Abstract
OBJECTIVE: To compare the risk of cardiovascular-related hospitalization, statin adherence, and direct (medical and drug) and indirect (disability and medically related absenteeism) costs in US employees in whom atorvastatin or simvastatin was newly prescribed.
PATIENTS AND METHODS: Active employees aged 18 to 64 years with a new atorvastatin or simvastatin prescription were identified from a deidentified claims database for 23 privately insured US companies from January 1, 1999, through December 31, 2006. Employees given atorvastatin were matched to those given simvastatin according to propensity scores based on patient characteristics, index statin dose, preindex cardiovascular events, and wage. Outcomes were compared between matched cohorts during the 2-year postindex period, including the risk of cardiovascular-related hospitalization, adherence to the index statin, use of other lipid-lowering drugs, direct medical costs for third-party payers, and indirect costs to employers. Indirect costs were computed as follows: Disability Payments + Daily Wage × Days of Medically Related Absenteeism. Atorvastatin and simvastatin drug costs were imputed using recent pricing to account for the availability of lower-cost generic simvastatin after the study period.
RESULTS: Among 13,584 matched pairs, treatment with atorvastatin vs simvastatin was associated with a reduced risk of cardiovascular-related hospitalization, higher adherence, and less use of other lipid-lowering drugs. The increase in statin costs associated with atorvastatin vs simvastatin therapy was almost completely offset by reductions in medical service and indirect costs.
CONCLUSION: In this study, treatment with atorvastatin compared with simvastatin was associated with a reduced risk of cardiovascular events, reduced indirect costs, and a minimal difference in total costs to employers.
Among 13,584 matched pairs in this study, treatment with atorvastatin compared with simvastatin was associated with a reduced risk of cardiovascular-related hospitalization, higher adherence, reduced indirect costs, and a minimal difference in total costs to employers.
Coronary heart disease (CHD) affects 13.2 million adults in the United States, resulting in total costs to society of $142.5 billion annually.1 Indirect costs attributed to CHD due to lost productivity, medically related absenteeism, or mortality account for almost half of this total, with CHD affecting substantial fractions of working-age adults.1 A population of almost 100 million adults in the United States1 is affected by high cholesterol levels (≥240 mg/dL; to convert to mmol/L, multiply by 0.0259), a major risk factor for CHD. More than half the costs attributed to high cholesterol levels are due to lost productivity.2
The primary treatments for lowering cholesterol levels, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors or statins, reduce the risk of CHD,3,4 reduce direct medical costs and indirect costs, and may even lead to net cost savings.5 The many statins indicated for the treatment of high cholesterol levels differ in how they are metabolized, their effects on other serum lipid components, bioavailability, and potency.6-10 The 2 most commonly prescribed statins in the United States are atorvastatin and simvastatin, with simvastatin being available as a lower-cost generic drug since mid-2006. Compared with simvastatin, atorvastatin results in a greater reduction in low-density lipoprotein cholesterol levels per milligram across all doses.11
In the only head-to-head clinical outcomes trial of atorvastatin vs simvastatin in patients with established CHD, high-dose atorvastatin and usual-dose simvastatin did not differ significantly in the primary end point of major coronary events, but high-dose atorvastatin was associated with a statistically significant reduction in several secondary end points relative to usual-dose simvastatin (nonfatal acute myocardial infarction, major cardiovascular events, and any coronary event).12 In observational studies of large US administrative insurance claims databases, atorvastatin treatment has been associated with a lower risk of cardiovascular-related hospital admissions compared with simvastatin treatment among all patients in whom statin therapy was initiated13 and among patients free of cardiovascular disease in whom statin therapy was initiated for primary prevention.14,15 Similarly, in analyses of a general practice research database in the Netherlands, new statin users who were prescribed atorvastatin experienced a lower risk of fatal and nonfatal cardiovascular and cerebrovascular events compared with those who were prescribed simvastatin and other statins.16 An editorial published in the December 2008 issue of Mayo Clinic Proceedings concluded that atorvastatin may be the better choice for prevention of cardiovascular disease but that it is unclear whether these benefits are considered fully cost-effective.17
For editorial comment, see pages 1059 and 1062
The aim of the current study was to compare clinical outcomes and economic outcomes from the employer perspective between patients in whom atorvastatin or simvastatin therapy was initiated. Given that indirect costs to employers are a substantial component of cardiovascular-related disease costs, the current study focused on a privately insured population of US employees in whom indirect costs to employers (ie, costs from missed work due to disability and medically related absenteeism) could be measured in addition to direct medical and pharmacy costs.
