Abstract
Understanding the mechanisms governing cytokine control of growth factor expression in smooth muscle cells would provide invaluable insight into the molecular regulation of vascular phenotypes and create future opportunities for therapeutic intervention. Here, we report that the proinflammatory cytokine interleukin (IL)-1β suppresses platelet-derived growth factor (PDGF)-D promoter activity and mRNA and protein expression in smooth muscle cells. NF-κB p65, induced by IL-1β, interacts with a novel element in the PDGF-D promoter and inhibits PDGF-D transcription. Interferon regulatory factor-1 (IRF-1) is also induced by IL-1β and binds to a different element upstream in the promoter. Immunoprecipitation and chromatin immunoprecipitation experiments showed that IL-1β stimulates p65 interaction with IRF-1 and the accumulation of both factors at the PDGF-D promoter. Mutation of the IRF-1 and p65 DNA-binding elements relieved the promoter from IL-1β-mediated repression. PDGF-D repression by IL-1β involves histone deacetylation and interaction of HDAC-1 with IRF-1 and p65. HDAC-1 small interfering RNA ablates complex formation with IRF-1 and p65 and abrogates IRF-1 and p65 occupancy of the PDGF-D promoter. Thus, HDAC-1 is enriched at the PDGF-D promoter in cells exposed to IL-1β and forms a cytokine-inducible gene-silencing complex with p65 and IRF-1.
Introduction
The platelet-derived growth factor (PDGF)2 family of ligands and receptors has been implicated in a wide variety of diseases involving aberrant migration and proliferation, including malignancy and atherogenesis (1–3). PDGF is synthesized by many different cell types, and its expression is broad. PDGF-D is one of two nonclassical PDGF ligand chains (other is PDGF-C) originally discovered in 2001 (4, 5). PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD act via two receptor tyrosine kinases, PDGF receptors α and β. PDGF-A and PDGF-B are activated during intracellular transport by exocytic secretion, whereas PDGF-C and PDGF-D are secreted as latent factors that require activation by extracellular proteases. PDGF-D has a two-domain structure similar to PDGF-C and is secreted as a disulfide-linked homodimer and is a specific agonistic ligand for PDGF receptor β (5). PDGF-D mRNA is widely expressed in organs such as the heart, pancreas, kidney, and ovary and has been linked to lung, prostate, and ovarian cancers. PDGF-D promotes tumor growth by accelerating tumor cell proliferation and stimulating tumor neovascularization (6, 7). PDGF-D has also been shown to induce Notch-1-dependent angiogenesis (8) and maturation of blood vessels during angiogenesis (9). PDGF-D is expressed upon by vascular injury and stimulates smooth muscle cell (SMC) proliferation, and induces cardiac fibrosis in transgenic mice (10, 11). The mechanisms regulating the PDGF-D promoter are poorly understood. We recently demonstrated that angiotensin II induces PDGF-D transcription in SMCs through Ets-1 and Sp1 and endogenous hydrogen peroxide generation (12, 13). More recently, we demonstrated that Sp1 regulation of PDGF-D promoter activity involves phosphorylation of multiple residues in the zinc finger of Sp1 (14). Whether histone modification in chromatin by enzymes such as histone deacetylases (HDACs) regulate PDGF-D transcription is also unexplored. HDACs play a central role in the epigenetic regulation of gene expression. Recent findings from our group indicate that IL-1β facilitates HDAC dissociation from the PDGF receptor α promoter and that HDAC inhibition potentiates induction of PDGF receptor α transcription (15).
Proinflammatory cytokines activate a complex network of intracellular signaling pathways that alter gene expression and cellular phenotype, which include nuclear factor-κB (NF-κB) and interferon regulatory factor-1 (IRF-1). NF-κB is a ubiquitous transcription factor that can be activated by diverse array of proatherogenic stimuli such as inflammatory cytokines, oxidant stress, and hemodynamic forces (16, 17). Interferon regulatory factor-1 (IRF-1) was isolated by virtue of its affinity to specific DNA sequences in the interferon-β promoter that mediate virus responsiveness. The IRFs are a family of factors that regulate cytokine signaling, cellular growth regulation, hematopoietic development, and pathogen response (18). Our understanding of NF-κB regulation of PDGF ligand expression is limited. KLF5 transactivation of PDGF-A involves cooperative interactions with NF-κB p50 but not Egr-1 (19, 20) in endothelial cells exposed to phorbol ester (21). We showed that NF-κB binds to the PDGF-B promoter in vascular endothelial cells exposed to fluid shear stress and activates the PDGF-B promoter (22, 23). Tumor necrosis factor-α activation of PDGF-B gene expression involves NF-κB (24). It is not known whether NF-κB controls PDGF-C or PDGF-D transcription in any cell type or whether IRF-1 is involved in transcription of any ligand chain. Here, we were surprised to find that IL-1β suppresses PDGF-D promoter activity, mRNA, and protein in SMCs, and this involves transcriptional repression by NF-κB p65 and IRF-1, both of which form a complex in response to the cytokine and interact with the PDGF-D promoter. This study provides the first direct link between NF-κB and the PDGF-D promoter and IRF-1 with any member of the PDGF family.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
Primary rat and human aortic SMCs were obtained from Cell Applications (San Diego, CA) and cultured in Waymouth's media containing 10% fetal bovine serum, 10 units/ml penicillin, and 10 μg/ml streptomycin at 37 °C in a humidified atmosphere of 5% CO2/air. Cells were used between passages 3 and 8. Cells were incubated for 24 h in serum-free media to achieve quiescence before being stimulated with indicated concentrations of IL-1β. Human recombinant IL-1β and oridonin were purchased from Calbiochem. Trichostatin was bought from Sigma. Rabbit polyclonal antibodies to NF-κB p65, NF-κB p50, c-Rel, RelB, IRF-1, and HDAC-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant human p65 protein was obtained from Active Motif. Recombinant HDAC-1 and IRF-1 were obtained from Abnova Corp. Protein A/G agarose was bought from Amersham Biosciences. Mutant PDGF-D promoter constructs were generated using the QuikChange site-directed mutagenesis kit (Stratagene).
