Abstract
The vesicular inhibitory amino acid transporter (VIAAT) is a synaptic vesicle protein responsible for the vesicular storage of γ-aminobutyrate (GABA) and glycine which plays an essential role in GABAergic and glycinergic neurotransmission. The transport mechanism of VIAAT remains largely unknown. Here, we show that proteoliposomes containing purified VIAAT actively took up GABA upon formation of membrane potential (Δψ) (positive inside) but not ΔpH. VIAAT-mediated GABA uptake had an absolute requirement for Cl− and actually accompanied Cl− movement. Kinetic analysis indicated that one GABA molecule and two Cl− equivalents were transported during one transport cycle. VIAAT in which Glu213 was specifically mutated to alanine completely lost the ability to take up both GABA and Cl−. Essentially the same results were obtained with glycine, another substrate of VIAAT. These results demonstrated that VIAAT is a vesicular Cl− transporter that co-transports Cl− with GABA or glycine in a Δψ dependent manner. It is concluded that Cl− plays an essential role in vesicular storage of GABA and glycine.
Introduction
Vesicular storage and subsequent exocytosis of GABA3 and glycine comprise the major pathway for inhibitory signal transmission in the central nervous system (for review, see Refs. 1 and 2). GABAergic inhibitory signaling occurs in the brain, whereas both GABAergic and glycinergic signaling occur primarily in spinal cord and brain stem. Like other neurotransmitters such as l-glutamate and acetylcholine, GABA and glycine are actively accumulated in synaptic vesicles through vesicular neurotransmitter transporters (3–7). Currently, only one type of transporter, SLC32A1, is known to be responsible for this process and is referred to as either vesicular GABA transporter or vesicular inhibitory amino acid transporter (VIAAT) (2, 8, 9). VIAAT is an ortholog of the unc-47 gene product, which has been identified as the protein responsible for the GABAergic signal transmission in Caenorhabditis elegans (10). The putative secondary structure of VIAAT contains either 10 or 9 transmembrane helices with a large (∼130 amino acids) hydrophilic N-terminal domain dependent on the analysis (supplemental Fig. S1), which is completely different from plasma membrane GABA transporters and other vesicular neurotransmitter transporters such as vesicular glutamate transporter (2, 8, 9, 11). VIAAT is present in synaptic vesicles from GABAergic and glycinergic neurons (12–14). VIAAT knock-out mice exhibit a partial loss of GABAergic as well as glycinergic neurotransmission (15).
Despite the well accepted significance of VIAAT in inhibitory neurotransmission, elucidation of the molecular mechanism of the transporter has been hampered mainly due to the lack of an in vitro assay system to assess the transport of recombinant VIAAT. Recently, we developed a procedure to assess the transport activity of recombinant vesicular glutamate transporter, vesicular nucleotide transporter, and vesicular excitatory amino acid transporter, comprising expression of wild type and mutant transporters in insect cells and their purification and reconstitution into proteoliposomes (16–18). In the present work, using the procedure, we characterized the transport activity of purified VIAAT and found an unexpected feature of VIAAT as a Cl− co-transporter.
EXPERIMENTAL PROCEDURES
Expression
Recombinant baculoviruses containing wild type and mutant rat VIAAT cDNA (14), which was kindly donated by Dr. Shigeo Takamori (Tokyo Dental and Medical University), were constructed using the Bac-to-Bac baculovirus expression system (Invitrogen) according to the manufacturer's protocol. Rat VIAAT cDNA was amplified by PCR using the primers 5′-CACCATGGCCACCCTGCTCCGC-3′ and 5′-CCTCTAGACTAGTCCTCTGCGTTG-3′ and ligated into the pENTR/D-TOPO vector. VIAAT cDNA was transferred from the pENTR/D-TOPO vector to a destination vector and named pDEST10-VIAAT. The resulting cloned VIAAT gene also encoded an N-terminal His6 tag. DH10Bac cells carrying bacmid DNA were transformed with pDEST10-VIAAT. Recombinant bacmid was isolated from DH10Bac cells and used for transfecting High Five cells for the expression of VIAAT protein. High Five cells (1 × 107 cells/10-cm dish) were grown in Express Five medium (Invitrogen) supplemented with 2 mm l-glutamine and 10 μg/ml gentamycin at 27 °C. High Five cells were infected by recombinant baculoviruses at a multiplicity of infection of 1 and grown for an additional 48 h. Afterward, the cells were harvested for membrane preparation. Upon infection, the insect cells expressed His-tagged VIAAT as revealed by Western blot analysis (16). Maximum expression was obtained 48 h after infection. The extent of enrichment, which was roughly estimated by Western blotting analysis, was 4-fold with recoveries of ∼30%.
