Abstract
Coronary artery disease (CAD) is the single most common cause of death in the developed world, responsible for about 1 in every 5 deaths. The morbidity, mortality, and socioeconomic importance of this disease make timely accurate diagnosis and cost-effective management of CAD of the utmost importance. This comprehensive review of the literature highlights key elements in the diagnosis, risk stratification, and management strategies of patients with chronic CAD. Relevant articles were identified by searching the PubMed database for the following terms: chronic coronary artery disease or stable angina. Novel imaging modalities, pharmacological treatment, and invasive (percutaneous and surgical) interventions have revolutionized the current treatment of patients with chronic CAD. Medical treatment remains the cornerstone of management, but revascularization continues to play an important role. In the current economic climate and with health care reform very much on the horizon, the issue of appropriate use of revascularization is important, and the indications for revascularization, in addition to the relative benefits and risks of a percutaneous vs a surgical approach, are discussed.
BMS = bare metal stent; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCS = Canadian Cardiovascular Society; CT = computed tomography; DES = drug-eluting stent; FFR = fractional flow reserve; LAD = left anterior descending artery; LBBB = left bundle branch block; LV = left ventricular; MI = myocardial infarction; MRI = magnetic resonance imaging; OMT = optimal medical therapy; PCI = percutaneous coronary intervention; SYNTAX = Synergy Between PCI With TAXUS and Cardiac Surgery
Chronic coronary artery disease (CAD) is estimated to affect 16.8 million people in the United States; of these, 9.8 million have angina pectoris, and nearly 8 million have had a myocardial infarction (MI).1 In 2005, CAD was the single most frequent cause of death in American men and women, causing 607,000 deaths (about 1 in every 5 deaths).1 In 2006, 1.76 million patients were discharged from US hospitals with a diagnosis of CAD. The estimated direct and indirect economic cost of CAD in the United States for 2009 is $165.4 billion.1 Worldwide, cardiovascular disease is becoming pandemic as developing countries experience the epidemiologic transition described by Omran from pestilence and famine to receding pandemics and degenerative diseases.2 In 2002, out of 57 million deaths worldwide, approximately 16.7 million were due to cardiovascular disease (as compared with approximately 5 million due to tuberculosis, human immunodeficiency virus, and malaria combined), and 80% of these cardiovascular deaths were in the developing world.3 Coronary artery disease (including acute MI) is responsible for about half of these cardiovascular deaths.4 Mortality from cardiovascular disease is predicted to reach 23.4 million in 2030. Moreover, in the developing world, cardiovascular disease tends to affect people at a younger age and thus could negatively affect the workforce and economic productivity.5 The morbidity, mortality, and socioeconomic importance of CAD make its diagnosis and management fundamental for all practicing physicians.
The article provides a state-of-the-art review of the literature on chronic CAD for interested physicians; appropriate articles were identified by searching the PubMed database for the following terms: chronic coronary artery disease or stable angina. This article highlights key points in diagnosis and risk stratification and delineates evidence-based management strategies for patients with chronic CAD, with particular emphasis on the indications for revascularization and the preferred method for each patient.
DIAGNOSIS OF CHRONIC CAD
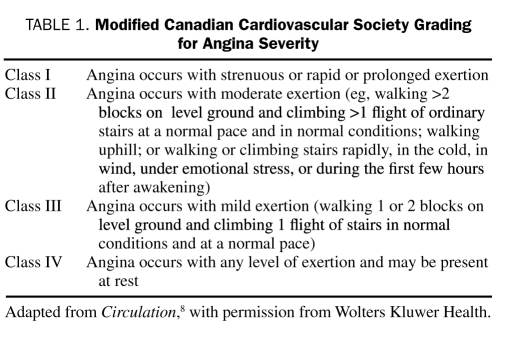
Chronic stable angina, the initial manifestation of CAD in approximately 50% of all patients,6 is usually caused by the obstruction of at least 1 large epicardial coronary artery by atheromatous plaque. Angina is due to the mismatch between myocardial oxygen demand and supply, resulting in myocardial ischemia. Angina pectoris is characterized by substernal discomfort, heaviness, or a pressure-like feeling, which may radiate to the jaw, shoulder, back, or arm and which typically lasts several minutes. These symptoms are usually brought on by exertion, emotional stress, cold, or a heavy meal and are relieved by rest or nitroglycerin within minutes. Symptoms can be classified as characteristic of typical angina, atypical angina, or noncardiac chest pain, depending on whether the chest pain characteristics meet all 3, 2, or less than 2 of the aforementioned criteria, respectively (Diamond classification7). The Canadian Cardiovascular Society (CCS)'s grading for angina severity8 has gained widespread popularity (Table 1).