PATIENTS AND METHODS
Data were obtained from Ingenix Employer Solutions, a privately insured claims database covering approximately 8 million beneficiaries (including employees, spouses, and dependents) from 23 large employers under various insurers, from January 1, 1998, through December 31, 2006. Together, these employers have operations throughout the United States in a broad array of industries and job classifications. The data contain deidentified information on patient demographics (eg, age, sex), health plan eligibility, and medical and pharmacy claims. Medical service use is recorded with a date of service, associated diagnoses, procedures, and paid reimbursed amounts. Paid amounts for patient co-payments are recorded. Pharmacy claims are associated with National Drug Codes and contain dates of prescription fills, days of supply, strengths, quantities, and reimbursed amounts. The 23 employers also provide data on wages, disability-related work loss, and disability payments to employees. The current study included only employed members younger than 65 years to avoid third-party payments under Medicare coverage.
To identify patients in whom statin therapy was newly prescribed, we initially included patients with at least 1 prescription for atorvastatin or simvastatin preceded by at least 1 year free of lipid-lowering therapy with any statin or with ezetimibe. The index date was defined as the date of the first prescription fill for atorvastatin or simvastatin (excluding single-pill combinations with ezetimibe). Study patients were further required to be active, full-time employees aged 18 to 64 years on the index date and to be continuously enrolled in their health plan for 1 year before the index date (baseline period). For the primary analyses, selected patients were required to be continuously eligible in their health plans for 2 years after the index date to ensure equal lengths of follow-up for parallel comparisons of health care resource use and cost outcomes. For a sensitivity analysis, patients were selected using the less restrictive criteria of at least 30 days of continuous enrollment in their health plan after the index date. Although patients in this secondary cohort were required to have 30 days of continuous enrollment, all subsequent available continuous enrollment was included for analysis.
Study Measures
Patient characteristics were assessed during the 1-year baseline period, including demographics (eg, age, sex), average wage, initial dose of index drug, selected comorbidities (including diabetes mellitus, obesity, hypertension, and chronic kidney disease, identified using the International Classification of Diseases, Ninth Revision, Clinical Modification codes associated with inpatient or outpatient medical services), outpatient cardiovascular-related diagnoses, inpatient cardiovascular events, medical service use (outpatient, inpatient, and emergency department visits), medication use, work loss, indirect costs, and all-cause and cardiovascular-related prescription and medical service costs.
In the primary analyses with 2 years of continuous postindex enrollment available for all patients, the primary study outcomes were the rate of inpatient cardiovascular events and total costs to employers. Cardiovascular-related events were defined through inpatient diagnoses and procedures, indicating ischemic heart disease, myocardial infarction, peripheral vascular disease, aortic aneurysm, angioplasty, coronary artery bypass graft, transient ischemic attack, cerebrovascular disease, and congestive heart failure. A similar grouping of cardiovascular events has been studied in prior analyses of atorvastatin and simvastatin on the basis of different sources of administrative claims data.14 The total cost outcomes examined in the current study include all-cause medical costs (services and prescription drugs) in addition to indirect costs incurred through work loss and disability payments and thus represent total costs from an employer perspective. To provide a complete characterization of outcomes for the study cohorts, rates of medical service use (outpatient, inpatient, and emergency department visits), medication use, work loss hours, and all-cause and cardiovascular-related prescription costs were also studied.
Initial doses of the index drug were classified as high, medium, or low. For atorvastatin, low dose was defined as 10 mg/d or less, medium dose as 11 through 20 mg/d, and high dose as more than 20 mg/d; for simvastatin, low dose was defined as 20 mg/d or less, medium dose as 21 through 40 mg/d, and high dose as more than 40 mg/d.14 Patients were considered adherent with the index prescription if their medication possession ratio was 80% or greater; nonadherence was defined as a medication possession ratio of less than or equal to 20%. Work loss was defined as days of disability (total time covered by disability claims plus an employer-specific period preceding short-term disability) and as days of medically related absenteeism identified from medical service claims. Indirect costs were identified from employer payments associated with disability claims and from the days of medically related absenteeism multiplied by daily wage.18,19 Medical service costs were identified as reimbursements from the insurer or managed care plan to health care professionals and inflated to year 2006 dollars. Prescription drug costs were identified as reimbursed amounts for pharmacy claims inflated to year 2006 dollars. Inflation used the medical component of the Consumer Price Index. Because simvastatin is currently available as a generic medication, paid amounts for simvastatin and atorvastatin were imputed using the average reimbursed amounts in 2007 by dose and days of supply.
