Abstract
Reactive oxygen species (ROS) generation, particularly by the endothelial NADPH oxidase family of proteins, plays a major role in the pathophysiology associated with lung inflammation, ischemia/reperfusion injury, sepsis, hyperoxia, and ventilator-associated lung injury. We examined potential regulators of ROS production and discovered that hyperoxia treatment of human pulmonary artery endothelial cells induced recruitment of the vesicular regulator, dynamin 2, the non-receptor tyrosine kinase, c-Abl, and the NADPH oxidase subunit, p47phox, to caveolin-enriched microdomains (CEMs). Silencing caveolin-1 (which blocks CEM formation) and/or c-Abl expression with small interference RNA inhibited hyperoxia-mediated tyrosine phosphorylation and association of dynamin 2 with p47phox and ROS production. In addition, treatment of human pulmonary artery endothelial cells with dynamin 2 small interfering RNA or the dynamin GTPase inhibitor, Dynasore, attenuated hyperoxia-mediated ROS production and p47phox recruitment to CEMs. Using purified recombinant proteins, we observed that c-Abl tyrosine-phosphorylated dynamin 2, and this phosphorylation increased p47phox/dynamin 2 association (change in the dissociation constant (Kd) from 85.8 to 6.9 nm). Furthermore, exposure of mice to hyperoxia increased ROS production, c-Abl activation, dynamin 2 association with p47phox, and pulmonary leak, events that were attenuated in the caveolin-1 knock-out mouse confirming a role for CEMs in ROS generation. These results suggest that hyperoxia induces c-Abl-mediated dynamin 2 phosphorylation required for recruitment of p47phox to CEMs and subsequent ROS production in lung endothelium.
Introduction
Vascular endothelial cell (EC)2 barrier integrity is critical to normal vessel homeostasis with defects in the EC barrier contributing to inflammation, tumor angiogenesis, atherosclerosis, and acute lung injury (1). The generation of reactive oxygen species (ROS) in the vasculature plays a major role in EC activation and barrier function (2). Of the several potential sources of ROS in the vasculature, the endothelial NADPH oxidase family of proteins is a major contributor of ROS, and accumulation of ROS is associated with lung inflammation, ischemia-reperfusion injury, sepsis, hyperoxia, and ventilator-associated lung injury (3). Activation of phagocytic NADPH oxidase requires the assembly of the cytosolic p47phox, p67phox, p40phox, and Rac2 with membrane-associated cytochrome b558 reductase, which consists of p22phox and Nox2 (gp91phox). Vascular cells express similar subcomponents to phagocytic NADPH oxidase subunits, including Rac1, p47phox, and Nox2 (2, 3). We have recently demonstrated that exposure of human pulmonary artery endothelial cells (HPAECs) to hyperoxia (95% O2) increases the ROS production that is dependent on NADPH oxidase activation and independent of the mitochondrial electron transport or xanthine/xanthine oxidase systems (4).
Regulation of NADPH oxidase activation in phagocytes requires serine phosphorylation of the cytosolic p47phox, p67phox, and p40phox components, assembly of the phosphorylated subunits with Rac2, and translocation to phagosomes for association with cytochrome b558 in which one-electron reduction of molecular O2 to O2˙̄ occurs with NADPH as the electron donor (5). In human pulmonary EC, tumor necrosis factor-α induces NADPH oxidase activation through a mechanism regulated by phosphatidylinositol 3-kinase and protein kinase Cζ (2, 6). In neutrophils and macrophages, FcγR-mediated activation of NADPH oxidase and ROS production is regulated by the Vav guanine nucleotide exchange factor and phosphatidylinositol 3-kinase-dependent phosphorylation of p40phox (2, 7). In addition, in HPAECs, activation of NADPH oxidase is regulated by Src-dependent tyrosine phosphorylation of p47phox and the association of p47phox, but not p67phox, with Src (8). However, angiotensin II-mediated activation of Src in vascular smooth muscle cells results in serine phosphorylation of p47phox, translocation from the cytosol to the membrane, and increased ROS production (9). Thus, phosphorylation of p47phox is an important post-translational modification that regulates the assembly, translocation, and activation of NADPH oxidase in vascular cells (2, 9–11). However, the mechanisms of assembly and translocation of p47phox with the membrane components of NADPH oxidase are yet to be completely defined.
In endothelial cells, as in many other cell types, there exist specialized sterol- and sphingolipid-enriched domains called lipid rafts, which have been implicated in NADPH oxidase signaling (2, 3, 12, 13). In addition, there exists a subset of lipid rafts, which are 50–100 nm plasma membrane microdomains containing a specific scaffolding protein called caveolin-1, termed caveolin-enriched microdomains (CEMs) (13–16). Recent studies show that these cholesterol-enriched detergent-resistant membrane microdomains play an important role in formylmethionylleucylphenylalanine-, phorbol myristate acetate-, Fcγ-, and angiotensin II-induced activation of NADPH oxidase by triggering the recruitment of key NADPH oxidase subunits (2, 17, 18). We have demonstrated that CEMs are critical for Rac1 activation of barrier-enhancing stimuli, including hyaluronan and hepatocyte growth factor (14, 15). Hepatocyte growth factor-induced Rac1 activation requires recruitment of the vesicular regulator, dynamin 2, to CEM (15). In addition, caveolin-1 is essential for the activation of Rac1 and NADPH oxidase after angiotensin II stimulation of vascular smooth muscle cells (17). Although certain stimuli induce formation of signaling platforms in CEMs, very little is known about the assembly of NADPH oxidase subunits and cytoskeletal proteins in CEMs and the mechanisms of ROS generation.