RNA Extraction and Reverse Transcription-PCR
Serum-starved SMCs were treated with 10 ng/ml of IL-1β at the indicated time or with different concentrations of cytokine for 4 h. For overexpression studies, cells were transfected with 20 or 40 μg of p65 expression plasmid or empty vector. Total RNA was prepared using TriZOL reagent, and 2 μg was used for synthesis of cDNA in a 25 μl volume in the presence of 0.5 μg of oligo dT primer (Sigma), 1 μm of dNTP mix (Roche), 40 units of RNase inhibitor (Promega), 200 units of SuperScriptTM II reverse transcriptase (Invitrogen), and 4 μl of 5× first strand buffer (Invitrogen) in diethyl pyrocarbonate-treated water. Reactions were allowed to proceed at 42 °C for 50 min and at 70 °C for 15 min. 1 μl of cDNA was amplified for PDGF-D fragment in a final volume of 20 μl. The primers for amplifying PDGF-D were: forward, 5′-GTG CAG AGT CCT ACT ATT CCC-3′ and reverse, 5′-GAG GTG GTC TTG AGC TGC AG-3′. PCR was performed by denaturing at 94 °C for 5 min; 94 °C for 1 min; 59 °C for 1 min; 72 °C for 1 min; and extending at 72 °C for 7 min. The primers for rat VCAM-1 were: forward, 5′-GGT CGC GAT CTT CGG AGC-3′ and reverse, 5′-CTT GTA GTT CTC TGA CAG TCT CCC TTT CTT-3′. The cycling conditions were: 94 °C 2 min; 96 °C for 30 s; 60 °C for 30 s; 72 °C for 1 min; and 72 °C for 2 min. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the internal control. The primers for GAPDH were: forward, 5′-ACC ACA GTC CAT GCC ATCAC-3′ and reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′. The amplification conditions for GAPDH were: 94 °C, 1 min; 94 °C, 30 s; 58 °C, 10 s; 72 °C, 1 min for 20 cycles; and 72 °C, 4 min.
siRNA
ON-TARGETplus SMARTpool siRNA targeting IRF-1, HDAC-1, and the ON-TARGETplus nonspecific siRNA were purchased from Dharmacon Technologies. NF-κB p65 siRNA and nonspecific siRNA were obtained from Santa Cruz Biotechnology.
Real-time Quantitative PCR
Quantitative PCR was carried out using Rotor-Gene 3000 (Corbett Life Science). The reaction was set in a final volume of 10 μl containing 1 μl of cDNA, 5 μl of 2× SYBR Green Master Mix (Applied Biosystems), 0.2 μl of 10 μm of forward and reverse primer (Sigma) and 3.6 μl of DNase-free water. Primers used were: rat PDGF-D: (forward) 5′-ATC GGG ACA CTT TTG CGA CT-3′, (reverse) 5′-GTG CCT GTC ACC CGA ATG TT-3′; and rat GAPDH: (forward) 5′-ACA AGA TGG TGA AGG TCG GTG-3′, (reverse) 5′-AGA AGG CAG CCC TGG TAA CC-3′. The PCR conditions were: 95 °C for 10 min, followed by 40 cycles at 95 °C for 20 s, 60 °C for 45 s, and 72 °C for 20 s.
Transient Transfection Assays
SMCs in 100-mm petri dishes at 60–70% confluence were transfected with 10 μg of the indicated luciferase reporter construct. 1 μg of pRL-null was also transfected to normalize transfection efficiencies. Transfection was performed using FuGENE6 reagent (Roche). After a 24-h incubation, cells were harvested, and cell lysates were prepared for luciferase activity assessment using Dual Luciferase assay reporter system (DLR) (Promega) on a luminometer (model TD-20/20; Turner Designs, Quantum Science). Firefly luciferase activity was normalized to Renilla. In some experiments, cells were arrested in serum-free medium for 6 h before overnight transfection of plasmid and then treated with IL-1β.
Western Blotting
Whole cell lysates and nuclear protein were prepared as described (12). The concentration of protein in both whole cell and nuclear extracts were determined with a BCA protein assay kit (Pierce). 20 μg total cell lysates or 50 μg nuclear extracts were resolved in 12% SDS-polyacrylamide gel and were transferred onto Immobilon polyvinylidene difluoride transfer membranes (Millipore). Membranes were blocked for 1 h at 22 °C with 5% skim milk in 0.05% Tween 20 phosphate-buffered saline and then incubated overnight with primary antibody at 4 °C. Primary antibodies were used at a final dilution of 1:300–500. Membranes were washed three times with 0.05% Tween 20 phosphate-buffered saline followed by incubation with swine anti-rabbit or rabbit anti-goat IgG conjugated with horseradish peroxidase (diluted at 1:2000) for 1 h at room temperature. Membranes were then incubated with chemiluminescence (PerkinElmer Life Sciences) and then exposed to film for 1–15 min.
Electrophoretic Mobility Shift Assays (EMSA)
Typically, 15 μg of nuclear extracts from untreated or IL-1β-treated SMCs were incubated with 150,000 cpm of 32P-labeled double-stranded oligonucleotide bearing a NF-κB or IRF-1 binding site in binding buffer (10 mm Tris, pH 7.5, 1 mm dithiothreitol, 1 mm EDTA, 5% glycerol, 1 mg/ml salmon sperm DNA, 1 mg/ml poly(dI·dC)) at 22 °C for 30 min. In supershift experiments, nuclear extracts were incubated with 2 μl (4 μg) of the rabbit polyclonal antibody in binding buffer for 20 min at room temperature before adding probe and then incubated with probe for another 30 min. The reaction mixture was run on 8% nondenaturing polyacrylamide gels in Tris borate-EDTA (TBE) buffer at 120V for 3 h at room temperature; the gel was vacuum dried and exposed to x-ray film for autoradiography (25, 26).
For EMSA using recombinant human proteins, reactions were conducted in binding buffer (10 mm Tris, pH 7.5, 50 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, 5% glycerol, 0.2% Nonidet P-40, 1 mg/ml bovine serum albumin) for 30 min at 22 °C. Oligo sequences were: Oligo D−481/−457, 5′-AAG AAG TGC TGG GAT TCT ACC CTC TC-3′ (putative NF-κB binding site underlined); mOligo D−481/−457, 5′-AAG AAG TGC TGT TCG TCT ACC CTC TC-3′; Oligo D−847/−823, 5′-AGT TCG CGA AAA GTG AAA GCT ACG C-3′ (putative IRF-1 binding site underlined); and mOligo D−847/−823, 5′-AGT TCG CGA CAA GTG ACT GCT ACG C-3′ (mutations italicized). Oligo PD-κB, 5′-CAA CGG CAG GGG AAT TCC CCT CTC CTT-3′ (NF-κB site underlined) (22).
Immunoprecipitation
500 μg of whole cell lysates or 200 μg of nuclear extracts from growth quiescent or IL-1β-stimulated SMCs were used in immunoprecipitation analysis. Protein G-Sepharose beads were precleaned with radioimmune precipitation assay buffer containing protease inhibitor mixture. Precleaned whole cell lysates were incubated with 5 μg of primary antibody overnight at 4° C with gentle rotating followed by incubation with 30 μl of bead suspension overnight at 4° C. Sepharose bound immunoprecipitates were washed, boiled in sample SDS buffer, and separated on 12% SDS-PAGE and then immunoblotted as described above.
