Abstract
OBJECTIVE: To assess bleeding and activated partial thromboplastin time (APTT) in relation to body mass index (BMI) in patients prescribed weight-based dosing of intravenous unfractionated heparin (UFH) for cardiac indications without a maximum (dose-capped) initial bolus or capped initial infusion rate.
PATIENTS AND METHODS: Consecutive patients admitted to an academic medical center from February 1, 2002, through November 31, 2003, who were treated with a UFH nomogram consisting of a 60-U/kg intravenous bolus plus an initial continuous intravenous infusion of 12 U/kg hourly and titrated to a goal APTT range corresponding to thromboplastin-adjusted target heparin levels of 0.3 to 0.7 U/mL by anti-Xa assay were evaluated for this retrospective cohort study. Patients were excluded if they concomitantly received a fibrinolytic, glycoprotein IIb/IIIa inhibitor, or any other antithrombotic agent (except warfarin). Study patients were divided into quartiles by BMI.
RESULTS: Of the 1054 patients included in the study, 807 (76.6%) had an initial bolus dose higher than 4000 U, and 477 (45.3%) had an initial infusion rate higher than 1000 U/h. Despite a significant difference among BMI quartiles in proportion of supratherapeutic first APTT values (P<.001), no statistically significant difference was found in bleeding frequency (P=.26) or frequency of first APTT within the goal range (P=.27). Logistic regression analyses revealed that BMI was not a significant predictor of bleeding or first APTT within the goal range.
CONCLUSION: We did not find any difference in the proportion of first APTT values in the goal range or an increased risk of bleeding in obese patients treated with UFH without a capped initial dose. Our data demonstrate the safe use of weight-based UFH without a capped initial bolus dose or capped initial infusion rate in patients with medical cardiac conditions.
The authors did not find any difference in the proportion of first activated partial thromboplastin time values in the goal range or an increased risk of bleeding in obese patients treated with unfractionated heparin without a capped initial dose.
APTT = activated partial thromboplastin time; BMI = body mass index; CI = confidence interval; IV = intravenous; OR = odds ratio; PCI = percutaneous coronary intervention; UFH = unfractionated heparin
Unfractionated heparin (UFH) is useful for prevention of clot formation or progression of both arterial and venous thromboembolic disorders. A weight-based regimen for determining the dose of UFH has been used for more than 15 years1; however, the effect of this dosing regimen on obese patients is still poorly understood. The volume of distribution of heparin approximates that of the blood volume (40-70 mL/kg),2 with obese patients intuitively requiring larger doses of heparin.3 In contrast, the blood volume of adipose tissue is less than that of lean tissue,2 and thus maximum (dose-capped) initial bolus and initial infusion rates for UFH have been suggested. Although guidelines from the American College of Cardiology and American Heart Association for ST-segment elevation myocardial infarction4 and non—ST-segment elevation myocardial infarction or unstable angina5 advocate institution of UFH with an initial intravenous (IV) bolus dose of 60 U/kg (with a capped initial bolus of 4000 U) and initial IV infusion rate of 12 U/kg hourly (capped initial infusion rate of 1000 U/h), neither guideline contains explicit rationale for the recommended capped initial bolus or capped initial infusion rate. Previous studies3,6 have attempted to clarify the confusion regarding the effect of obesity on activated partial thromboplastin time (APTT) values. These studies recommended actual body weight for calculation of initial heparin doses; however, they were relatively small and did not evaluate the effect of obesity on bleeding outcomes. We designed the current study to evaluate APTT results and bleeding outcomes in patients in whom UFH was prescribed for medical cardiac indications without a capped initial bolus or capped initial infusion rate regardless of weight.
