Abstract
The effects of altering N-cadherin N-glycosylation on several cadherin-mediated cellular behaviors were investigated using small interfering RNA and site-directed mutagenesis. In HT1080 fibrosarcoma cells, small interfering RNA-directed knockdown of N-acetylglucosaminyltransferase V (GnT-V), a glycosyltransferase up-regulated by oncogene signaling, caused decreased expression of N-linked β(1,6)-branched glycans expressed on N-cadherin, resulting in enhanced N-cadherin-mediated cell-cell adhesion, but had no effect on N-cadherin expression on the cell surface. This effect on adhesion was accompanied by decreased cell migration and invasion, opposite of the effects observed when GnT-V was overexpressed in these cells (Guo, H. B., Lee, I., Kamar, M., and Pierce, M. (2003) J. Biol. Chem. 278, 52412–52424). A detailed study using site-directed mutagenesis demonstrated that three of the eight putative N-glycosylation sites in the N-cadherin sequence showed N-glycan expression. Moreover, all three of these sites, located in the extracellular domains EC2 and EC3, were shown by leucoagglutinating phytohemagglutinin binding to express at least some β(1,6)-branched glycans, products of GnT-V activity. Deletion of these sites had no effect on cadherin levels on the cell surface but led to increased stabilization of cell-cell contacts, cell-cell adhesion- mediated intracellular signaling, and reduced cell migration. We show for the first time that these deletions had little effect on formation of the N-cadherin-catenin complex but instead resulted in increased N-cadherin cis-dimerization. Branched N-glycan expression at three sites in the EC2 and -3 domains regulates N-cadherin-mediated cell-cell contact formation, outside-in signaling, and cell migration and is probably a significant contributor to the increase in the migratory/invasive phenotype of cancer cells that results when GnT-V activity is up-regulated by oncogene signaling.
Introduction
Cadherins are single-pass transmembrane receptors that mediate calcium-dependent cell-cell adhesion at adherens junctions and play an essential role in regulating major cellular behaviors, including cell growth, motility, and differentiation (1, 2). Several cadherins, including E-cadherin and N-cadherin, have in common an extracellular domain with five segments of repeated sequences and regulate cell-cell adhesion in a homotypic manner through their association of amino-terminal extracellular domains, such as EC1 (3, 4). Calcium binding to the extracellular domain triggers a conformation that initiates the homotypic binding of cadherin between cells. The conserved cytoplasmic domain of cadherin interacts with various proteins, collectively termed catenins, that link cadherins to the actin-based cytoskeleton and promote strong cell-cell adhesion (5). Evidence indicates that the formation and tyrosine phosphorylation of the cadherin-catenin complex are critical for the maintenance of the stabilization of cell-cell adhesion (6, 7). It has been well documented that cadherins are implicated in the regulation of tumor invasiveness and metastatic potential. For example, loss of E-cadherin in epithelial tumors results in reduced tumor cell-cell adhesion, leading to a less adhesive, more motile, and invasive phenotype (8, 9). By contrast, up-regulation of N-cadherin expression has been demonstrated in some invasive tumor cell lines and human tumor tissues, including mammary and esophageal squamous cell carcinoma (10–12), and its expression is associated with tumor aggressiveness and increased metastatic potential in these tumors. Expression of N-cadherin in human mammary tumor cells leads to increased cell migration and invasion (13, 14) through a sustained activation of the mitogen-activated protein kinase signaling pathway (15). These results show that N-cadherin expression is also associated with tumor invasiveness and may contribute to tumor progression.
One of the most important posttranslational modifications of cadherins is N-glycosylation, which results from the action of many glycosyltransferases. There is an increasing body of evidence showing that aberrant N-glycosylation affects the location and stability of cadherin, cadherin-mediated cell-cell adhesion, and intracellular signal transduction, leading to tumor progression and metastasis (16–21). For example, removal of several N-glycans from E-cadherin was reported to increase interaction of E-cadherin-catenin complexes and stabilized cell-cell contacts (20), although the mechanism was not explored in detail. Moreover, overexpression of GnT-III,3 an enzyme catalyzing formation of “bisected” N-linked glycans, in B16 mouse melanoma cells resulted in an altered glycosylation of E-cadherin. This change in glycosylation was associated with a reduced E-cadherin turnover rate at the cell surface and reduced metastatic potential of the melanoma cells by increasing cell-cell adhesion (16, 17). A more recent study showed that dense cultures of human salivary epidermoid carcinoma A253 cells exhibited elevated expression of DPAGT1, the gene that initiates protein N-glycosylation (22). Partial inhibition of DPAGT1 with small interfering RNA reduced the complex N-glycans of E-cadherin and increased the abundance of α-catenin and stabilizing proteins in adherens junctions (22). Altered adhesion has also been observed for N-cadherin-expressing cells when N-glycosylation was altered. A study from an N-cadherin-expressing line subcloned from Madin-Darby canine kidney cells showed that incubation of these cells with tunicamycin, an inhibitor of N-glycosylation, or treatment with the O-glycosylation inhibitor BAG showed a lower molecular weight N-cadherin only after tunicamycin treatment, interpreted to mean that N-cadherin is N- but not O-glycosylated in these cells. Deletion of N-glycans by tunicamycin treatment had no impact on N-cadherin trafficking to the plasma membrane but altered its binding to catenins, consequently affecting the formation of cell-cell junctions (23).
Our laboratory reported that N-cadherin function was modulated by expression levels of GnT-V (19), a glycosyltransferase that functions in the synthesis of multiantennary N-glycans during glycoprotein biosynthesis. GnT-V catalyzes formation of the β(1,6)-branched N-acetylglucosamine on N-glycans (24), one of the most commonly up-regulated N-glycan structures during malignant transformation (25). In HT1080 human fibrosarcoma cells, overexpression of GnT-V caused increased β(1,6)-GlcNAc on N-cadherin and reduced cell-cell adhesion by promoting phosphorylation of catenins through EGFR and Src signaling pathways, resulting in a more motile phenotype. Moreover, GnT-V-deficient embryo fibroblasts isolated from GnT-V homozygous null mice (GnT-V(−/−)) showed significantly increased levels of N-cadherin-based cell-cell adhesion compared with those from GnT-V(+/+) mice (19). These results indicated that levels of particular N-linked glycans can modulate N-cadherin-associated, homotypic cell-cell adhesion and signaling, which play a crucial role in regulating cellular motility and invasiveness.
N-cadherin is widely present in mesenchymal and neural cells, endothelia, and skeletal myocytes. Human N-cadherin contains eight putative N-linked glycosylation sites, although it is not known which of these sites are utilized. In the present study, we present a detailed investigation of the effects of N-cadherin N-glycosylation on cadherin-mediated cellular behaviors by using siRNA and site-directed mutagenesis strategies. Three of eight potential N-glycosylation sites on N-cadherin were identified to be N-glycosylated, present in domains EC2 and EC3, in contrast to the report on E-cadherin N-glycans (20). Complex N-glycans with β(1,6) branching were identified at all of these three sites, as evidenced by binding by the lectin, L-PHA. In further contrast to the E-cadherin study, we provide evidence that inhibition or deletion of the N-glycosylation on N-cadherin had no significant effects on either N-cadherin expression on the cell surface or formation of cadherin-catenin complexes but instead led to increased cis-dimerization of N-cadherins, which led to stabilization of cell-cell contacts, increased cell-cell adhesion-mediated intracellular (outside-in) signaling, and reduced cell migration.
EXPERIMENTAL PROCEDURES
Cell Lines and Materials
Chinese hamster ovary cells (CHO-K1), HT1080 human fibrosarcoma cells (CCL-121), MCF-7 human breast carcinoma cells, and HEK293 cells were obtained from ATCC; Pro-5 CHO cells were provided by Dr. Pamela Stanley (Albert Einstein College of Medicine); bovine serum albumin (BSA), Dulbecco's modified Eagle's medium (DMEM), swainsonine, tunicamycin, and function-blocking monoclonal antibodies against N-cadherin (A-CAM, clone GC-4) and E-cadherin (DECMA-1) were products of Sigma. LipofectamineTM 2000 reagent was from Invitrogen. The human N-cadherin cDNA clone (Puc19/N-cadherin) was a gift from Dr. Keith R. Johnson (Nebraska Medical Center); Ncad-Fc fusion pRK5 clone was provided by Dr. Takeshi Sakurai (Mount Sinai School of Medicine); the QuikChangeTM site-directed mutagenesis kit was from Stratagene; NHS-LS-biotin, streptavidin-horseradish peroxidase was obtained from Rockland. Biotinylated L-PHA and streptavidin-agarose were products of Vector Laboratories. Mouse anti-N-cadherin (clone 3B9) was from Zymed Laboratories Inc.. Protein A-agarose; polyclonal antibody against N-cadherin, E-cadherin, β-catenin, and ERK; monoclonal anti-phospho-ERK; and horseradish peroxidase-labeled anti-rabbit IgG and anti-mouse IgG were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Construction of pcDNA3.1/N-cadherin Expression Plasmid and Its N-Glycosylation Mutants
A cDNA fragment encoding the human N-cadherin (26) was excised from Puc19/N-cadherin plasmid using BamHI, SmaI, and AhdI and subcloned into pcDNA3.1 vector digested with EcoRI and BamHI, forming pcDNA3.1/N-cadherin expression plasmid. The QuikChange site-directed mutagenesis kit was used to mutate eight putative N-glycosylation sites with pcDNA3.1/N-cadherin as the template by using the mutagenesis primers listed in Table 1, according to the manufacturer's instructions. These primers contained a change of asparagine into glutamine in the middle of their sequences. pcDNA3.1/N-cadherin with multiple N-glycosylation sites mutated was created using pcDNA3.1/N-cadherin with single N-glycosylation mutation as template in sequential rounds of mutagenesis. All mutations were confirmed by DNA sequencing.
