Abstract
In the past 20 years, management of primary myelofibrosis (PMF) has incorporated new treatment approaches, but survival benefits have not been confirmed in controlled studies. This retrospective study includes 176 consecutive patients younger than age 60 years in whom PMF was diagnosed during a 30-year period (1976-2005). Median age at diagnosis was 50 years (range, 18-59 years), and 98 patients (55%) were men. At the time of this report, 99 patients (56%) had died; the 77 surviving patients were followed up for a median of 8 years (range, 4-24 years). Overall median survival was 9.2 years, and 15- and 20-year survival rates were 32% and 20%, respectively. According to the Dupriez Prognostic Scoring System (PSS), median survivals were 12.7, 4.8, and 2.4 years in low- (n=117), intermediate- (n=44) and high- (n=15) risk patients (P<.001). According to the International PSS, median survivals were 13.4, 9.7, 3.3, and 2.4 years in low- (n=76), intermediate-1 (n=50), intermediate-2 (n=29), and high-risk patients (n=8; P<.001). To examine the effect of decade of diagnosis on survival, we divided study patients into 3 groups by year of diagnosis: 1976-1985 (n=36), 1986-1995 (n=45), and 1996-2005 (n=95). The corresponding median survivals were 4.8, 7.3, and “not reached” (P=.003), and the difference in survival was significant during multivariable analysis that included risk scores according to the aforementioned PSSs and age as covariates. The improvement in survival in recent years was most apparent in patients with high/intermediate-risk disease (P<.002), not in those with low-risk disease (P=.42). These observations are encouraging and suggest a salutary effect from modern therapeutic approaches in PMF.
allo-HCT = allogeneic hematopoietic cell transplant; CIC = conventional intensity conditioning; PMF = primary myelofibrosis; PSS = Prognostic Scoring System; RIC = reduced-intensity conditioning
Primary myelofibrosis (PMF) is a hematologic malignancy currently classified as a myeloproliferative neoplasm.1 Pathogenetic mechanisms in PMF include stem cell—derived clonal myeloproliferation and recurrent but nonspecific cytogenetic and molecular abnormalities.2-4 Clinical manifestations include anemia, marked hepatosplenomegaly, constitutional symptoms, cachexia, and extramedullary hematopoiesis.5 Blood and bone marrow changes include leukoerythroblastosis seen in the peripheral blood smear, bone marrow fibrosis, and osteosclerosis.5 Primary myelofibrosis should be distinguished from post—polycythemia vera myelofibrosis or post—essential thrombocythemia myelofibrosis,6 although management of all 3 is similar.
Primary myelofibrosis is associated with both short-ened survival and poor quality of life.7 Causes of death include progression to blast phase disease.8 Currently, 5 main treatment approaches are available for PMF9: (1) observation alone for asymptomatic disease without severe anemia or marked splenomegaly; (2) conventional drug therapy for either anemia (eg, androgen preparations, danazol, cortico steroids, erythropoiesis-stimulating agents, thalidomide, or lenalidomide) or splenomegaly, thrombocytosis, or leukocytosis (eg, hydroxyurea)10; (3) use of experimental drugs such as pomalidomide11 or JAK2 inhibitors12; (4) palliative surgery (eg, splenectomy) or radiation therapy for symptomatic extramedullary hematopoiesis; and (5) allogeneic hematopoietic cell transplant (allo-HCT).13
Currently, allo-HCT is the only treatment approach for PMF (or post—polycythemia vera or post—essential thrombocythemia myelofibrosis) that is potentially curative.14 The first series of reports on the value of con ventional intensity conditioning (CIC) allo-HCT for myelofibrosis appeared in the 1990s and included a seminal paper published in 1999.15 Since then, reduced-intensity conditioning (RIC) regimens have been used and promoted as being less toxic and as effective as CIC regimens.16 The overall value of both RIC and CIC allo-HCT is undermined by high incidences of treatment-related mortality and morbidity, and survival benefit has not been shown in a controlled setting.17 Similarly, the first report on the use of thalidomide for PMF appeared in 2000,18 but its value, as well as that of other new drugs for PMF,19 in terms of survival has not been systematically analyzed. Furthermore, an increasing number of patients with PMF are participating in clinical trials. Considering all these issues, it is reasonable to examine survival trends in PMF during the past few decades.