Mutagenesis
Mutation (E213A) was introduced to pDEST10-VIAAT by PCR using the primer 5′-AGATCATCGCCCTGGTGATGAC-3′. The sequence was confirmed by nucleotide sequencing. The E213A mutant was also expressed and used in the study after purification and reconstitution as follows.
Purification
Insect cells (1∼2 × 107 cells) were suspended in a buffer containing 20 mm Tris-HCl (pH 8.0), 0.1 m potassium acetate, 10% glycerol, 0.5 mm dithiothreitol, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin and disrupted by sonication with a TOMY UD200 tip sonifier. Cell lysates were centrifuged at 700 × g for 10 min to remove debris, and the resultant supernatant was centrifuged at 160,000 × g for 1 h. The pellet (membrane fraction) was suspended in buffer containing 20 mm MOPS-Tris (pH 7.0), 10% glycerol, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin at ∼1.5 mg of protein/ml. The membrane fraction was solubilized with 2% octylglucoside. After centrifugation at 260,000 × g for 30 min, the supernatant was added to 1 ml of nickel-nitrilotriacetic acid Superflow resin (Qiagen) and incubated for 4 h at 4 °C. The resin was washed with 10 ml of 20 mm MOPS-Tris (pH 7.0), 5 mm imidazole, 10% glycerol, and 1% octylglucoside in a column. VIAAT was eluted from the resin with 3 ml of the same buffer containing 60 mm imidazole. The eluate containing purified VIAAT was stored at −80 °C, where it was stable without loss of activity for at least a few months. Bacterial F-ATPase was expressed in DK8/pBWU13 Escherichia coli cells and purified by glycerol density gradient centrifugation as described previously (19).
Reconstitution
Co-reconstitution of purified recombinant VIAAT and bacterial F-ATPase into liposomes was carried out by the freeze-thaw method described elsewhere (16). In brief, 10 μg of VIAAT was mixed with 90 μg of F-ATPase and liposomes (0.5 mg of lipid), frozen at −80 °C, and left at this temperature for at least 5 min. The mixture was thawed quickly by holding the sample tube in the hand and diluted 60-fold with reconstitution buffer (20 mm MOPS-Tris (pH 7.0), 0.5 mm dithiothreitol, 0.1 m potassium acetate, and 5 mm magnesium acetate). The buffer composition was changed as necessary. Reconstituted proteoliposomes were pelleted by centrifugation at 200,000 × g for 1 h at 4 °C and suspended in 0.4 ml of 20 mm MOPS-Tris (pH 7.0) containing 10 mm KCl, 0.1 m potassium acetate, and 5 mm magnesium acetate. If VIAAT was reconstituted alone in proteoliposomes, 10 μg of protein was mixed with asolectin liposomes (0.5 mg of lipid) and processed as usual. The reconstitution buffer was 20 mm MOPS-Tris (pH 7.0), 0.5 mm dithiothreitol, 0.15 m sodium acetate, and 5 mm magnesium acetate. After sedimentation, the VIAAT-containing proteoliposomes were suspended in 0.2 ml of 20 mm MOPS-Tris (pH 7.0), containing 0.15 m sodium acetate and 5 mm magnesium acetate. Asolectin liposomes were prepared as follows. Soybean lecithin (20 mg; Sigma type IIS) was suspended in 2 ml of 20 mm MOPS-NaOH (pH 7.0) containing 0.5 mm dithiothreitol. The mixture was sonicated in a bath-type sonicator until clear, divided into small aliquots, and stored at −80 °C until use (19).
