Abstract
IL-8 produced by prostate cancer cells may be responsible for the androgen-independent growth of advanced prostate cancers. Accumulating evidence from microarray analyses and animal genetic models highlights the central involvement of the transcription factor early growth response-1 (EGR-1) in prostate carcinoma progression. It is unknown, however, whether knockdown of EGR-1 inhibits IL-8 production and IL-8-mediated tumor metastasis. Here we show that EGR-1 knockdown by a specific shRNA-Egr1 inhibited gene transcription and production of IL-8 by the human prostate cancer cell line DU145. Conversely, enforced expression of EGR-1 in EGR-1-lacking PC3 prostate cancer cells markedly enhanced IL-8 transcription and secretion. By using wild type and a series of mutant IL-8 promoter luciferase constructs, we found that the NF-κB binding site is important for EGR-1 regulation of IL-8. Furthermore, silencing EGR-1 suppressed a synergistically functional interaction between EGR-1 and NF-κB. Consequently, knockdown of EGR-1 inhibited IL-8-mediated tumor colony formation and invasion. Thus, targeted knockdown of EGR-1 could be an effective therapeutic approach against prostate cancer.
Introduction
Prostate cancer is currently the most prevalent noncutaneous cancer in men in the Western world and is the second leading cause of male death from cancer. There is considerable evidence from experimental models and studies conducted on patient samples to support a role for the pro-inflammatory chemokine interleukin 8 (IL-8)2 in the promotion of prostate cancer progression (1, 2). Several studies have now confirmed elevated expression of IL-8 and its associated receptors in prostate cancer (3–6), although these independent studies suggest markedly different distribution patterns for IL-8 and its receptors. By using immunohistochemistry staining, IL-8 expression was detected in glandular epithelial cells of prostate cancer tissue, with little or no IL-8 staining hypertrophy or normal prostate epithelium (7, 8). In contrast, Huang et al., reported that IL-8 was expressed solely by neuroendocrine rather than epithelial cells. Their analysis of benign and malignant prostate tissue cores confirmed an increased IL-8 expression that correlated with progressive disease (9). These studies suggested that there is a consistent trend of increased and concurrent expression of IL-8 and its two receptors in prostate cancer tissue; thus, indicating that prostate cancer cells are subject to a continuous autocrine/paracrine stimulus. In addition, several studies have reported the detection of increased IL-8 levels in the serum of patients with either localized or metastatic prostate cancer relative to control patients or patients with benign prostatic hypertrophy (10, 11). It is evident that future research providing a more comprehensive understanding of the transcriptional, translational, and post-translational signaling basis for IL-8-promoted cell motility and cell invasion will be required to identify viable and effective therapeutic strategies to attenuate the disease-progressing effects of IL-8.
EGR-1 expression results in either promotion or regression of cell proliferation, depending on the cellular context (12–14). Accumulating evidence indicates that EGR-1 plays a significant part in the development and progression of prostate cancer (15–21). There is a direct correlation between the level of EGR-1 expression and the prostate tumor Gleason grade (19). In addition, loss of the EGR-1 corepressor NAB2 enhances the EGR-1 levels in prostate cancer. Although there is no EGR-1 binding motif in the promoter of the interleukin 8 gene, EGR-1-associated IL-8 production was investigated in combination with three central transcription factors such as NF-κB, C/EBP, or AP-1 (22). The expression of EGR-1 can be induced by a range of stimuli, which in many cases overlap with those known to be capable of inducing NF-κB expression. NF-κB and EGR-1 have been shown to cooperatively stimulate the NF-κB promoter (23) despite a report that EGR-1 specifically represses NF-κB transcriptional activity in a regulatory way by preventing its interaction with promoter target elements (24). In addition, it has been reported that EGR-1 regulates proinflammatory cytokine gene expression by synergistic interaction with other transcription factors such as nuclear factors of activated T cells (NFAT) (25, 26). AP-1 is also positive downstream of EGR-1 in a cooperative way (27). It is unknown, however, whether knockdown of EGR-1 inhibits IL-8 production and IL-8-mediated tumor metastasis.
The proinflammatory chemokine IL-8 is undetectable in androgen-responsive prostate cancer cells (e.g. LNCaP and LAPC-4), but it is highly expressed in androgen-independent metastatic prostate cancer tissue (9) and cell lines such as DU145. Therefore, IL-8 is associated with chemoresistance, tumor growth, and angiogenesis in androgen-independent prostate carcinoma (28–31). In the present study, we used DU145/sh-EGR1 and DU145/sh-Control stable cell lines to evaluate the effect of EGR-1 knockdown on IL-8 transcription and production by human prostate cancer cells. Furthermore, it appears that EGR-1 functions cooperatively with NF-κB to stimulate IL-8 transcription and production. Our data demonstrate knockdown of EGR-1-inhibited tumor colony formation and invasion may be through the down-regulation of IL-8. Thus, targeted knockdown of EGR-1 could be an effective therapeutic approach against prostate cancer.