Chromatin Immunoprecipitation
Human SMCs in a 75 cm2 flask (80∼90% confluent) were arrested in serum-free media for 24 h and then stimulated with or without IL-1β for 1 h. After cross-linking with 37% formaldehyde and shearing with sonicator, the cells were washed twice with phosphate-buffered saline, pH 7.4 before chromatin immunoprecipitation (ChIP) using the appropriate antibody. PCR was carried out in presence of 1 mm of MgCl2, 0.1 mm of dNTPs, 0.1 μm primers, and 1 unit of platinum Taq polymerase (Invitrogen) and 2 μl of cDNA. Amplification conditions were as follows: 94 °C, 5 min; 94 °C, 1 min; 59 °C, 45 s; and 72 °C, 45 s for 35 cycles; and 72 °C, 4 min. Primers for PDGF-D−863/−445: forward, 5′-CCC TGA CTC ATC CTG GAG TT-3′; reverse: 5′-GGA GGA AGT GGG AGA GGG TA-3′. Primers for −863PDGF-D−674: forward, 5′-CCC TGA CTC ATC CTG GAG TT-3′, reverse, 5′-GCT CCC ATC AGT GTG ACC TT-3′. Primers used for −689PDGF-D−445: forward, 5′-TCA CAC TGA TGG GAG CTC AA-3′, reverse, 5′-GGA GGA AGT GGG AGA GGG TA-3′.
RESULTS
Proinflammatory Cytokine IL-1β Reduces Levels of PDGF-D mRNA, Promoter Activity, and Protein Expression
Because PDGF-D and IL-1β are both generated in response to vascular injury and regulate SMC proliferation (10, 11, 27–30) and because other members of the PDGF family are induced by IL-1β, we began this study with the hypothesis that PDGF-D expression would be controlled by IL-1β. Growth-quiescent rat aortic SMCs were incubated with IL-1β (10 ng/ml) for various times (0–24 h) and PDGF-D mRNA levels were measured by reverse-transcription PCR. Surprisingly, the cytokine caused a progressive decrease in levels of PDGF-D mRNA despite unbiased loading (Fig. 1A). In contrast, using the same samples, we found that IL-1β stimulates VCAM-1 mRNA expression (Fig. 1A). VCAM-1 is a well recognized IL-1β-dependent gene, positively regulated by IL-1β in an NF-κB-dependent manner in multiple cell types (31, 32). Quantitative real-time PCR and transient transfection analysis (with −1168-PDGF-D-luciferase, a PDGF-D promoter-luciferase construct bearing −1168/+72 of the PDGF-D promoter in pGL3-basic) confirmed IL-1β suppression of PDGF-D mRNA and promoter activity, respectively (Fig. 1B). Further expression analysis with increasing concentrations of IL-1β (0.1–50 ng/ml) revealed that cytokine inhibition of PDGF-D mRNA levels was dose-dependent (Fig. 1C) with no biphasic effect. The decline in PDGF-D levels at this concentration of IL-1β is not due to toxic effects of the cytokine; the y axis of Fig. 1C indicates the ratio of PDGF-D relative to GAPDH mRNA expression. Western blot analysis demonstrated that PDGF-D protein levels (50 and 21 kDa) (12) were down-regulated by IL-1β (Fig. 1D).
FIGURE 1.
Interleukin-1β reduces levels of PDGF-D mRNA, promoter activity, and protein expression. A, time course of IL-1β suppression of PDGF-D mRNA expression. SMCs were treated with 10 ng/ml of IL-1β for the times indicated, mRNA was extracted, and semi-quantitative reverse transcription-PCR was performed for PDGF-D and VCAM-1. GAPDH was also amplified as a loading control. B, quantitative real-time PCR (left) and transient transfection analysis (right) confirms IL-1β suppression of PDGF-D mRNA levels and PDGF-D promoter activity, respectively. In the latter, SMCs were transfected with 10 μg of p1168D-luciferase (luc) construct and 1 μg of pRL-null and then exposed to IL-1β for 8 h. firefly luciferase activity was normalized to Renilla. C, dose-response of IL-1β suppression of PDGF-D mRNA levels. SMCs were treated with different amounts of IL-1β for 4 h prior to extraction of total RNA and assessment of PDGF-D mRNA levels by real-time quantitative PCR. D, IL-1β suppression of PDGF-D protein expression. SMCs were treated with IL-1β for various times, and 20 μg total protein was used in Western blot analysis with PDGF-D antibodies. No Addition refers to samples that did not receive IL-1β. Data are representative of at least three experiments. Error bars represent S.E. performed in triplicate. An asterisk denotes p < 0.05.
NF-κB p65, Induced by IL-1β in SMCs, Interacts with a Novel Element in the PDGF-D Promoter and Inhibits PDGF-D Transcription
The transcription factor NF-κB plays a major role in coordinating innate and adaptive immunity, proliferation, and apoptosis. Inspection of the PDGF-D promoter revealed the existence of a putative NF-κB binding element at position −471GGGATTCTAC−462 (Table 1). EMSA, using a double-stranded oligonucleotide spanning this element (32P-labeled Oligo D−481/−457) in NF-κB binding conditions (32), revealed that IL-1β induced the formation of two distinct nucleoprotein complexes within 1 h, A and B (Fig. 2A, left). Antibody elimination analysis revealed that Complex A contains NF-κB p65 and HDAC-1, whereas Complex B contains p65 but no HDAC-1 (Fig. 2A). No supershifts were obtained with antibodies to p50, c-Rel, RelB, or IRF-1 (Fig. 2A, left). Mutation of the −470GGAT−467 sequence to −470TTCG−467 in 32P-labeled mOligo D−481/−457 disrupted complex formation in EMSA with control or IL-1β-treated nuclear extracts (Fig. 2A, upper right). Moreover, wild-type oligonucleotide bound recombinant p65-p65 homodimers in a dose-dependent manner, whereas the mutant probe failed to interact (Fig. 2A, lower right). Western blotting of cytoplasmic and nuclear extracts of untreated and IL-1β-treated cells confirmed the existence of immunoreactive p65, p50, c-Rel, RelB, IRF-1, and HDAC-1 in the extracts (Fig. 2B, left). Of these, levels of p65 and IRF-1 were increased in the nucleus. EMSA performed with 32P-labeled Oligo PD-κB, which bears a high affinity consensus palindromic binding element for NF-κB (22), revealed that the nucleoprotein complex that formed with these IL-1β-treated extracts contained p65 but not p50, c-Rel, or RelB (Fig. 2B, right).
TABLE 1.
Proximal region of the human PDGF-D promoter
The NF-κB and IRF-1 binding sites are indicated in blue. ChIP amplicon (−863 to −445) is indicated in red. Four putative Ets sites described previously (12) (Ets-D1, -D2, -D3, and -D4) are in bold and underlined. The transcriptional start site is italicized and bolded (ccAGCGC). The primers originally used for primer extension analysis (12) are bolded/underlined and labeled as primer A and B. Capital letters represent 5′-untranslated region (UTR). atg denotes the predicted translational start site. Putative binding sites for other factors are indicated.
FIGURE 2.