TABLE 1.
Individual N-glycosylation mutants of human N-cadherin
The mutated nucleotides are shown in italic type and underlined.
| Name | Mutation primers | Consensus sequence disrupted |
|---|---|---|
| Ncad190 | 5′-GGTCTGATAGAGATAAACAGCTTTCACTGCGGTACAG-3′ | Asn190 → Gln |
| Ncad273 | 5′-CTTACACCAGGTTTGGCAGGGGACAGTTCCTGAGG-3′ | Asn273 → Gln |
| Ncad325 | 5′-CATGTTTACAATCAACCAGGAGACTGGTGACATC-3′ | Asn325 → Gln |
| Ncad402 | 5′-GGTAGACATCATAGTAGCTCAGCTAACTGTGACCGATAAGG-3′ | Asn402 → Gln |
| Ncad572 | 5′-GAAAAACAATATATATCAGGCTACTTTCCTTGC-3′ | Asn572 → Gln |
| Ncad622 | 5′-CAGACCCCAATTCAATTCAGATTACAGCACTTG-3′ | Asn622 → Gln |
| Ncad651 | 5′-CCAGTGACTATTAAGAGACAGTGGACCATCACTCGG-3′ | Asn651 → Gln |
| Ncad692 | 5′-GGTAATCCTCCCAAATCACAGATTTCCATACTGCGCGTG-3′ | Asn692 → Gln |
Cell Culture and Transfection
All cells were grown at 37 °C in 5% CO2 in DMEM containing 10% fetal bovine serum and 2 mm l-glutamine. Cell transfections were performed with LipofectamineTM 2000 according to the manufacturer's instructions using 4 μg of recombinant pcDNA3.1/N-cadherin, different mutants, and pSUPER GnT-V siRNA (27) in a 6-well plate. 24 h after transfection, cells were selected for 3 weeks under G418 (800 μg/ml). Non-clonal populations of transfected cells were used for all experiments.
Expression and Purification of Ncad-Fc Fusion Protein
N-cadherin-Fc (Ncad-Fc) fusion protein was produced as described previously (28) using plasmid pRK5 fused with a cDNA encoding the human Fc region of IgG, to which the fragment of N-cadherin corresponds to the EC1 to -4 domains of the extracellular region, including its peptide sequence and cleavable prodomain region. The construct was co-transfected with a pEGFP-N1 plasmid into HEK293 cells using Lipofectamine 2000 (Invitrogen), and stable transfectants were isolated by selection with G418 (0.8 mg/ml). The culture medium was collected, and Ncad-Fc fusion protein was then detected by Western blotting. The positive cells expressing Ncad-Fc were grown in large scale to subconfluence, and culture media were replaced with low serum-containing media. The culture media were collected 2–3 days later and kept at −20 °C. Purification of Ncad-Fc was deployed as described previously (29) using protein A columns. SDS-PAGE was carried out to estimate the purification. Purified protein was dialyzed against PBS with 1 mm CaCl2 and stocked at −70 °C until use.
Western Blotting, Lectin Blotting, and Immunoprecipitation
Subconfluent cells were harvested and lysed. 20 and 500 μg of total cell lysate protein were used for Western (lectin) blotting and immunoprecipitation, respectively, as described in our earlier reports (27, 30).
Cell Surface Labeling and Immunoprecipitation
Subconfluent cells were washed and detached using 2 mm EDTA. Cells were then washed twice with ice-cold PBS and incubated with 1 mg/ml NHS-LC-biotin in PBS for 20 min at 4 °C on a rocking platform. After washing with PBS, cells were lysed, and cell surface proteins were precipitated using 50 μl of streptavidin-agarose at 4 °C overnight and detected by Western blotting as described before (31).
Cell Aggregation Assay
Cell aggregation assays were performed as described previously (19). Briefly, subconfluent cells were washed with PBS and then detached with HCMF buffer (HEPES-buffered Ca2+-, Mg2+-free Hanks' solution) containing 0.02% trypsin and 2 mm CaCl2. Single cell suspensions were prepared, washed, and resuspended in HCMF buffer containing 2 mm CaCl2 at a concentration of 105 cells/ml. 300-μl aliquots of cell suspension were added to the wells of 24-well plates precoated with 1% BSA and incubated for different times at 37 °C with 80 rpm agitation. For some experiments, function blocking antibodies against β1 integrin (mAb13), N-cadherin (anti-A-CAM, 50 μg/ml), and E-cadherin (DECMA-1, 1:100) were added into the cell suspension, respectively. For swainsonine-and tunicamycin-treated cells, 1 μg/ml swainsonine or tunicamycin was added into the culture media for 24 h before cells were prepared for the aggregation assay. Cell aggregation was measured using an inverted phase-contrast microscope by counting the number of cells in aggregates (three or more cells) in each field over a total of five randomly chosen fields and calculating the mean values of aggregation percentage.
Assay of Mitogen-activated Protein Kinase Activation Mediated by Cell-Cell Adhesion
Cadherin-mediated cell-cell adhesion was performed as described previously (19, 32). Cells were grown to confluence and serum-starved overnight in DMEM culture media containing 10 mm Hepes. Cells were then incubated with DMEM containing 4 mm EGTA for 20 min (2 h for CHO-K1 cells) at 37 °C. Then Ca2+-free medium was removed, and CaCl2 was adjusted back to 1.8 mm (Ca2+ switch) to induce cadherin-mediated intercellular interactions. Cells were harvested at different time points after the addition of Ca2+ and then lysed for assay of phospho-ERK by Western blotting using the monoclonal antibody against phospho-ERK.
Ncad-Fc Fusion Protein-mediated Adhesion Assay
96-well plates were coated with 10 μg/ml protein A in a coating buffer (0.02 m carbonate-bicarbonate (pH 9.4)), incubated at 4 °C overnight, washed with HCMF, and blocked with 1% BSA/HCMF for 1 h at room temperature. Ncad-Fc protein (10 μg/ml) was coated on the immobilized protein A for 1 h at 37 °C and then washed with HCMF. 5 × 104 suspended cells in 1% BSA/HCMF were loaded onto the coated area with 1 mm Ca2+ and allowed to adhere to the substrate for 30 min at 37 °C (29). The plate was gently washed three times with HCMF, and adherent cells were counted.
In Vitro Cell Migration and Invasion Assay
Cell migration assays were performed using 12-well Transwell units with 8-μm pore size polycarbonate inserts as described (19). Confluent cells were harvested and washed with serum-free DMEM. Cells (3 × 104) suspended in 0.5 ml of DMEM plus 0.1% BSA were added to the upper compartment of the Transwell unit, and 0.75 ml of fibroblast-conditioned medium was added into the lower chamber. Cells were allowed to migrate for 12 h at 37 °C in a humidified atmosphere containing 5% CO2. The cells on the upper side of the membrane were removed using a cotton swab, whereas the cells that migrated to the underside were fixed and stained with crystal violet. The number of cells on the underside of the membrane was counted in five different fields with a light microscope at ×100 magnification, and the mean ± S.D. was calculated. Using Matrigel-coated 24-well Boyden chambers, invasion assays were performed using the same procedure as for the migration assays, except that the incubation time of the experiment was prolonged to 21 h. Wound healing assays were performed as described previously (19). Cells were cultured to confluence in 30-mm dishes, and wounds were made on the cell monolayer with a 200-μl sterile yellow plastic tip. Migration of cells into the wounded area was evaluated with an inverted microscope.
Flow Cytometry Analysis
Cells were grown to subconfluence and detached with 2 mm EDTA in PBS. Cells (106) were washed, resuspended in 100 μl of flow cytometry buffer (PBS containing 1% BSA and 0.01% sodium azide), and then incubated with rabbit antibody against N-cadherin (1:100) at 4 °C for 30 min, followed by incubation with biotinylated anti-rabbit IgG (1:100). After washing with flow cytometry buffer, cells were labeled with phycoerythrin-conjugated streptavidin (10 μl) at 4 °C for 30 min. Analysis was then performed using the FACSCalibur (BD Biosciences) instrument.