PATIENTS AND METHODS
The Mayo Clinic database of adult patients with PMF (age ≥18 years) was used to select consecutive patients younger than 60 years who were seen from 1976 through 2005 (30-year period). Permission was obtained from the Mayo Clinic Institutional Review Board to examine the medical records of all study patients. Follow-up information was updated in September 2009. Primary myelofibrosis was diagnosed according to the World Health Organization criteria after re-review of both clinical information and bone marrow histologic findings.20
Descriptive and statistically analyzed data were based on parameters collected at the time of initial diagnosis. Conventional statistical procedures were used, and all data were analyzed with StatView (SAS Institute, Cary, NC). All P values were 2-tailed, and statistical significance was set at P<.05. Kaplan-Meier survival curves were used to estimate overall and leukemia-free survival. Cox proportional hazards regression models were used to assess associations of risk factors with survival. The multivariate Cox models were designated a priori to include factors considered to have potential association with patient survival. These factors included age at diagnosis, sex, decade of diagnosis, Dupriez risk group, and International Prognostic Scoring System (PSS) risk group.
RESULTS AND DISCUSSION
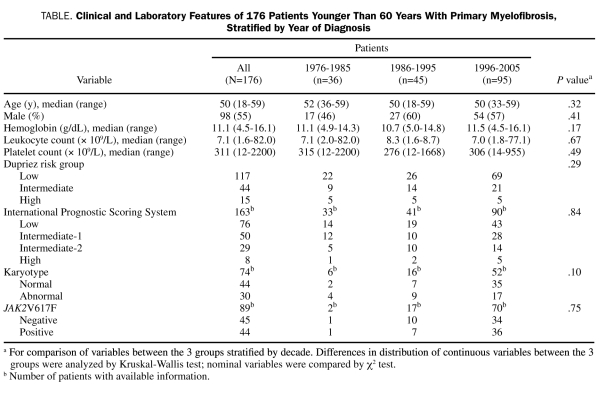
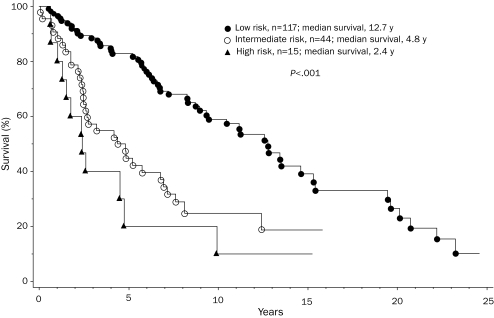
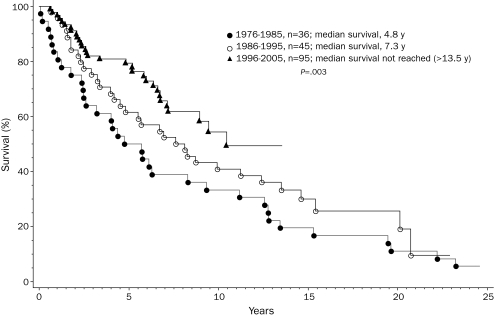
This study consists of 176 young adults (median age, 50 years; range, 18-59 years; 98 men) with World Health Organization—defined PMF. Their clinical and laboratory details are outlined in the Table. Risk distribution according to the Dupriez21 PSS was low in 117 patients (66%), intermediate in 44 (25%), and high in 15 (9%); Figure 1 shows Dupriez PSS-stratified survival data (P<.001), which confirm the representative nature of the study population. In addition, sufficient information was available for 163 patients, which allowed stratification according to the International PSS; this was even more effective in delineating prognostically different patient groups (Figure 2; P<.001).22
TABLE.
Clinical and laboratory Features of 176 Patients Younger Than 60 Years With Primary Myelofibrosis, Stratified by Year of Diagnosis
FIGURE 1.
Survival data for 176 patients younger than 60 years with primary myelofibrosis, according to their risk assignment per the Dupriez Prognostic Scoring System.21
FIGURE 2.
Survival data for 163 patients younger than 60 years with primary myelofibrosis, according to their risk assignment per the International Prognostic Scoring System.22
At the time of this report, 99 patients (56%) had died (median time to death, 4.4 years; range, 0.1-23.2 years). Overall median survival was 9.2 years, and 15- and 20-year survival rates were 32% and 20%, respectively. During the disease course, 25 patients (14%) developed blast phase disease, which was uniformly fatal; the respective incidences of blast phase disease were 22%, 13%, and 12% in patients in whom the diagnosis was made in the 3 consecutive decades from 1976 through 2005; leukemia-free survival was similar among the 3 groups (P=.68). Among the 77 surviving patients, median follow-up was 8 years (range, 4-24 years). Treatment administered to more than 10 patients included hydroxyurea in 59 patients, systemic corticosteroids in 51, erythropoiesis-stimulating agents in 30, androgen preparations in 29, thalidomide in 20, subcutaneous interferon in 16, anagrelide in 15, danazol in 11, and pirfenidone in 11. Thirty-eight patients (21%) had undergone splenectomy; 27%, 33%, and 14% in the 3 consecutive decades, respectively; these numbers should not be compared because, like leukemic transformation, the need for splenectomy is a time-dependent variable. Twelve patients had undergone allo-HCT.