In the present study, we evaluated the role of CEMs in hyperoxia-induced translocation of p47phox to CEMs, activation of NADPH oxidase, and generation of ROS. Our results demonstrate that (i) hyperoxia treatment of HPAECs promotes recruitment of NADPH oxidase subunits, dynamin 2 and c-Abl, to CEMs; (ii) small interfering RNAs (siRNA) for caveolin-1, dynamin 2, or c-Abl are able to block hyperoxia-mediated ROS production; (iii) hyperoxia induces tyrosine phosphorylation of dynamin 2 and the interaction between dynamin 2 and p47phox; (iv) knockdown of c-Abl with siRNA decreases hyperoxia-induced phosphorylation of dynamin 2 and the association between p47phox and dynamin 2; and (v) caveolin-1 knock-out mice decreases hyperoxia-mediated ROS production, c-Abl activation, dynamin 2 association with p47phox, and pulmonary leak. These results suggest that CEMs are important platforms to organize the assembly of NADPH oxidase components during the generation of ROS in response to hyperoxia both in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Materials
HPAECs, endothelial cell basal medium-2 (EBM-2), and Bullet kits were obtained from Cambrex (Walkersville, MD). Unless otherwise specified, reagents were obtained from Sigma. Dihydroethidium (hydroethidine), 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA), Alexa Fluor 568 phallacidin, and Alexa Fluor 488 or 568 mouse, rabbit, or goat secondary antibodies were purchased from Molecular Probes (Eugene, OR). An enhanced chemiluminescence (ECL) kit was from Amersham Biosciences. Polyclonal antibodies to Src, p47phox, dynamin 2, Rac1, cortactin, p22phox, gp91phox (Nox-2), protein A/G plus-agarose, and siRNA-targeting caveolin-1, dynamin 2, and c-Abl were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit phospho-Src antibody was purchased from BIOSOURCE (Camarillo, CA). Polyclonal antibodies to caveolin-1, c-Abl and pY245 c-Abl, were purchased from Cell Signaling Technology (Danvers, MA). Recombinant active c-Abl protein was purchased from Millipore. Glass bottom micro-well dishes were obtained from MatTek Corp. (Ashland, MA).
Endothelial Cell Culture
HPAECs at passages 5–8 in endothelial cell growth medium-2 (EGM-2) with 10% fetal bovine serum, 100 units/ml penicillin, and streptomycin were grown to contact-inhibited monolayers with a typical cobblestone morphology in a 37 °C incubator under a 5% CO2, 95% air atmosphere as described previously (19). Cells were detached from T-75 flasks with 0.05% trypsin and resuspended in fresh complete medium, then cultured in 35- or 60-mm dishes or on glass coverslips for immunofluorescence studies. All cells were starved overnight in EGM-2 medium containing 1% fetal bovine serum prior to exposure to normoxia or hyperoxia.
Exposure of Cells to Hyperoxia
HPAECs (at ∼90% confluence) in complete EGM-2 medium were placed in a humidity-controlled airtight modulator incubator chamber (Billups-Rothenberg, Del Mar, CA) and flushed continuously with 95% O2, 5% CO2 for 30 min until the oxygen level inside the chamber reached ∼95%. HPAECs were then placed in a cell culture incubator at 37 °C for the desired length of time (1–3 h). The concentration of O2 inside the chamber was monitored with a digital oxygen monitor. The buffering capacity of the cell culture medium did not change significantly during the period of hyperoxic exposure and was maintained at pH 7.4.
CEM Isolation
CEMs were isolated from human lung ECs as we have previously described (14–16). Briefly, ECs were scraped in PBS, centrifuged at 2,000 rpm at 4 °C, and lysed with 0.2 ml of TN solution (25 mm Tris-HCl (pH 7.5), containing 150 mm NaCl, 1 mm dithiothreitol, protease inhibitors, 10% sucrose, 1% Triton X-100) for 30 min on ice. Triton X-100-insoluble materials were then mixed with 0.6 ml of cold 60% OptiPrepTM and overlaid with 0.6 ml of 40%, 30, and 20% OptiPrepTM in TN solution. The gradients were centrifuged at 35,000 rpm in an SW-60 rotor for 12 h at 4 °C, and different fractions were collected. Cellular proteins associated with the 20% OptiPrepTM fraction were either immunoprecipitated or directly analyzed by SDS-PAGE plus Western blotting.
Transfection of HPAECs with siRNA
To optimize conditions for efficient transfection, HPAECs were transfected with Fl-luciferase GL2 duplex siRNA (target sequence: 5′-CGTACGCGGAATACTTCGA-3′, Dharmacon) as a positive control. HPAECs grown to ∼60–70% confluence in 35-mm dishes were transfected with Gene Silencer® (Gene Therapy System, Inc., San Diego, CA)-transfecting agent plus caveolin-1, dynamin 2, or c-Abl siRNA (Santa Cruz Biotechnology and/or Dharmacon, 50 nm) in serum-free EBM-2 medium according to the manufacturer's recommendations. At 3 h post-transfection, 1 ml of fresh complete EGM-2 medium containing 10% fetal bovine serum was added, and the cells were cultured for an additional 48 h for analysis of caveolin-1, dynamin 2, or c-Abl protein expression by Western blotting.
Determination of Hyperoxia-induced Production of ROS
Total ROS production in HPAECs exposed to either normoxia or hyperoxia was determined by DCFDA or DHE fluorescence methods (20). Briefly, HPAECs (∼90% confluent in 35-mm dishes) were loaded with 10 μm DCFDA or 1 μm DHE in EGM-2 basal medium and incubated at 37 °C for 30 min. Hyperoxia-induced ROS formation in cells was quantified by fluorescence microscopy. HPAECs (∼90% confluent) in 35-mm dishes were loaded with DCFDA or DHE (10 μm) in EBM-2 basal medium for 30 min at 37 °C in a 95% air, 5% CO2 environment. After 30 min of loading, the medium containing DCFDA or DHE was aspirated; cells were rinsed once with EGM-2 complete medium; cells were preincubated with agents for the indicated time periods followed by exposure to either normoxia (95% air, 5% CO2) or hyperoxia (95% O2, 5%CO2) for 3–72 h. At the end of the incubation, cells were washed twice with PBS at room temperature and were examined under a Nikon Eclipse TE 2000-S fluorescence microscope with Hamamatsu digital charge-coupled device camera (Japan) using a 20× objective lens and MetaVue software (Universal Imaging Corp.). Total pixel density was quantitated.
Immunofluorescence Microscopy
HPAECs grown on gelatinized 9-mm coverslips to ∼95% confluence were exposed to either normoxia or hyperoxia for 1–3 h. Coverslips were washed twice with PBS at room temperature, permeabilized for 2 min in PBS containing 0.25% Triton X-100 and 3.7% formaldehyde, rinsed once with PBS, and fixed in 3.7% formaldehyde or 3% paraformaldehyde for 20 min at room temperature. The cells were rinsed three times in PBS and incubated for 30 min at room temperature in TBST blocking buffer containing 1% bovine serum albumin. Cells were incubated with primary antibodies (1:200 dilution in blocking buffer, 1 h), then thoroughly rinsed with TBST and stained with Alexa Fluor secondary antibodies (1:200 dilution in blocking buffer for 1 h). Cells were examined with a Nikon Eclipse TE 2000-S fluorescence microscope and Hamamatsu digital camera (Japan), using a ×60 oil immersion objective lens. Some images were captured with a Coolsnap HQ charge-coupled device camera (Roper, Duluth, GA) and Openlab software (Improvision, Lexington, MA).