NF-κB p65 induced by IL-1β interacts with a novel element in the PDGF-D promoter and inhibits PDGF-D transcription. A, left, EMSA was performed with 32P-labeled Oligo D−481/−457 and nuclear extracts from SMCs treated with IL-1β for 1 h. Two inducible complexes were formed, A and B. The upper inset, from a longer exposure of the autoradiogram, more clearly indicates Complex A. These complexes were eliminated with 4 μg of (supershift grade) antibodies to p65, p50, c-Rel, RelB, IRF-1, or HDAC-1 for 20 min prior to the addition of the probe. Middle, EMSA with 32P-labeled mOligo D−481/−457 bearing a mutation in the putative NF-κB element demonstrates no inducible complex with nuclear extracts. Right, EMSA with recombinant human (rh) p65 protein and 32P-labeled Oligo D−481/−457 or 32P-labeled mOligo D−481/−457. ns denotes nonspecific band. B, 50 μg of cytoplasmic or nuclear extract from SMCs treated with or without IL-1β for 1 h were used for Western blotting. C, p65 inhibits PDGF-D transcription (left) and mRNA expression (right). SMCs were co-transfected with 10 μg of p1168D-luciferase (luc) and 3 μg of the expression plasmid (left). Alternatively, SMCs were transfected with 20 or 40 μg of p65-pcDNA3 (made up to total 40 μg with pcDNA3). After 24 h, luciferase activity was measured in the lysates, or total RNA was extracted, and real-time quantitative PCR for PDGF-D was performed. D, left, p50 has no effect on PDGF-D transcription, either alone or in conjunction with p65. Transient transfection was performed with various combinations of p65-pcDNA3 (3 μg), p50-pUNO (3 μg), and 10 μg of p1168D-luciferase. Right, p65 activates the control reporter construct, pNF-κB-luc (22). E, SMCs were pre-treated with 10 μg/ml of the p65 inhibitor Oridonin for 1 h prior to incubation with 10 ng/ml of IL-1β for 4 h. Total RNA was extracted, and real-time quantitative PCR was performed to assess PDGF-D mRNA levels. F, SMCs were transfected with p1168D-luciferase and 100 nm siRNA, and after 24 h, incubated with 10 ng/ml IL-1β for 8 h prior to harvest and assessment of luciferase activity (left) or total p65 levels by Western blotting (right). Data are representative of at least two independent experiments. Band density was quantified by scanning densitometry. siRNAns, nonspecific siRNA. No Addition refers to samples that did not receive IL-1β. An asterisk denotes p < 0.05.
To provide functional evidence for the influence of p65 on PDGF-D promoter activity, we overexpressed p65 in a transient transfection system with the PDGF-D promoter-luciferase construct. p65 suppressed PDGF-D promoter activity (Fig. 2C) and inhibited PDGF-D mRNA levels (Fig. 2C). p50 has no effect on PDGF-D transcription, either alone or in conjunction with p65 (Fig. 2D). Additionally, the diterpenoid oridonin, which inhibits p65 DNA binding (33), rescued the PDGF-D promoter from IL-1β suppression (Fig. 2E). p65 siRNA inhibits IL-1β-suppression of PDGF-D transcription, whereas nonsense siRNA has no effect (Fig. 2F, top). Western blotting confirmed that the siRNA, but not the nonsense siRNA, inhibits cellular p65 levels (Fig. 2F, bottom).
IRF-1 Is Induced by IL-1β in SMCs and Interacts with a Second Novel Binding Element in the PDGF-D Promoter
Further inspection of the PDGF-D promoter revealed a putative IRF- 1 recognition element in the PDGF-D promoter at −840GAAAAGTGAAA−830. Western blotting demonstrated that IRF-1 accumulated in SMC nuclei within 1 h of exposure to IL-1β (Fig. 3A, right). EMSA studies with 32P-labeled Oligo D−847/−823 spanning this element revealed IL-1β-inducible nucleoprotein complex formation within 1 h (Fig. 3A, left). Mutation of the −840GAAAAGTGAAA−830 sequence to −840GACAAGTGACT−830 in 32P-labeled mOligo D−847/−823 abrogated an IL-1β-inducible complex formation (Fig. 3A). Antibody elimination experiments demonstrated the presence of IRF-1 in this IL-1β-inducible complex (Fig. 3B). This is the first association of IRF-1 with any PDGF promoter. The EMSA also showed that this complex, formed with 32P-labeled Oligo D−847/−823 contains HDAC-1 (Fig. 3B). HDACs play a central role in the epigenetic regulation of gene expression. HDAC-1 is a predominantly nuclear 482-amino acid HDAC isoform (34). Germline deletion of HDAC-1 results in embryonic lethality, which cannot be sufficiently compensated by HDAC2 (35), and like several other HDACs, is a drug target in cancer (34). HDAC-1 is also a component of the p65/32P-labeled Oligo D−481/−457 complex in Fig. 2A (left).
FIGURE 3.
IRF-1 is induced by IL-1β and interacts with an element in the PDGF-D promoter. A, left, EMSA was performed by incubating 32P-labeled Oligo D−847/−823 and nuclear extracts of SMCs treated with IL-1β for various times, or 32P-labeled Oligo D−847/−823 bearing a mutation in the putative IRF-1 binding motif. Alternatively, Western blot analysis (right) demonstrates the presence of IRF-1 in the extracts. B, EMSA was performed using nuclear extracts of SMCs exposed to IL-1β for 1 h incubated with antibodies (Ab; 4 μg) to IRF-1, HDAC-1, and the indicated factors for 20 min prior to the addition of 32P-labeled Oligo D−847/−823. ns, non-specific. No Addition refers to samples that did not receive IL-1β. Data are representative of at least two independent experiments.