Statistical Analyses
Baseline characteristics and outcomes were compared for the unmatched atorvastatin cohort vs the simvastatin cohort using Wilcoxon rank sum tests for continuous variables and χ2 tests for categorical variables. Statistical significance was defined as P<.05.
To adjust for imbalance in baseline characteristics between the atorvastatin and simvastatin cohorts with 2 years of continuous postindex enrollment, patients in the atorvastatin cohort were matched 1-to-1 to simvastatin users for (1) initial drug dose (low, medium, high), (2) baseline inpatient cardiovascular events, (3) average wage within ±$5500, and (4) a propensity score within ±0.05. All 4 criteria were required to be satisfied for each match. Propensity scores were generated from a logistic regression model predicting membership in the atorvastatin vs simvastatin cohort using a model developed by entering all studied baseline characteristics into a stepwise selection algorithm. To assess match quality, baseline characteristics were compared between the matched atorvastatin and simvastatin patients using Wilcoxon signed rank tests for continuous variables and the McNemar test for dichotomous variables. Outcomes were similarly compared between the matched atorvastatin vs simvastatin cohorts.
As a sensitivity analysis, time to the first cardiovascular-related hospitalization, the primary clinical outcome, was studied using a multivariate Cox proportional hazards model based on the patient sample with at least 30 days of continuous postindex enrollment. The time to first event outcome was defined as the time from the index date to the first cardiovascular-related hospitalization, with patients who had a gap in health plan enrollment of more than 30 days treated as censored. Thus, these analyses used all available follow-up during which a patient was continuously enrolled after the index date. All baseline characteristics were entered as potential predictors into a stepwise selection procedure to develop the final multivariate Cox model.
RESULTS
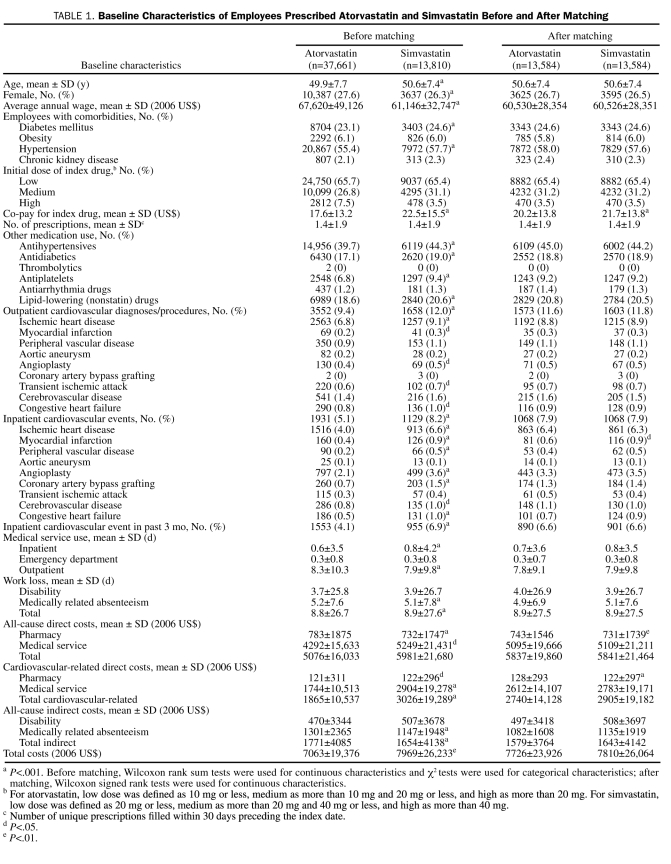
Before matching, sample selection identified 37,661 employees in whom atorvastatin was prescribed and 13,810 patients in whom simvastatin was prescribed. Atorvastatin-treated patients were slightly younger on average and more likely to be female, earned a higher average wage, had lower co-payments for their index drug, and were less likely to be using other medications. Fewer atorvastatin vs simvastatin-treated patients had prior diagnoses of diabetes or hypertension or prior outpatient or inpatient cardiovascular diagnoses. Atorvastatin vs simvastatin-treated patients had lower baseline direct medical costs but higher baseline indirect costs and overall lower total costs.