Assay of Cis-dimer Formation of N-cadherin Using Chemical Cross-linker
Cells were grown in 6-well plates to confluence. After they were washed with ice-cold PBS, the cells were cross-linked by adding BS3 (final concentration 2.5 mm in PBS) on ice for 2 h. After 1 m Tris (pH 7.5) was added to a final concentration of 10 mm and incubated for another 15 min on ice, cells were then lysed with 1% Nonidet P-40, and N-cadherin cis-dimer formation was detected by immunoblotting after SDS-PAGE.
Glycosidase Treatment of Cell Lysates
Deglycosylation of N-cadherin was performed with whole cell lysates (23). Cell extracts containing 30 μg of protein were mixed with denaturing buffer containing SDS (5%) and β-mercaptoethanol (10%) and incubated at 100 °C for 10 min. Samples were incubated at 37 °C with 500 milliunits of endoglycosidase H for 2 h or peptide N-glycosidase F (PNGase F) overnight. The deglycosylated proteins were subjected to SDS-PAGE and immunoblotting with anti-N-cadherin.
RESULTS
Reduced Expression Levels of N-Linked β(1,6) Branching Increased N-cadherin-mediated Cell-Cell Adhesion and Inhibited Invasiveness-related Phenotypes
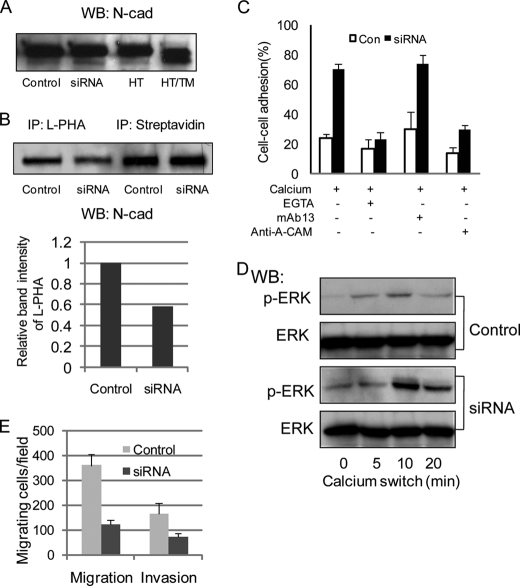
Studies from our laboratory showed that increased expression of GnT-V activity in two fibroblastic cell types, HT1080 and NIH3T3, caused increased levels of N-linked β(1,6) branching on N-cadherin, a concomitant decrease in the rates of homophilic cell-cell adhesion mediated by N-cadherin, and a stimulation of cadherin-mediated cell migration (19). We first asked if reduced expression of GnT-V in these cells renders the opposite effect on the N-cadherin-mediated cell phenotypes. To this end, HT1080 cells expressing an siRNA targeting GnT-V were used, in which GnT-V activity and levels of its β(1,6)-branched products were reduced by at least 50% (33). When treated with tunicamycin, which results in the deletion of N-linked β(1,6) oligosaccharide expression, SDS-PAGE revealed that the N-cadherin in HT1080 cells showed a significant shift corresponding to a lower molecular weight (Fig. 1A, right two lanes), indicating that N-cadherin is highly N-glycosylated in these cells. The levels of N-cadherin expression on the cell surface were not affected after GnT-V siRNA expression, as detected by biotinylation of the cell surface, followed by streptavidin precipitation and immunoblotting with anti-N-cadherin antibody (Fig. 1B, top right two lanes). Similar results were obtained by immunoblotting using total cell lysates (Fig. 1A, left two lanes). When the β(1,6) glycan branching of the N-cadherin from GnT-V knockdown cells was compared with that of control cells using L-PHA precipitation, blotting, and anti-N-cadherin antibody staining, a decrease of about 40% was observed (Fig. 1B, top left two lanes and bottom), demonstrating that knockdown of GnT-V expression caused decreased levels of β(1,6)-branched glycans on the N-cadherin.
FIGURE 1.
Knockdown of N-linked β(1,6) branching by GnT-V siRNA expression increased N-cadherin-mediated cell-cell adhesion in HT1080 cells. A, expression levels of N-cadherin were detected by Western blotting using cell lysates from control and GnT-V siRNA-transfected cells (left two lanes) and parental cells with and without pretreatment with tunicamycin (1 μg/ml) for 24 h (right two lanes). WB, Western blotting; Control, control siRNA-transfected; siRNA, GnT-V siRNA-transfected; HT, HT1080; TM, tunicamycin. B, cells were labeled with NHS-LC-biotin (1 mg/ml), followed by precipitation with streptavidin-agarose, SDS-PAGE, and blotting with anti-N-cadherin (top right two lanes). Cell lysates were immunoprecipitated with L-PHA, followed by precipitation with streptavidin-agarose, SDS-PAGE, and blotting with anti-N-cadherin (top left two lanes). Relative band intensity of L-PHA binding (bottom) was calculated versus N-cadherin surface expressions (right two lanes). IP, immunoprecipitation. C, cells were removed from culture plates and separated into single cells, followed by constant agitation in calcium-containing media at 37 °C for 30 min with different inhibitors, including EGTA, anti-N-cadherin (Anti-A-CAM), and anti-β1 integrin (mAb13). Aggregated cells were scored visually under light microscopy. Each error bar represents the mean ± S.D. percentage of cell-cell adhesion from five randomly selected fields. D, calcium switch experiments where cells were serum-starved for 24 h, incubated in DMEM containing 4 mm EGTA at 37 °C for 20 min, and shifted to calcium-containing media for various times, followed by processing for phospho-ERK (p-ERK) and total ERK detection by immunoblotting. E, cell migration was assayed using a Transwell apparatus for 12 h using NIH3T3 serum-free culture media as the chemoattractant in the lower chamber. Migrating cells found on the underside of the membrane were fixed, stained, and counted by microscopy. For the cell invasion assay, Transwell membrane was coated on the upper sides with Matrigel, and cells were allowed to migrate through Matrigel for 21 h. Each bar represents the mean ± S.D. number of migrating cells from five randomly selected fields.
To test if reduced expression of β(1,6)-branched glycans on N-cadherin was associated with aberrant N-cadherin function, a quantitative measurement of the rates of cell-cell adhesion was performed. As shown in Fig. 1C, compared with mock-transfected cells, homotypic cell-cell aggregation was significantly increased in GnT-V knockdown HT1080 cells. Increased cell-cell adhesion observed in knockdown cells was calcium-dependent and unaffected by an antibody directed against integrin β1 (mAb13). By contrast, a function-blocking antibody directed against N-cadherin (anti-A-CAM) significantly inhibited the calcium-dependent cell-cell adhesion of GnT-V knockdown cells, demonstrating that the most significant calcium-dependent, cell-cell adhesion process measured by these aggregation assays was mediated by N-cadherin, not integrin. Consistent with increased cell-cell adhesion, cell adhesion-mediated phosphorylation of ERK in GnT-V knockdown cells, detected by a calcium switch experiment, was significantly enhanced (Fig. 1D), indicating increased outside-in signaling pathways induced by calcium-dependent cell-cell contacts (19, 34, 35). Because N-cadherin-mediated cell-cell adhesion has been shown to regulate cancer cell motility and invasiveness (19, 36), experiments were then designed to determine whether reduced expression of GnT-V also affected cell migration and invasion. In the Boyden chamber (transwell) migration assay system, GnT-V knockdown cells showed a decrease of more than 60% in rate of migration after 12 h compared with control cells (Fig. 1E). Moreover, in vitro invasive migration through a reconstituted Matrigel basement membrane was also significantly inhibited in GnT-V knockdown cells after 21 h, as shown in Fig. 1E, indicating a less invasive phenotype resulting from knockdown of GnT-V. These results suggested that reduced expression of GnT-V caused aberrant N-glycosylation of N-cadherin and reduced invasiveness, which resulted, at least in part, from increased cell-cell adhesion mediated by N-cadherin.