EXPERIMENTAL PROCEDURES
Reagent and Antibodies
All reagents and chemicals and affinity-purified anti-rabbit IgG and anti-mouse IgG coupled to horseradish peroxidase or fluorescent tags were purchased from Sigma. The following specific antibodies were used in this study: anti-NF-κB p65 and p50 (Upstate Biotech); anti-EGR-1, anti-actin (Santa Cruz Biotechnology), The IL-8 ELISA kit was purchased from R&D Systems. QCMTM 24-Well Cell Invasion Assay kit was purchased from Chemicon Corp.
shRNA Expression, siRNA Duplex, and Reporter Gene Constructs
The empty pU6 + 27, shRNA-control and shRNA-p65 vectors were purchased from Panomics Corp. (Fremont, CA). A 21-nucleotide sequence coding for amino acids 413–419 of human Egr-1 was selected as the targets for RNAi according to the manufacturer's instructions. In all cases, the corresponding sequences were scrambled to generate a control vector. In initial studies, we used empty vector (pU6 + 27) and scrambled shRNA as controls. Double-strand siRNAs targeting NF-κB (p65) and scramble control were synthesized using the following sequences: siRNA-p65: forward 5′-GAU UGA GGA GAA ACG UAA AdTdT, reverse 5′-UUU ACG UUU CUC CUC AAU CdTdT; siRNA-scramble: forward 5′-AUG AAC GUG AAU UGC UCA AdTdT, Reverse 5′-UUG AGC AAU UCA CGU UCA UdTdT. siRNA duplexes were purchased from Invitrogen.
To construct the IL-8 promoter luciferase reporter, a 350-bp DNA fragment from the promoter region of the human IL-8 gene, including −323 to +27 nt upstream of the start ATG, was obtained by PCR using human genomic DNA as the template and primer pair IL-8-pf: 5′- CGA CGC GTC ACC TGC CAC TCT AGT ACT A-3′ and IL-8-pr: 5′- CCG CTC GAG AAG CTT GTG TGC TCT GCT G-3′, where the restriction enzyme sites for cloning are indicated by underlines. The PCR products were inserted into the pGL3-basic vector (Promega, Madison, MI) and designated as pGL3-IL-8-wt. The AP-1, CEBP, and NF-κB binding sites were each mutated using site-directed mutagenesis as indicated in Fig. 3A. All constructs were verified by sequencing. The Egr-1 expression plasmids containing a full-length cDNA coding for human EGR-1 were generous gifts from Dr. Jie Du, Baylor College of Medicine, Houston, TX.
FIGURE 3.
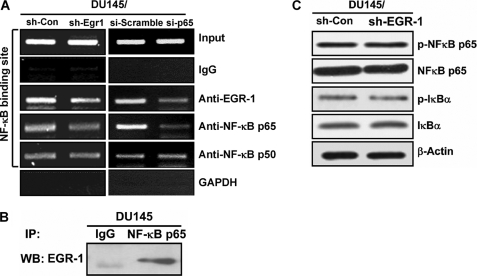
EGR-1 and NF-κB forms a protein-protein complex that binds to the NF-κB site within the IL-8 promoter. A, ChIP assay was performed with cell lysates from DU145/sh-EGR-1 and vector control cells (left panel) or DU145/si-p65 and scramble control cells (right panel). The cells were cross-linked with 1% formaldehyde, and chromatin was immunoprecipitated with anti-EGR-1, -NF-κB p65, and -NF-κB p50 antibodies or normal IgG that served as a negative control. The genomic fragments associated with immunoprecipitated DNA were isolated and amplified by PCR using specific primers flanking the NF-κB binding site within the IL-8 promoter. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. The input was used as a positive control showing equal loading of the cell lysates, whereas GAPDH is the negative control of the ChIP assay. B, immunoprecipitation assay with cell lysates from parent DU145 cells. C, effect of EGR-1 on expression and phosphorylation of NF-κB measured by Western blot.
Cell Culture and Transfection
Human prostate cancer cell lines, DU145 and PC3, were purchased from ATCC and cultured at 37 °C in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 2 mm l-glutamine. Lipofectamine-2000 (Invitrogen) was employed for transfection. The stably transfected cell lines were obtained after being selectively screened by G418 (800 μg/ml, GIBCO) for 3–4 weeks.
RT-PCR and Real-time PCR
Total RNA was isolated by TRIzol (Invitrogen) according to the manufacturer's instructions, and 1 μg was converted to cDNA using the Superscript III reverse transcriptase (Invitrogen). The following primer sets were used for RT-PCR and real-time PCR with Cyber-Green incorporation: huIL-8-RT- Re: TGG TGG CGC AGT GTG GTC CA, huIL-8-RT- Fw: AAG CTG GCC GTG GCT CTC TT; β-ACTIN-Re: GTC ACA CTT CAT GAT GGA GTT GAA GG, β-ACTIN-Fw: GAC CTG ACT GAC TAC CTC ATG AAG AT; Egr-1-Re: TGG GTG CCG CTG AGT AAA TG, Egr-1-Fw: CTG ACC GCA GAG TCT TTT CCT G.