After matching atorvastatin-treated patients 1-to-1 to simvastatin-treated patients on the basis of the propensity score, presence of inpatient cardiovascular events, initial dose (high, medium, or low), and average wage, 13,584 matched pairs with 2 years of continuous postindex enrollment were obtained. The matched sample included more than 98% (13,584/13,810) of the patients in the smaller simvastatin cohort. Baseline characteristics were well balanced between the matched cohorts, although statistically significant differences remained, with atorvastatin patients having slightly higher preindex all-cause and cardiovascular-related pharmacy costs compared with simvastatin patients (by $11 and $6, respectively) and a lower rate of prior myocardial infarction (0.60% vs 0.85%) (Table 1). The average baseline characteristics of the matched cohorts better represent the average baseline characteristics of the smaller simvastatin cohort before matching than those of the atorvastatin cohort before matching. Simvastatin and atorvastatin drug costs were imputed according to the dose and days of supply using 2007 pharmacy claims costs observed after generic availability of simvastatin. For example, the average reimbursed amounts for a 30-day (90-day) supply of generic simvastatin were $12.60 ($65.26) for 10 mg, $19.88 ($112.48) for 20 mg, $19.07 ($110.98) for 40 mg, and $18.65 ($108.28) for 80 mg.
TABLE 1.
Baseline Characteristics of Employees Prescribed Atorvastatin and Simvastatin Before and After Matching
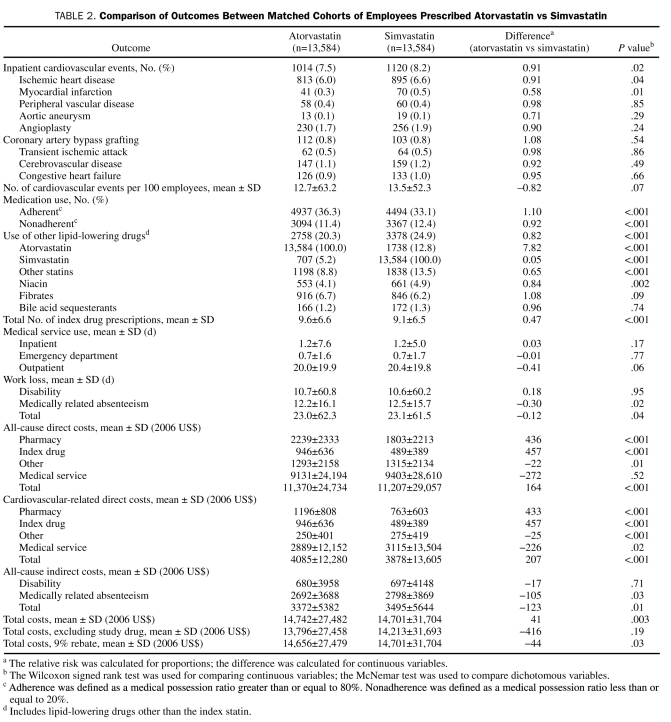
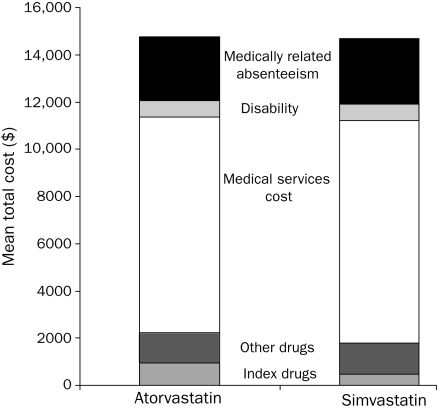
During the 2-year outcome period (Table 2), atorvastatin vs simvastatin patients experienced significantly lower rates of total inpatient cardiovascular events (7.5% vs 8.2%; P=.02). Treatment with atorvastatin vs simvastatin was also associated with fewer days of medically related absenteeism (12.2 vs 12.5; P=.02) and fewer total work loss days (23.0 vs 23.1; P=.04), higher rates of medication adherence (36.3% vs 33.1%; P<.001), and lower rates of nonadherence (11.4% vs 12.4%; P<.001), as well as lower concomitant use of other lipid-lowering medication (20.3% vs 24.9%; P<.001). As expected, pharmacy costs derived from index drug costs remained higher for atorvastatin because of the generic simvastatin ($946 vs $489; P<.001), but atorvastatin patients had slightly lower nonindex drug costs for all other drugs ($1293 vs $1315; P=.01) and for other cardiovascular-related drugs ($250 vs $275; P<.001) (Table 2). Treatment with atorvastatin vs simvastatin was also associated with lower cardiovascular-related medical service costs ($2889 vs $3115; P=.02), lower medically related absenteeism costs ($2692 vs $2798; P=.03), and lower total indirect costs ($3372 vs $3495; P=.01). In total, taking into account the direct costs of medical services and prescription drugs and indirect costs to employers due to disability and medically related absenteeism, treatment with atorvastatin was associated with a $41 (0.30%) higher total cost to the employer compared with simvastatin ($14,742 vs $14,701; P=.003) during the 2-year follow-up period (Figure and Table 2).