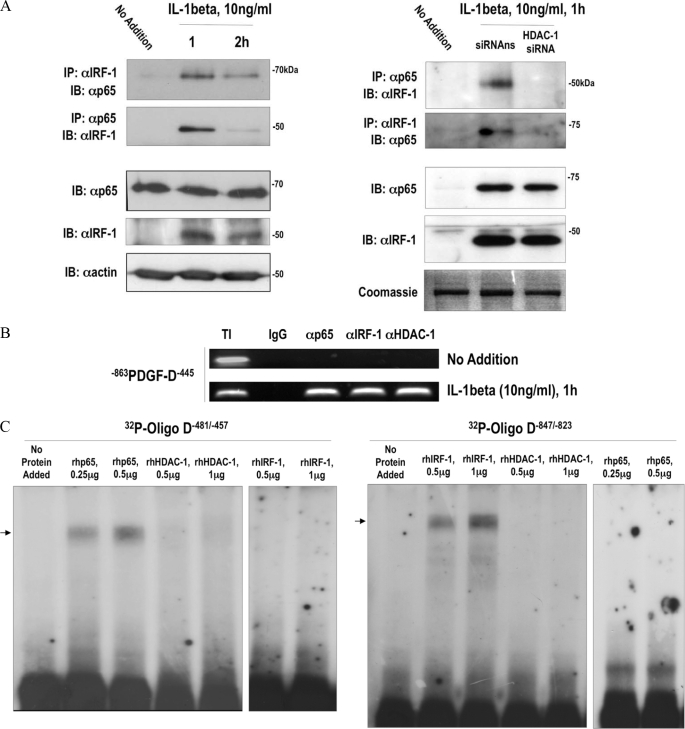
IL-1β Induces Interaction of p65 and IRF-1 Together and with the PDGF-D Promoter
Previous studies in certain other genes, such as VCAM-1 (36), have demonstrated that p65 and IRF-1 cooperatively activate gene expression. To the best of our knowledge, this has not been shown in SMCs nor is there any report of negative cooperation between these factors. We hypothesized that these factors bind not only one another but also the PDGF-D promoter in response to SMC exposure to IL-1β. Immunoprecipitation analysis and Western blotting was performed with lysates of cells that had been exposed to IL-1β for various times (0–2 h). We pulled down with IRF-1 antibodies and then immunoblotted for p65. Alternatively, we immunoprecipitated with p65 antibodies and then probed for IRF-1. p65 and IRF-1 formed a complex within 1 h of IL-1β exposure using either of these conditions, and complex formation was transient (Fig. 4A, left). ChIP analysis was performed to demonstrate the inducible interaction of endogenous proteins with the authentic PDGF-D promoter. IRF-1, p65, and HDAC-1 were not detected at the PDGF-D promoter under basal conditions but were both bound to the promoter within 1 h of SMC exposure to IL-1β (Fig. 4B). EMSA with recombinant proteins revealed that 32P-labeled Oligo D−481/−457 directly bound p65, whereas HDAC-1 and IRF-1 did not bind (Fig. 4C). Conversely, 32P-labeled mOligo D−847/−823 was bound by recombinant IRF-1, but not p65 or HDAC-1 (Fig. 4C). These binding data with recombinant proteins, taken together with the ChIP and EMSA studies, indicate that HDAC-1 occupancy of the PDGF-D promoter is dynamically regulated by IL-1β and suggest that it partners both IRF-1 and p65 without directly contacting the IRF-1 and p65 binding elements.
FIGURE 4.
IL-1β induces physical interaction of p65 and IRF-1 with each other and with the PDGF-D promoter. A, left, immunoprecipitation (IP) and immunoblotting (IB) or immunoblotting alone were performed with total cell lysates or nuclear extracts of SMCs treated with IL-1β for various times. Right, HDAC-1 siRNA was used at 100 nm. SMCs were treated with 100 nm HDAC-1 siRNA for 24 h then incubated with 10 ng/ml IL-1β for 1 h. No Addition refers to samples that did not receive IL-1β. B, ChIP analysis was performed using primers amplifying the −863/−445 region of the human PDGF-D promoter containing NF-κB and IRF-1 elements, with antibodies against p65, IRF-1, and HDAC-1. Total input (TI) and IgG serve as positive and negative controls. C, EMSA with 32P-labeled Oligo D−481/−457 and 32P-labeled mOligo D−847/−823 with recombinant human (rh) p65, HDAC-1, and IRF-1. Data are representative of at least two independent experiments. siRNAns, nonspecific siRNA. Arrow denotes nucleoprotein complex.
NF-κB and IRF-1 DNA-binding Elements in the PDGF-D Promoter Are Required for NF-κB, IRF-1, and IL-1β Repression of PDGF-D Promoter Activity
Transient transfection and mutational analysis revealed that IRF-1 suppression of the PDGF-D promoter was dependent on the −840GAAAAGTGAAA−830 element and that p65 suppression of PDGF-D promoter activity was dependent on the −471GGGATTCTAC−462 element (Fig. 5A, left). IL-1β repression of the PDGF-D promoter was reversed by mutation in either site (Fig. 5A, right).
FIGURE 5.
IRF-1 and p65 are required for IL-1β-mediated repression of the PDGF-D promoter. A, left, SMCs were transfected with 10 μg of promoter plasmid construct p1168D-luciferase (luc), p1168DmKBluc (mutation in the NF-κB binding site), p1168DmIRF1-luciferase (mutation in the IRF-1 binding site), 1 μg of pRL-null, together with 3 μg pcDNA3, p65-pcDNA3, or IRF-1-pcDNA3. Luciferase activity was determined after 24 h. Right, SMCs 24 h after being transfected with 10 μg of promoter construct and 1 μg of pRL-null were treated with IL-1β for 8 h prior to assessment of luciferase activity in the lysates. B, upper, SMCs were transfected with 10 μg of p1168D-luciferase and IRF-1 siRNA or nonspecific siRNA. After 16 h, the cells were exposed to IL-1β for a further 8 h. Luciferase activity in the lysates was determined after 8 h. Error bars represent S.E. performed in triplicate. An asterisk denotes p < 0.05 compared with no treatment control. Lower panel, Western blot analysis for IRF-1 in cells transfected with IRF-1 siRNA and exposed to 10 ng/ml IL-1β for 1 h. No Addition refers to samples that did not receive IL-1β. Data are representative of at least two independent experiments. siRNAns, nonspecific siRNA.
To demonstrate the requirement of endogenous IRF-1 in IL-1β repression of the PDGF-D promoter, we performed an additional transient transfection experiment. IL-1β-mediated suppression of PDGF-D transcription was rescued by 100 nm of IRF-1 siRNA, but not by an identical concentration of irrelevant siRNA (Fig. 5B, upper). Western blotting confirmed that the siRNA, but not the nonsense siRNA, inhibited IL-1β-inducible IRF-1 expression (Fig. 5B, lower).
Repression of PDGF-D Transcription by IL-1β Involves Histone Deacetylation and Interaction of HDAC-1 with IRF-1 and p65
The role of the chromatin environment in regulation of IL-1β PDGF-D expression was next investigated. Rat aortic SMCs were pretreated for 18 h with trichostatin A, a HDAC inhibitor, and then treated with IL-1β for another 4 h. Repression of PDGF-D mRNA expression by IL-1β was completely blocked by trichostatin A (Fig. 6A, left). In contrast, trichostatin A had no effect on PDGF-D levels in the absence of IL-1β (Fig. 6A, left). This data implicates HDACs in the negative control of PDGF-D in SMCs exposed to the cytokine. Co-immunoprecipitation experiments revealed that IL-1β indeed triggers the inducible interaction between IRF-1, p65, and HDAC-1 (Fig. 6A, right).
FIGURE 6.