In CHO-K1 Cells Expressing Exogenous N-cadherin, N-cadherin-mediated Cell-Cell Adhesion and Migration Were Regulated by Its N-Glycosylation
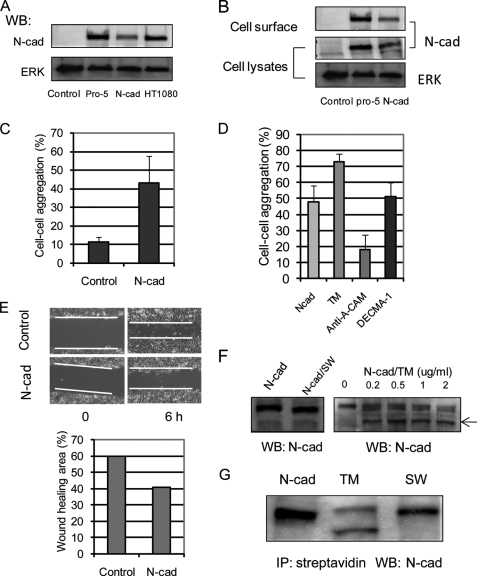
To test further if changes in N-glycosylation of N-cadherin regulated cadherin function, we exogenously expressed N-cadherin in Chinese hamster ovary cells (CHO-K1), because CHO-K1 cells do not express endogenous cadherins. The exogenous expression of N-cadherin in CHO-K1 cells was established by Western blot (Fig. 2A) using HT1080 and a clone of CHO cells (Pro-5) (37) that does express endogenous N-cadherin as controls. To validate that exogenously expressed N-cadherin was expressed on the cell surface, cell surface biotinylation was performed, and N-cadherin levels on the cell surface were determined after pull-down of biotinylated proteins using streptavidin-agarose beads and Western blotting using anti-N-cadherin. As shown in Fig. 2B, as in CHO Pro-5 cells used as controls, N-cadherin was detected on the cell surface of CHO-K1 cells after transfection. Compared with mock-transfected CHO-K1 cells, which displayed a fibroblast-like morphology, CHO-K1 cells with N-cadherin expression were more spread and epithelial-like, similar to control Pro-5 cells with endogenous expression of N-cadherin (data not shown). Calcium-dependent cell aggregation was significantly enhanced in N-cadherin-expressing CHO-K1 cells, determined using the cell-cell aggregation assay (Fig. 2C). Increased cell-cell adhesion observed in the N-cadherin-expressing CHO-K1 cells was significantly inhibited by a function-blocking antibody directed against N-cadherin (anti-A-CAM) but not against E-cadherin (DECMA-1; Fig. 2D), demonstrating that the observed increase in cell-cell adhesion was mediated by N-cadherin. Consistent with enhanced cell-cell adhesion, cell migration into scratch wound areas was inhibited in N-cadherin-expressing CHO-K1 cells compared with control cells (Fig. 2E). These results demonstrate that active and functional N-cadherin was expressed in CHO-K1 cells after transfection (38, 39).
FIGURE 2.
Inhibition of N-glycans caused increased cell-cell adhesion in CHO-K1 cells with exogenous expression of N-cadherin. A, exogenous expression of N-cadherin was detected by Western blotting (WB) using anti-N-cadherin in CHO-K1 cells. Control, CHO-K1 with mock transfection; N-cad, CHO-K1 with N-cadherin transfection. B, cells were labeled with NHS-LC-biotin, followed by precipitation with streptavidin-agarose, SDS-PAGE, and blotting with anti-N-cadherin (top). Cell lysates were Western blotted using anti-N-cadherin (middle) and ERK (bottom; used as loading control). C, cells were removed from culture plates and separated into single cells, followed by constant agitation in calcium-containing media at 37 °C for 30 min. Aggregated cells were scored visually under light microscopy. D, N-cadherin-expressing CHO-K1 cells were pretreated with tunicamycin (TM) overnight, or anti-N-cadherin (anti-A-CAM) and anti-E-cadherin (DECMA-1) were added into the cell suspension before it was subjected to the cell-cell adhesion assay. Each error bar represents the mean ± S.D. percentage of cell-cell adhesion from five randomly selected fields. E, cells were seeded into a 6-well plate, and the monolayer was then scratched with a yellow plastic pipette tip. After incubation for 6 h at 37 °C, migration of cells into the “wounded area” was imaged (top), and the “healed area” was quantified (bottom). F, N-cadherin-expressing CHO-K1 cells were treated with swainsonine (SW) and tunicamycin overnight, and cells were then collected and subjected to Western blotting using anti-N-cadherin. The arrow indicates a band of N-cadherin deglycosylated by tunicamycin treatment. G, cells were treated with swainsonine and tunicamycin overnight and labeled with NHS-LC-biotin, followed by precipitation (IP) with streptavidin-agarose, SDS-PAGE, and blotting with anti-N-cadherin.
We next investigated the effects of modifying N-glycosylation on N-cadherin function. When the N-cadherin-expressing CHO-K1 cells were treated with swainsonine, N-cadherin in CHO-K1 cells showed a significant shift to a lower molecular weight (Fig. 2F, left) detected by Western blotting, as expected, whereas after treatment with tunicamycin, N-cadherin showed two bands with higher mobility than the untreated sample (Fig. 2F, right), as reported (23). These results indicated that N-cadherin expressed in CHO-K1 cells was significantly N-glycosylated with complex N-linked glycans. The expression of N-cadherin on the cell surface was not significantly affected by inhibition of branched N-glycosylation, confirmed by cell surface biotinylation (Fig. 2G). However, the removal of N-glycans by treatment of cells with tunicamycin increased cell-cell aggregation mediated by N-cadherin (Fig. 2D). These results were consistent with those obtained with HT1080 cells that showed reduced expression of branched N-glycan by GnT-V siRNA expression and suggested that both decreased N-glycosylation (by tunicamycin) and reduced N-linked β(1,6) branching (by swainsonine or GnT-V siRNA) had similar effects of increased cell-cell contact mediated by N-cadherin.
Identification of N-linked Glycosylation Sites on N-cadherin
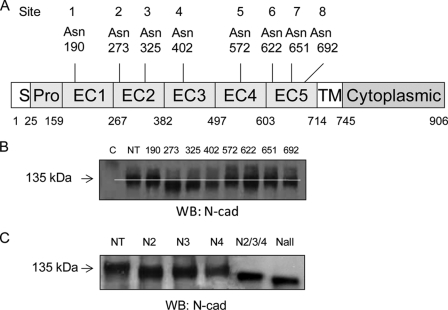
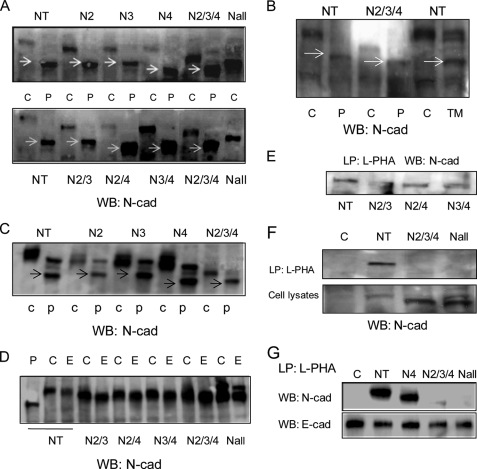
To study in more detail the N-glycan regulation of cadherin-mediated cell-cell adhesion and migration by identifying individual N-glycosylation site occupancy during cell-cell contact formation, N-cadherin variants that lacked specific N-glycosylation site(s) were generated using site-directed mutagenesis. Analysis of human N-cadherin cDNA identified eight N-glycosylation sequons located within the extracellular domains EC1-EC5: Asn at 190, 273, 325, 402, 572, 622, 651, and 692 (Fig. 3A). Constructs were created by substitution of the Asn of the consensus site, Asn-Xaa-Ser/Thr, for Gln, where the least perturbation was expected (20, 40). The mutant constructs were each expressed in CHO-K1 cells, and the mobility of each expressed construct was determined after SDS-PAGE and detection by Western blotting, along with native N-cadherin controls. As shown in Fig. 3B, each of the variant N-cadherins with one site mutated at either Asn273 (N2), Asn325 (N3), or Asn402 (N4) showed a consistent and reproducible increase in mobility over at least three separate experiments, compared with that of the native (NT) construct, indicating that all these three sites were N-glycosylated. By contrast, Asn190, Asn572, Asn622, Asn651, and Asn692 did not appear to express N-glycans, because mutations at these sites did not cause a significant and reproducible change in the mobility of these N-cadherin variants compared with the native sequence. Based on these results, the triple variant with changes at Asn273/Asn325/Asn402 (N2/3/4) and all sites (Nall), with all eight sites mutated, were also constructed. Compared with constructs with single site mutations (N2, N3, and N4), the triple variant N-cadherin showed an even greater increase in mobility (Fig. 3C). Interestingly, with all eight sites mutated, the variant Nall still showed a shift in mobility compared with the N2/3/4 variant, suggesting that one or more of the remaining sequons, Asn190, Asn572, Asn622, Asn651, and Asn692, which were not originally found to be glycosylated, were now glycosylated in this variant in which all three sites that are utilized in the native sequence have been mutated (40).
FIGURE 3.
Identification of occupied N-glycosylation sites of N-cadherin. A, schematic of putative N-glycosylation sites in five extracellular domains (EC1–EC5). S, signal peptide; Pro, pro-region; TM, trans-membrane domain. B, both native and N-glycosylation mutants of N-cadherin were expressed in CHO-K1 cells, and expression of N-cadherins and their shifts in rate of migration were detected by Western blotting (WB) using anti-N-cadherin. C, control with mock transfection. NT, native type. C, total expression levels of N-cadherin were detected in CHO-K1 cells transfected with native N-cadherin and N-cadherins with mutations in sites N2, N3, N4, all three sites (N2/3/4), and all putative sites (Nall) by Western blotting with anti-N-cadherin.