TABLE 2.
Comparison of Outcomes Between Matched Cohorts of Employees Prescribed Atorvastatin vs Simvastatin
FIGURE.
Total 2-year costs and cost components for matched patients treated with atorvastatin or simvastatin.
Agreements between the employer and the insurer or the pharmacy benefits management company can involve manufacturer rebates that reduce drug acquisition costs. Because these rebates are not included in the data source used for the current study, cost comparisons for the matched atorvastatin and simvastatin cohorts were recalculated using representative rebate amounts paid by manufacturers of brand-name but not generic agents. Although the rebate rate fluctuates on the basis of the specific contract between employers and health care professionals, 9% functions as a simplistic and conservative estimate that should encompass most health care plans. After applying a 9% rebate to the atorvastatin pharmacy cost in the 2-year sample, atorvastatin therapy was cost saving by $44 compared with simvastatin therapy ($14,656 vs $14,701) (Table 2).
Multivariate Cox Proportional Hazards Model
The study sample included 59,370 active full-time employees aged 18 to 64 years in whom atorvastatin was initiated and 23,325 in whom simvastatin was initiated, with continuous health plan enrollment for at least 30 days after the index date. In the multivariate Cox proportional hazards model obtained via stepwise selection, treatment with atorvastatin vs simvastatin was associated with a statistically significant 11% reduction in the hazard of cardiovascular-related hospitalization (hazard ratio, 0.89; 95% confidence interval, 0.85-0.95; P=.001). Baseline characteristics that were significantly associated with an increased hazard of inpatient cardiovascular events included older age, male sex, lower wage, baseline diabetes, hypertension, chronic kidney disease, use of medium (vs high) dose of the index statin, use of antihypertensives, use of antiplatelets, use of antidiabetics, use of antiarrhythmics, prior inpatient diagnoses of peripheral vascular disease, and prior cardiovascular events in the past 3 months.
DISCUSSION
In this observational study of an employed population, treatment with atorvastatin vs simvastatin was associated with a reduced risk of cardiovascular-related hospitalization and a minimal difference in total costs to employers. This study suggests that, despite a substantial difference in the drug acquisition costs between the brand-name atorvastatin and the generic simvastatin, atorvastatin treatment leads to only minimally higher costs to employers and may result in cost savings if drug rebates are included. The higher drug acquisition costs for atorvastatin were largely offset by reduced costs for medical services and reduced indirect costs.
These findings are important to payers and self-insured employers attempting to reduce overall costs by reducing a pharmacy budget. Although it can be relatively straightforward to implement programs designed to increase the use of generic statins over more expensive brand-name agents and to measure the impact of such programs on pharmacy costs, our findings suggest that, in the case of atorvastatin and simvastatin, cost savings predicted by this type of silo budget analysis would not materialize in bottom-line costs to employers. Another example of how the pharmacy budget may be a misleading proxy for overall health care costs is a recent analysis concluding that, for patients who had either no or minimal drug coverage, the increase in pharmacy spending as a result of enrollment in Medicare part D was essentially offset by reductions in other medical spending.20 Appropriate pharmaceutical treatment of other chronic diseases has also been associated with increased productivity and reduced indirect costs to employers.21
The clinical explanation for reduced resource use in atorvastatin-treated patients could not be determined in the current study. Better adherence to atorvastatin may contribute to this explanation.14,22,23 In addition, evidence suggests that the 2 agents may differ in their nonlipid effects, such as the reduction in inflammation.24 The association between inflammatory markers and cardiovascular events has received increased attention after the publication of JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin), in which rosuvastatin therapy was shown to benefit patients with normal low-density lipoprotein cholesterol levels (<130 mg/dL) but elevated levels of the inflammatory marker C-reactive protein.25 Multiple studies have shown that atorvastatin significantly reduces high-sensitivity C-reactive protein levels,26-29 whereas a study of simvastatin found reductions of smaller magnitude,30 and a comparative study found that atorvastatin was associated with greater high-sensitivity C-reactive protein reductions compared with simvastatin.31
Previous observational studies of statin therapy in the general (not necessarily employed) population for both primary prevention and all new statin users13-15 have consistently shown a relative risk reduction associated with atorvastatin therapy of 10% to 13%, which is roughly in line with the 9% relative risk reduction in our analysis. Taken together, the consistency across studies suggests a robust association between initiation with atorvastatin and reduced cardiovascular risk that is not sensitive to differences across administrative claims databases, statistical methods (Cox regression vs propensity score matching), employment status, or small variations in sample selection and study design. It would be informative to assess whether the risk reduction and corresponding cost offsets could be replicated in a prospective, randomized cardiovascular outcomes trial with comparable doses.