Preparation of Cell Lysates, Immunoprecipitation, and Western Blotting
HPAECs grown on 100-mm dishes grown to confluence were serum-deprived for ∼16–18 h in EBM-2 containing 1% fetal bovine serum; all subsequent incubations were carried out in serum-free minimal essential medium. Cells were scraped into 1 ml of modified lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm Na3VO4, 1 mm NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 μg/ml pepstatin), sonicated on ice with a probe sonicator (3 times for 15 s), and centrifuged at 5000 × g in a microcentrifuge (4 °C for 5 min). Protein concentrations of the supernatants were determined using a Pierce protein assay kit. Equal volumes of the supernatants, adjusted to 1 mg of protein/ml (cell lysates), were denatured by boiling in 6× SDS sample buffer for 5 min, and samples were separated on 10% SDS-PAGE gels and analyzed by Western blotting. For immunoprecipitation, cell lysates (0.5–1 mg of protein) were incubated overnight with anti-Src or anti-cortactin antibody conjugated to agarose for 2 h at 4 °C, then centrifuged at 5000 × g in a microcentrifuge, dissociated by boiling in 2× SDS sample buffer for 5 min, and separated on 10% SDS-PAGE precast gels. Protein bands were transferred overnight (24 V at 4 °C) on a polyvinylidene difluoride membrane (Millipore), probed with primary and secondary antibodies according to the manufacturer's protocol, and detected using the Enhanced Chemiluminescence kit (Amersham Biosciences). The blots were scanned (UMAX Power Lock II) and quantified by an automated digitizing system UN-SCAN-IT GEL (Silk Scientific Corp.).
In Vitro Binding of p47phox to Dynamin 2
This procedure was performed as we have previously described (21) with the following modifications. Briefly, dynamin 2 from untreated (control) HPAECs was immunoprecipitated with Sepharose beads conjugated with anti-dynamin 2 antibody. In some cases, the dynamin 2-conjugated beads were tyrosine-phosphorylated with recombinant active c-Abl. Recombinant p47phox protein containing an Myc and FLAG epitope was purified from HPAECs transfected with a human p47phox overexpression vector (Origene) and anti-FLAG antibody-conjugated agarose beads (Sigma). Aliquots (0.5–1 ng of protein) of purified dynamin 2-conjugated Sepharose beads were incubated in 0.5 ml of binding buffer (20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.1% bovine serum albumin, and 0.05% Triton X-100) containing biotinylated recombinant human p47phox in the presence of various concentrations of unlabeled p47phox ranging from 10−12 to 10−5 m. Following the binding, the dynamin 2-conjugated beads were washed extensively in binding buffer. Biotinylated p47phox associated with dynamin 2-conjugated beads was detected by ExtrAvidin peroxidase reaction. Nonspecific binding, which was ∼20% of the total binding, was always subtracted from the total binding.
Hyperoxia Treatment of Mice: Animal Procedures
Male Caveolin−/− mice (background C57BL/6) and C57BL/6J mice were obtained from Jackson Laboratories, Bar Harbor, ME. At 8 weeks of age, animals were exposed to either normoxia or hyperoxia for 72 h in a Plexiglas chamber designed for animal procedures. The chamber was supplied with 95% oxygen and 5% CO2, and oxygen saturation inside the chamber was monitored by an oxygen sensor (Pro ox 110, BioSpherix Inc., Redfield, NY). Mice were supplied with fresh water and a Harlan Teklad diet. Animals that were weak and showed symptoms of loss of weight were excluded from the study. Experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85-23) and were approved by the Institutional Animal Care and Use Committee of The University of Chicago. Bronchoalveolar lavage (BAL) was performed on mice with Hanks' balanced salt solution via a polyurethane catheter placed in the trachea. BAL fluid was collected on ice; cells from the lavage were analyzed by cytological techniques. At the end of the experiment mice were anesthetized and lungs were perfused with fresh PBS several times, and whole lungs without trachea were stored in liquid nitrogen. These samples were further processed for paraffin embedment, sectioning, and staining for hematoxylin & eosin by the histological core facility at the University of Chicago.
Statistics
Analysis of variance and the Student-Newman-Keul test were used to compare means of two or more different treatment groups. The level of significance was set to p < 0.05 unless otherwise stated. Data are expressed as mean ± S.E.
RESULTS
CEM Regulates Hyperoxia-mediated Human Lung EC NADPH Oxidase Assembly and ROS Production
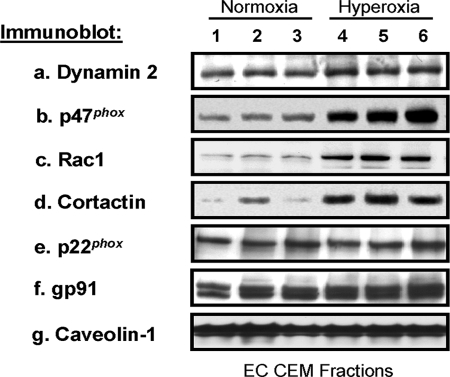
The role of CEMs in hyperoxia-mediated NADPH oxidase activation and ROS production is unknown. Our data indicate that silencing (siRNA) the CEM scaffolding protein, caveolin-1 (Fig. 1A), blocked hyperoxia-mediated ROS/superoxide production in human lung ECs (Fig. 1, B–G). Down-regulation of caveolin-1 with two different sources of siRNA gave similar reduction in ROS/superoxide production to hyperoxia confirming the specificity of caveolin-1 siRNA. Immunoblot analysis of CEMs isolated from normoxia- and hyperoxia-treated human lung ECs revealed that hyperoxia induced recruitment of the vesicular regulator, dynamin 2, the small GTPase, Rac1, the actin cytoskeletal regulatory protein, cortactin (10), and the NADPH oxidase subunit, p47phox, to join pre-existing NADPH subunits, p22phox and Nox2, in CEMs (Fig. 2). Recruitment of these proteins to CEM suggests a role for CEMs in the assembly of cytoskeletal proteins and NADPH oxidase components for increased ROS production in response to hyperoxia.
FIGURE 1.