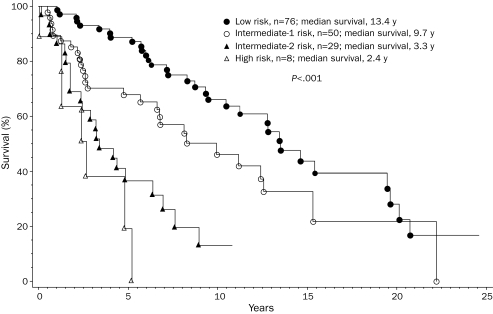
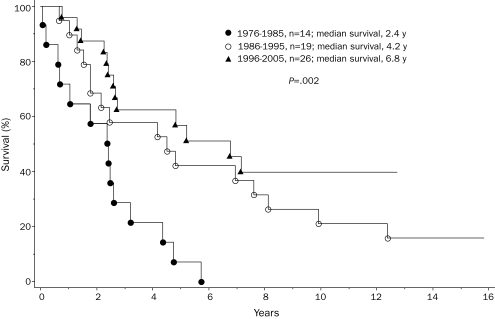
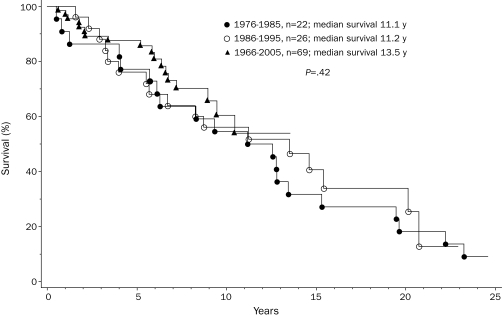
To assess possible changes in life expectancy during recent years, we stratified the study cohort by year of diagnosis into 3 groups in whom the diagnosis was made between 1976 and 1985 (n=36), 1986 and 1995 (n=45), and 1996 and 2005 (n=95). These decade-stratified patient groups were similar in age, sex, and clinical stage distributions (Table). However, they differed significantly in survival (Figure 3; P=.003). Hazard ratios (95% confidence intervals) were 2.4 (1.4-4.0) and 1.7 (1.1-2.9) when survival was compared in the first 2 decades, respectively, with those of the most recent decade. The independent effect of decade of diagnosis on survival was confirmed by multivariate analysis that included age and staging according to conventional PSSs.21,22 However, the survival benefit in the more recently diagnosed patient groups was most apparent for those with high/intermediate-risk (Figure 4; P=.002) compared with those with low-risk disease (Figure 5; P=.42). Censoring patients at time of transplant had minimal effect on the overall results. Among those diagnosed in the most recent decade, 70 patients had information on JAK2V617F mutation status; survival was similar between mutation-positive (n=36) and mutation-negative (n=34) patients (P=.12).
FIGURE 3.
Survival data for 176 patients younger than 60 years with primary myelofibrosis, stratified into 3 consecutive decades according to their year of diagnosis.
FIGURE 4.
Survival data for 59 patients younger than 60 years with intermediate/high-risk (Dupriez Prognostic Scoring System21) primary myelofibrosis, stratified into 3 consecutive decades according to their year of diagnosis.
FIGURE 5.
Survival data for 117 patients younger than 60 years with low-risk (Dupriez Prognostic Scoring System21) primary myelofibrosis, stratified into 3 consecutive decades according to their year of diagnosis.
In this study of mature survival data for 176 consecutive patients with PMF diagnosed over a 30-year period, most patients (56%) were followed to death, and the minimum follow-up for surviving patients was 4 years (maximum, 24 years). The findings suggest that high/intermediate-risk patients in whom the diagnosis was made after 1986 (ie, 1986-1995 or 1996-2005) lived longer than those in whom the diagnosis was made earlier (ie, 1976-1985). The improvement in survival was most impressive for patients in whom the diagnosis was made in the most recent decade (ie, 1996-2005), in which median survival was not reached (Figure 3). These results are somewhat different from those of another single institutional study in which survival was found to be similar among 109 patients (age range, 17-89 years) in whom the diagnosis was made before 1986 (ie, 1975-1986) or between 1987 and 1997.23 The 2 studies cannot be directly compared because of the different age groups and the comparatively smaller sample size of the informative patient group (high/intermediate-risk) in the Spanish study. One could also argue that increased access to investigational therapy might have contributed to the positive findings in the current study. However, the number of patients treated with one particular treatment modality (eg, allo-HCT or thalidomide) was not large enough to credit specific treatment approaches for the observed improvement in survival. In other words, although the results of the current study should encourage patients to participate in clinical trials, prospective randomized studies are necessary to confirm associated survival benefit and, according to the observations from the current study, historical controls cannot be used as a substitute to estimate the benefit of new treatment modalities.