Although our findings are based on a mixed primary and secondary prevention population, they are consistent with the health economic analyses of the IDEAL (Incremental Decrease in Endpoints Through Aggressive Lipid Lowering) trial, which investigated the cost-effectiveness of high-dose atorvastatin therapy relative to regular-dose simvastatin therapy among patients with established coronary heart disease in several European populations; this study also found substantial indirect cost savings associated with atorvastatin vs simvastatin.19 Although statin acquisition costs for atorvastatin therapy are significantly more expensive compared with generic simvastatin therapy, this difference was partially offset by reduced medical and indirect costs. However, atorvastatin therapy was only moderately cost-effective relative to simvastatin in the IDEAL trial, possibly because the generic simvastatin daily drug cost, ranging from €0.07 to €0.30 for simvastatin at 20 mg, or approximately $2.90 to $12.30 for a 30-day supply, was assumed to be significantly lower compared with the amount paid by a typical US employer in the current study (using the generic simvastatin pricing identified after 2007). Although the time frame for the current study extended only 6 months past the initial approval of generic simvastatin, generic simvastatin drug prices were imputed throughout to better represent the current reimbursement environment for privately insured US employers.
Our study consists of a large number of patients, but administrative claims data have limitations. As in all nonrandomized, observational studies, it is necessary to adjust for patient characteristics that may be associated with treatment choice and outcomes.32 We attempted to adjust for all potentially important confounding variables that were in the data source, including prior cardiovascular events, but it is possible that unmeasured confounding variables were present.
The measurement of indirect cost outcomes in the current study was limited and may underestimate the total indirect costs to employers. It was not possible to compare losses in productivity or presenteeism for statin-treated patients or the costs to employers of lost productivity of coworkers or of hiring replacement staff for patients with absenteeism due to cardiovascular events, early retirement, or early mortality.33,34 Also, because disability payments were not associated with particular diagnoses or dates of absence from work, classification of disability payments as cardiovascular related was not possible. The average annual wage observed in this patient cohort (approximately $60,000) is somewhat higher than the median annual wage of US employees, which may limit the generalizability of the findings, particularly because wage data are used to calculate indirect costs. Because the matching-adjusted analyses primarily adjusted the atorvastatin cohort to match the baseline characteristics of the simvastatin cohort, the treatment effects of atorvastatin vs simvastatin pertain to patients typically treated with simvastatin during the study period. As with any analysis of administrative claims, data may contain errors in coding or underrepresentation of certain comorbidities. For example, the rates of recorded obesity diagnoses in this study were much lower than would be expected for the general US population. However, because even inaccurate diagnoses can be associated with cardiovascular risk, ensuring balance between study groups for all relevant recorded comorbidities can provide helpful adjustment for confounding variables. Moreover, claims data may not contain all clinical assessments of interest. For example, in the current study, laboratory values, such as serum lipid levels, were not available and could not be directly adjusted between the atorvastatin and simvastatin cohorts. The 2-year follow-up period may not have been long enough to capture all outcomes associated with the initial statin prescription. Long-term differences in health care resource use, work loss, and costs could persist as lifelong consequences of cardiovascular events experienced during the 2-year study period. An additional limitation of the data source was that mortality, which could differ between treatment cohorts given the differences in cardiovascular event rates, could not be assessed. Finally, the primary outcomes were inpatient cardiovascular event rates and total costs to employers. Multiple other outcomes were analyzed to support the interpretation of the primary outcomes and to provide hypotheses for potential future research, but no statistical adjustment was made for multiple comparisons.