TABLE 1.
Modified Canadian Cardiovascular Society Grading for Angina Severity
Anginal “equivalents,” such as epigastric discomfort, dyspnea, fatigue, or faintness, may be the dominant symptom in some patients, particularly elderly ones. Coronary artery disease may be asymptomatic or present with such complications as an acute coronary syndrome (unstable angina or MI), congestive heart failure, cardiac arrhythmias, or sudden death.
Physical examination is often unrevealing in patients with stable angina. Nonetheless, examination to check for the presence of such comorbid conditions as hypertension, tobacco stains, chronic lung disease (smoking), xanthelasma (hyperlipidemia), and evidence of noncoronary atherosclerotic disease (decreased peripheral pulses, carotid or renal artery bruits, abdominal aortic aneurysm) is essential because these findings may be important in determining the risks and benefits of a comprehensive treatment strategy and the need for additional investigations. Cardiac auscultation, particularly during an episode of chest pain, can reveal a third or fourth heart sound due to transient left ventricular (LV) dysfunction or a mitral regurgitation murmur due to papillary muscle dysfunction during myocardial ischemia. Bibasilar rales may be indicative of congestive heart failure.
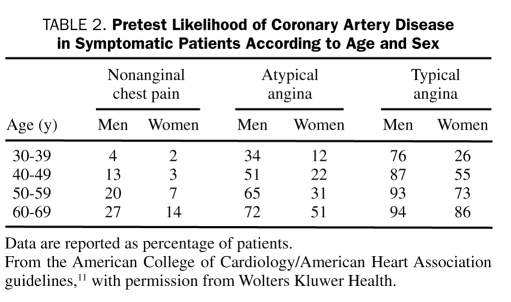
The importance of estimating the probability of substantial CAD by obtaining a detailed history and performing a risk factor assessment and focused physical examination cannot be overemphasized. Knowing the prevalence of CAD in the population helps the physician estimate pretest probability9,10 (Table 2). Risk factors, such as smoking, hypertension, diabetes, hyperlipidemia, and a family history of MI before age 60 years, increase the likelihood of CAD.12,13 Resting electrocardiography should be performed in all patients with suspected angina, although findings may be normal in approximately half of patients with stable angina, including those with severe CAD,14 particularly in the setting of preserved LV function.15 Electrocardiographic evidence of ST-T wave changes or LV hypertrophy (even though nonspecific) favor the diagnosis of angina, and prior Q wave MI on electrocardiography is highly suggestive of underlying CAD.16 Various conduction disturbances, most frequently left bundle branch block (LBBB) and left anterior fascicular block, may occur in patients with stable angina and are often associated with impairment of LV function and reflect multivessel disease or previous myocardial damage. During an episode of angina pectoris, 50% of patients with normal findings on resting electrocardiography develop electrocardiographic abnormalities, with the most common finding being ST-segment depression. However, ST-segment elevation and normalization of previous resting ST-T wave depression or inversion (pseudonormalization) may also develop.
TABLE 2.