Immunoblot Analyses
For preparing the whole cell extract, cells were harvested and lysed in the Nonidet P-40 cell lysis buffer containing 50 mm Tris-Cl, pH 6.8, 150 mm NaCl, 1 mm EGTA, 1% Nonidet P-40, and freshly added proteinase inhibitors, including 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin (Sigma), 1 μg/ml pepstatin (Sigma), and 1 μg/ml aprotinin (Sigma). Lysates were subjected to immunoblot analysis. Horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence detection (Pierce Chemical Co.) was used to detect specific immunoreactive proteins.
Immunoprecipitation
Cellular lysates were prepared using a radioimmune precipitation assay buffer (50 mm Tris-Cl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mm Na3VO4, and 1 mm NaF). Equivalent amount of total protein was incubated with 2 μg of specific antibody or control IgG at 4 °C overnight. The immune complexes were captured by incubation with protein A-agarose at 4 °C for 2 h, and the immunoprecipitated proteins were subjected to Western blotting with specific antibodies.
Chromatin Immunoprecipitation Assay (ChIP)
Cells were seeded 24 h prior to fixation with 1% formaldehyde at 37 °C for 7 min. The cells were harvested in lysis buffer (50 mm Tris-Cl, pH 8.1, 10 mm EDTA, 1% SDS) and sonicated to shear the chromatin (∼500 bp). The soluble fraction was collected by centrifugation and incubated with specific antibodies or control IgG at 4 °C overnight. The immune complexes were captured with protein A-agarose beads. After extensive washing, the bound DNA fragments were eluted and purified (32, 33). DNA from these samples was subjected to PCR analyses with NF-κB-BS-pf: 5′-CAT CAG TTG CAA ATC GTG GA-3′ and NF-κB-BS-pr: 5′-GAA GCT TGT GTG CTC TGC TG-3′; GAPDH-pf: 5′-GTA TTC CCC CAG GTT TAC AT-3′ and GAPDH-pr: 5′-TTC TGT CTT CCA CTC ACT CCT-3′. The PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining. The GAPDH promoter fragment acted as the internal negative control for this assay.
Luciferase Reporter Assays
Briefly, an appropriate amount of the IL-8 promoter-luciferase reporters, together with Renilla luciferase plasmids, which served as the internal control, were co-transfected into cells. 24 h later, cellular lysates were subjected to Dual-luciferase Reporter Assay (Promega) according to the manufacturer's instructions. Luciferase activities for the promoter reporters were normalized to Renilla luciferase activities. Data are presented as the ratio of IL8-Wt in DU145/sh-Con cells. Data represent at least three independent experiments, and in each experiment, there were triplicate tests for all samples.
Tumor Colony Formation Assay
Forty-eight hours after transfection with siRNA, 5 × 103 cells were suspended in 1.5 ml of Dulbecco's modified Eagle's medium with 0.35% agar and 10% fetal bovine serum and seeded in 6-well plates precoated with the same medium used for suspending the cells except containing 0.5% agar. The cells were allowed to form colonies for 14 days. At the end, the colonies were stained with 0.1% crystal violet for 10 min at 37 °C and counted. The experiments were repeated at least twice with three or six replicates in each experiment, and the data are presented as the mean of multiple tests. The Student's t test was used for statistical analysis.
ELISA
ELISA assays were performed using IL-8 ELISA kits from R&D Systems according to the manufacturer's instructions. Cell culture supernatants were collected and diluted 5–160-fold with deionized water before assaying. The data represent the mean of at least two experiments and triplicate samples for each experiment.
Cell Invasion Assay
Stable transfected DU145/sh-con and DU145/sh-Egr1 cells were transiently transfected with either si-Scramble or si-IL-8 RNA duplexes (Invitrogen) before incubation for 48 h; the cells were plated onto Matrigel-coated wells and cultured for another 48 h. By the end, the cells in the lower chamber of the transwells were collected, and cell numbers were detected by CyQUANT@GR Dye (QCMTM 24-Well Cell Invasion Assay kit from Chemicon Corp.) per the manufacturer's instruction. Data are presented as relative luminescence units and the mean of three samples in each experiment.