Transport Assay
Assays were carried out by the gel-permeation procedure as described previously (16–18). In more detail, for ATP-driven transport, proteoliposomes containing both VIAAT and F-ATPase (5 μg of total protein) were suspended in 20 mm MOPS-Tris (pH 7.5), 5 mm magnesium acetate, 4 mm KCl, and 0.1 m potassium acetate and incubated for 3 min at 27 °C. ATP at 2 mm was added, and the mixture was incubated for a further 3 min. The assay was initiated by addition of 100 μm [2,3-3H]GABA (0.5 MBq/μmol) or 2 mm [2-3H]glycine (0.5 MBq/μmol). Aliquots (130 μl) were taken at the times indicated and centrifuged through a Sephadex G-50 (fine) spin column at 760 × g for 2 min. Radioactivity and protein concentration of the eluate were measured. For Val-induced transport assays, proteoliposomes containing VIAAT (0.5 μg of protein/assay) were suspended in 500 μl of 20 mm MOPS-Tris (pH 7.0), 5 mm magnesium acetate, 10 mm KCl, and 0.15 m potassium acetate and incubated for 3 min at 27 °C. Val was added to a final concentration of 2 μm, and the mixture was incubated for a further 3 min. A radiolabeled substrate [2,3-3H]GABA (0.5 MBq/μmol) or [2-3H]glycine (0.5 MBq/μmol) was then added to a final concentration of 100 μm or 2 mm, respectively, to initiate assays. For Cl− transport, proteoliposomes containing VIAAT (0.5 μg of protein/assay) were suspended in 500 μl of 20 mm MOPS-Tris (pH 7.0), 5 mm magnesium acetate, 5 mm GABA, and 0.15 m potassium acetate and incubated for 3 min at 27 °C. Three minutes after the addition of Val, radiolabeled 36Cl− (740 MBq/g) was added to a final concentration of 5 or 10 mm. For measurement of Δψ, proteolipsomes were incubated in the standard assay condition in the presence of 20 μm [14C]SCN for 2 min, and internal concentration of [14C]SCN was quantified as described. Internal volumes of proteoliposomes were also determined by measuring of exclusive volume with [14C]mannitol. Then, Δψ was calculated by the formula Δψ = RT/F × ln([internal SCN−]/[external SCN−]), where R is the gas constant, T is absolute temperature, and F is the Faraday constant.
Fluorescence Measurement and Protein Concentration Determination
ΔpH and Δψ were measured by fluorescence quenching of acridine orange and oxonol-V, respectively, as described (16, 17). In determining protein concentration, bovine serum albumin was used as the reference (20).
Data Analysis
All numerical values are shown as the mean ± S.E.; n = 3–6. Statistical significance was determined by Student's t test.
RESULTS AND DISCUSSION
Purification and Reconstitution of Wild Type and Mutant VIAATs
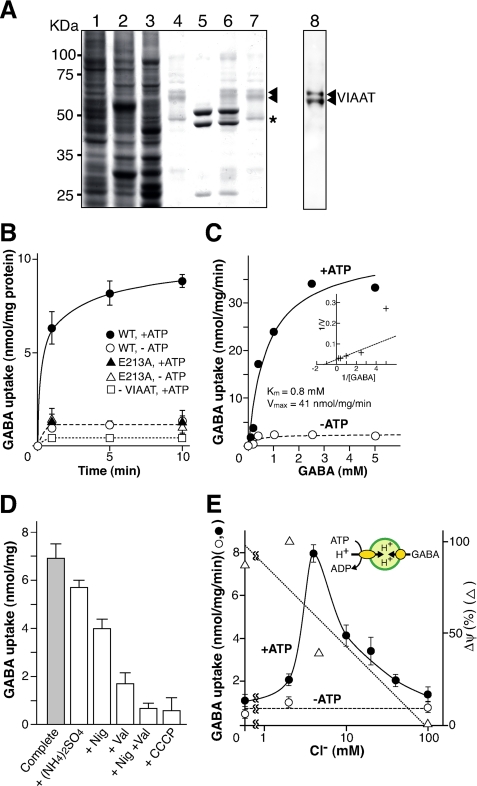
An in vitro assay system for VIAAT includes a baculovirus overexpression system for the heterologous expression and purification of His-tagged wild type and mutant VIAAT. After solubilization of the insect cell membrane fraction with octylglucoside, VIAAT was purified by nickel-nitrilotriacetic acid column chromatography (Fig. 1A, lane 4). Three polypeptides with apparent molecular masses of 63, 59, and 49 kDa were concomitantly purified. Western blot analysis revealed that the upper two polypeptides corresponded to VIAAT (Fig. 1A, lane 8), which is consistent with previous observations (14, 21). Minor polypeptide with a molecular mass of 49 kDa (Fig. 1A, asterisk) is contaminated from insect cells. Glu213, a conserved charged amino acid residue in transmembrane domain 2 in all VIAATs (supplemental Fig. S1), was mutated into alanine. Other charged residues in transmembrane region such as Arg351 and His344 are not fully conserved, suggesting functional importance of Glu213. The E213A VIAAT could be purified to near homogeneity (Fig. 1A, lane 7).