Caveolin-1 regulation of hyperoxia-mediated human lung EC ROS production. A, immunoblot analysis of siRNA-treated or untreated HPAECs. Cellular lysates from untransfected (control, no siRNA), scramble siRNA (siRNA that does not target any known human mRNA), caveolin-1 siRNA#1 (Santa Cruz Biotechnology), or caveolin-1 siRNA#2 (ON-TARGETplus siRNA, Dharmacon) transfection were analyzed using immunoblotting with anti-caveolin-1 (a), anti-c-Abl (b), anti-dynamin 2 (c), or anti-actin (d) antibody. B, fluorescent microscopy image of HPAEC ROS production. Lung ECs were treated with either scramble siRNA (Sc) or caveolin-1 siRNA#1 (Santa Cruz Biotechnology), loaded with DCFDA, and exposed to either normoxia or hyperoxia for 3 h. C, graphical representation of data described in Panel B with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, scramble siRNA). **, a statistically significant change (p < 0.05) between scramble siRNA, hyperoxia and caveolin-1 siRNA, hyperoxia. D, fluorescent microscopy image of HPAEC ROS production. Lung ECs were treated with either scramble siRNA (Sc) or caveolin-1 siRNA#2 (ON-TARGETplus siRNA, Dharmacon), loaded with DCFDA and exposed to either normoxia or hyperoxia for 3 h. E, graphical representation of data described in Panel D with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, scramble siRNA). **, a statistically significant change (p < 0.05) between scramble siRNA, hyperoxia and caveolin-1 siRNA, hyperoxia. F, fluorescent microscopy image of HPAEC ROS production. Lung ECs were treated with either scramble siRNA (Sc) or caveolin-1 siRNA#2 (ON-TARGETplus siRNA, Dharmacon), loaded with DHE, and exposed to either normoxia or hyperoxia for 3 h. G, graphical representation of data described in Panel F with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, scramble siRNA). **, a statistically significant change (p < 0.05) between scramble siRNA, hyperoxia and caveolin-1 siRNA, hyperoxia.
FIGURE 2.
Hyperoxia regulation of proteins in human lung EC CEMs. Immunoblot analysis of CEMs isolated from human lung ECs exposed to either normoxia or hyperoxia for 3 h. Cellular material was solubilized in 1% Triton X-100 at 4 °C and soluble and insoluble fractions were obtained. The Triton X-100-insoluble fraction was overlaid with 60%, 40%, 30, and 20% OptiPrepTM and centrifuged at 35,000 rpm in an SW-60 rotor for 12 h at 4 °C as described under “Experimental Procedures.” The 20% OptiPrepTM fractions containing CEMs were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-dynamin 2 (a), anti-p47phox (b), anti-Rac1 (c), anti-cortactin (d), anti-p22phox (e), anti-gp91 (f), and anti-caveolin-1 (g) antibodies. The results indicate samples from three independent experiments for normoxia (1–3) and hyperoxia (4–6).
The CEM Component, Dynamin 2, Regulates ROS Production in Hyperoxia-challenged Human Lung ECs
Our results in Fig. 2 indicate that dynamin 2 is present in CEMs isolated from control (normoxia) human lung ECs, and hyperoxia treatment induced recruitment of additional dynamin 2 to CEMs. Given our previous published data indicating CEM-associated dynamin 2 is involved in activation of the NADPH oxidase regulator, Rac1 (15), we examined whether dynamin 2 can regulate hyperoxia-mediated NADPH oxidase activation and ROS production. Silencing (siRNA) dynamin 2 (Fig. 3A) or inhibiting the GTPase activity of dynamin (Dynasore) attenuated hyperoxia-mediated ROS production in human lung ECs (Fig. 3, B–D).
FIGURE 3.
Regulation of ROS production in hyperoxia-challenged human lung ECs by the CEM component, dynamin 2. A, immunoblot analysis of siRNA-treated or untreated HPAECs. Cellular lysates from non-transfected (control, no siRNA), scramble siRNA, dynamin 2 siRNA#1 (Santa Cruz Biotechnology), or dynamin 2 siRNA#2 (ON-TARGETplus siRNA, Dharmacon) transfection were analyzed using immunoblotting with anti-dynamin 2 (a), anti-c-Abl (b), anti-caveolin-1 (c), or anti-actin (d) antibody. B, graphical representation of fluorescent intensity utilizing HPAECs transfected with scramble siRNA or dynamin 2 siRNA#1, loaded with DCFDA, and exposed to normoxia or hyperoxia (3 h) with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, scramble siRNA). **, a statistically significant change (p < 0.05) between scramble siRNA, hyperoxia and dynamin 2 siRNA, hyperoxia. C, graphical representation of fluorescent intensity utilizing HPAECs transfected with scramble siRNA or dynamin 2 siRNA#2, loaded with DHE, and exposed to normoxia or hyperoxia (3 h) with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, scramble siRNA). **, a statistically significant change (p < 0.05) between scramble siRNA, hyperoxia and dynamin 2 siRNA, hyperoxia. D, graphical representation of fluorescent intensity utilizing HPAECs treated with vehicle control or Dynasore, loaded with DCFDA, and exposed to normoxia or hyperoxia (3 h) with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, vehicle control). **, a statistically significant change (p < 0.05) between vehicle control, hyperoxia and Dynasore, hyperoxia. E, HPAECs were transfected with either scramble siRNA or caveolin-1 siRNA and treated with normoxia or hyperoxia (3 h). The EC were then solubilized in IP buffer (50 mm HEPES (pH 7.5), 150 mm NaCl, 20 mm MgCl2, 1% Nonidet P-40, 0.4 mm Na3VO4, 40 mm NaF, 50 μm okadaic acid, 0.2 mm phenylmethylsulfonyl fluoride, CompleteTM protease inhibitor mixture (Roche Applied Science)) and immunoprecipitated with anti-dynamin 2 antibody. The resulting Immunobeads were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phospho-tyrosine (a), anti-p47phox (b), or anti-dynamin 2 (c) antibody. The results indicate samples from three independent experiments for scramble siRNA treatment, normoxia (1–3) and hyperoxia (4–6) and caveolin-1 siRNA treatment, normoxia (7–9) and hyperoxia (10–12).
Dynamin 2 GTPase activity can be regulated by post-translational modifications, including tyrosine phosphorylation (22, 23). In addition, dynamin 2 can bind to certain proteins containing the phox homology domain (24). Our data indicate that hyperoxia treatment of HPAECs resulted in tyrosine phosphorylation of dynamin 2 and formation of a dynamin 2-p47phox complex. Silencing of caveolin-1 with siRNA attenuated hyperoxia-induced tyrosine phosphorylation of dynamin 2 and association of p47phox with dynamin 2 (Fig. 3E). These results suggest a role for dynamin 2 in ROS production and caveolin-1-dependent tyrosine phosphorylation and association of dynamin 2 with p47phox in response to hyperoxia in human lung ECs.