Mutation of N-Glycosylation Sites Had No Significant Effect on Cell Surface Levels of N-cadherin but Stimulated N-cadherin-mediated Adhesion and Outside-in ERK Signaling and Inhibited Cell Migration
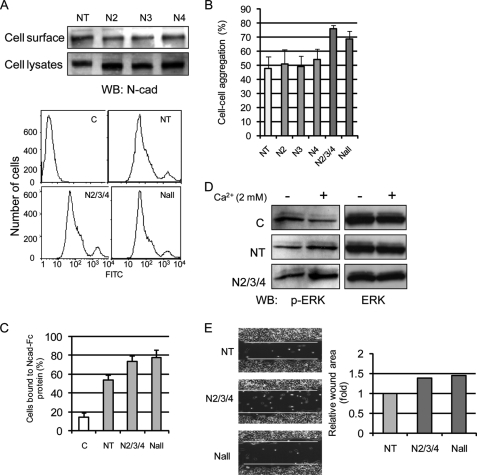
To detect if mutation of the N-glycosylation sites of N-cadherin affected its display on the cell surface, cells expressing either native or variant N-cadherins were subjected to surface biotinylation and flow cytometry analysis. Similar to the exogenously expressed N-cadherin with no mutations, five variant N-cadherins were expressed on the cell surface of CHO-K1 cells (Fig. 4A), indicating that N-cadherin cell surface levels were not significantly affected by mutation of N-glycosylation sites, consistent with the results from tunicamycin-treated, N-cadherin-expressing CHO-K1 cells (Fig. 2) and in agreement with studies of cadherin trafficking in other cell types (23).
FIGURE 4.
Removal of N-glycosylation sites had no effect on N-cadherin trafficking to cell surface but stimulated cell-cell adhesion and outside-in ERK signaling and reduced cell migration. A, cells were labeled with NHS-LC-biotin, followed by precipitation with streptavidin-agarose, SDS-PAGE, and blotting with anti-N-cadherin (top). Cell surface expression of N-cadherin was detected by flow cytometry using anti-N-cadherin antibody (bottom). C, control with mock transfection; NT, native type. B, a single cell suspension was prepared for a cell-cell adhesion assay, followed by constant agitation in calcium-containing media at 37 °C for 30 min, and cell aggregation was scored. C, cells were detached, spread into Ncad-Fc (10 μg/ml)-coated 96-well plates, and incubated for 30 min at 37 °C. The plates were gently washed three times with HCMF, and adherent cells were counted. The percentage of cell binding represents the number of cells bound to plates after washes over the number of total cells before washing. D, calcium switch experiments where cells were serum-starved for 24 h, incubated in DMEM containing 4 mm EGTA at 37 °C for 2 h, and shifted to calcium-containing media for 5 min, followed by processing for phospho-ERK (p-ERK) and total ERK detection by immunoblotting. E, cells were seeded onto 6-well plates, and the monolayer was then scratched with a yellow plastic pipette tip. After incubation for 6 h at 37 °C, migration of cells into the “wounded area” was imaged (left), and the wounded area was quantified (right).
To determine the effects of mutation of N-glycosylation sites on cadherin's adhesive function, the cell-cell aggregation assay was performed using CHO-K1 cells expressing either the native or variant N-cadherins. As shown in Fig. 4B, calcium-dependent cell-cell adhesion was not significantly affected by a mutation of one of the three N-glycosylation sites observed to express N-glycans, although variants N2 and N4 seemed to cause a slight increase in cell-cell adhesion. By contrast, deletion of all three sites (N2/3/4 and Nall) from N-cadherin resulted in a significant increase in cell-cell adhesion. These results were further confirmed by a binding assay that measured adhesion of the cells to the extracellular domain of native N-cadherin (Ncad-Fc protein), which was used to coat assay plates to serve as a potential ligand (Fig. 4C). In this case, compared with cells expressing native N-cadherin (NT), cells expressing the N-cadherin variant with deletion of the three occupied N-linked sites showed a significant increase in binding to wells coated with the extracellular domain of N-cadherin. Supporting increased cell-cell contact formation, cell-cell contact-induced outside-in ERK activation was also significantly increased, detected by a calcium switch experiment (Fig. 4D). Consistent with the observed increase in cell-cell adhesion, mutant N2/3/4 and Nall showed a reduced ability to migrate into the scratch wound area after 6 h compared with cells expressing native N-cadherin (Fig. 4E). Taken together, these results suggest that deletion of occupied N-glycosylation sites resulted in increased N-cadherin-mediated cell-cell contact, outside-in ERK signaling, and, consequently, an inhibition of their ability to migrate into the scratch wound area.
Cis-dimerization of N-cadherin Was Affected in N-Glycosylation Mutants
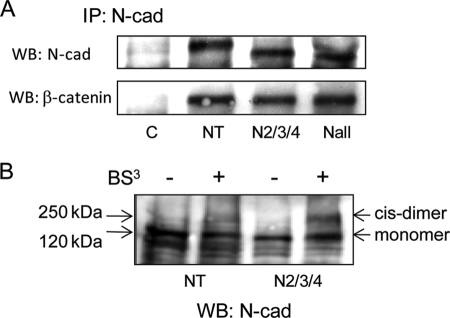
We next explored the possible mechanisms whereby deletion of N-glycosylation caused aberrant N-cadherin adhesive function in CHO-K1 cells. Cadherin-based adhesion is regulated by the formation of the cadherin-catenin complex (6, 41). To determine if deletion of N-cadherin N-glycosylation had effects on the formation of N-cadherin-catenin complexes, co-immunoprecipitation experiments were carried out using an anti-N-cadherin antibody. As shown in Fig. 5A, almost identical levels of β-catenin in complex were observed after deletion of N-glycosylation sites, indicating that the glycosylation state of N-cadherin had no significant impact on complex formation between cadherin and catenin. Increased cell-cell adhesion caused by mutation of N-glycosylation sites was, therefore, not due to aberrant formation of the cadherin-catenin complex.
FIGURE 5.
Cis-dimerization of N-cadherin was affected in N-glycosylation mutants. A, cells were collected for co-immunoprecipitation (IP) using anti-N-cadherin, followed by SDS-PAGE and blotting (WB) with anti-N-cadherin and anti-β-catenin, respectively. C, CHO-K1 with mock transfection; NT, native type of N-cadherin. B, cells were grown to confluence and cross-linked with BS3 at 4 °C for 2 h, followed by lysis, SDS-PAGE, and blotting for N-cadherin.
Studies have shown that cis (lateral)-interaction of cadherins may play an important role in regulating cadherin adhesive function (42, 43). To determine if cis-dimer formation of N-cadherin was affected by deletion of N-glycosylation sites, a membrane-impermeable chemical cross-linker, BS3, was used for labeling cell surface cadherins. As shown in Fig. 5B, cis-dimer formation of N-cadherin with an approximate molecular mass of ∼250 kDa was observed after treatment of a monolayer of cells with BS3. However, in mutant N2/3/4 cells, the level of cis-dimer formation of N-cadherin was significantly increased. These results indicated that increased cell-cell adhesion caused by deletion of N-glycosylation sites in CHO-K1 cells could be due at least in part to enhanced formation of N-cadherin cis-dimers.
Analysis of N-Glycan Types on N-cadherin
To characterize the class of N-glycan at each individual site, we used first N-glycosidase digestion and then L-PHA precipitation. To this end, the N-cadherin constructs with a combination of mutations at two sites, N2/3 (Asn274/Asn325), N2/4 (Asn274/Asn402), and N3/4 (Asn325/Asn402), were generated and expressed in CHO-K1 cells. As expected, the variants with double site mutations showed even faster migration in the SDS-polyacrylamide gels compared with variants with single site mutations, as detected by Western blotting (data not shown). PNGase F cleaves diverse vertebrate N-glycans, including high mannose, hybrid, and complex structures. Incubation of membrane extracts from different mutants with PNGase F produced a lower molecular weight band of N-cadherin with increased mobility. As shown in Fig. 6A, mutants N2, N3, and N2/3 showed the same mobility as native N-cadherin (NT) after PNGase F digestion, whereas the mutants in which Asn402 was mutated (N4, N2/4, N3/4, and N2/3/4) showed even greater mobility after PNGase F digestion. These altered mobilities were similar to that of native N-cadherin from cells treated with tunicamycin (Fig. 6B), indicating that digestion of N-cadherin remained incomplete under the standard digestion conditions when Asn402 was glycosylated. A similar observation was made when MCF-7 cells expressing both native and different N-cadherin mutants were analyzed similarly. The same three occupied sites and mobility after PNGase F digestion, in which the mutant sequences with deletion of N-glycosylation on Asn402 (N4 and N2/3/4) showed faster migration on SDS-PAGE, were also observed (Fig. 6C). Compared with PNGase F, endoglycosidase H digestion caused little, if any, change in mobility of both native type and mutant N-cadherins (Fig. 6D), indicating that N-cadherin expresses fully processed complex N-glycans.
FIGURE 6.