FIGURE 1.
Purification and reconstitution of recombinant wild type and mutant VIAAT. A, SDS-PAGE, Coomassie Brilliant Blue staining, and Western blot analysis (19) of recombinant VIAAT-containing samples. Lane 1, 200 μg of membrane protein from control cells; lane 2, 200 μg of membrane protein containing VIAAT; lane 3, 40 μg of solubilized membrane protein; lane 4, 10 μg of purified wild type VIAAT; lane 5, 20 μg of purified F-ATPase; lane 6, proteoliposomes after reconstitution (30 μg of total protein); lane 7, 10 μg of purified E213A mutant VIAAT. The positions of molecular mass markers are indicated. Lane 8, Western blot of purified wild type VIAAT (2 μg) after probing with monoclonal anti-VIAAT antibody (14). The positions of VIAAT are marked. B, time course of GABA uptake after reconstitution of purified wild type (WT; circles) or mutant (triangles) VIAAT with bacterial F-ATPase (complete system). The reaction was started by adding radioactive GABA to a final concentration of 100 μm in the presence (filled symbols) or absence (open symbols) of ATP. GABA uptake by proteoliposomes lacking VIAAT is also shown (squares), which was similar to that of GABA uptake by liposomes (background). C, concentration dependence of GABA uptake. Sample was taken after 1 min. Inset, Lineweaver-Burk plot. D, effect of various compounds on GABA uptake. Additions: ammonium sulfate at 10 mm, valinomycin (Val) at 2 μm, nigericin (Nig) at 2 μm, and carbonyl cyanide m-chlorophenylhydrazone (CCCP) at 2 μm. E, effect of Cl− on the GABA uptake by proteoliposomes containing both VIAAT and F-ATPase. The assay was carried out at various Cl− concentrations in the presence or absence of ATP. The uptake values at 5 min are shown. Formation of Δψ under the same conditions is also indicated.
The wild type and mutant VIAAT were then co-reconstituted with bacterial F-ATPase into liposomes according to the procedure employed in the reconstitution of vesicular glutamate transporter (16) (Fig. 1A, lane 6). The resultant proteoliposomes established a stable Δψ and/or ΔpH upon the addition of ATP and facilitated GABA uptake in a time-dependent manner (Fig. 1B). Omission of wild type VIAAT reduced uptake to background level, indicating that VIAAT was an essential factor for GABA uptake. Omission of either F-ATPase or ATP also reduced GABA uptake to an extent similar to omitting VIAAT, suggesting that the contribution of facilitated diffusion through VIAAT could not account for the observed level of GABA uptake. VIAAT containing the E213A mutation lacked GABA uptake activity, indicating an essential role of Glu213 in GABA transport (Fig. 1B). In subsequent experiments, we used this E213A mutant as one of the negative controls. The ATP-dependent uptake of GABA was dependent on the concentration of GABA and became saturated at Km and Vmax of about 0.8 mm and 41 nmol/min/mg of protein, respectively (Fig. 1C). The ATP-dependent GABA uptake was sensitive to carbonyl cyanide m-chlorophenylhydrazone, indicating that ΔμH+ drove the uptake (Fig. 1D). Val, an electrogenic K+ ionophore, significantly reduced GABA uptake to 24% of the control, whereas nigericin, an electroneutral H+/K+ exchanger, in the presence of K+, produced a limited effect. A combination of Val and nigericin that dissipate a proton-motive force, abolished the activity, suggesting that ΔpH contributes the uptake to some extent. Consistently, ammonium sulfate, a dissipator of ΔpH but not of Δψ, slightly decreased GABA uptake. From these results, we concluded that VIAAT preferentially used Δψ as the driving force for the uptake of GABA. Glycine, another substrate of VIAAT (5, 7), was also taken up by reconstituted VIAAT with properties similar to those of GABA uptake (supplemental Figs. S2–S4). l-Glutamate, l-aspartate, and serotonin, known substrates of other vesicular neurotransmitter transporters, were not taken up by the proteoliposomes (data not shown).