Dynamin 2 Tyrosine Phosphorylation by c-Abl Regulates p47phox Complex Formation and Hyperoxia-mediated ROS Production
Dynamin 2 can be tyrosine-phosphorylated by Src (23), a tyrosine kinase important in NADPH oxidase activation (2, 8). Further, the tyrosine kinase, c-Abl, is associated with Src and ROS signaling (25–28). We examined a potential role for c-Abl and observed that hyperoxia treatment of HPAECs resulted in caveolin-1-dependent c-Abl activation (pY245 phosphorylation) (Fig. 4, A and B). Importantly, silencing (siRNA) c-Abl expression (Fig. 4C) blocked hyperoxia-mediated dynamin 2 tyrosine phosphorylation, dynamin 2 complex formation with p47phox, and ROS production in HPAECs (Fig. 4, D–F). Further, recombinant active c-Abl directly tyrosine-phosphorylated dynamin 2 immunoprecipitated from HPAECs (Fig. 5, A and B), and this phosphorylation increased recombinant human p47phox protein binding to dynamin 2 (change in the dissociation constant (Fig. 5C)).
FIGURE 4.
Hyperoxia-induced c-Abl activation promotes dynamin 2-p47phox complex formation and ROS production in human lung ECs. A, HPAECs were transfected with either scramble siRNA or caveolin-1 siRNA#1 (Santa Cruz Biotechnology) and treated with normoxia or hyperoxia (3 h). EC lysates were obtained, run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine 245 c-Abl (a), anti-c-Abl (b), or anti-actin (c) antibody. B, graphical representation of data described in Panel A with n = 3 experiments; error bars = ±S.D. There is a statistically significant change (p < 0.05) between normoxia and hyperoxia with scramble siRNA, but not caveolin-1 siRNA. C, immunoblot analysis of siRNA-treated or untreated HPAECs. Cellular lysates from non-transfected (control, no siRNA), scramble siRNA, c-Abl siRNA#1 (Santa Cruz Biotechnology), or c-Abl siRNA#2 (ON-TARGETplus siRNA, Dharmacon) transfection were analyzed using immunoblotting with anti-c-Abl (a), anti-caveolin-1 (b), anti-dynamin 2 (c), or anti-actin (d) antibody. D, HPAECs were transfected with either scramble siRNA or c-Abl siRNA#1 and treated with normoxia or hyperoxia (3 h). The ECs were then solubilized in IP buffer and immunoprecipitated with anti-dynamin 2 antibody. The resulting Immunobeads were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine (a), anti-p47phox (b), or anti-dynamin 2 (c) antibody. E, graphical representation of fluorescent intensity utilizing HPAECs transfected with scramble siRNA or c-Abl siRNA#1, loaded with DCFDA, and exposed to normoxia or hyperoxia (3 h) with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, scramble siRNA). **, a statistically significant change (p < 0.05) between scramble siRNA, hyperoxia and c-Abl siRNA, hyperoxia. F, graphical representation of fluorescent intensity utilizing HPAECs transfected with scramble siRNA or c-Abl siRNA#2, loaded with DHE, and exposed to normoxia or hyperoxia (3 h) with n = 3 experiments; error bars = ±S.D. *, a statistically significant change (p < 0.05) from control (normoxia, scramble siRNA). **, a statistically significant change (p < 0.05) between scramble siRNA, hyperoxia and c-Abl siRNA, hyperoxia.
FIGURE 5.
Dynamin 2 tyrosine phosphorylation by c-Abl promotes association with p47phox. A, recombinant human p47phox (10 nm) was incubated for 1 h in binding buffer (20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.1% bovine serum albumin, and 0.05% Triton X-100) with anti-dynamin 2 antibody-conjugated beads alone (control, no EC lysates), immunoprecipitated dynamin 2 (from serum-free (1 h) EC lysates)-conjugated Immunobeads, or immunoprecipitated dynamin 2 Immunobeads previously phosphorylated with recombinant active c-Abl. The resulting Immunobeads were run on SDS-PAGE, transferred to nitrocellulose and immunoblotted with anti-p47phox (a), anti-phospho-tyrosine (b), or anti-dynamin 2 (c) antibody. B, graphical representation of p47phox and dynamin 2 immunoblot data described in Panel A with n = 3 experiments; error bars = ±S.D. There is a statistically significant change (p < 0.05) between p47phox binding to non-phosphorylated dynamin 2 versus c-Abl-phosphorylated dynamin 2. C, aliquots (0.5–1 ng of protein) of purified dynamin 2- or c-Abl-phosphorylated dynamin 2-conjugated Immunobeads were incubated in 0.5 ml of binding buffer (see above) containing biotinylated recombinant human p47phox in the presence of various concentrations of unlabeled p47phox ranging from 10−12 to 10−5 m. Following the binding, the dynamin 2-conjugated beads were washed extensively in binding buffer. Biotinylated p47phox associated with dynamin 2-conjugated beads was detected by ExtrAvidin peroxidase reaction. Nonspecific binding, which was ∼20% of the total binding, was always subtracted from the total binding. The results are expressed as % of maximal binding (y axis) with n = 3 per condition.
Caveolin-1 Regulates Hyperoxia-mediated c-Abl Activation, Dynamin 2-p47phox Complex Formation, ROS Production, and Pulmonary Leakiness in Vivo
Given our in vitro results indicating caveolin-1 and CEMs regulate hyperoxia-mediated NADPH oxidase assembly and ROS production, we examined whether caveolin-1 regulates hyperoxia-mediated assembly of cytoskeletal proteins and NADPH oxidase components in vivo. Fig. 6 (A and B) demonstrates that, similar to our in vitro results, hyperoxia induces c-Abl tyrosine phosphorylation (pY245) in isolated mouse lungs, which was blocked in the caveolin-1 knock-out mouse. In addition, hyperoxia-mediated dynamin 2 tyrosine phosphorylation and p47phox complex formation were blocked in the caveolin-1 knock-out mouse (Fig. 6, C and D). The increased levels of hydrogen peroxide (Fig. 6E) and total protein (Fig. 6F) in BAL fluid of hyperoxia-treated mice were significantly attenuated in the caveolin-1 knock-out mouse. Further, histochemical analysis of mouse lungs indicates hyperoxia induces caveolin-1-dependent accumulation of margination of neutrophil infiltration into the alveolar spaces. Further, alveolar walls are significantly thickened, and alveolar spaces are edematous, confirming the vascular leakiness from capillaries (Fig. 6G). These results suggest caveolin-1 is an important regulator of hyperoxia-mediated ROS production via c-Abl and dynamin 2 in regulating pulmonary vascular permeability in vivo.