CONCLUSION
This study found that active employees in whom lipid-lowering therapy with atorvastatin, rather than simvastatin, was initiated experienced a lower risk of cardiovascular-related hospitalizations, consistent with previous observational studies. Initiation of statin therapy with the less-expensive generic simvastatin did not result in any meaningful total cost savings to employers. The higher drug acquisition costs for atorvastatin were largely offset by reduced cardiovascular-related medical service costs and reduced indirect costs. The net total cost difference of $41 indicates that atorvastatin, relative to simvastatin, is a cost-effective intervention in this patient population and, after assuming typical rebates, may even be cost saving.
Footnotes
Data from this study were presented as an abstract at the American Heart Association Annual Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke; April 23-25, 2009; Washington, DC.
Research for this study was supported by Pfizer Inc.
REFERENCES
- 1.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 update: a report from the American heart association statistics committee and stroke statistics subcommittee [published corrections appear in Circulation. 2006;113(14):e696 and 2006;114(23):e630] Circulation 2006February14;113(6):85-151 Epub 2006 Jan 11 [DOI] [PubMed] [Google Scholar]
- 2.Müller-Nordhorn J, Englert H, Wegscheider K, et al. Productivity loss as a major component of disease-related costs in patients with hypercholesterolemia in Germany. Clin Res Cardiol. 2008March;97(3):152-159 Epub 2007 Dec 1 [DOI] [PubMed] [Google Scholar]
- 3.Sever PS, Dahlöf N, Poulter NR, et al. ASCOT Investigators Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149-1158 [DOI] [PubMed] [Google Scholar]
- 4.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364(9435):685-696 [DOI] [PubMed] [Google Scholar]
- 5.Grover SA, Ho V, Lavoie F, Coupal L, Zowall H, Pilote L. The importance of indirect costs in primary cardiovascular disease prevention. Arch Intern Med. 2003;163(3):333-339 [DOI] [PubMed] [Google Scholar]
- 6.Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341(7):498-511 [DOI] [PubMed] [Google Scholar]
- 7.Bakker-Arkema RG, Davidson MH, Goldstein RJ, et al. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA 1996;275(2):128-133 [PubMed] [Google Scholar]
- 8.Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol [published correction appears in Lancet. 1997;349(9046):214] Lancet 1996;348(9034):1079-1082 [DOI] [PubMed] [Google Scholar]
- 9.Hsu I, Spinler SA, Johnson NE. Comparative evaluation of the safety and efficacy of HMG—CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann Pharmacother. 1995;29(7-8):743-759 [DOI] [PubMed] [Google Scholar]
- 10.Mason RP. Molecular basis of differences among statins and a comparison of antioxidant vitamins. Am J Cardiol. 2006;December4;98(11A):34P-41P Epub 2006 Oct 10 [DOI] [PubMed] [Google Scholar]
- 11.Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the Curves study) [published correction appears in Am J Cardiol. 1998;82(1):128] Am J Cardiol. 1998;81(5):582-587 [DOI] [PubMed] [Google Scholar]
- 12.Pedersen TR, Faergeman O, Kastelein JJP, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized clinical trial [published correction appears in JAMA. 2005;294(24):3092] JAMA 2005;294(19):2437-2445 [DOI] [PubMed] [Google Scholar]
- 13.Willke R, Zhou S, Vogel R. Differences in cardiovascular event rates between atorvastatin and simvastatin among new users: managed-care experience. Curr Med Res Opin. 2008;10(24):2873-2882 [DOI] [PubMed] [Google Scholar]
- 14.Jacobson TA, Wertz DA, Hoy T, Kuznik A, Grouchulski D, Cziraky M. Comparison of cardiovascular event rates in patients without cardiovascular disease in whom atorvastatin or simvastatin was newly initiated [published correction appears in Mayo Clin Proc. 2009;84(2):208] Mayo Clin Proc. 2008;83(12):1316-1325 [DOI] [PubMed] [Google Scholar]
- 15.Foody JM, Joyce AT, Rudolph AE, Liu LZ, Benner JS. Cardiovascular outcomes among patients newly initiating atorvastatin or simvastatin therapy: a large database analysis of managed care plans in the United States. Clin Ther. 2008;30(1):195-205 [DOI] [PubMed] [Google Scholar]