PATIENTS AND METHODS
This retrospective cohort analysis was performed at Saint Marys Hospital in Rochester, MN, which is a 1157-bed, tertiary care academic medical center. Consecutive patients with medical cardiac conditions aged 15 years or older who were prescribed a computerized weight-based UFH nomogram7,8 consisting of a 60-U/kg initial IV bolus followed by an initial continuous IV infusion rate of 12 U/kg hourly for medical cardiac indications from February 1, 2002, through November 31, 2003, were included in this study. Excluded patients were those who received UFH for less than 6 hours and without an APTT at 6 hours, those who did not provide authorization for their information to be used for research purposes, those with a documented allergy to UFH (including heparin-induced thrombocytopenia), and those treated with a fibrinolytic, glycoprotein IIb/IIIa inhibitor, or any other antithrombotic (except warfarin). All dosing data and APTT values were collected concurrent to therapy, but patients were identified and screened and bleeding frequency was assessed retrospectively. The study protocol was approved by the Mayo Clinic Institutional Review Board, which waived the need for informed consent.
Definitions
In the primary analysis, patients were divided into near-equal quartiles by body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) according to the following ranges: quartile 1 (Q1, 15.9-25.9 kg/m2), quartile 2 (Q2, 26.0-29.4 kg/m2), quartile 3 (Q3, 29.5-34.2 kg/m2), and quartile 4 (Q4, >34.2 kg/m2). In an exploratory analysis, patients were also divided into obese (BMI ≥30) and nonobese (BMI <30) groups. We chose BMI for analysis vs actual body weight to coincide with the World Health Organization definitions of obesity.9 Bleeding was prospectively defined as the occurrence of any of the following criteria from the initiation of the UFH infusion through 24 hours after the infusion ended: (1) non—operation-related transfusion of at least 1 U of packed red blood cells, (2) decrease in hemoglobin of 2 g/dL or greater within 24 hours, (3) bleeding identified by radiographic study (eg, intracranial hemorrhage, retroperitoneal hemorrhage), or (4) bleeding related to heparin therapy documented in the patient's discharge summary. Patients identified as meeting bleeding criteria were placed in mutually exclusive categories in the following priority: (1) radiographic bleeding, (2) decrease in hemoglobin concentration, (3) transfusion, and (4) medical record—documented bleeding. We noted whether the patient underwent diagnostic coronary angiography or percutaneous coronary intervention (PCI) between 24 hours before the initiation of UFH therapy through 24 hours after discontinuation of UFH therapy.
Heparin Dosing and Monitoring
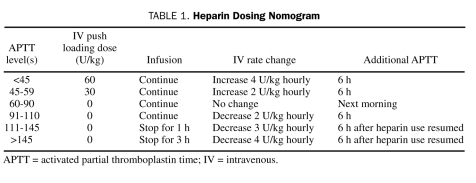
A first APTT was measured 6 hours after initiation of UFH therapy, with infusion rates adjusted per nomogram based on APTT (Table 1). A goal APTT range of 60 to 90 seconds was used for thromboplastin-adjusted target heparin levels of 0.3 to 0.7 U/mL by anti-Xa assay,4,5 with the goal range verified when the reagent lot number was changed. The APTT reagent used by our hematology laboratory during this study period was Platelin L (bioMérieux, Durham, NC). During the study, the UFH dose used in the coronary angiography suite for patients not receiving a glycoprotein IIb/IIIa inhibitor was 70 to 100 U/kg to target an activated clotting time of 250 to 300 seconds.
TABLE 1.
Heparin Dosing Nomogram
Outcomes
The primary objectives of this study were to assess the effect of BMI quartile on (1) the percentage of first APTT values within the goal range (60-90 seconds) and (2) bleeding frequency. Secondary objectives included evaluation of overall effects of BMI on mean and median first APTT and evaluation of first APTT and bleeding frequency in the obese vs nonobese groups.