FIGURE 6.
Caveolin-1 regulates hyperoxia-mediated c-Abl activation, dynamin 2-p47phox complex formation, ROS production, and pulmonary leakiness in vivo. For Panels A–D, male C57BL/6J wild-type and caveolin-1 knock-out mice were treated with normoxia or hyperoxia for 72 h. Then, mice were anesthetized, BAL fluid was collected, and lungs were extracted. A, wild-type and caveolin-1 knock-out lungs with normoxia or hyperoxia treatment were homogenized in homogenization buffer (50 mm HEPES (pH 7.5), 150 mm NaCl, 20 mm MgCl2, 1% Triton X-100, 0.2% SDS, 0.8 mm Na3VO4, 80 mm NaF, 100 μm okadaic acid, 0.4 mm phenylmethylsulfonyl fluoride, CompleteTM protease inhibitor mixture (Roche Applied Science)), run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine 245 c-Abl (a), anti-c-Abl (b), or anti-actin (c) antibody. B, graphical representation of data described in Panel A with n = 3 experiments; error bars = ±S.D. There is a statistically significant change (p < 0.05) in c-Abl activation (tyrosine phosphorylation) between normoxia and hyperoxia in wild type, but not caveolin-1 knock-out, mice. C, samples as described in Panel A were solubilized in IP buffer and immunoprecipitated with anti-dynamin 2 antibody. The resulting Immunobeads were run on SDS-PAGE, transferred to nitrocellulose, and immunoblotted with anti-phosphotyrosine (a), anti-p47phox (b), or anti-dynamin 2 (c) antibodies. D, graphical representation of data described in Panel C with n = 3 experiments; error bars = ±S.D. There is a statistically significant change (p < 0.05) in p47phox association with dynamin 2 between normoxia and hyperoxia in wild-type, but not caveolin-1 knock-out, mice. E, graphical representation of the -fold increase of H2O2 in BAL fluid of wild-type and caveolin-1 knock-out mice with normoxia or hyperoxia treatment. There is a statistically significant difference (p < 0.01) between normoxia and hyperoxia. Further, there is a statistically significant difference (p < 0.005) between hyperoxia treatment of wild-type mice and hyperoxia treatment of caveolin-1 knock-out mice. F, graphical representation of the -fold increase of total protein in BAL fluid of wild-type and caveolin-1 knock-out mice with normoxia or hyperoxia treatment. There is a statistically significant difference (p < 0.01) between normoxia and hyperoxia. Further, there is a statistically significant difference (p < 0.005) between hyperoxia treatment of wild-type mice and hyperoxia treatment of caveolin-1 knock-out mice. G, C57BL/6J WT mice or Cav1−/− mice were exposed to normoxia (room air/21% oxygen) or hyperoxia (95% oxygen) for 72 h. Mice were anesthetized by intraperitoneal injection of 30 μl of ketamine (100 mg/kg)/xylazine (10 mg/kg). G shows the histological staining of the lung tissue (40×) from mice exposed to normoxia or hyperoxia. Arrows represent the marginal filtration of neutrophils (yellow arrow) and collapse and thickening of alveolar walls (orange arrow). Lung tissues were stored in formalin for 24 h before processing to cut and mount the section for staining with hematoxylin & eosin. Shown is a representative staining of the lung tissue from three independent determinations.
DISCUSSION
This study presents several novel observations, including the finding that caveolin-1 regulates hyperoxia-mediated c-Abl activation and dynamin 2-p47phox interactions, both in human lung ECs and in mouse lungs. Further, our data indicate hyperoxia induces recruitment of NADPH oxidase subunits, p22phox and p47phox, as well as Rac1, dynamin 2, and c-Abl to CEMs, events required for ROS production in human ECs (Fig. 7).
FIGURE 7.
Proposed model of CEM regulation of hyperoxia-mediated NADPH oxidase assembly and ROS production in human ECs. Hyperoxia treatment of human ECs induces c-Abl activation and c-Abl-mediated tyrosine phosphorylation of dynamin 2, which promotes p47phox-dynamin 2 interactions and recruitment of p47phox to CEMs. This recruitment promotes NADPH oxidase functional assembly and consequent ROS production.
An interesting and novel observation is the hyperoxia-enhanced association between p47phox and dynamin 2. Dynamin 2 is a ∼96-kDa GTPase implicated in lipid raft internalization, modulation of cell shape, and regulation of podosomal adhesion (22, 29). We and others have previously reported that dynamin 2 regulates cortical actin dynamics through its interaction with cortactin (15, 22) and Rac1 cellular localization and activation (15, 30). Further, dynamin 2 interactions with caveolin-1 promote cortactin recruitment to CEMs (31). We have previously demonstrated that down-regulation of cortactin with cortactin siRNA blocked hyperoxia-dependent translocation of p47phox to the cell periphery as well as ROS formation, indicating a role for cortactin in NADPH oxidase assembly and activation (10). Our results in the present study indicate hyperoxia induces cortactin recruitment to CEMs. We are currently investigating cortactin regulation of hyperoxia-mediated p47phox-dynamin 2 interactions.
Another novel finding is the role of c-Abl in enhancing tyrosine phosphorylation of dynamin 2 and increasing the affinity of dynamin 2 for p47phox following exposure of HPAECs to hyperoxia. Further, hyperoxia-mediated ROS production was dependent on caveolin- 1-mediated c-Abl activation. Our results are in concurrence with an earlier study demonstrating a requirement for caveolin-1/lipid rafts in angiotensin subtype-1 receptor mediated ROS production in vascular smooth muscle cells (13). Although the one or more upstream signaling mechanisms regulating c-Abl activation have not been fully defined, c-Abl has been identified as an effector of Src and Src family kinases such as Fyn (32, 33). We have previously demonstrated that hyperoxia activates Src and Src-dependent tyrosine phosphorylation of p47phox in HPAECs (8). The non-receptor tyrosine kinase, c-Abl, shares similar SH2 and SH3 domain structure to Src and often functions downstream of Src signaling (25, 34, 35); however, the role of Src in hyperoxia-induced activation of c-Abl in human lung ECs has not been established. Also, it is unclear if c-Abl, in addition to Src, can stimulate tyrosine phosphorylation of p47phox in response to hyperoxia in human lung ECs. Interestingly, c-Abl can be activated by ROS and is an important mediator of oxidative stress-induced caveolin-1 tyrosine phosphorylation (28).