Analysis of N-glycan types of N-cadherin using glycosidases and L-PHA precipitation. A, CHO-K1 cell lysates expressing native and mutant N-cadherins were digested with PNGase F, followed by SDS-PAGE and Western blotting (WB) using anti-N-cadherin antibody. C, undigested control. P, PNGase F-digested. The arrows indicate deglycosylated band of N-cadherin by PNGase F digestion. B, cells were digested with PNGase F or pretreated with tunicamycin overnight before being subjected to SDS-PAGE and Western blotting for N-cadherin. TM, tunicamycin. The arrows indicate deglycosylated band of N-cadherin by PNGase F digestion or tunicamycin treatment. C, MCF-7 cell lysates expressing native and mutant N-cadherins were digested with PNGase F, followed by SDS-PAGE and Western blotting using anti-N-cadherin antibody. D, CHO-K1 cell lysates containing native and mutant N-cadherins were digested with endoglycosidase H, followed by SDS-PAGE and Western blotting using anti-N-cadherin antibody. C, undigested control; P, PNGase F-digested; E, endoglycosidase H-digested. E and F, CHO-K1 cell lysates were used for precipitation with biotinylated L-PHA, followed by the addition of streptavidin-agarose, SDS-PAGE, and Western blotting for N-cadherin. LP, lectin precipitation. G, MCF-7 cell lysates were used for precipitation with biotinylated L-PHA, followed by the addition of streptavidin-agarose, SDS-PAGE, and Western blotting for N-cadherin. E-cadherins were detected as control. E-cad, E-cadherin.
To determine the expression of N-linked β(1,6)-branched glycans on the three N-linked sequons of N-cadherin shown to be glycosylated in CHO-K1 cells, L-PHA was used to precipitate β(1,6)-branched glycoproteins from lysates of native type and mutant cells. As shown in Fig. 6E, after L-PHA pull-down, significant amounts of N-cadherin were detected in the cells expressing non-mutated cadherin, indicating that native N-cadherin contained N-linked β(1,6) branching, as expected. After combinations of two of the three occupied sites were deleted (N2/3, N2/4, and N3/4), N-cadherin could still be precipitated by L-PHA (Fig. 6E), indicating the possible presence of β(1,6) branching at each of the three sites. By contrast, only insignificant amounts of N-cadherin were precipitated by L-PHA from cells expressing the triple deletion variant N2/3/4 and Nall cells (Fig. 6F). Similar results were also observed with MCF-7 cells expressing the same N-cadherin variants (Fig. 6G). These findings indicate that at least some GnT-V-modified N-glycans were expressed at each of the three sites of N-cadherin that natively express N-glycans, and no significant L-PHA-binding glycans were found after all three sites were deleted.
DISCUSSION
In the present study, a detailed investigation of the regulation of N-cadherin-mediated function by changes in N-glycosylation was performed using siRNA and site-directed mutagenesis of exogenously expressed N-cadherin. In addition, we dissected individual N-glycosylation site occupancy of N-cadherin and focused on the effects of deleting sites that expressed N-glycans, individually and in groups, on N-cadherin-related adhesive functions. Our results revealed consistently that inhibition of N-glycan expression on N-cadherin had no apparent effect on N-cadherin expression on the cell surface but stimulated N-cadherin-mediated cell-cell adhesion and suppressed migration. Moreover, we identified three potential N-glycosylation sites in extracellular domains EC2 and EC3 that expressed N-glycans that were bound by L-PHA, demonstrating that they contained β(1,6)-branched glycans.
Oncogenesis is often associated with decreased cell-cell adhesion, alterations in adhesion-mediated signaling pathways, and changes in organization of the cytoskeleton that influence cell migration and invasiveness. Studies have shown that cadherin-induced cell-cell adhesion can be regulated by the level of N-glycosylation, including β(1,6) branching formed by GnT-V (16–21). GnT-V, a rate-limiting and oncogene-regulated enzyme in the processing of multiantennary N-glycans during glycoprotein biosynthesis, catalyzes the formation of (GlcNAcβ(1,6)Man) branches on N-glycans (24, 25). Both in vitro and in vivo studies have implicated GnT-V in regulating tumor invasiveness and, in some cases, metastatic potential (19, 31, 44–47). In an earlier study, we found that N-cadherin glycans were modified by GnT-V, and increased GnT-V expression in HT1080 cells inhibited clustering of cell surface N-cadherin. This effect on receptor clustering, in turn, enhanced the susceptibility of β-catenin and p120ctn phosphorylation by growth factors and the src oncogene, leading to decreased cell-cell adhesion, consistent with the increased migratory phenotype observed for GnT-V-overexpressing cells (19). These results suggested that the level of β(1,6) branching positively regulated tumor invasiveness-related phenotypes by altering cell-cell aggregation. Supporting this conclusion, further evidence was presented in the present study that reduced levels of GnT-V activity, resulting from GnT-V siRNA expression, enhanced homotypic cell-cell adhesion and adhesion-mediated ERK signaling and reduced cell migration/invasion of HT1080 cells. Knockdown of GnT-V in these cells had no effect on N-cadherin cell surface expression but did cause decreased expression levels of β(1,6) branching on N-cadherin. These results indicated that altered cell-cell adhesion was most likely due to changes in adhesive properties of N-cadherin regulated by GnT-V expression levels (19) rather than changes in the cell surface turnover of cadherin, which was noted for E-cadherin when GnT-III was overexpressed in melanoma cells (17).
The regulation of N-cadherin-mediated cell-cell contact and cell migration by expression of N-glycans was further confirmed in CHO-K1 cells when exogenous N-cadherin was expressed. In CHO-K1 cells expressing exogenous N-cadherin, cell migration was inhibited due to increased cell-cell contact mediated by N-cadherin. Treatment of N-cadherin-expressing CHO-K1 cells with the N-glycan inhibitor, tunicamycin, resulted in promotion of cell-cell adhesion without affecting trafficking of N-cadherin to the cell surface, consistent with a previous report that treatment with tunicamycin induced an epithelioid phenotype in a Madin-Darby canine kidney subclonal line, clone-YH, by stabilizing homotypic N-cadherin-mediated cell junctions (23). Although cell surface expression of N-cadherin was not affected by treatment of cells with N-glycosylation inhibitors, cell migration was significantly reduced, indicating that N-glycan expression can regulate N-cadherin-mediated migratory behavior. Inhibition of N-glycosylation or decreased expression of β(1,6) branching on N-cadherin both led to increased cell-cell contact and reduced cell migration. A recent study has shown that N-glycosylation of E-cadherin was cell density-dependent (20). The complexity of N-glycans on E-cadherin, detected by N-glycosidase treatments and lectin binding, was decreased in dense cultures of Madin-Darby canine kidney cells with more stable adhesion junction formation, compared with sparse cultures. Another study, also using Madin-Darby canine kidney cells, showed that inhibition of N-glycan branching by swainsonine treatment tightened and stabilized cell-cell junctions (48). The normal development of cell-cell adhesion in these cells was associated with reduced complexity of E-cadherin N-glycans as well as those on the Na/K-ATPase β1 subunit, a membrane transport enzyme that has been found important for intercellular adhesion. Interestingly, these changes were found to be associated with increased expression of GnT-III, which forms bisecting GlcNAc on hybrid or complex N-glycan structures and consequently reduces branched glycan formation, but decreased expression of GnT-IV and GnT-V mRNA. Our results are in agreement with these observations and suggest that the regulation of cell-cell contact formation by changes in N-glycosylation or reduction of expression of GnT-V-modified N-glycans may be a general means to affect cadherin function that includes N- and E-cadherin and other adhesion-related membrane proteins, such as Na/K-ATPase.