Cl− Activates VIAAT Activity
Because both GABA and glycine are neutral under physiological conditions, the fact that inside positive Δψ acted as the driving force suggested the association of a net uptake of negative charge or extrusion of positive charge during VIAAT-mediated transport. Because in previous reports it was observed that mm concentrations of Cl− stimulate ATP-dependent GABA uptake by proteoliposomes containing reconstituted synaptic vesicle proteins (6), we wondered whether Cl− was involved in VIAAT-mediated transport.
To test this working hypothesis, we assessed the effect of Cl− on GABA uptake by proteoliposomes containing VIAAT and F-ATPase. We found that Cl− was absolutely required for VIAAT activity: essentially no transport activity was observed in the absence of Cl−, although the proteoliposomes retained the maximum level of Δψ (Fig. 1E). ATP-dependent uptake activity reached a maximum at ∼5 mm Cl− and decreased with increasing concentrations of Cl− (Fig. 1E). The decrease in GABA uptake activity at concentrations higher than 5 mm Cl− may be due to a decrease in Δψ, as observed for l-glutamate transport by proteoliposomes containing vesicular glutamate transporter and F-ATPase (16). In contrast, cations including K+, Na+, and choline at 0.1 m did not show any significant effect. These results indicated that Cl− was somehow involved in VIAAT-mediated transport.
Absolute Requirement of Cl− in VIAAT Activity
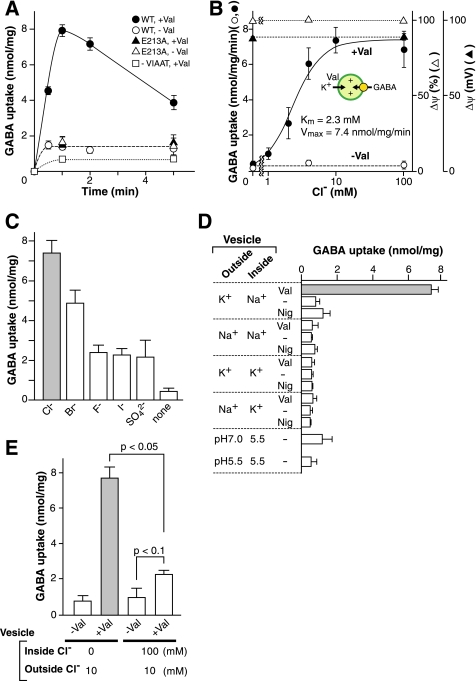
To obtain conclusive results on the role(s) of Cl− in VIAAT-mediated GABA transport, a more refined assay system without F-ATPase was necessary because Δψ and ΔpH, components of an electrochemical gradient of protons established by F-ATPase, are dependent on the concentration Cl− as shown above, and this made interpretation of results difficult. Therefore, we prepared proteoliposomes containing VIAAT as the only protein constituent in the absence of K+ and then suspended them in buffer containing K+. Addition of Val drove K+ movement from the extravesicular space into the intravesicular space, established a K+ diffusion potential (inside positive Δψ, around 90 mV), and triggered GABA uptake when the Cl− concentration in the assay medium was greater than 2 mm (Fig. 2A). Background level of GABA transport activity was observed in liposomes containing no VIAAT.
FIGURE 2.