Although the mechanisms that govern the interactions between dynamin 2 and p47phox are as yet unclear, it is reasonable to speculate that the novel phox homology domain of p47phox plays a role in dynamin 2 association (24). Previous studies have demonstrated that the phox homology domain of phospholipase D binds to dynamin (24). In addition, this binding promotes dynamin GTPase activity (24). Using the dynamin GTPase inhibitor, Dynasore, our present data indicate that dynamin GTPase activity is important for hyperoxia-mediated ROS production. Our recent study demonstrates a role for PLD in hyperoxia-mediated IQGAP1 activation through Rac1 on tyrosine phosphorylation of Src and cortactin as well as p47phox translocation and ROS formation in HPAECs (36). We are currently examining the role of phospholipase D in hyperoxia-induced activation of c-Abl regulating association of p47phox with dynamin 2 and ROS production in human lung ECs.
Whereas phagocytes generate excess O2˙̄ by activation of phagocytic NADPH oxidase, recent studies suggest that the vascular endothelial and smooth muscle cells are fully capable of ROS production (37–39). Furthermore, ROS generated by the vascular cells have emerged as important second messengers modulating signal transduction pathways regulating vascular growth, cell motility, cytoskeletal reorganization, and barrier function (2, 40, 41). Although mitochondria seem to play a major role in 4-hydroxynonenal- or hypoxia/reoxygenation-induced ROS formation in vascular smooth muscle cells and human umbilical vein ECs (2, 42), the present results suggest that CEMs are important for hyperoxia-mediated ROS generation via NADPH oxidase activation both in HPAECs and in mouse lungs.
Hyperoxia induces disruption of pulmonary endothelial and epithelial barriers resulting in leakage of fluid and protein into the alveolar space (43). Utilizing caveolin-1 knock-out mice, our data indicate that hyperoxia-mediated pulmonary ROS production and barrier disruption (as measured by BAL protein concentration and lung histochemistry) is dependent on caveolin-1 expression. These results are in agreement with a recent report that indicates caveolin-1 knock-out mice are protected from oxidative lung injury and death via up-regulation of heme oxygenase 1 (43). However, the one or more mechanisms of regulation of ROS production by NADPH oxidase requiring caveolin-1 have not been well defined. Here, we show for the first time that caveolin-1 regulates hyperoxia-mediated c-Abl activation, dynamin 2 tyrosine phosphorylation, and p47phox complex formation in mouse lungs and human lung ECs. A similar role for caveolin-1 in activation of Rac1, increased ROS production, and vascular hypertrophy after angiotensin II type 1 receptor activation has been suggested in vascular smooth muscle cells (17). Although both epithelial and endothelial cells express caveolin-1 (44, 45), there is substantial evidence that lack of endothelial caveolin-1 plays a major role in the pathophysiology observed in caveolin-1 knock-out mice (46, 47). Therefore, it is reasonable to speculate that the pulmonary effects we observe with hyperoxia treatment of mice are through a preferential endothelial-dependent, rather than epithelial-dependent, mechanism (46, 47).
In summary, the data presented in this study demonstrate an essential role for caveolin-1, c-Abl, and dynamin 2 in hyperoxia-induced translocation of p47phox to CEMs and generation of ROS. The interactions between caveolin-1, dynamin 2, and p47phox may form the basis of a protein platform in CEMs for the assembly of NADPH oxidase components and the generation of ROS in the endothelium. Hyperoxia mediates lung injury in animals and humans and is a relevant model of acute lung injury/acute respiratory distress syndrome; therapeutic targeting of caveolin-1 and its interacting proteins involved in NADPH oxidase assembly and ROS generation may provide new avenues in the management of acute lung injury patients.
Acknowledgment
We thank Donghong He for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-HL 095723 (to P. A. S.), RO1 HL 085553 (to V. N.), and PO1-HL 58064 (to J. G. N., V. N., and P. A. S.). This work was also supported by American Heart Association National Scientist Development Grant 0730277N (to P. A. S.) and by American Lung Association National Biomedical Research Grant RG-75229-N (to P. A. S.).
- EC
- endothelial cell
- HPAEC
- human pulmonary artery endothelial cell
- DCFDA
- 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate
- DHE
- dihydroethidium
- ROS
- reactive oxygen species
- siRNA
- small interfering RNA
- CEM
- caveolin-enriched microdomain
- EGM-2
- endothelial cell growth medium-2
- PBS
- phosphate-buffered saline
- BAL
- bronchoalveolar lavage.
REFERENCES
- 1.Pries A. R., Kuebler W. M. (2006) Handbook of Experimental Pharmacology, pp. 1–40, Springer Publishing Co., New York: [DOI] [PubMed] [Google Scholar]
- 2.Pendyala S., Usatyuk P., Gorshkova I. A., Garcia J. G., Natarajan V. (2008) Antioxi. Redox. Signal. 11, 841–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ushio-Fukai M. (2006) Sci. STKE 2006, re8. [DOI] [PubMed] [Google Scholar]
- 4.Parinandi N. L., Kleinberg M. A., Usatyuk P. V., Cummings R. J., Pennathur A., Cardounel A. J., Zweier J. L., Garcia J. G., Natarajan V. (2003) Am. J. Physiol. Lung Cell Mol. Physiol. 284, L26–L38 [DOI] [PubMed] [Google Scholar]
- 5.Robinson J. M. (2008) Histochem. Cell Biol. 130, 281–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadwallader K. A., Condliffe A. M., McGregor A., Walker T. R., White J. F., Stephens L. R., Chilvers E. R. (2002) J. Immunol. 169, 3336–3344 [DOI] [PubMed] [Google Scholar]