Pretest Likelihood of Coronary Artery Disease in Symptomatic Patients According to Age and Sex
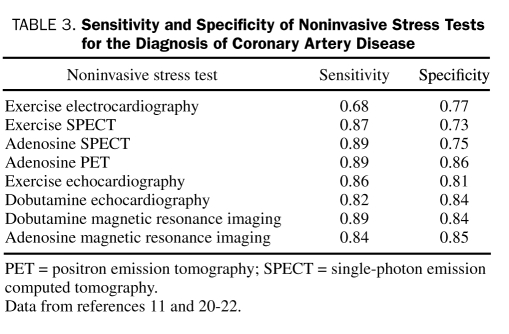
Noninvasive stress tests, although extremely helpful tools, are often underused in the United States and the United Kingdom in patients undergoing percutaneous coronary intervention (PCI)17,18; however, they may perhaps be overused in other situations.19 These tests are most useful in patients with an intermediate pretest probability of CAD because in such patients the results of the stress test, whether positive or negative, will have the greatest effect on the posttest probability (according to Bayesian principles) and consequently on clinical management. Several noninvasive stress tests are available; sensitivity and specificity of the various tests are shown in Table 3.11,20-22 These data have been obtained from small studies with catheterization laboratory referral biases with a high pretest likelihood of CAD. Exercise electrocardiography is a good initial choice in patients who can exercise and who have normal electrocardiographic findings at rest11; however, in many other situations an imaging technique is preferred. Imaging studies are recommended for patients whose findings on resting electrocardiography make the relevance of changes with stress (LBBB, ST-segment depression ≥1 mm, ventricular paced rhythm, or Wolff-Parkinson-White syndrome) difficult to assess, for patients who have had previous coronary revascularization, and for patients in whom clinical evaluation and exercise electrocardiography have provided insufficient information to guide management. A pharmacological imaging test is required if the patient is unable to exercise (due to orthopedic limitations, frailty, deconditioning, symptomatic heart failure, cardiac arrhythmia, acute pulmonary embolus, acute aortic dissection, acute myopericarditis, and possibly acute MI within the previous 48 hours). The choice between stress nuclear imaging vs stress echocardiography in many cases should depend on the local expertise of the laboratory. Adenosine or dipyridamole nuclear perfusion imaging is the preferred test for patients with LBBB or ventricular paced rhythm because of increased false-positive findings with exercise or dobutamine echocardiography. In obese patients or women with large breasts, positron emission tomography stress may be superior to conventional myocardial perfusion imaging because of its ability to perform attenuation correction. Magnetic resonance imaging (MRI) is an exciting new stress imaging technique that may be used for both adenosine perfusion and dobutamine wall motion imaging; however, it is not widely available.
TABLE 3.
Sensitivity and Specificity of Noninvasive Stress Tests for the Diagnosis of Coronary Artery Disease
The American College of Cardiology/American Heart Association guidelines11 discourage use of noninvasive testing for CAD in asymptomatic patients, except in those with evidence of possible myocardial ischemia on ambulatory electrocardiography or with severe coronary calcification on electron-beam computed tomography (CT). Screening of asymptomatic patients with type 2 diabetes does not reduce MI or death and is not recommended.23
Invasive coronary angiography may be indicated for diagnostic purposes in all patients who have survived sudden cardiac death, in patients with a high pretest probability of having left main or 3-vessel disease, and in patients who cannot undergo noninvasive testing. Other indications include patients with uncertain diagnosis on noninvasive testing, high-risk occupational requirements (eg, pilots), clinically suspected nonatherosclerotic causes of ischemia or possible vasospasm with need for provocative testing, multiple hospital admissions, or an overriding patient desire for definitive diagnosis of the presence or absence of obstructive disease.11
Risk Stratification of Patients With Chronic CAD
The major clinical and angiographic predictors of survival of patients with CAD are as follows: (1) LV function, (2) anatomic extent and severity of coronary atherosclerosis, (3) severity of ischemia, (4) tempo and severity of angina and/or the presence of recent plaque rupture, and (5) the patient's general health and noncoronary comorbid conditions. Other noncardiovascular factors that may be determinants of overall mortality, including ethnicity (south Asian), socioeconomic status, drug adherence, depression, and modification of risk factors, are not addressed in this section but nonetheless may exert a substantial contributory influence on prognosis.