Statistical Analyses
Patient baseline characteristics and bleeding outcomes were evaluated either by the Mantel-Haenszel χ2 test or by the regression analysis for the association between BMI and a continuous variable. First APTT values in the a priori defined ranges were compared among BMI quartiles with the Mantel-Haenszel χ2 test. Kruskal-Wallis overall test and pairwise Wilcoxon rank sum test were also used for comparison of UFH duration and mean APTT values among the 4 quartiles. To further explore the effect of BMI on bleeding and first APTT in the goal range, we compared BMI between groups who met these end points and those who did not meet the end points with the t test. For the end points of first APTT within each APTT range and bleeding, logistic multivariate regression analysis was used. The following factors were given a priori consideration in the multivariate analysis for first APTT within the goal range: BMI, age, sex, diabetes mellitus, tobacco use, PCI, angiogram, and indication for UFH. For bleeding, the following factors were given a priori consideration in the multivariate model: BMI, age, sex, diabetes, tobacco use, angiogram, percentage of overall APTTs greater than 110 seconds, and first APTT. Any predicting variable with P<.10 at the univariate stage was included in a full model, and a stepwise elimination process was used to find the predictors. Age, sex, and BMI were retained in the model, and age- and sex-adjusted odds ratios (ORs) for each predictor were calculated with 95% confidence intervals (CIs). In the exploratory analysis comparing obese and nonobese patients, first APTT in the goal range and bleeding outcome were assessed using the Pearson χ2 test, whereas the t test was used to compare mean APTT values. All analyses were 2-sided and considered statistically significant at P<.05. A power analysis was not performed for this study; data were collected from a convenience sample of consecutive patients treated with the computerized weight-based UFH dosing nomogram. All statistics were computed using SAS/STAT software, version 9 (SAS Institute, Cary, NC).
RESULTS
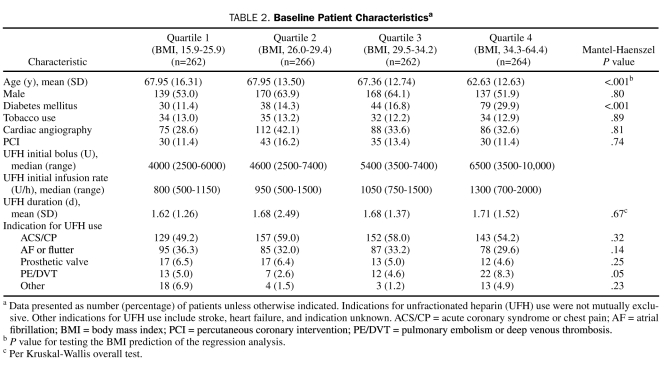
Primary Analysis
A total of 2411 patients met the inclusion criteria for this study, and 1357 patients were excluded for various reasons (750 patients received a fibrinolytic or glycoprotein IIb/IIIa inhibitor, 460 did not have an APTT at 6 hours, and 147 did not provide research authorization); thus, 1054 patients were included for analysis. Of these 1054 patients, 807 (76.6%) had an initial bolus dose higher than 4000 U, and 477 (45.3%) had an initial infusion rate higher than 1000 U/h. Significant differences were found between baseline characteristics among the BMI quartiles for many variables (Table 2), including age, presence of diabetes, and frequency of UFH for pulmonary embolism or deep venous thrombosis.
TABLE 2.