Properties of GABA uptake by Val-evoked Δψ. Proteoliposomes containing purified VIAAT were prepared, and GABA uptake was initiated by the addition of Val. A, time courses of proteoliposomes containing wild type (WT) or mutant VIAAT or liposomes containing no VIAAT (control). B, GABA uptake was assayed in the presence of different concentrations of Cl−. Levels of Δψ as measured with oxonol-V fluorescence and SCN− uptake are also shown, and expressed as percentage of maximum quenching and mV, respectively. C, effect of other anions. Val-evoked GABA uptake was assayed in the presence of 10 mm potassium salt of the indicated anions. D, proteoliposomes containing Na+ or K+ were prepared and incubated in buffer containing K+ or Na+ as indicated. GABA uptake was measured 3 min after the addition of valinomycin (Val) at 2 μm or nigericin (Nig) at 2 μm or ethanol (−). For some experiments, proteoliposomes were prepared at pH 5.5, incubated in buffer at either pH 7.0 or 5.5, and assayed after 1 min. E, trans-inhibition of GABA uptake. Proteoliposomes were prepared in the presence or absence of KCl (100 mm) and incubated in buffer containing 10 mm Cl−. Val-evoked GABA uptake was measured.
Consistent with proteoliposomes containing both VIAAT and F-ATPase, GABA transport was also not observed when Cl− was omitted from the assay buffer of VIAAT-containing proteoliposomes even though the maximum level of Δψ was maintained (Fig. 2B). Significantly, Cl− dependence showed a plateau and did not decrease with increasing concentrations of Cl− (Fig. 2B). Km and Vmax were 2.3 mm and 7.4 nmol/min/mg of protein, respectively (Fig. 2B). Replacing Cl− with Br− partially compensated for transport activity (Fig. 2C). Neither inside negative Δψ nor pH gradient (both inside and outside acidic) supported uptake (Fig. 2D). Essentially the same results were obtained when glycine was used as the substrate (supplemental Fig. S5). These results confirmed that VIAAT used both Δψ (positive inside) and Cl− for transport activity under the assay conditions employed. Because Δψ-mediated GABA uptake was reduced when high concentrations (0.1 m) of Cl− were present internally (Fig. 2E), an outwardly directed Cl− gradient may prevent GABA uptake. These results suggested that VIAAT co-transported GABA or glycine with Cl−.
VIAAT Mediates Cl− Transport
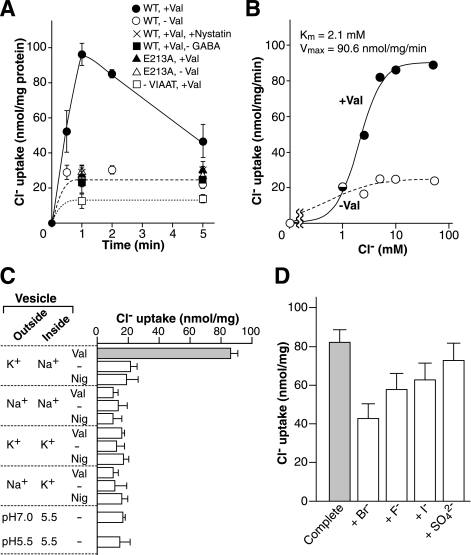
Finally, we tested the ability of VIAAT to transport Cl− by direct isotope tracing. We measured VIAAT-mediated uptake of radiolabeled Cl− directly. Upon the formation of Δψ (inside positive), proteoliposomes containing wild type VIAAT took up radioactive Cl− with saturating kinetics. Km and Vmax were 2.1 mm and 90.6 nmol/min/mg of protein, respectively (Fig. 3, A and B). The uptake was not observed when either Δψ (inside negative) or ΔpH (acidic inside) was imposed (Fig. 3C). Omission of GABA or glycine or VIAAT prevented uptake (Fig. 3A). Nystatin inhibited the uptake (Fig. 3A). Proteoliposomes containing the E213A mutant did not show any Δψ-induced Cl− uptake (Fig. 3A). Cl− uptake induced by Δψ and GABA was partially sensitive to halide anions with Br− being much more inhibitory than I− and F− (Fig. 3D). Essentially, the uptake characteristics in the presence of glycine were similar to that of GABA (supplemental Fig. S6). Taken together, we concluded that VIAAT co-transports Cl− with GABA or glycine.
FIGURE 3.