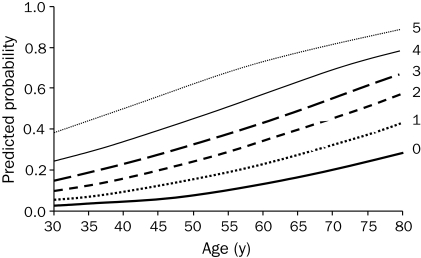
Despite a growing reliance on noninvasive or invasive testing, history and physical examination continue to be helpful in assessing the severity of CAD. Pryor et al12 identified 11 clinical characteristics—typical angina, previous MI, age, sex, duration of symptoms, hypertension, diabetes, hyperlipidemia, smoking, carotid bruit, and chest pain frequency—and formulated a model using these characteristics to accurately estimate the likelihood of severe disease in a patient. An easy to use 5-point cardiac risk score was developed by Hubbard et al24 using male sex, typical angina, history or electrocardiographic evidence of MI, diabetes, and use of insulin as risk factors for predicting severe CAD (3-vessel or left main) at different ages (Figure 1).
FIGURE 1.
Composite graph estimating the probability of severe coronary artery disease on the basis of a 5-point risk score that awards 1 point for each of the following variables: male sex, typical angina, history or electrocardiographic evidence of myocardial infarction, diabetes, and use of insulin. Each curve shows the probability of severe disease as a function of age for a given risk score.
From Arch Intern Med,24 with permission. Copyright ©1992 American Medical Association. All rights reserved.
Resting electrocardiography is helpful in risk stratification. The prognosis of patients with normal findings on electrocardiography is usually excellent because normal electrocardiographic findings imply normal LV function.15 In contrast, such abnormalities as Q waves, ST-T changes, LV hypertrophy,25 LBBB, bifascicular block, second and third degree atrioventricular block, atrial fibrillation, and ventricular arrhythmias26 are associated with a worse prognosis.
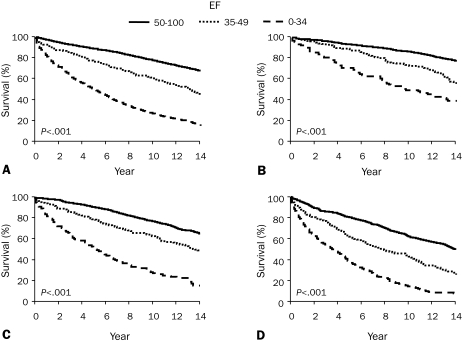
Left ventricular function is a major predictor of long-term survival in patients with CAD27 (Figure 2, A), and end-systolic LV volume was found to be the best predictor of survival after MI.28 Assessment of LV function, usually with echocardiography, is appropriate in patients with symptoms or signs of heart failure, a history of MI, or pathologic Q waves on electrocardiography.
FIGURE 2.
Survival of medically treated patients with coronary artery disease according to ejection fraction (EF) and number of diseased vessels. A, Patients with 1-, 2-, or 3-vessel disease by EF; B, patients with 1-vessel disease by EF; C, patients with 2-vessel disease by EF; and D, patients with 3-vessel disease by EF. From Circulation,27 with permission from Wolters Kluwer Health.
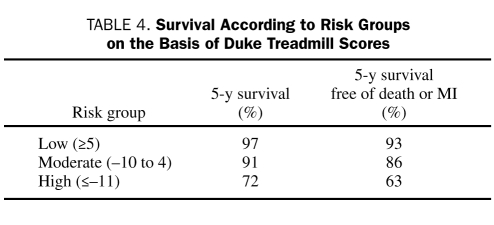
Exercise electrocardiographic testing is recommended as the first choice for all patients with an intermediate or high probability of CAD, except for those who cannot exercise or have electrocardiographic abnormalities that compromise interpretation or those for whom the information is unlikely to alter management. Risk should also be stratified for patients with known chronic CAD who have a marked change in the severity of cardiac symptoms with exercise electrocardiography. A useful tool for calculating risk is the Duke treadmill score,29 which incorporates exercise capacity, ST-segment deviation, and angina as major risk determinants. The score is calculated using the following formula: Exercise Time in Minutes — (5 × the Maximum ST-Segment Deviation in Millimeters) — (4 × the Angina Index [0, no pain; 1, angina; and 2, angina that caused discontinuation of the test]) (Table 4). Other risk factor determinants include extensive and prolonged ST-segment depression, transient ST-segment elevation, abnormal heart rate recovery, and delayed systolic blood pressure response to exercise.11
TABLE 4.