Repression of PDGF-D mRNA by IL-1β involves enrichment of HDAC-1 at the PDGF-D promoter by IRF-1 and p65. A, left, SMCs were arrested overnight, pretreated with 1 μm trichostatin A for 18 h before incubation with 10 ng/ml of IL-1β for another 4 h. Total RNA was extracted and real-time quantitative PCR was performed for PDGF-D mRNA. An asterisk denotes p < 0.05 compared with no treatment control. Right, SMCs were arrested for 24 h and treated with 10 ng/ml of IL-1β for 1 h, and total protein was extracted. 500 μg of protein was used for the co-immunoprecipitation (IP) with 5 μg of anti-IRF-1 or anti-p65 antibody, prior to immunoblot (IB) for HDAC-1. B, ChIP analysis was performed using two separate PDGF-D promoter amplicons, −689PDGF-D−445 and −863PDGF-D−674 with cells transfected with 100 nm siRNA and exposed to 10 ng/ml IL-1β for 1 h. Total input (TI) and IgG serve as positive and negative controls, respectively. C, time-dependent enrichment of HDAC-1 at the PDGF-D promoter in response to IL-1β. Total input and IgG serve as positive and negative controls. No Addition refers to samples that did not receive IL-1β. D, HDAC-1 siRNA rescues the PDGF-D promoter from IL-1β repression. SMCs were transfected with p1168D-luciferase (luc) and 100 nm siRNA, and after 24 h, incubated with 10 ng/ml IL-1β for 8 h prior to harvest, and assessment of luciferase activity (upper). Alternatively, Western blot showing that HDAC-1 siRNA reduces HDAC-1 expression (lower). Data are representative of at least two independent experiments. No Addition refers to samples that did not receive IL-1β. E, schematic depicting the formation of a cytokine-inducible gene silencing complex involving HDAC-1, p65, and IRF-1. The ChIP amplicon −689PDGF-D−445 is indicated in blue, whereas amplicon −863PDGF-D−674 is indicated in red.
Negative regulation of PDGF-D expression by IL-1β is clearly an unusual phenomenon for this cytokine, which is well known for its stimulatory roles. The present study demonstrates the requirement of HDAC-1 in this process. IL-1β induces HDAC-1 complex formation with IRF-1 and HDAC-p65 (Fig. 6A, right) and the enrichment of HDAC-1, p65, and IRF-1 at the PDGF-D promoter (Fig. 4B).
To provide further insight on the role of HDAC-1 in IL-1β-dependent transcriptional repression, we performed additional ChIP experiments, this time using two separate PDGF-D promoter amplicons. One amplicon (−689PDGF-D−445) spans the NF-κB recognition motif (−471GGGATTCTAC−462), and the other amplicon (−863PDGF-D−674) spans the IRF-1 binding site further upstream (−840GAAAAGTGAAA−830) (Table 1). Our findings demonstrate that IL-1β stimulates the accumulation of p65, IRF-1, and HDAC-1 to both of these regions of the PDGF-D promoter (Fig. 6B). HDAC-1 associates with the promoter within 15 min (Fig. 6C). HDAC-1 levels are unchanged in cells exposed to IL-1β for 1 h (Fig. 6D, lower). Yet, HDAC-1 siRNA, which reduces basal HDAC-1 expression (Fig. 6D, lower), abrogates cytokine-inducible occupancy of HDAC-1, and that of p65 and IRF-1, at both regions of the promoter (Fig, 6B). HDAC-1 siRNA rescues the PDGF-D promoter from IL-1β repression (Fig. 6D, upper). Importantly, HDAC-1 siRNA also blocks the IL-1β-inducible interaction of IRF-1 with p65, as demonstrated by either pulling down with IRF-1 antibodies and immunoblotting with p65 antibodies or pulling down with p65 antibodies and immunoblotting for IRF-1 (Fig. 4A, right). These results indicate that HDAC-1 is enriched at the PDGF-D promoter in cells exposed to IL-1β and forms a cytokine-inducible gene silencing complex with p65 and IRF-1 (Fig. 6E).
DISCUSSION
In this study, we have shown that proinflammatory cytokine IL-1β down-regulates PDGF-D expression in a time- and dose-dependent manner. This contrasts with levels of VCAM-1, which, as expected, are induced by the cytokine. p65, induced by IL-1β, interacts with a novel element in the PDGF-D promoter and inhibits PDGF-D transcription. IRF-1 is also induced by IL-1β and binds to a second novel element in the PDGF-D promoter. ChIP experiments with two separate sets of primers, one amplicon (−689PDGF-D−445) spanning the NF-κB recognition motif (−471GGGATTCTAC−462), with the other (−863PDGF-D−674) spanning the IRF-1 binding site further upstream (−840GAAAAGTGAAA−830). p65, IRF-1, and HDAC-1 are enriched at each region of the PDGF-D promoter in cells exposed to IL-1β (Fig. 6E). Co-immunoprecipitation studies demonstrate that IRF-1 and p65 form a complex containing HDAC-1 within 1 h of exposure to IL-1β (Figs. 4A, left and 6A, right). Moreover, HDAC-1 siRNA ablates complex formation with IRF-1 and p65 (Fig. 4A, right) and in ChIP analysis, abrogates IRF-1 and p65 occupancy of the PDGF-D promoter (Fig. 6B). NF-κB and IRF-1 are transcription factors activated by a variety of proinflammatory cytokines including IL-1β and play a positive regulatory role in many genes implicated in the pathogenesis of vascular disease. These include VEGF (37), VCAM-1 (36), and iNOS (38) in SMCs. Cooperativity between NF-κB and IRF-1 has been reported previously. For example, recent studies demonstrate that IRF-1 and NF-κB form a functional complex at the LTR-κB sites in the human immunodeficiency virus type 1 enhancer, which is abrogated by specific mutations in NF-κB sites in the enhancer region (39). Similarly, cooperation between IRF-1 and NF-κB p65 positively regulates IL-6 (40) and iNOS (41) transcription. In the present study, IL-1β simulates p65·IRF-1 complex formation, but rather than activating PDGF-D transcription, p65 and IRF-1 bind to and repress the PDGF-D promoter. HDAC-1 is enriched at the PDGF-D promoter in cells exposed to IL-1β and forms a cytokine-inducible gene silencing complex with p65 and IRF-1 (Fig. 6E). We used IRF-1 antibodies in the EMSA shown in Fig. 2A (left) (with 32P-labeled Oligo D−481/−457, containing the p65 binding site); however, this did not perturb formation of either IL-1β-inducible Complex A or B. Conversely, p65 antibodies used in the EMSA (with 32P-labeled Oligo D−843/−827, containing the IRF-1 binding site) shown in Fig. 3B did not affect formation of the IL-1β-inducible complex. Although p65 and IRF-1 each bound their respective sites (Figs. 2A, left, and 3B), HDAC-1 was the only nuclear protein bound by either factor (Figs. 2A, left, and 3B). However, it is unreasonable to expect that more than two proteins could be successfully supershifted/eliminated when short oligonucleotides such as those employed in this study are used. The split ChIP nonetheless revealed that p65, IRF-1, and HDAC-1 were inducibly enriched at the PDGF-D promoter (Fig. 6B). p65 and IRF-1 form a complex containing HDAC-1 within 1 h of exposure to IL-1β (Figs. 4A, left, and 6A, right). Moreover, HDAC-1 siRNA ablates complex formation with IRF-1 and p65 (Fig. 4A, right) and abrogates IRF-1 and p65 occupancy of the PDGF-D promoter (Fig. 6B). These data, in combination, indicate that HDAC-1 is enriched at the PDGF-D promoter in cells exposed to IL-1β and forms a cytokine-inducible gene silencing complex with p65 and IRF-1 (Fig. 6E). This is consistent with the emerging role of HDAC-1 as a “transcriptional switch,” shifting from active gene expression to repression as a consequence of its interactions with other proteins. For example, ZBP-89, which can either activate or a repress gene expression, serves as a repressor of vimentin expression by recruiting HDAC-1 to the vimentin promoter (42). HDAC-1 is thus a critical component of an IL-1-inducible gene silencing complex that includes transcription factors better known for their ability to activate transcription.