VIAAT-mediated Cl− transport. A, time course of Cl− uptake by proteoliposomes containing wild type (WT; circles) or E213A mutant (triangles) VIAAT in the presence or absence of valinomycin (Val). Samples were taken at the indicated times. Cl− uptakes by wild type VIAAT in the absence of GABA (squares) and in the presence of nystatin 250 μm (×) are also shown. B, dose-dependent Cl− uptake after 1 min in the indicated concentrations of Cl−. C, Na+- or K+-trapped VIAAT-containing proteoliposomes were incubated as indicated with valinomycin at 2 μm or nigericin (Nig) at 2 μm or ethanol (−) for 3 min. Then, Cl− was added at 10 mm. The uptake after 1 min was measured. D, effect of various anions. Cl− uptake at 10 mm after 1 min was measured in the presence of the listed sodium salts at 5 mm.
VIAAT Is a Cl−/GABA (Glycine) Co-transporter
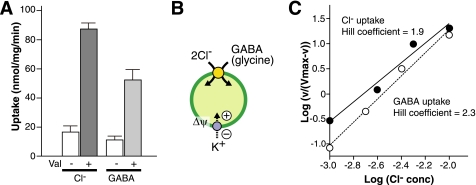
In the present study, we have elucidated a significant property of VIAAT as a vesicular co-transporter of GABA or glycine with Cl−. This transport mechanism explains why VIAAT accumulates electroneutral substrates using Δψ as a driving force. Kinetic analysis of GABA and Cl− transport through VIAAT indicated that two Cl− equivalents and one GABA were transported in one cycle of VIAAT (Fig. 4, A and B). This stoichiometry was supported by the Hill coefficient of both Cl−-dependent GABA uptake and GABA-dependent Cl− uptake, which were calculated to be 2.3 and 1.9, respectively (Fig. 4C). Thus, transport of GABA and Cl− transport through VIAAT are tightly coupled in nature. Based on stoichiometry and Δψ, VIAAT could establish an approximately 50-fold concentration gradient across synaptic vesicle membranes, which roughly corresponds to the GABA concentration difference observed between the synaptic vesicle intravesicular space and cytoplasm (22, 23). It is well expected that Cl− is essential for plasma membrane GABA transporter and GABAA receptor Cl− channel (24–26). The present study demonstrated an essential role of Cl− in vesicular storage of inhibitory amino acids. Taken together, it is concluded that Cl− is primarily important not only for signal termination and reception but also for vesicular release in GABAergic or glycinergic neurotransmission. The simple in vitro assay system developed here for VIAAT is useful for studying the molecular mechanism of co-transport of Cl− with GABA or glycine.
FIGURE 4.
Stoichiometry of VIAAT-mediated Cl− and GABA uptake. A, stoichiometry was measured at 1 min under 5 mm GABA and 10 mm Cl−. The ratio of Cl− and GABA was 1.7. B, proposed mechanism of GABA transport by VIAAT. C, Hill plot of Cl− and GABA uptake. Cl− concentration dependence of GABA and Cl− uptake was replotted from the data shown in Figs. 2B and 3B, and the Hill coefficient was calculated from the slope of the fitted line.
It should be stressed that vesicular glutamate transporter (16), vesicular nucleotide transporter (17), and vesicular excitatory amino acid transporter (18), which are members of the SLC17 anion transporter family, all require Cl− for transport activity. Although it is unknown at present whether these proteins also co-transport Cl−, it is quite probable that Cl− has a wider physiological significance in excitatory and inhibitory neurotransmission than currently expected.
This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan and the Ajinomoto 3A Research grant (to Y. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- GABA
- γ-aminobutyrate
- Δψ
- membrane potential
- ΔpH
- transmembrane pH gradient
- MOPS
- 3-morpholinopropane sulfonic acid
- VIAAT
- vesicular inhibitory amino acid transporter
- Val
- valinomycin
- SCN
- thiocyanate.