RESULTS
Knockdown of EGR-1 Decreases Transcription and Secretion of IL-8 by Prostate Cancer Cells
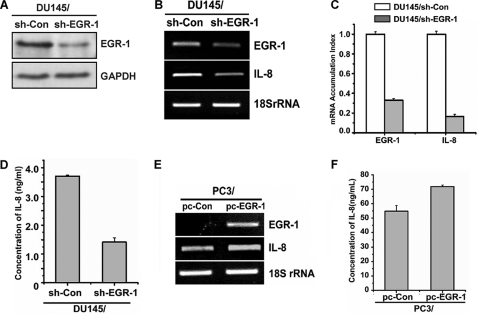
To determine whether EGR-1 regulates transcription and secretion of IL-8 by prostate cancer cells, DU145 cells were transfected with shRNA-EGR-1 plasmids or control plasmids to generate the DU145/sh-EGR-1 and DU145/sh-Con stable cell lines, respectively. The level of EGR-1 expression in both cell lines was analyzed by Western blotting, RT-PCR, and real-time PCR. As show in Fig. 1A, a significant reduction of EGR-1 expression was observed, confirming the knockdown of Egr-1 in DU145/sh-EGR-1 stable prostate cancer cells. RT-PCR (Fig. 1B) and real-time PCR (Fig. 1C) showed that the level of transcripts of IL-8 was reduced in the DU145 cell with EGR-1-knockdown compared with the control cells. Furthermore, IL-8 secretion by the above cell lines was also measured by ELISA. Knockdown of EGR-1 resulted in a significant decrease of IL-8 production (Fig. 1D).
FIGURE 1.
The expression of IL-8 is correlated with the level of EGR-1 in prostate cancer cells. A, B, and C, whole cell lysates or total RNA were prepared from DU145 cells stably transfected with either the control vector or shRNA targeting EGR-1 (designated as DU145/sh-Con and DU145/sh-EGR-1, respectively). The expression of EGR-1 was monitored by Western blot (A), RT-PCR, and real-time PCR, whereas the level of IL-8 transcripts in the same sample was measured by RT-PCR (B) and real-time PCR (C). D, accumulated secretion of IL-8 was assayed by ELISA with the cell culture supernatants collected after a period of culture of 48 h. E and F, transcript levels of EGR-1 and IL-8 in PC3 cells that were either stably transfected with pcDNA3 control vector or EGR-1 expression plasmid (designated as PC3/pc-Con and PC3/pc-EGR1, respectively) were monitored by RT-PCR (E), whereas the accumulated production of IL-8 was assayed by ELISA (F).
Because PC3, another prostate cancer cell line, expresses little detectable EGR-1 mRNA, we used PC3 cells as a model system to directly assess the actual role of EGR-1 expression in the EGR-1-mediated IL-8 production by prostate cancer cells. The PC3 cells were transfected with plasmids containing either cDNA coding for full-length human EGR-1 wild type or empty vector pcDNA3.1. Compared with the control PC3 cells, the level of IL-8 transcription and secretion was significantly higher in the EGR1-overexpressing cells (Fig. 1, E and F). These results suggested that expression and secretion of IL-8 by prostate cancer cells are correlated with the level of EGR-1.
Importance of NF-κB Binding Site for EGR-1 Regulation of IL-8
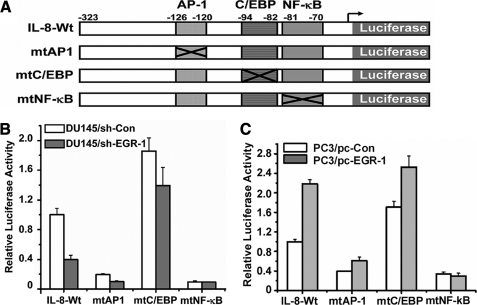
Although there is no EGR-1 binding motif in the promoter of the interleukin 8 gene, EGR-1-associated IL-8 production may be regulated by other transcription factors such as NF-κB, C/EBP, or AP-1 because the IL-8 promoter contains these binding sites. We used wild type and a series of mutant IL-8 promoter luciferase constructs to assess whether EGR-1 affects activation of IL-8 promoter mediated by the above transcription factor binding sites. DU145/sh-Con and DU145/sh-EGR-1 cells were transiently transfected with the −323 bp IL-8 promoter construct and mutation constructs each containing AP-1 (−126 to −120), C/EBP (−94 to −81), or NF-κB site mutations (−80 to −69) (Fig. 2A) for 48 h and assayed for luciferase activity. As shown in Fig. 2B, compared with the control DU145 cells, knockdown of EGR-1 resulted in a 60% loss in IL-8 promoter luciferase activity of transfected cells with a wild-type construct, confirming that EGR-1 regulates IL-8 promoter activity in prostate cancer cells. When cells were transfected with an IL-8 promoter construct containing a single C/EBP site mutant (−94 to −81) or AP-1 site mutant (−126 to −120), IL-8 promoter activation was affected by knockdown of EGR-1. However, in the case of the transfected cells with the construct with a single mutation of the NF-κB site, there was a lower level of IL-8 promoter luciferase activity than that of the wild-type construct. In addition, no difference in transactivation of the IL-8 promoter containing an NF-κB binding site mutation between DU145/sh-EGR-1 and control cells was observed, demonstrating that EGR-1-regulating transactivation of IL-8 promoter is associated with NF-κB.