Baseline Patient Characteristicsa
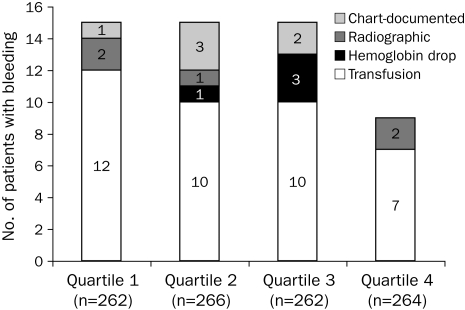
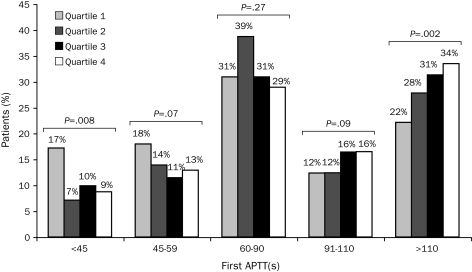
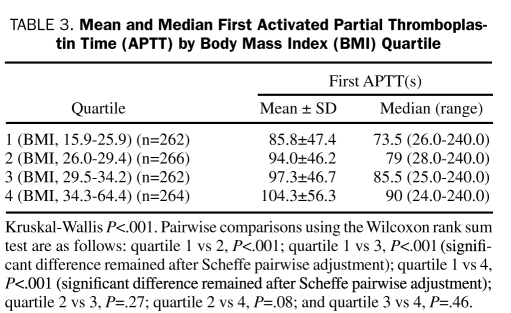
No statistically significant difference was found in bleeding frequency among the 4 quartiles (P=.26; Figure 1). Furthermore, the proportion of first APTT values within the goal range (60-90 seconds) was not significantly different among the BMI quartiles (P=.27; Figure 2). However, significant differences were found among BMI quartiles in the proportion of first APTT values of less than 45 seconds (P=.008) and greater than 110 seconds (P=.002; Figure 2). In addition, a significant difference was found in overall mean and median first APTT among BMI quartiles (P<.001 and P=.002, respectively; Table 3). The mean first APTT was significantly lower for quartile 1 compared with all other quartiles, whereas no difference was found among the other 3 quartiles (Table 3). No significant differences were found in mean BMI between patients with their first APTT in the goal range and those without (30.4 vs 30.7; P=.50) and patients who did or did not met the bleeding outcome (30.0 vs 30.6; P=.53).
FIGURE 1.
Effect of body mass index (BMI) on bleeding outcome. Over all P=.26 (Mantel-Haenszel χ2 test).
FIGURE 2.
Effect of body mass index quartile on first activated partial thromboplastin time (APTT). Significance testing per the Mantel-Haenszel χ2 test.
TABLE 3.
Mean and Median First Activated Partial Thromboplastin Time (APTT) by Body Mass Index (BMI) Quartile
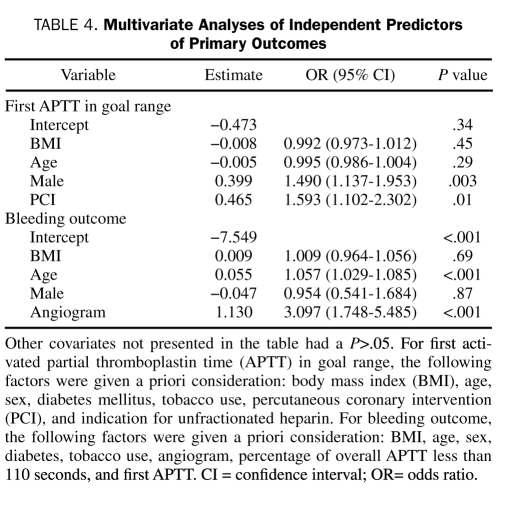
Logistic regression analysis revealed that male sex and PCI were independent predictors of first APTT within the goal range, whereas independent predictors of bleeding included age and cardiac angiography (Table 4). Lower BMI was also an independent predictor of first APTT of less than 45 seconds (OR, 0.954; 95% CI, 0.923-0.986) and first APTT of 45 to 59 seconds (OR, 0.965; 95% CI, 0.938-0.993), whereas higher BMI was an independent predictor of first APTT of more than 110 seconds (OR, 1.044; 95% CI, 1.023-1.066).
TABLE 4.
Multivariate Analyses of Independent Predictors of Primary Outcomes
Exploratory Analysis
When patients were divided into obese and nonobese groups, the results were similar. No statistically significant difference was found in bleeding frequency between the obese (4.5%) and the nonobese groups (5.7%; P=.39). The mean first APTT was higher in the obese group (100.9 vs 90.8 seconds; P=.001), but the proportion of first APTT values within the goal range was not statistically different between the groups (29.6% in the obese group vs 34.8% in the nonobese group; P=.07).