- 16.Dieleman JP, van Wyk JT, van Wijk MA, et al. Differences between statins on clinical endpoints: a population-based cohort study. Curr Med Res Opin. 2005;21(9):1461-1468 [DOI] [PubMed] [Google Scholar]
- 17.Krasuski RA. Primary prevention and statins: is it just about going to class [editorial]? Mayo Clin Proc. 2008;83(12):1313-1315 [DOI] [PubMed] [Google Scholar]
- 18.Birnbaum HG, Barton M, Greenberg P, et al. Direct and indirect costs of rheumatoid arthritis to an employer. J Occup Environ Med. 2006;42(6):588-596 [DOI] [PubMed] [Google Scholar]
- 19.Lindgren P, Graff J, Olsson AG, Pedersen TJ, Jönsson B, IDEAL Trial Investigators Cost-effectiveness of high-dose atorvastatin compared with regular dose simvastatin. Eur Heart J. 2007June;28(12):1448-1453 Epub 2007 Mar 19 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Donohue JM, Lave JR, O'Donnell G, Newhouse JP. The effect of Medicare Part D on drug and medical spending. N Engl J Med. 2009;361(1):52-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldfarb N, Weston C, Hartmann CW, et al. Impact of appropriate pharmaceutical therapy for chronic conditions on direct medical costs and workplace productivity: a review of the literature. Dis Manag. 2004;7(1):62-75 [DOI] [PubMed] [Google Scholar]
- 22.Lachaine J, Rinfret S, Merikle EP, Tarride JE. Persistence and adherence to cholesterol lowering agents: evidence from Régie de l'Assurance Maladie du Québec data. Am Heart J. 2006;152(1):164-169 [DOI] [PubMed] [Google Scholar]
- 23.Huser M, Evans T, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22(2):163-171 [DOI] [PubMed] [Google Scholar]
- 24.Blanco-Colio LM, Martin-Ventura JL, de Teresa E, et al. ACTFAST Investigators Elevated ICAM-1 and MCP-1 plasma levels in subjects at high cardiovascular risk are diminished by atorvastatin treatment: Atorvastatin on Inflammatory Markers study: a substudy of Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration. Am Heart J. 2007;153(5):881-888 [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Danielson E, Fonseca FAH, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008November20;359(21):2195-2207 Epub 2008 Nov 9 [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Gerique JA, Ros E, Olivan J, et al. ATOMIX Investigators Effect of atorvastatin and bezafibrate on plasma levels of C-reactive protein in combined (mixed) hyperlipidemia. Atherosclerosis 2002;162(2):245-251 [DOI] [PubMed] [Google Scholar]
- 27.Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. Targeting vascular risk in patients with metabolic syndrome but without diabetes. Metabolism 2005;54(8):1065-1074 [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Kent SM, Flaherty PJ, et al. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation 2002;106(16):2055-2060 [DOI] [PubMed] [Google Scholar]
- 29.Kinlay S, Schwartz GG, Olsson AG, et al. Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators Effect of atorvastatin on risk of recurrent cardiovascular events after an acute coronary syndrome associated with high soluble CD40 ligand in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study. Circulation 2004July27;110(4):386-391 Epub 2004 Jul 19 [DOI] [PubMed] [Google Scholar]
- 30.Meredith KG, Horne BD, Pearson RR, et al. Comparison of effects of high (80 mg) vs low (20 mg) dose of simvastatin on C-reactive protein and lipoproteins in patients with angiographic evidence of coronary arterial narrowing. Am J Cardiol. 2007January15;99(2):149-153 Epub 2006 Nov 16 [DOI] [PubMed] [Google Scholar]
- 31.van Wissen S, Trip M, Smilde T, et al. Differential hs-CRP reduction in patients with familial hypercholesterolemia treated with aggressive or conventional statin therapy. Atherosclerosis 2002;165(2):361-366 [DOI] [PubMed] [Google Scholar]
- 32.Walker AM. Confounding by indication [editorial]. Epidemiology 1996;7(4):335-336 [PubMed] [Google Scholar]
- 33.Burton WN, Chen CY, Conti DJ, Schultz AB, Pransky G, Edington DW. The association of health risks with on-the-job productivity. J Occup Environ Med. 2005;47(8):769-777 [DOI] [PubMed] [Google Scholar]
- 34.Goetzel RZ, Long SR, Ozminkowski RJ, et al. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med. 2004;46(4):398-412 [DOI] [PubMed] [Google Scholar]