FIGURE 2.
EGR-1 regulates IL-8 requiring the NF-κB binding site. A, histograph represents the IL-8 promoter reporters used in the study. The location of each binding site on the promoter of IL-8 is indicated and labeled. The crossed boxes indicate the mutation sites on each construct and are designated as mtAP1, mtC/EBP, and mtNF-κB for AP-1, C/EBP, and NF-κB binding sites, respectively. IL8-wt contains a wild-type promoter sequence from the transcription initiation nucleotide as +27 to −323 bp of the IL-8 proximal promoter. B, above, IL-8-Wt and -mutant promoter reporters were transfected into the DU145/Sh-EGR-1 or control cells for 24 h for the luciferase activity assay. C, above, IL-8 Wt and mutant promoter reporters were transfected into the PC3/pc-EGR-1 or control cells for 48 h for the luciferase activity assay.
Furthermore, similar experiments were performed on EGR-1-overexpressing PC3 cells and their parent cells (Fig. 2C). Overexpression of EGR-1 in PC3 prostate cancer cells markedly enhanced IL-8 promoter activity, but did not affect activity of IL-8 promoter with a single NF-κB site mutation. These data indicate that the NF-κB site is the most important positive regulatory element and the target site for EGR-1 activation of IL-8 promoter transactivation in prostate cancer cells.
The EGR1-NF-κB Complex Binding to the IL-8 Promoter
We used ChIP to directly assess whether EGR-1 binds to the NF-κB binding site of IL-8 promoter. Stably transfected DU145/sh-con and DU145/sh-EGR-1 cells were harvested for the preparation of chromatin, which was immunoprecipitated with anti-EGR-1, -NF-κB p65, and -NF-κB p50 antibodies or normal IgG. Genomic fragments associated with immunoprecipitated DNA were amplified by PCR using specific primers flanking the NF-κB binding site within the IL-8 promoter (Fig. 3A, left panel). Not only did NF-κB bind to its specific binding site in the IL-8 promoter, but also the occupancy by EGR-1 of the NF-κB site was detected. Transfection with sh-Egr1 resulted in the decrease in such EGR-1 occupancy of NF-κB site in IL-8 promoter.
To further examine whether the binding of EGR-1 to IL-8 promoter is dependent on NF-κB, another ChIP assay was performed using control and p65 knockdown cells (Fig. 3A, right panel). Knockdown of p65 decreased the association of EGR-1 to a region (−500 bp) that contains the κB site in IL-8 promoter, demonstrating that the molecular interplay between EGR-1 and NF-κB in the regulation of IL-8 is dependent on p65.
Similar results were observed in co-immunoprecipitation analysis performed with cell lysates from parent DU145 cells using anti-NF-κB p65 monoclonal antibody for immunoprecipitation and anti-EGR-1 for blotting (Fig. 3B). The data indicated that EGR-1 formed a complex with NF-κB binding to the IL-8 promoter.
Next, we examined whether EGR-1 affected expression and phosphorylation of NF-κB using Western blot. As shown in Fig. 3C, neither the level of expression, nor phosphorylation of NF-κB was affected by knockdown of EGR-1. This suggested that EGR-1 regulates IL-8 through facilitating NF-κB regulatory activity on the IL-8 promoter.
Silencing EGR-1 Suppressed a Synergistically Functional Interaction between EGR-1 and NF-κB
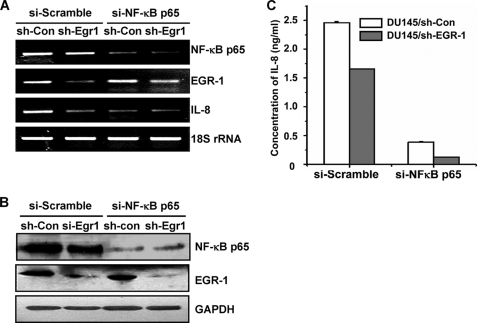
To further investigate how the EGR-1/NF-κB complex affects the expression of IL-8, DU145/sh-EGR-1 stable cell line and its parent cell line were transfected with RNA duplexes siRNA-p65 or siRNA scramble that either specifically targeted the NF-κB p65 coding region or served as control, respectively. In contrast to scramble transfections, transcription and expression of both NF-κB p65 and EGR-1 were significantly reduced in DU145/sh-Egr1 cells transfected with siRNA-p65 determined by RT-PCR (Fig. 4A) and Western blot (Fig. 4B), confirming double knockdown of NF-κB p65 and EGR-1. IL-8 secretion by the same cells was measured by ELISA (Fig. 4C). Comparing the DU145 cells transfected with either sh-Egr1 or si-p65 alone, double knockdown of EGR-1 and NF-κB p65 resulted in a remarkable loss of IL-8 production. This suggested that EGR-1 functions cooperatively with NF-κB to stimulate IL-8 transcription and production. Silencing EGR-1 suppressed a synergistically functional interaction between EGR-1 and NF-κB.