Survival According to Risk Groups on the Basis of Duke Treadmill Scores
The incremental value of imaging tests as the initial testing modality vs exercise electrocardiography is controversial,30 but they are the first choice in patients with electrocardiographic abnormalities that preclude interpretation of the exercise tracing or in patients who are taking digoxin. Imaging tests may provide additional information regarding the extent, severity, and location of myocardial jeopardy; an estimate of the extent of irreversible scar tissue; and LV function. Stress imaging studies are also indicated for assessment of the functional implications of coronary lesions in planning PCI.11 Risk stratification on the basis of the results of noninvasive stress testing is shown in Table 5.
TABLE 5.
Risk Stratification on the Basis of Noninvasive Testing
Coronary angiography, which helps stratify risk in patients on the basis of the extent and location of atherosclerosis, is indicated in patients who have high-risk criteria on noninvasive testing, patients who have angina and signs and symptoms of congestive heart failure, patients who have survived sudden cardiac arrest or serious ventricular arrhythmias, and as a first test in patients with CCS class III or IV angina despite medical therapy. Coronary angiography is acceptable for patients with CCS class I or II angina who are intolerant to medication, whose lifestyle is still impaired by these symptoms, who have LV dysfunction, or whose risk status is uncertain after noninvasive testing. A low threshold for angiographic evaluation is recommended for patients with angina who have undergone previous revascularization and in patients with a history of MI.31
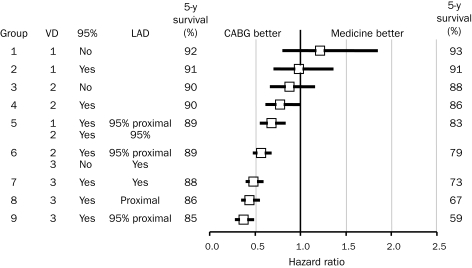
The extent and severity of coronary atherosclerotic disease and LV dysfunction identified on cardiac catheterization are the most powerful predictors of long-term outcome27,32 (Figure 2, B-D). Several prognostic indices have been used to quantify the extent of severity of CAD, but the simplest classification into 1-, 2-, or 3-vessel CAD or left main CAD is the most widely used and is effective.33 Additional risk stratification is provided by the severity of obstruction and its location, with proximal lesions predicting reduced survival rate (Figure 3).32 Quantifying the extent of coronary disease, including nonobstructive lesions, also adds to risk stratification.34
FIGURE 3.
Five-year survival rate in patients according to severity and proximity of coronary artery lesions and adjusted hazard ratios for coronary artery bypass grafting (CABG) vs medical treatment. 95% = 95% coronary artery stenosis; LAD = left anterior descending artery; VD = number of diseased vessels.
From J Thorac Cardiovasc Surg,32 with permission from Elsevier.
Cardiac Ct and Mri
Coronary artery calcium scanning with CT is a screening tool that has no role in patients with established CAD in whom the presence of coronary artery calcification is a given. Furthermore, the specificity of the coronary calcium score for obstructive coronary lesions is low.11,35 Although CT coronary angiography is showing promise for noninvasive detection of obstructive CAD in major epicardial arteries, it is still limited by a high number of false-positive results (up to 50% with severe calcification and coronary stents), specific patient selection (heart rate must be regular and <70 beats/min; patient must hold breath for 15 seconds), and high-dose radiation exposure.36,37 Magnetic resonance imaging may be used for stress perfusion or stress wall motion imaging (Table 3) as well as noninvasive coronary angiography.22,38 Most heart valve prostheses and vascular stents are compatible with MRI; however, MRI cannot be used in the presence of certain implanted metal objects or medical devices, such as pacemakers or implantable cardioverter-defibrillators.39 However, electronic rhythm management devices and other cardiovascular devices are being developed that could be compatible with MRI.