REFERENCES
- 1.Maycox P. R., Hell J. W., Jahn R. (1990) Trends Neurosci. 13, 83–87 [DOI] [PubMed] [Google Scholar]
- 2.Gasnier B. (2004) Pfluegers Arch. 447, 756–759 [DOI] [PubMed] [Google Scholar]
- 3.Hell J. W., Maycox P. R., Stadler H., Jahn R. (1988) EMBO J. 7, 3023–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fykse E. M., Christensen H., Fonnum F. (1989) J. Neurochem. 52, 946–951 [DOI] [PubMed] [Google Scholar]
- 5.Kish P. E., Fischer-Bovenkerk C., Ueda T. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 3877–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hell J. W., Maycox P. R., Jahn R. (1990) J. Biol. Chem. 265, 2111–2117 [PubMed] [Google Scholar]
- 7.Hell J. W., Edelmann L., Hartinger J., Jahn R. (1991) Biochemistry 30, 11795–11800 [DOI] [PubMed] [Google Scholar]
- 8.McIntire S. L., Reimer R. J., Schuske K., Edwards R. H., Jorgensen E. M. (1997) Nature 389, 870–876 [DOI] [PubMed] [Google Scholar]
- 9.Sagné C., El Mestikawy S., Isambert M. F., Hamon M., Henry J. P., Giros B., Gasnier B. (1997) FEBS Lett. 417, 177–183 [DOI] [PubMed] [Google Scholar]
- 10.McIntire S. L., Jorgensen E. M., Horvitz H. R. (1993) Nature 364, 334–337 [DOI] [PubMed] [Google Scholar]
- 11.Martens H., Weston M. C., Boulland J. L., Grønborg M., Grosche J., Kacza J., Hoffmann A., Matteoli M., Takamori S., Harkany T., Chaudhry F. A., Rosenmund C., Erck C., Jahn R., Härtig W. (2008) J. Neurosci. 28, 13125–13131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhry F. A., Reimer R. J., Bellocchio E. E., Danbolt N. C., Osen K. K., Edwards R. H., Storm-Mathisen J. (1998) J. Neurosci. 18, 9733–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumoulin A., Rostaing P., Bedet C., Lévi S., Isambert M. F., Henry J. P., Triller A., Gasnier B. (1999) J. Cell Sci. 112, 811–823 [DOI] [PubMed] [Google Scholar]
- 14.Takamori S., Riedel D., Jahn R. (2000) J. Neurosci. 20, 4904–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojcik S. M., Katsurabayashi S., Guillemin I., Friauf E., Rosenmund C., Brose N., Rhee J. S. (2006) Neuron 50, 575–587 [DOI] [PubMed] [Google Scholar]
- 16.Juge N., Yoshida Y., Yatsushiro S., Omote H., Moriyama Y. (2006) J. Biol. Chem. 281, 39499–39506 [DOI] [PubMed] [Google Scholar]
- 17.Sawada K., Echigo N., Juge N., Miyaji T., Otsuka M., Omote H., Yamamoto A., Moriyama Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5683–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyaji T., Echigo N., Hiasa M., Senoh S., Omote H., Moriyama Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11720–11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moriyama Y., Iwamoto A., Hanada H., Maeda M., Futai M. (1991) J. Biol. Chem. 266, 22141–22146 [PubMed] [Google Scholar]
- 20.Schaffner W., Weissmann C. (1973) Anal. Biochem. 56, 502–514 [DOI] [PubMed] [Google Scholar]
- 21.Bedet C., Isambert M. F., Henry J. P., Gasnier B. (2000) J. Neurochem. 75, 1654–1663 [DOI] [PubMed] [Google Scholar]
- 22.Burger P. M., Hell J., Mehl E., Krasel C., Lottspeich F., Jahn R. (1991) Neuron 7, 287–293 [DOI] [PubMed] [Google Scholar]
- 23.Patel A. J., Hunt A. (1985) J. Neurochem. 44, 1816–1821 [DOI] [PubMed] [Google Scholar]
- 24.Zomot E., Bendahan A., Quick M., Zhao Y., Javitch J. A., Kanner B. I. (2007) Nature 449, 726–730 [DOI] [PubMed] [Google Scholar]
- 25.Forrest L. R., Tavoulari S., Zhang Y.-W., Rudnick G., Honig B. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12761–12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieghart W. (1992) Trends Pharmacol. Sci. 13, 446–450 [DOI] [PubMed] [Google Scholar]