DISCUSSION
Weight-based dosing of UFH has been used for more than 15 years, centered on the landmark study by Raschke et al.1 These authors showed the benefit of dosing UFH on the basis of body weight vs conventional dosing for deep venous thrombosis, unstable angina, or arterial thromboembolism. Although Spinler et al10 compared the safety and efficacy of enoxaparin to UFH for non—ST-segment elevation acute coronary syndromes in a subgroup of obese patients from the ESSENCE (Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events) and TIMI (Thrombolysis In Myocardial Infarction) 11B trials, they did not compare the effects of UFH between obese and nonobese patients. Use of several different methods to determine the “correct” weight on which to base UFH dosing has been debated in the literature. Previous studies have calculated UFH dosing on the basis of actual body weight,6 ideal body weight,11 and even an adjusted body weight12 for obese patients, but all used a capped dose because of concern of bleeding complications.
In the current study, we evaluated both APTT values and bleeding complications for patients treated with UFH dosing on actual body weight without a capped dose. We found that both the mean and the median first APTT values were significantly different overall among BMI quartiles. Of note, however, the median first APTT was within the goal range for all quartiles. The mean first APTT was significantly lower in quartile 1 compared with the other 3 quartiles. Capped dose limitations are not intended to affect these patients and would not address the lower APTT values in this quartile. Despite significantly different mean first APTT values among quartiles, no difference was found in the proportion of first APTT values within the goal range among BMI quartiles. Moreover, BMI was not a significant predictor of first APTT value in the goal range on logistic regression analysis. However, significant differences were found in first APTT of less than 45 seconds and first APTT of greater than 110 seconds among the BMI quartiles, and BMI remained a significant independent predictor of first APTT in both these categories on multivariate analysis. This difference in first APTT, however, did not equate to a difference in bleeding among the BMI quartiles. Both Yee and Norton6 and Spruill et al3 demonstrated the utility of dosing UFH on the basis of actual body weight for obese patients, but Yee and Norton advocated a capped initial bolus of 10,000 U and capped initial infusion rate of 1500 U/h for safety reasons. Neither of these studies assessed clinical outcomes of UFH use, such as bleeding frequency.
Bleeding is the most frequent and worrisome complication of anticoagulant therapies, with clinically important bleeding during therapy developing in 5.1% of patients in the current study and ranging from 4% to 8% in the literature.13 Markers of degree of anticoagulation, such as APTT, are often used to assess both efficacy of therapy and a patient's risk of bleeding. Although subgroup analyses of large randomized trials have suggested an association between the incidence of bleeding and APTT values,14-18 data to support this association are not definitive.13 Bleeding during heparin therapy can occur regardless of the APTT value, even when the APTT is within the therapeutic range.13 Moreover, in the bleeding risk index devised by Landefeld et al,19 patient-specific comorbidities at initiation of therapy and age of the patient had higher hazard ratios for bleeding than the maximal prothrombin time or partial thromboplastin time ratio and thus were assigned a higher point weight in the model. Despite the noticeable differences in APTT values in the current study, no differences were found among BMI quartiles in bleeding frequency. Furthermore, first APTT, percentage of APTT values greater than 110 seconds, and BMI were not independent predictors of bleeding on multivariate analysis. Therefore, the APTT value alone may be an insensitive marker to predict bleeding, as reflected in the current study.
In a recently published cohort study of 101 patients, Barletta et al20 noted higher APTT values in morbidly obese patients (BMI ≥40) treated with a standardized weight-based UFH nomogram without capped initial boluses or capped initial infusion rates. On the basis of these laboratory findings, the authors suggested that a dose-capping strategy may be indicated in morbidly obese patients. However, the study may not have adequately assessed clinical sequelae associated with supratherapeutic APTT values. In fact, of the 4 patients who had a bleeding event, only 2 had a supratherapeutic APTT value, and none were morbidly obese. The current study evaluated both laboratory and clinical end points and found that, despite elevated APTT values, obese patients did not exhibit a higher incidence of bleeding.