The literature points toward PDGF-D and IL-1β each playing a regulatory role in SMC growth. PDGF-D is expressed upon vascular injury and stimulates SMC proliferation (10, 11). Balloon injury of porcine coronary arteries induces IL-1β production in the vessel wall (27), and administration of IL-1β to porcine coronary arteries causes neointima formation (28). The absence of IL-1 receptor antagonist in mice results in a 250% increase in neointima formation after injury (29), while IL-1 receptor antagonist infusion after balloon injury reduces intimal thickening (30). The irony here is our present demonstration of IL-1β repression PDGF-D expression.
Cytokine or growth factor suppression of growth factor expression is an interesting but not well understood phenomenon. TNF-α represses insulin-like growth factor-1 expression (43). TNF-α and IL-1β down-regulate the dipeptidyl carboxypeptidase angiotensin-converting enzyme, generates angiotensin II from ATI (44). Angiotensin II and transforming growth factor-β (TGF-β) negatively regulate hepatocyte growth factor (45). IL-1β inhibits VEGF-D expression (46). We showed that FGF-2 inhibits expression of PDGF receptor α transcription (47). Antagonism between PDGF and IL-1β has been observed. Li and colleagues (48) have demonstrated that migration and proliferation of retinal pigment epithelial cells induced by PDGF-CC and PDGF-DD are abolished by the presence of IL-1β, TNF-α, and interferon-γ. Moreover, IL-1β can block baboon aortic SMC migration and proliferation induced by PDGF (49, 50). These reports, combined with the present findings, suggest that IL-1β suppression of PDGF-D expression through IRF-1/p65/HDAC-1 may represent a negative regulatory mechanism in the vessel wall. Our data provides new insights into the mechanisms governing cytokine transcriptional control of growth factor gene expression. In the biological context, PDGF-D by IL-1β, or other factors, proinflammatory cytokine may help constrain the extent of intimal hyperplasia. This need not be confined to SMCs. For example, PDGF-D regulates numerous cellular processes, including proliferation, transformation, invasion, and angiogenesis. PDGF-D deregulation has been implicated in tumorigenesis and progression to metastatic disease (51). Down-regulation of PDGF-D results in inhibition of cell growth and angiogenesis through inactivation of Notch-1 and NF-κB signaling (8). Thus, IL-1β control of PDGF-D expression may represent an autoregulatory mechanism whereby PDGF-D-dependent biological processes are restrained by the cytokine.
This work was supported by grants from the National Health and Medical Research Council of Australia, Australian Research Council, and National Heart Foundation of Australia.
- PDGF
- platelet-derived growth factor
- IL
- interleukin
- IRF-1
- interferon regulatory factor-1
- HDAC
- histone deacetylase
- siRNA
- small interfering RNA
- HDAC
- histone deacetylases
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- EMSA
- electrophoretic mobility shift assays
- ChIP
- chromatin immunoprecipitation.
REFERENCES
- 1.Raines E. W., Bowen-Pope D. F., Ross R. (1990) in Handbook of Experimental Pharmacology: Peptide Growth Factors and Their Receptors (Sporn M. B., Roberts A. B. eds.) pp. 173–262, Springer-Verlag, Berlin [Google Scholar]
- 2.Khachigian L. M., Chesterman C. N. (1992) Platelets 4, 304–315 [DOI] [PubMed] [Google Scholar]
- 3.Raines E. W. (2004) Cytokine Growth Factor Rev. 15, 237–254 [DOI] [PubMed] [Google Scholar]
- 4.LaRochelle W. J., Jeffers M., McDonald W. F., Chillakuru R. A., Giese N. A., Lokker N. A., Sullivan C., Boldog F. L., Yang M., Vernet C., Burgess C. E., Fernandes E., Deegler L. L., Rittman B., Shimkets J., Shimkets R. A., Rothberg J. M., Lichenstein H. S. (2001) Nat. Cell Biol. 3, 517–521 [DOI] [PubMed] [Google Scholar]
- 5.Bergsten E., Uutela M., Li X., Pietras K., Ostman A., Heldin C. H., Alitalo K., Eriksson U. (2001) Nat. Cell Biol. 3, 512–516 [DOI] [PubMed] [Google Scholar]
- 6.Changsirikulchai S., Hudkins K. L., Goodpaster T. A., Volpone J., Topouzis S., Gilbertson D. G., Alpers C. E. (2002) Kidney Int. 62, 2043–2054 [DOI] [PubMed] [Google Scholar]
- 7.LaRochelle W. J., Jeffers M., Corvalan J. R., Jia X. C., Feng X., Vanegas S., Vickroy J. D., Yang X. D., Chen F., Gazit G., Mayotte J., Macaluso J., Rittman B., Wu F., Dhanabal M., Herrmann J., Lichenstein H. S. (2002) Cancer Res. 62, 2468–2473 [PubMed] [Google Scholar]
- 8.Wang Z., Kong D., Banerjee S., Li Y., Adsay N. V., Abbruzzese J., Sarkar F. H. (2007) Cancer Res. 67, 11377–11385 [DOI] [PubMed] [Google Scholar]
- 9.Uutela M., Wirzenius M., Paavonen K., Rajantie I., He Y., Karpanen T., Lohela M., Wiig H., Salven P., Pajusola K., Eriksson U., Alitalo K. (2004) Blood 104, 3198–3204 [DOI] [PubMed] [Google Scholar]
- 10.Pontén A., Folestad E. B., Pietras K., Eriksson U. (2005) Circ. Res. 97, 1036–1045 [DOI] [PubMed] [Google Scholar]
- 11.Chen J., Han Y., Lin C., Zhen Y., Song X., Teng S., Chen C., Chen Y., Zhang Y., Hui R. (2005) Biochem. Biophys. Res. Commun. 329, 976–983 [DOI] [PubMed] [Google Scholar]
- 12.Liu M. Y., Eyries M., Zhang C., Santiago F. S., Khachigian L. M. (2006) Blood 107, 2322–2329 [DOI] [PubMed] [Google Scholar]
- 13.Kavurma M. M., Bobryshev Y., Khachigian L. M. (2002) J. Biol. Chem. 277, 36244–36252 [DOI] [PubMed] [Google Scholar]