- 7.Utomo A., Cullere X., Glogauer M., Swat W., Mayadas T. N. (2006) J. Immunol. 177, 6388–6397 [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury A. K., Watkins T., Parinandi N. L., Saatian B., Kleinberg M. E., Usatyuk P. V., Natarajan V. (2005) J. Biol. Chem. 280, 20700–20711 [DOI] [PubMed] [Google Scholar]
- 9.Touyz R. M., Yao G., Schiffrin E. L. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 981–987 [DOI] [PubMed] [Google Scholar]
- 10.Usatyuk P. V., Romer L. H., He D., Parinandi N. L., Kleinberg M. E., Zhan S., Jacobson J. R., Dudek S. M., Pendyala S., Garcia J. G., Natarajan V. (2007) J. Biol. Chem. 282, 23284–23295 [DOI] [PubMed] [Google Scholar]
- 11.El-Benna J., Dang P. M., Gougerot-Pocidalo M. A., Marie J. C., Braut-Boucher F. (2009) Exp. Mol. Med. 41, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X. A., Everson W. V., Smart E. J. (2005) Trends Cardiovasc. Med. 15, 92–96 [DOI] [PubMed] [Google Scholar]
- 13.Parton R. G., Simons K. (2007) Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
- 14.Singleton P. A., Dudek S. M., Ma S. F., Garcia J. G. (2006) J. Biol. Chem. 281, 34381–34393 [DOI] [PubMed] [Google Scholar]
- 15.Singleton P. A., Salgia R., Moreno-Vinasco L., Moitra J., Sammani S., Mirzapoiazova T., Garcia J. G. (2007) J. Biol. Chem. 282, 30643–30657 [DOI] [PubMed] [Google Scholar]
- 16.Singleton P. A., Chatchavalvanich S., Fu P., Xing J., Birukova A. A., Fortune J. A., Klibanov A. M., Garcia J. G., Birukov K. G. (2009) Circ. Res. 104, 978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo L., Ushio-Fukai M., Ikeda S., Hilenski L., Patrushev N., Alexander R. W. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1824–1830 [DOI] [PubMed] [Google Scholar]
- 18.Hu G., Ye R. D., Dinauer M. C., Malik A. B., Minshall R. D. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L178–L186 [DOI] [PubMed] [Google Scholar]
- 19.Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. (2001) J. Clin. Invest. 108, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pendyala S., Gorshkova I. A., Usatyuk P., He D., Pennathur A., Lambeth J. D., Thannickal V. J., Natarajan V. (2008) Antioxid. Redox. Signal. 11, 747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton P. A., Bourguignon L. Y. (2004) Exp. Cell Res. 295, 102–118 [DOI] [PubMed] [Google Scholar]
- 22.Schafer D. A. (2004) Traffic 5, 463–469 [DOI] [PubMed] [Google Scholar]
- 23.Shajahan A. N., Timblin B. K., Sandoval R., Tiruppathi C., Malik A. B., Minshall R. D. (2004) J. Biol. Chem. 279, 20392–20400 [DOI] [PubMed] [Google Scholar]
- 24.Lee C. S., Kim I. S., Park J. B., Lee M. N., Lee H. Y., Suh P. G., Ryu S. H. (2006) Nat. Cell Biol. 8, 477–484 [DOI] [PubMed] [Google Scholar]
- 25.Sirvent A., Benistant C., Roche S. (2008) Biol. Cell 100, 617–631 [DOI] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M., Zuo L., Ikeda S., Tojo T., Patrushev N. A., Alexander R. W. (2005) Circ. Res. 97, 829–836 [DOI] [PubMed] [Google Scholar]
- 27.Sun X., Majumder P., Shioya H., Wu F., Kumar S., Weichselbaum R., Kharbanda S., Kufe D. (2000) J. Biol. Chem. 275, 17237–17240 [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti A. R., Mastick C. C. (2003) Cell. Signal. 15, 289–298 [DOI] [PubMed] [Google Scholar]
- 29.Kruchten A. E., McNiven M. A. (2006) J. Cell Sci. 119, 1683–1690 [DOI] [PubMed] [Google Scholar]
- 30.Schlunck G., Damke H., Kiosses W. B., Rusk N., Symons M. H., Waterman-Storer C. M., Schmid S. L., Schwartz M. A. (2004) Mol. Biol. Cell 15, 256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Q., Chen J., Cao H., Orth J. D., McCaffery J. M., Stan R. V., McNiven M. A. (2005) J. Mol. Biol. 348, 491–501 [DOI] [PubMed] [Google Scholar]
- 32.Plattner R., Kadlec L., DeMali K. A., Kazlauskas A., Pendergast A. M. (1999) Genes Dev. 13, 2400–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furstoss O., Dorey K., Simon V., Barilà D., Superti-Furga G., Roche S. (2002) EMBO J. 21, 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J., Arlinghaus R. (2008) Oncogene 27, 4385–4391 [DOI] [PubMed] [Google Scholar]
- 35.Hantschel O., Superti-Furga G. (2004) Nat. Rev. Mol. Cell Biol. 5, 33–44 [DOI] [PubMed] [Google Scholar]
- 36.Usatyuk P. V., Gorshkova I. A., He D., Zhao Y., Kalari S. K., Garcia J. G., Natarajan V. (2009) J. Biol. Chem. 284, 15339–15352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selemidis S., Sobey C. G., Wingler K., Schmidt H. H., Drummond G. R. (2008) Pharmacol. Ther. 120, 254–291 [DOI] [PubMed] [Google Scholar]
- 38.Lau A. T., Wang Y., Chiu J. F. (2008) J. Cell. Biochem. 104, 657–667 [DOI] [PubMed] [Google Scholar]
- 39.Cave A. C., Brewer A. C., Narayanapanicker A., Ray R., Grieve D. J., Walker S., Shah A. M. (2006) Antioxid. Redox Signal. 8, 691–728 [DOI] [PubMed] [Google Scholar]
- 40.Yung L. M., Leung F. P., Yao X., Chen Z. Y., Huang Y. (2006) Cardiovasc. Hematol. Disord. Drug Targets 6, 1–19 [DOI] [PubMed] [Google Scholar]
- 41.Ray R., Shah A. M. (2005) Clin. Sci. 109, 217–226 [DOI] [PubMed] [Google Scholar]
- 42.Zhang D. X., Gutterman D. D. (2007) Am. J. Physiol. Heart Circ. Physiol. 292, H2023–H2031 [DOI] [PubMed] [Google Scholar]
- 43.Jin Y., Kim H. P., Chi M., Ifedigbo E., Ryter S. W., Choi A. M. (2008) Am. J. Respir. Cell Mol. Biol. 39, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank P. G., Hassan G. S., Rodriguez-Feo J. A., Lisanti M. P. (2007) Curr. Pharm. Des. 13, 1761–1769 [DOI] [PubMed] [Google Scholar]
- 45.Williams T. M., Lisanti M. P. (2004) Ann. Med. 36, 584–595 [DOI] [PubMed] [Google Scholar]
- 46.Murata T., Lin M. I., Huang Y., Yu J., Bauer P. M., Giordano F. J., Sessa W. C. (2007) J. Exp. Med. 204, 2373–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wunderlich C., Schober K., Schmeisser A., Heerwagen C., Tausche A. K., Steinbronn N., Brandt A., Kasper M., Schwencke C., Braun-Dullaeus R. C., Strasser R. H. (2008) J. Mol. Cell Cardiol. 44, 938–947 [DOI] [PubMed] [Google Scholar]