By designing our heparin nomogram to dose UFH on the basis of actual body weight without a capped initial bolus or initial infusion rate, we placed a higher value on achieving a first APTT above the therapeutic threshold than on concern of bleeding from supratherapeutic APTT values. The risk of not achieving a therapeutic APTT value early in heparin therapy may result in clot progression and recurrence of thromboembolic events.1 Provision of a capped initial bolus and infusion rate exposes obese patients to the potential for subtherapeutic APTT values and subsequent complications.21 Our heparin nomogram was implemented before publication of the ST-segment elevation myocardial infarction and non—ST-segment elevation myocardial infarction/unstable angina guidelines4,5 and was not changed for fear of these compli-cations. Despite higher first APTT values in obese patients in the current study, we found no increased bleeding risk in these patients. Thus, we recommend initiation of UFH on the basis of actual body weight without a capped initial bolus or initial infusion rate.
The large sample size of the current study increases the applicability of the data to other institutions. In addition, all patients were treated strictly per protocol according to our heparin nomogram, which was controlled by a computer-based system requiring adherence to the nomogram. This system ensured that each APTT was ordered at the correct time, interpreted the result of the APTT, and alerted the nurse of an action to take regarding the heparin infusion (including provision of the correct calculated dose). As such, this protocol eliminated many of the common problems with UFH infusions, such as APTT values drawn at incorrect times and miscalculated infusion rates, which may lead to complications in therapy.
As with any retrospective analysis, our study has the intrinsic limitations of this study design. Additionally, we did not directly compare initiation of UFH without a capped initial bolus or capped initial infusion rate to a regimen with these limits. Moreover, we did not assess efficacy outcomes of the nomogram, such as additional thromboembolic events; however, our study was underpowered to assess these end points because of the relatively infrequent event rate of these complications. Furthermore, we were unable to determine whether some patients were exposed to UFH in the emergency department or at another institution before initiation of the heparin nomogram. Because of the large sample size, however, we expect this frequency to be nearly equal among BMI quartiles. Anticoagulants other than UFH (such as aspirin, clopidogrel, and warfarin) may have played a role in the bleeding frequency of our patients. No statistically significant differences were found among BMI quartiles in the proportion of patients prescribed UFH for acute coronary syndrome or chest pain or frequency of PCI, which may be surrogate markers for aspirin and clopidogrel use, respectively. From the available data, we cannot conclude that use of anticoagulants other than UFH was similar among BMI quartiles.
CONCLUSION
Despite a noticeable difference in APTT values, we did not demonstrate an increased risk of bleeding in obese patients treated with UFH without a capped initial dose. Our data demonstrate the safe use of weight-based UFH without a capped initial bolus or capped initial infusion rate and do not support a need for capped initial bolus doses or capped initial infusion rates in patients with medical cardiac conditions who are prescribed weight-based UFH and who are not concomitantly treated with a fibrinolytic, glycoprotein IIb/IIIa inhibitor, or any other antithrombotic (except warfarin).
Footnotes
This study was supported by departmental funding from the Department of Pharmacy Services, Mayo Clinic, Rochester, MN.