An increasing number of studies have explored the effects of altering the N-glycosylation of particular cell surface adhesion molecules using site-directed mutagenesis, including integrins (49–51), cadherins (20), and Na,K-ATPase β1 (48). Structural studies have shown that cadherin-induced cell-cell contacts are mediated by the extracellular portion of cadherins, which is divided into five homologous repeats (∼110 amino acids) of extracellular cadherin domains (EC1–EC5) (52). EC1 and EC2 have been most implicated in being responsible for cadherin adhesive activity via different mechanisms (53, 54). EC1 was also found to be responsible for the binding specificity of cadherins (55). EC4 is believed to play a major role in regulating N-cadherin-mediated epithelial to mesenchymal transition and increased motility (56). Although the amino acid sequence of N-cadherin contains eight putative N-linked glycosylation sites through EC1–EC5, no information was available on the number of these sites that are glycosylated. To examine the site occupancy of N-glycosylation, we generated different N-glycosylation mutants in which Asn of the consensus site, Asn-Xaa-Ser/Thr, was mutated to Gln, where the least perturbation was expected (20, 40), using site mutagenesis. Based on the observation of consistent molecular shifts of mutant N-cadherins compared with the native form, measured from multiple SDS-PAGE experiments, we found that three of eight putative sites were N-glycosylated, Asn207 and Asn325 in EC2 and Asn402 in EC3. By contrast, mutation of the remaining sites did not reveal consistent band shifts upon SDS-PAGE, although these sites were located in the adhesion-regulating domain EC1 (Asn190) and migration-regulating domain EC4 (Asn572) or were conserved (Asn572 and Asn651) among all six of the common human cadherins. Interestingly, the mutant Nall, in which all eight potential sites were mutated, still showed a faster migration of N-cadherin on the gel (Fig. 3C) when compared with that of the N2/3/4 mutant, indicating that some of sites that were not glycosylated in the native protein may acquire N-glycosylation in a mutant in which all glycosylation sites that are normally utilized have been mutated. This compensatory N-glycosylation has been observed (40) and may suggest a cellular response to disruption of N-glycosylation of proteins or simply an alteration in protein structures that makes available normally non-utilized sites. Results from glycosidase digestions of N-cadherins showed that both native N-cadherins and N-cadherins in which at least one N-glycan was expressed were all PNGase F-sensitive, with little or no sensitivity to endoglycosidase H, indicating that N-cadherin expressed complex N-glycans. Interestingly, PNGase F digestion of N-cadherins seemed to be incomplete (Fig. 6). However, when Asn402 was mutated (mutants N4, N2/4, N3/4, and N2/3/4), deglycosylated N-cadherins showed increased mobility on SDS-polyacrylamide gel in two different cell lines (CHO-K1 and MCF-7), which was identical to the mobility of N-cadherins from tunicamycin-treated cells (Fig. 6, A and B), indicating that the resistance of N-glycosylation on Asn402 to PNGase F digestion was not cell type-dependent and either that this glycosylation site might contain some specific N-glycan structures that resist PNGase F digestion or that its removal may render some conformation changes that consequently make mutants more sensitive to PNGase F digestion. The finding that the three utilized sites in N-cadherin expressed complex N-glycans was further confirmed by L-PHA precipitation experiments in both CHO-K1 and MCF-7 cells, showing that all three active sites contained complex N-glycans with β(1,6) branching.
We also found that mutation of single employed N-glycosylation sites did not significantly increase N-cadherin-mediated aggregation of CHO-K1 cells expressing N-cadherin. This result is probably due to the insensitivity of the assay employed (visual inspection). However, removal of the three utilized sites and all putative sites caused a remarkable enhancement of cell-cell adhesion, calcium-dependent cell-cell contact-mediated outside-in ERK signaling, and a consequent reduction in the rate of wound closure in the cell migration assay. These results are consistent with our observations on the effects on cell-cell adhesion of HT1080 cells with reduced branched N-glycan expression from expression of siRNA of GnT-V and the results of N-glycan inhibitor-treated CHO cells expressing N-cadherin, confirming the hypothesis that reduced N-glycosylation branching or deletion stabilizes cell-cell contact and inhibits cell migration.
A previous study showed that overexpression of GnT-III in B16 mouse melanoma cells resulted in an altered glycosylation of E-cadherin (increased levels of the “bisected” N-linked glycan but decreased expression of the N-linked β(1,6) branch) that consequently delayed the E-cadherin turnover rate at the cell surface and reduced the lung metastatic potential of the melanoma cells (17). Another recent report, however, showed a reduction in total levels of E-cadherin expression after N-glycosylation sites were mutated (20). In our study, by contrast, N-cadherin expression levels were not significantly affected by either GnT-V-directed siRNA expression or deletion of N-glycosylation by either N-glycan inhibitor treatment or site mutation, suggesting that altered N-cadherin adhesive function was not due to changes in N-cadherin expression but most likely due to changes in cell-cell cadherin binding.
Moreover, in contrast to the work on E-cadherin, our results with N-cadherin demonstrate that the three sites with N-glycosylation are in domains EC2 and EC3, not domains EC4 and EC5 (20), and that the mechanism of regulation does not involve catenin-cadherin interactions; rather, the regulation point appears to be in dimerization of the cadherin monomers prior to the trans-binding of dimers on apposing cell surfaces. It is generally believed that cis-dimerization of cadherins yields the functional unit of cadherin adhesion, formed by binding of two cadherin extracellular domains on the same cell surface. The interaction of a cadherin cis-dimer on one cell with a cis-dimer on an adjacent cell leads to formation of low affinity trans-dimers of cadherin, which initiate cell-cell adhesion (57, 58). EC1 and EC2 have been reported to be involved in cadherin cis- and trans-dimer formation (59–61). Furthermore, cadherins form the adherens junction via association of their cytoplasmic tails with several intracellular proteins known as the catenins, which link cadherins to the actin-based cytoskeleton and promote stronger cell-cell adhesion (62, 63). Although studies showed that deletion of N-glycans caused increased interaction of cadherin with catenins and vinculin (20, 23), in the present study, the interaction of cadherin and catenins appeared to be unaffected by inhibition of N-glycosylation.
To test the hypothesis that the effects of N-glycan expression on N-cadherin function involved cis-dimer formation, chemical cross-linking experiments were performed. The results showed that increased cell-cell contact caused by inhibition of N-glycosylation resulted most likely from altered cis-dimer formation of N-cadherin after inhibition of N-glycosylation.
A recent study from our laboratory using SH-SY5Y neuroblastoma cells showed that increased activity of GnT-Vb, a paralog of GnT-V whose expression is restricted mainly to neural cells, promoted the addition of the O-mannosyl-linked HNK-1 modification found on the developmentally regulated and neuron-specific receptor protein-tyrosine phosphatase β (64). These changes in glycosylation resulted in decreased cell-cell adhesion and increased rates of migration on laminin, accompanied by increased phosphorylated β-catenin. Expression of siRNA directed toward GnT-Vb transcripts in these cells had the opposite effects, as expected. Moreover, overexpression of GnT-V in HT1080 human fibrosarcoma cells caused increased β(1,6)-GlcNAc on N-cadherin and reduced cell-cell adhesion by enhancing phosphorylation of catenins (19). Altered phosphorylation of catenins, therefore, appears to be a common mechanism by which changes in GnT-V and GnT-Vb expression result in altered cell-cell adhesion and enhanced migration.
In conclusion, our results, along with those from E-cadherin N-glycan studies, strongly support the conclusion that N-glycosylation plays a prominent role in regulating cadherin-mediated adhesion and its intracellular signaling pathways, which can contribute to the increase in the migratory/invasive phenotype of cancer cells that results when GnT-V activity is up-regulated by oncogene signaling.
Acknowledgments
We thank Dr. Keith R. Johnson from the University of Nebraska Medical Center for kindly providing the human N-cadherin cDNA clone (Puc19/N-cadherin), Dr. Takeshi Sakurai at the Mount Sinai School of Medicine for providing the Ncad-Fc fusion pRK5 clone, and Dr. Pamela Stanley for providing the Pro-5 CHO cells. We also thank Julie Nelson for help with the flow cytometry and Drs. Bing Zhang, Jin-kyu Lee, and Gerardo Alvarez-Manilla for informative discussions.
This work was supported in part by National Institutes of Health, NCI, Grant RO1CA64462.
- GnT-III and -V
- N-acetylglucosaminyltransferase III and V (Mgat5
- EC 2.4.1.155)
- respectively
- siRNA
- small interfering RNA
- L-PHA
- leucoagglutinating phytohemagglutinin
- DMEM
- Dulbecco's modified Eagle's medium
- NHS-LS-biotin
- sulfosuccinimidyl-b-[biotin-amido]hexanoate
- BS3
- bis(sulfosuccinimidyl) suberate
- ERK
- extracellular signal-regulated kinase
- BSA
- bovine serum albumin
- PBS
- phosphate-buffered saline
- Ncad
- N-cadherin
- PNGase F
- peptide N-glycosidase F.
