
- 11.Zerial M., McBride H. (2001) Nat. Rev. Mol. Cell Biol. 2, 107–117 [DOI] [PubMed] [Google Scholar]
- 12.Stankovic T., Byrd P. J., Cooper P. R., McConville C. M., Munroe D. J., Riley J. H., Watts G. D., Ambrose H., McGuire G., Smith A. D., Sutcliffe A., Mills T., Taylor A. M. (1997) Genomics 40, 267–276 [DOI] [PubMed] [Google Scholar]
- 13.Cheng H., Ma Y., Ni X., Jiang M., Guo L., Ying K., Xie Y., Mao Y. (2002) Cytogenet. Genome Res. 97, 72–75 [DOI] [PubMed] [Google Scholar]
- 14.Creagh E. M., Brumatti G., Sheridan C., Duriez P. J., Taylor R. C., Cullen S. P., Adrain C., Martin S. J. (2009) PLoS One 4, e5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miggin S. M., Pålsson-McDermott E., Dunne A., Jefferies C., Pinteaux E., Banahan K., Murphy C., Moynagh P., Yamamoto M., Akira S., Rothwell N., Golenbock D., Fitzgerald K. A., O'Neill L. A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 3372–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello C. A. (2009) Annu. Rev. Immunol. 27, 519–550 [DOI] [PubMed] [Google Scholar]
- 17.Martinon F., Agostini L., Meylan E., Tschopp J. (2004) Curr. Biol. 14, 1929–1934 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Chen T., Han C., He D., Liu H., An H., Cai Z., Cao X. (2007) Blood 110, 962–971 [DOI] [PubMed] [Google Scholar]
- 19.Yao M., Liu X., Li D., Chen T., Cai Z., Cao X. (2009) J. Immunol. 183, 1751–1758 [DOI] [PubMed] [Google Scholar]
- 20.Mariathasan S., Newton K., Monack D. M., Vucic D., French D. M., Lee W. P., Roose-Girma M., Erickson S., Dixit V. M. (2004) Nature 430, 213–218 [DOI] [PubMed] [Google Scholar]
- 21.Feldmeyer L., Keller M., Niklaus G., Hohl D., Werner S., Beer H. D. (2007) Curr. Biol. 17, 1140–1145 [DOI] [PubMed] [Google Scholar]