FIGURE 4.
Silencing EGR-1 suppressed a synergistically functional interaction between EGR-1 and NF-κB. Total RNAs and cell lysates were prepared from DU145/si-con and DU145/si-Egr1 cells transfected with scramble RNA and siRNA targeting NF-κB p65, respectively, and subjected to RT-PCR (A) and Western blotting (B) to monitor the expression levels of NF-κB p65 and EGR-1. The expression level of IL-8 was assessed by RT-PCR (A) and ELISA (C), which was performed the same as described in the legend to Fig. 1.
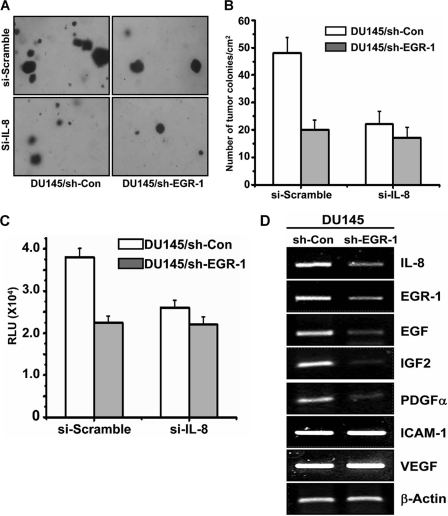
Knockdown of EGR-1 Inhibited IL-8-mediated Tumor Colony Formation and Invasion
Because IL-8 has been reported to be involved in tumor colony formation and invasion, we investigated whether EGR-1 might further regulate IL-8-mediated tumor colony formation and invasion. Stably transfected DU145/sh-EGR-1 and DU145/sh-Con cells were transiently transfected with either siRNA-IL-8 or siRNA-Scramble. A human tumor colony-forming assay (Fig. 5, A and B) and in vitro chemoinvasion assay (Fig. 5C) were performed with the cells under different conditions. When the level of IL-8 in DU145 cells was reduced by transfection with siRNA-IL-8, both the tumor colony number and invasion activity of DU145 cells were significantly dropped off, confirming that IL-8 stimulates tumor colony formation and invasion of prostate cancer cells. In DU145 cells with knockdown of EGR-1, the number of tumor colonies and invasion of cells through the extracellular matrix were significantly decreased compared with the control DU145 cells (Fig. 5, A, B, and C, respectively). Silencing IL-8 also attenuated EGR-1 knockdown-caused inhibition of the tumor colony number and invasion activity on DU145 cells. The data indicated that knockdown of EGR-1 inhibited tumor colony formation and invasion that might be through down-regulation of IL-8.
FIGURE 5.
Knockdown of Egr-1 inhibited IL-8-mediated tumor colony formation and invasion. A and B, tumor colony formation was performed with DU145/si-con and DU145/si-Egr1 cells that were further transfected with siRNA silencing IL-8 or scramble RNA. The cells were plated in a 6-well plate (1 × 104 cells/well) and cultured for 14 days. Cell colonies were visualized under a microscope (A), and the number was counted and presented as number/cm2 (B). C, for the chemoinvasion assay, the transfected cells were plated onto Matrigel-coated wells and cultured for 48 h. By the end, the cells in the lower chamber of the transwell were collected and measured by CyQUANT@GR Dye (QCMTM 24-Well Cell Invasion Assay kit) per the manufacturer's instruction. The data are presented as relative luminescence units and the mean of three samples in each experiment. D, gene expression profile of other growth factors and invasion proteins measured by RT-PCR.
Because other growth factors in addition to IL-8 may also participate in tumor invasion, we further examined the gene expression profile in EGR-1 knockdown prostate cells. In DU145/sh-EGR1 cells, the expression levels of EGF, PGDF-α, and IGF2 were significantly less than that in control DU145 cells (Fig. 5D). However, the expression of ICAM-1 and VEGF was not affected by EGR-1 knockdown. Therefore, besides IL-8, the role of other growth factors in tumor invasion needs to be further investigated.
DISCUSSION
The expression of IL-8 has been shown to correlate with the angiogenesis, tumorigenicity, and metastatic potential of prostate cancer cells. Inhibiting these pronounced effects of IL-8 signaling within the tumor microenvironment may have significant therapeutic potential in modulating disease progression (1, 31). This study revealed that EGR-1 knockdown resulted in significant reduction in the expression of IL-8 gene transcription and protein production. Small interfering RNA-mediated knockdown of EGR-1 in DU145 cells dramatically decreased IL-8-mediated tumor forming and invasion. Because the majority of clinical studies confirm overexpression of IL-8 in the most advanced stages of disease, this suggests that suppressing the effects of IL-8 may have important implications for the systemic treatment of aggressive and metastatic disease.