REFERENCES
- 1.Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram: a randomized controlled trial. Ann Intern Med. 1993;119(9):874-881 [DOI] [PubMed] [Google Scholar]
- 2.Schaefer DC, Hufnagle J, Williams L. Rapid heparin anticoagulation: use of a weight-based nomogram. Am Fam Physician 1996;54(8):2517-2521 [PubMed] [Google Scholar]
- 3.Spruill WJ, Wade WE, Huckaby WG, Leslie RB. Achievement of anticoagulation by using a weight-based heparin dosing protocol for obese and nonobese patients. Am J Health Syst Pharm. 2001;58(22):2143-2146 [DOI] [PubMed] [Google Scholar]
- 4.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) [published correction appears in Circulation. 2005;111(15):2013] Circulation 2004;110(5):588-636 [DOI] [PubMed] [Google Scholar]
- 5.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction). [published correction appears in Circulation. 2008;117(9):e180] Circulation 2007August14;116(7):e48-e304 Epub 2007 Aug 6 [DOI] [PubMed] [Google Scholar]
- 6.Yee WP, Norton LL. Optimal weight base for a weight-based heparin dosing protocol. Am J Health Syst Pharm. 1998;55(2):159-162 [DOI] [PubMed] [Google Scholar]
- 7.Oyen LJ, Nishimura RA, Ou NN, Armon JJ, Zhou M. Effectiveness of a computerized system for intravenous heparin administration: using information technology to improve patient care and patient safety. Am Heart Hosp J. 2005;3(2):75-81 [DOI] [PubMed] [Google Scholar]
- 8.Wilson JW, Oyen LJ, Ou NN, et al. Hospital rules-based system: the next generation of medical informatics for patient safety. Am J Health Syst Pharm. 2005;62(5):499-505 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452 [PubMed] [Google Scholar]
- 10.Spinler SA, Inverso SM, Cohen M, Goodman SG, Stringer KA, Antman EM, ESSENCE and TIMI 11B studies Safety and efficacy of unfractionated heparin versus enoxaparin in patients who are obese and patients with severe renal impairment: analysis from the ESSENCE and TIMI 11B studies. Am Heart J. 2003;146(1):33-41 [DOI] [PubMed] [Google Scholar]
- 11.Kershaw B, White RH, Mungall D, Van Houten J, Brettfeld S. Computer-assisted dosing of heparin: management with a pharmacy-based anticoagulation service. Arch Intern Med. 1994;154(9):1005-1011 [PubMed] [Google Scholar]
- 12.Holliday DM, Watling SM, Yanos J. Heparin dosing in the morbidly obese patient [letter]. Ann Pharmacother. 1994;28(9):1110-1111 [DOI] [PubMed] [Google Scholar]
- 13.Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3)(suppl):287S-310S [DOI] [PubMed] [Google Scholar]
- 14.The urokinase pulmonary embolism trial. A national cooperative study. Circulation 1973;47(2 suppl):II1-II108 [PubMed] [Google Scholar]
- 15.Anand SS, Yusuf S, Pogue J, Ginsberg JS, Hirsh J, Organization to Assess Strategies for Ischemic Syndromes (OASIS) Investigators Relationship of activated partial thromboplastin time to coronary events and bleeding in patients with acute coronary syndromes who receive heparin. Circulation 2003June17;107(23):2884-2888 Epub 2003 Jun 9 [DOI] [PubMed] [Google Scholar]
- 16.Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation 1996;93(5):870-878 [DOI] [PubMed] [Google Scholar]
- 17.Norman CS, Provan JL. Control and complications of intermittent heparin therapy. Surg Gynecol Obstet. 1977;145(3):338-342 [PubMed] [Google Scholar]
- 18.Wilson JE, III, Bynum LJ, Parkey RW. Heparin therapy in venous thromboembolism. Am J Med. 1981;70(4):808-816 [DOI] [PubMed] [Google Scholar]
- 19.Landefeld CS, McGuire E, III, Rosenblatt MW. A bleeding risk index for estimating the probability of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med. 1990;89(5):569-578 [DOI] [PubMed] [Google Scholar]
- 20.Barletta JF, DeYoung JL, McAllen K, Baker R, Pendleton K. Limitations of a standardized weight-based nomogram for heparin dosing in patients with morbid obesity. Surg Obes Relat Dis. 2008Nov–Dec;4(6):748-753 Epub 2008 Jun 30 [DOI] [PubMed] [Google Scholar]
- 21.Badawi O, Oyen LJ, Haines ST. Dosing of unfractionated heparin in acute coronary syndromes. Pharmacotherapy 2004;24(12):1681-1691 [DOI] [PubMed] [Google Scholar]