Supplementary Material
REFERENCES
- 1.Vardiman JW, Brunning RD, Arber DA, et al. Introduction and overview of the classification of the myeloid neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 4 th ed.Lyon, France: International Agency for Research on Cancer; 2008:18-30 [Google Scholar]
- 2.Hussein K, Huang J, Lasho T, et al. Karyotype complements the International Prognostic Scoring System for primary myelofibrosis. Eur J Haematol. 2009;82(4):255-259 [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A. Molecular drug targets in myeloproliferative neoplasms: mutant ABL1, JAK2, MPL, KIT, PDGFRA, PDGFRB and FGFR1. J Cell Mol Med. 2009;13(2):215-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tefferi A, Pardanani A, Lim KH, et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia 2009;23(5):905-911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342(17):1255-1265 [DOI] [PubMed] [Google Scholar]
- 6.Barosi G, Mesa RA, Thiele J, et al. International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment [letter]. Leukemia 2008;22(2):437-438 [DOI] [PubMed] [Google Scholar]
- 7.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109(1):68-76 [DOI] [PubMed] [Google Scholar]
- 8.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood 2005;105(3):973-977 [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A. Essential thrombocythemia, polycythemia vera, and myelofibrosis: current management and the prospect of targeted therapy. Am J Hematol 2008;83(6):491-497 [DOI] [PubMed] [Google Scholar]
- 10.Cervantes F, Mesa R, Barosi G. New and old treatment modalities in primary myelofibrosis. Cancer J. 2007;13(6):377-383 [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Verstovsek S, Barosi G, et al. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol. 2009;27(27):4563-4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia 2008;22(1):23-30 [DOI] [PubMed] [Google Scholar]
- 13.Kerbauy DM, Gooley TA, Sale GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant 2007;13(3):355-365 [DOI] [PubMed] [Google Scholar]
- 14.Kröger N, Badbaran A, Holler E, et al. Monitoring of the JAK2-V617F mutation by highly sensitive quantitative real-time PCR after allogeneic stem cell transplantation in patients with myelofibrosis. Blood 2007;109(3):1316-1321 [DOI] [PubMed] [Google Scholar]
- 15.Guardiola P, Anderson JE, Bandini G, et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European group for blood and marrow transplantation, Société Française de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center collaborative study. Blood 1999;93(9):2831-2838 [PubMed] [Google Scholar]
- 16.Devine SM, Hoffman R, Verma A, et al. Allogeneic blood cell transplantation following reduced-intensity conditioning is effective therapy for older patients with myelofibrosis with myeloid metaplasia. Blood 2002;99(6):2255-2258 [DOI] [PubMed] [Google Scholar]
- 17.Siragusa S, Passamonti F, Cervantes F, Tefferi A. Survival in young patients with intermediate-/high-risk myelofibrosis: estimates derived from data-bases for non transplant patients. Am J Hematol 2009;84(3):140-143 [DOI] [PubMed] [Google Scholar]
- 18.Tefferi A, Elliot MA. Serious myeloproliferative reactions associated with the use of thalidomide in myelofibrosis with myeloid metaplasia [letter]. Blood 2000;96(12):4007 [PubMed] [Google Scholar]
- 19.Tefferi A, Cortes J, Verstovsek S, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood 2006;108(4):1158-1164 [DOI] [PubMed] [Google Scholar]
- 20.Thiele J, Vardiman JW, Pierre R, Brunning RD, Imbert M, Flandrin G. Chronic idiopathic myelofibrosis. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of the Haematopoietic and Lymphoid Tissues Lyon, France: International Agency for Research on Cancer (IARC) Press; 2001:35-38 [Google Scholar]
- 21.Dupriez B, Morel P, Demory JL, et al. Prognostic factors in agnogenic myeloid metaplasia: a report on 195 cases with a new scoring system. Blood 1996;88(3):1013-1018 [PubMed] [Google Scholar]
- 22.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009;113(13):2895-2901 [DOI] [PubMed] [Google Scholar]
- 23.Cervantes F, Pereira A, Esteve J, Cobo F, Rozman C, Montserrat E. The changing profile of idiopathic myelofibrosis: a comparison of the presenting features of patients diagnosed in two different decades. Eur J Haematol. 1998;60(2):101-105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.