The present data, obtained from both knockdown and overexpression of EGR-1 experiments, demonstrate that EGR-1 suppresses transactivation of IL-8 via down-regulation of the IL-8 promoter in an EGR-1-dependent manner. Interfering of EGR-1 expression repressed IL-8 induction as well as EGR-1 production. Although there is no EGR-1 binding motif in the promoter of the interleukin-8 gene, EGR-1-associated IL-8 production may be regulated by other transcription factors in an indirect way. The transcriptional regulation of the IL-8 gene has been analyzed extensively at the level of the IL-8 promoter. Cis-acting elements for several transcription factors have been identified within this regulatory region (22). The factors that bind to these motifs include C/EBP, AP-1, and NF-κB. By using wild type and a series of mutant IL-8 promoter luciferase constructs, we have found that the NF-κB binding site is an important positive regulatory element for EGR-1 regulation of IL-8 (Fig. 2). It is also worthy of note that deletion of the κB site seems to be too destructive to the promoter with/without EGR-1 knockdown. By using ChIP assays, we have found the decreased binding of EGR-1 to the IL-8 promoter after treatment with shRNA-Egr-1 (Fig. 3A). Such molecular interplay between EGR-1 and NF-κB in the regulation of IL-8 is dependent on p65. We further show that EGR-1 interacts with NF-κB forming an NF-κB/EGR-1 complex, which was consistent with Cogswell et al. (23) observations in T cell activation. Moreover, IL-8 transcription (mediated synergistically by the NF-κB and EGR-1 transcription factors) was significantly reduced by knockdown of EGR-1 in prostate carcinoma cells.
Taken together, these data provided the evidence that targeting knockdown of EGR-1 resulted in the reduction of IL-8 production and IL-8-mediated tumor colony formation and invasion of prostate cancer cells through suppression of a synergistically functional interaction between EGR-1 and NF-κB. Such a model could highlight the importance of transcriptional cross-talk in IL-8-mediated prostate cancer growth and progression. EGR-1 may be the therapeutic molecular target in androgen-independent prostate cancer cells by targeting IL-8 gene expression.
This work was supported in part by grants from the National Natural Science Foundation of China (30771973, 30721002), National Basic Research Program of China (973 Program) (2007CB914503), Ministry of Science and Technology of China (KSCX1-YW-R-58 and KJCX2-YW-N38), and China Ministry of Education (20060358019).
- IL
- interleukin
- AP-1
- activator protein 1
- C/EBP
- CCAAT/enhancer-binding protein
- ChIP
- chromatin immunoprecipitation
- EGF
- epidermal growth factor
- EGR-1
- early growth response-1
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- NF-κB
- nuclear factor κB
- RT-PCR
- reverse transcriptase polymerase chain reaction
- shRNA
- short hairpin RNA
- siRNA
- short interference RNA
- ELISA
- enzyme-linked immunosorbent assay.
REFERENCES
- 1.Moore B. B., Arenberg D. A., Stoy K., Morgan T., Addison C. L., Morris S. B., Glass M., Wilke C., Xue Y. Y., Sitterding S., Kunkel S. L., Burdick M. D., Strieter R. M. (1999) Am. J. Pathol. 154, 1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie K. P. (2001) Cytokine Growth Factor Rev. 12, 375–391 [DOI] [PubMed] [Google Scholar]
- 3.Waugh D. J., Wilson C., Seaton A., Maxwell P. J. (2008) Front. Biosci. 13, 4595–4604 [DOI] [PubMed] [Google Scholar]
- 4.Lentsch A. B. (2006) Future Oncol. 2, 651–658 [DOI] [PubMed] [Google Scholar]
- 5.Waugh D. J., Wilson C. (2008) Clin. Cancer Res. 14, 6735–6741 [DOI] [PubMed] [Google Scholar]
- 6.Uehara H., Troncoso P., Johnston D., Bucana C. D., Dinney C., Dong Z., Fidler I. J., Pettaway C. A. (2005) Prostate 64, 40–49 [DOI] [PubMed] [Google Scholar]
- 7.Ferrer F. A., Miller L. J., Andrawis R. I., Kurtzman S. H., Albertsen P. C., Laudone V. P., Kreutzer D. L. (1998) Urology 51, 161–167 [DOI] [PubMed] [Google Scholar]