REFERENCES
- 1.Takeichi M. (1995) Curr. Opin. Cell Biol. 7, 619–627 [DOI] [PubMed] [Google Scholar]
- 2.Yagi T., Takeichi M. (2000) Genes Dev. 14, 1169–1180 [PubMed] [Google Scholar]
- 3.Shan W. S., Koch A., Murray J., Colman D. R., Shapiro L. (1999) Biophys. Chem. 82, 157–163 [DOI] [PubMed] [Google Scholar]
- 4.Koch A. W., Bozic D., Pertz O., Engel J. (1999) Curr. Opin. Struct. Biol. 9, 275–281 [DOI] [PubMed] [Google Scholar]
- 5.Hirano S., Kimoto N., Shimoyama Y., Hirohashi S., Takeichi M. (1992) Cell 70, 293–301 [DOI] [PubMed] [Google Scholar]
- 6.Irby R. B., Yeatman T. J. (2002) Cancer Res. 62, 2669–2674 [PubMed] [Google Scholar]
- 7.Conacci-Sorrell M., Zhurinsky J., Ben-Ze'ev A. (2002) J. Clin. Invest. 109, 987–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birchmeier W., Behrens J. (1994) Biochim. Biophys. Acta 1198, 11–26 [DOI] [PubMed] [Google Scholar]
- 9.Takeichi M. (1993) Curr. Opin. Cell Biol. 5, 806–811 [DOI] [PubMed] [Google Scholar]
- 10.Hazan R. B., Kang L., Whooley B. P., Borgen P. I. (1997) Cell Adhes. Commun. 4, 399–411 [DOI] [PubMed] [Google Scholar]
- 11.Nagi C., Guttman M., Jaffer S., Qiao R., Keren R., Triana A., Li M., Godbold J., Bleiweiss I. J., Hazan R. B. (2005) Breast Cancer Res. Treat. 94, 225–235 [DOI] [PubMed] [Google Scholar]
- 12.Li K., Wang X., He W., Lin N., Fan Q. X. (2009) Chin. J. Cancer 28, 8–13 [PubMed] [Google Scholar]
- 13.Nieman M. T., Prudoff R. S., Johnson K. R., Wheelock M. J. (1999) J. Cell Biol. 147, 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazan R. B., Phillips G. R., Qiao R. F., Norton L., Aaronson S. A. (2000) J. Cell Biol. 148, 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suyama K., Shapiro I., Guttman M., Hazan R. B. (2002) Cancer Cell 2, 301–314 [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura M., Nishikawa A., Ihara Y., Taniguchi S., Taniguchi N. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8754–8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura M., Ihara Y., Matsuzawa Y., Taniguchi N. (1996) J. Biol. Chem. 271, 13811–13815 [DOI] [PubMed] [Google Scholar]
- 18.Kitada T., Miyoshi E., Noda K., Higashiyama S., Ihara H., Matsuura N., Hayashi N., Kawata S., Matsuzawa Y., Taniguchi N. (2001) J. Biol. Chem. 276, 475–480 [DOI] [PubMed] [Google Scholar]
- 19.Guo H. B., Lee I., Kamar M., Pierce M. (2003) J. Biol. Chem. 278, 52412–52424 [DOI] [PubMed] [Google Scholar]
- 20.Liwosz A., Lei T., Kukuruzinska M. A. (2006) J. Biol. Chem. 281, 23138–23149 [DOI] [PubMed] [Google Scholar]
- 21.Zhao H., Sun L., Wang L., Xu Z., Zhou F., Su J., Jin J., Yang Y., Hu Y., Zha X. (2008) Acta Biochim. Biophys. Sin. 40, 140–148 [DOI] [PubMed] [Google Scholar]
- 22.Nita-Lazar M., Noonan V., Rebustini I., Walker J., Menko A. S., Kukuruzinska M. A. (2009) Cancer Res. 69, 5673–5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn Y. H., Hong J., Burke J. M. (2006) Invest. Ophthalmol. Vis. Sci. 47, 2675–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brockhausen I., Carver J. P., Schachter H. (1988) Biochem. Cell Biol. 66, 1134–1151 [DOI] [PubMed] [Google Scholar]
- 25.Hakomori S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10231–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomon D., Ayalon O., Patel-King R., Hynes R. O., Geiger B. (1992) J. Cell Sci. 102, 7–17 [DOI] [PubMed] [Google Scholar]
- 27.Guo H. B., Randolph M., Pierce M. (2007) J. Biol. Chem. 282, 22150–22162 [DOI] [PubMed] [Google Scholar]
- 28.Sakurai T., Lustig M., Nativ M., Hemperly J. J., Schlessinger J., Peles E., Grumet M. (1997) J. Cell Biol. 136, 907–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shan W., Yagita Y., Wang Z., Koch A., Fex Svenningsen A., Gruzglin E., Pedraza L., Colman D. R. (2004) J. Biol. Chem. 279, 55914–55923 [DOI] [PubMed] [Google Scholar]
- 30.Guo H. B., Lee I., Bryan B. T., Pierce M. (2005) J. Biol. Chem. 280, 8332–8342 [DOI] [PubMed] [Google Scholar]
- 31.Guo H. B., Lee I., Kamar M., Akiyama S. K., Pierce M. (2002) Cancer Res. 62, 6837–6845 [PubMed] [Google Scholar]
- 32.Li G., Satyamoorthy K., Herlyn M. (2001) Cancer Res. 61, 3819–3825 [PubMed] [Google Scholar]
- 33.Guo H. B., Johnson H., Randolph M., Lee I., Pierce M. (2009) Glycobiology 19, 547–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pece S., Gutkind J. S. (2000) J. Biol. Chem. 275, 41227–41233 [DOI] [PubMed] [Google Scholar]
- 35.Tran N. L., Adams D. G., Vaillancourt R. R., Heimark R. L. (2002) J. Biol. Chem. 277, 32905–32914 [DOI] [PubMed] [Google Scholar]
- 36.Rappl A., Piontek G., Schlegel J. (2008) J. Cell Sci. 121, 4089–4097 [DOI] [PubMed] [Google Scholar]
- 37.Chaney W., Sundaram S., Friedman N., Stanley P. (1989) J. Cell Biol. 109, 2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levenberg S., Sadot E., Goichberg P., Geiger B. (1998) Cell Adhes. Commun. 6, 161–170 [DOI] [PubMed] [Google Scholar]
- 39.Levenberg S., Yarden A., Kam Z., Geiger B. (1999) Oncogene 18, 869–876 [DOI] [PubMed] [Google Scholar]
- 40.Ray K., Clapp P., Goldsmith P. K., Spiegel A. M. (1998) J. Biol. Chem. 273, 34558–34567 [DOI] [PubMed] [Google Scholar]
- 41.Chothia C., Jones E. Y. (1997) Annu. Rev. Biochem. 66, 823–862 [DOI] [PubMed] [Google Scholar]
- 42.Foty R. A., Steinberg M. S. (2005) Dev. Biol. 278, 255–263 [DOI] [PubMed] [Google Scholar]
- 43.Chen C. P., Posy S., Ben-Shaul A., Shapiro L., Honig B. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 8531–8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demetriou M., Nabi I. R., Coppolino M., Dedhar S., Dennis J. W. (1995) J. Cell Biol. 130, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granovsky M., Fata J., Pawling J., Muller W. J., Khokha R., Dennis J. W. (2000) Nat. Med. 6, 306–312 [DOI] [PubMed] [Google Scholar]
- 46.Seelentag W. K., Li W. P., Schmitz S. F., Metzger U., Aeberhard P., Heitz P. U., Roth J. (1998) Cancer Res. 58, 5559–5564 [PubMed] [Google Scholar]
- 47.Yamamoto H., Swoger J., Greene S., Saito T., Hurh J., Sweeley C., Leestma J., Mkrdichian E., Cerullo L., Nishikawa A., Ihara Y., Taniguchi N., Moskal J. R. (2000) Cancer Res. 60, 134–142 [PubMed] [Google Scholar]
- 48.Vagin O., Tokhtaeva E., Yakubov I., Shevchenko E., Sachs G. (2008) J. Biol. Chem. 283, 2192–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato Y., Isaji T., Tajiri M., Yoshida-Yamamoto S., Yoshinaka T., Somehara T., Fukuda T., Wada Y., Gu J. (2009) J. Biol. Chem. 284, 11873–11881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isaji T., Sato Y., Fukuda T., Gu J. (2009) J. Biol. Chem. 284, 12207–12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isaji T., Sato Y., Zhao Y., Miyoshi E., Wada Y., Taniguchi N., Gu J. (2006) J. Biol. Chem. 281, 33258–33267 [DOI] [PubMed] [Google Scholar]
- 52.Takeichi M. (1990) Annu. Rev. Biochem. 59, 237–252 [DOI] [PubMed] [Google Scholar]
- 53.Chappuis-Flament S., Wong E., Hicks L. D., Kay C. M., Gumbiner B. M. (2001) J. Cell Biol. 154, 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pertz O., Bozic D., Koch A. W., Fauser C., Brancaccio A., Engel J. (1999) EMBO J. 18, 1738–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shan W. S., Tanaka H., Phillips G. R., Arndt K., Yoshida M., Colman D. R., Shapiro L. (2000) J. Cell Biol. 148, 579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J. B., Islam S., Kim Y. J., Prudoff R. S., Sass K. M., Wheelock M. J., Johnson K. R. (2000) J. Cell Biol. 151, 1193–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niessen C. M., Gottardi C. J. (2008) Biochim. Biophys. Acta 1778, 562–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gumbiner B. M. (2000) J. Cell Biol. 148, 399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boggon T. J., Murray J., Chappuis-Flament S., Wong E., Gumbiner B. M., Shapiro L. (2002) Science 296, 1308–1313 [DOI] [PubMed] [Google Scholar]
- 60.Häussinger D., Ahrens T., Aberle T., Engel J., Stetefeld J., Grzesiek S. (2004) EMBO J. 23, 1699–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Sivasankar S., Nelson W. J., Chu S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cavallaro U., Christofori G. (2004) Nat. Rev. Cancer 4, 118–132 [DOI] [PubMed] [Google Scholar]
- 63.Wheelock M. J., Johnson K. R. (2003) Curr. Opin. Cell Biol. 15, 509–514 [DOI] [PubMed] [Google Scholar]
- 64.Abbott K. L., Matthews R. T., Pierce M. (2008) J. Biol. Chem. 283, 33026–33035 [DOI] [PMC free article] [PubMed] [Google Scholar]