- 14.Tan N. Y., Midgley V. C., Kavurma M. M., Santiago F. S., Luo X., Peden R., Fahmy R. G., Berndt M. C., Molloy M. P., Khachigian L. M. (2008) Circ. Res. 102, e38–51 [DOI] [PubMed] [Google Scholar]
- 15.Zhang N., Khachigian L. M. (2009) J. Biol. Chem. 284, 27933–27943 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Perkins N. D. (2007) Nat. Rev. Mol. Cell Biol. 8, 49–62 [DOI] [PubMed] [Google Scholar]
- 17.Collins T., Cybulsky M. I. (2001) J. Clin. Invest. 107, 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamane Y., Heylbroeck C., Génin P., Algarté M., Servant M. J., LePage C., DeLuca C., Kwon H., Lin R., Hiscott J. (1999) Gene 237, 1–14 [DOI] [PubMed] [Google Scholar]
- 19.Mitchell A., Dass C. R., Sun L. Q., Khachigian L. M. (2004) Nucleic Acids Research 32, 3065–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delbridge G. J., Khachigian L. M. (1997) Circ. Res. 81, 282–288 [DOI] [PubMed] [Google Scholar]
- 21.Aizawa K., Suzuki T., Kada N., Ishihara A., Kawai-Kowase K., Matsumura T., Sasaki K., Munemasa Y., Manabe I., Kurabayashi M., Collins T., Nagai R. (2004) J. Biol. Chem. 279, 70–76 [DOI] [PubMed] [Google Scholar]
- 22.Khachigian L. M., Resnick N., Gimbrone M. A., Jr., Collins T. (1995) J. Clin. Invest. 96, 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resnick N., Yahav H., Khachigian L. M., Collins T., Anderson K. R., Dewey F. C., Gimbrone M. A., Jr. (1997) Adv. Exp. Med. Biol. 430, 155–164 [DOI] [PubMed] [Google Scholar]
- 24.Shi J., Wang X., Qiu J., Si Q., Sun R., Guo H., Wu Q. (2004) J. Cardiovasc. Pharmacol. 44, 26–34 [DOI] [PubMed] [Google Scholar]
- 25.Sumpio B. E., Du W., Galagher G., Wang X., Khachigian L. M., Collins T., Gimbrone M. A., Jr., Resnick N. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 349–355 [DOI] [PubMed] [Google Scholar]
- 26.Rafty L. A., Khachigian L. M. (1998) J. Biol. Chem. 273, 5758–5764 [DOI] [PubMed] [Google Scholar]
- 27.Chamberlain J., Gunn J., Francis S., Holt C., Crossman D. (1999) Cardiovasc. Res. 44, 156–165 [DOI] [PubMed] [Google Scholar]
- 28.Shimokawa H., Ito A., Fukumoto Y., Kadokami T., Nakaike R., Sakata M., Takayanagi T., Egashira K., Takeshita A. (1996) J. Clin. Invest. 97, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isoda K., Shiigai M., Ishigami N., Matsuki T., Horai R., Nishikawa K., Kusuhara M., Nishida Y., Iwakura Y., Ohsuzu F. (2003) Circulation 108, 516–518 [DOI] [PubMed] [Google Scholar]
- 30.Morton A. C., Arnold N. D., Gunn J., Varcoe R., Francis S. E., Dower S. K., Crossman D. C. (2005) Cardiovasc. Res. 68, 493–501 [DOI] [PubMed] [Google Scholar]
- 31.Neish A. S., Williams A. J., Palmer H. J., Whitley M. Z., Collins T. (1992) J. Exp. Med. 176, 1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khachigian L. M., Collins T., Fries J. W. U. (1995) Biochem. Biophys. Res. Comm. 206, 462–467 [DOI] [PubMed] [Google Scholar]
- 33.Leung C. H., Grill S. P., Lam W., Han Q. B., Sun H. D., Cheng Y. C. (2005) Mol. Pharmacol. 68, 286–297 [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanian S., Verner E., Buggy J. J. (2009) Cancer Lett. 280, 211–221 [DOI] [PubMed] [Google Scholar]
- 35.Montgomery R. L., Davis C. A., Potthoff M. J., Haberland M., Fielitz J., Qi X., Hill J. A., Richardson J. A., Olson E. N. (2007) Genes Dev. 21, 1790–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neish A. S., Read M. A., Thanos D., Pine R., Maniatis T., Collins T. (1995) Mol. Cell Biol. 15, 2558–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung Y. D., Liu W., Reinmuth N., Ahmad S. A., Fan F., Gallick G. E., Ellis L. M. (2001) Angiogenesis 4, 155–162 [DOI] [PubMed] [Google Scholar]
- 38.Jiang B., Brecher P., Cohen R. A. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1915–1920 [DOI] [PubMed] [Google Scholar]
- 39.Sgarbanti M., Remoli A. L., Marsili G., Ridolfi B., Borsetti A., Perrotti E., Orsatti R., Ilari R., Sernicola L., Stellacci E., Ensoli B., Battistini A. (2008) J. Virol. 82, 3632–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sancéau J., Kaisho T., Hirano T., Wietzerbin J. (1995) J. Biol. Chem. 270, 27920–27931 [DOI] [PubMed] [Google Scholar]
- 41.Saura M., Zaragoza C., Bao C., McMillan A., Lowenstein C. J. (1999) J. Mol. Biol. 289, 459–471 [DOI] [PubMed] [Google Scholar]
- 42.Wu Y., Zhang X., Salmon M., Zehner Z. E. (2007) Genes Cells 12, 905–918 [DOI] [PubMed] [Google Scholar]
- 43.Anwar A., Zahid A. A., Scheidegger K. J., Brink M., Delafontaine P. (2002) Circulation 105, 1220–1225 [DOI] [PubMed] [Google Scholar]
- 44.Saijonmaa O., Nyman T., Fyhrquist F. (2001) J. Vasc. Res. 38, 370–378 [DOI] [PubMed] [Google Scholar]
- 45.Nakano N., Morishita R., Moriguchi A., Nakamura Y., Hayashi S. I., Aoki M., Kida I., Matsumoto K., Nakamura T., Higaki J., Ogihara T. (1998) Hypertension 32, 444–451 [DOI] [PubMed] [Google Scholar]
- 46.Mountain D. J., Singh M., Singh K. (2008) J. Cell Physiol. 215, 337–343 [DOI] [PubMed] [Google Scholar]
- 47.Bonello M. R., Khachigian L. M. (2004) J. Biol. Chem. 279, 2377–2382 [DOI] [PubMed] [Google Scholar]
- 48.Li R., Maminishkis A., Wang F. E., Miller S. S. (2007) Invest. Ophthalmol. Vis. Sci. 48, 5722–5732 [DOI] [PubMed] [Google Scholar]
- 49.Kenagy R. D., Clowes A. W. (2000) J. Vasc. Res. 37, 381–389 [DOI] [PubMed] [Google Scholar]
- 50.Englesbe M. J., Deou J., Bourns B. D., Clowes A. W., Daum G. (2004) J. Vasc. Surg. 39, 1091–1096 [DOI] [PubMed] [Google Scholar]
- 51.Wang Z., Kong D., Li Y., Sarkar F. H. (2009) Curr. Drug Targets 10, 38–41 [DOI] [PubMed] [Google Scholar]