- 8.Murphy C., McGurk M., Pettigrew J., Santinelli A., Mazzucchelli R., Johnston P. G., Montironi R., Waugh D. J. (2005) Clin. Cancer Res. 11, 4117–4127 [DOI] [PubMed] [Google Scholar]
- 9.Huang J., Yao J. L., Zhang L., Bourne P. A., Quinn A. M., di Sant'Agnese P. A., Reeder J. E. (2005) Am. J. Pathol. 166, 1807–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veltri R. W., Miller M. C., Zhao G., Ng A., Marley G. M., Wright G. L., Jr., Vessella R. L., Ralph D. (1999) Urology 53, 139–147 [DOI] [PubMed] [Google Scholar]
- 11.Lehrer S., Diamond E. J., Mamkine B., Stone N. N., Stock R. G. (2004) Technol. Cancer Res. Treat. 3, 411–411 [DOI] [PubMed] [Google Scholar]
- 12.Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T., Le Beau M. M., Adamson E. D. (1988) Cell 53, 37–43 [DOI] [PubMed] [Google Scholar]
- 13.Liu C. T., Rangnekar V. M., Adamson E., Mercola D. (1998) Cancer Gene Therapy 5, 3–28 [PubMed] [Google Scholar]
- 14.Thiel G., Cibelli G. (2002) J. Cell. Physiol. 193, 287–292 [DOI] [PubMed] [Google Scholar]
- 15.Salah Z., Maoz M., Pizov G., Bar-Shavit R. (2007) Cancer Res. 67, 9835–9843 [DOI] [PubMed] [Google Scholar]
- 16.Xiao D., Chinnappan D., Pestell R., Albanese C., Weber H. C. (2005) Cancer Res. 65, 9934–9942 [DOI] [PubMed] [Google Scholar]
- 17.Mora G. R., Olivier K. R., Mitchell R. F., Jr, Jenkins R. B., Tindall D. J. (2005) Prostate 63, 198–207 [DOI] [PubMed] [Google Scholar]
- 18.Eid M. A., Kumar M. V., Iczkowski K. A., Bostwick D. G., Tindall D. J. (1998) Cancer Res. 58, 2461–2468 [PubMed] [Google Scholar]
- 19.Ahmed M. M., Chendil D., Lele S., Venkatasubbarao K., Dey S., Ritter M., Rowland R. G., Mohiuddin M. (2001) Am. J. Clin. Oncol. 24, 500–505 [DOI] [PubMed] [Google Scholar]
- 20.Baron V., Duss S., Rhim J., Mercola D. (2003) Ann. N.Y. Acad. Sci. 1002, 197–216 [DOI] [PubMed] [Google Scholar]
- 21.Thigpen A. E., Cala K. M., Guileyardo J. M., Molberg K. H., McConnell J. D., Russell D. W. (1996) J. Urol. 155, 975–981 [PubMed] [Google Scholar]
- 22.Moon Y., Yang H., Lee S. H. (2007) Biochem. Biophys. Res. Commun. 362, 256–262 [DOI] [PubMed] [Google Scholar]
- 23.Cogswell P. C., Mayo M. W., Baldwin A. S., Jr. (1997) J. Exp. Med. 185, 491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman N. R., Perkins N. D. (2000) J. Biol. Chem. 275, 4719–4725 [DOI] [PubMed] [Google Scholar]
- 25.Decker E. L., Skerka C., Zipfel P. F. (1998) J. Biol. Chem. 273, 26923–26930 [DOI] [PubMed] [Google Scholar]
- 26.Decker E. L., Nehmann N., Kampen E., Eibel H., Zipfel P. F., Skerka C. (2003) Nucleic Acids Res. 31, 911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uzzo R. G., Crispen P. L., Golovine K., Makhov P., Horwitz E. M., Kolenko V. M. (2006) Carcinogenesis 27, 1980–1990 [DOI] [PubMed] [Google Scholar]
- 28.Araki S., Omori Y., Lyn D., Singh R. K., Meinbach D. M., Sandman Y., Lokeshwar V. B., Lokeshwar B. L. (2007) Cancer Res. 67, 6854–6862 [DOI] [PubMed] [Google Scholar]
- 29.Seaton A., Scullin P., Maxwell P. J., Wilson C., Pettigrew J., Gallagher R., O'Sullivan J. M., Johnston P. G., Waugh D. J. (2008) Carcinogenesis 29, 1148–1156 [DOI] [PubMed] [Google Scholar]
- 30.MacManus C. F., Pettigrew J., Seaton A., Wilson C., Maxwell P. J., Berlingeri S., Purcell C., McGurk M., Johnston P. G., Waugh D. J. J. (2007) Mol. Cancer Res. 5, 737–748 [DOI] [PubMed] [Google Scholar]
- 31.Inoue K., Slaton J. W., Eve B. Y., Kim S. J., Perrotte P., Balbay M. D., Yano S., Bar-Eli M., Radinsky R., Pettaway C. A., Dinney C. P. N. (2000) Clin. Cancer Res. 6, 2104–2119 [PubMed] [Google Scholar]
- 32.Peters A. H., Kubicek S., Mechtler K., O'Sullivan R. J., Derijck A. A., Perez-Burgos L., Kohlmaier A., Opravil S., Tachibana M., Shinkai Y., Martens J. H., Jenuwein T. (2003) Mol. Cell. 12, 1577–1589 [DOI] [PubMed] [Google Scholar]
- 33.Martens J. H., O'Sullivan R. J., Braunschweig U., Opravil S., Radolf M., Steinlein P., Jenuwein T. (2005) EMBO J. 24, 800–812 [DOI] [PMC free article] [PubMed] [Google Scholar]