MANAGEMENT OF CHRONIC CAD
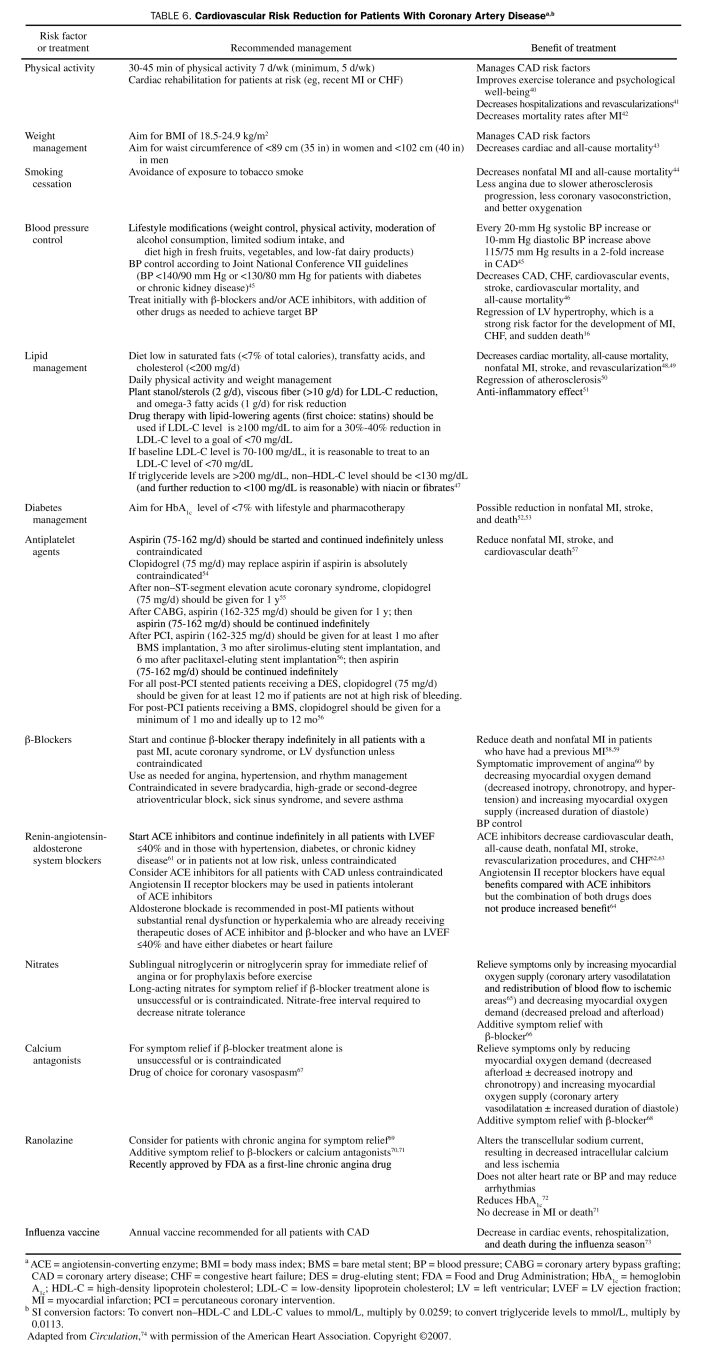
Management of CAD has 2 main goals: to reduce symptoms and ischemia and to prevent MI and death. These are modulated by different mechanisms: symptoms and ischemia, by the insufficient oxygen supply/demand ratio (usually due to coronary atherosclerosis); and MI and death, usually by unstable coronary artery plaque rupture. Medical management is pivotal in all patients with CAD. The first step is to identify and treat any associated diseases that can precipitate angina by increasing myocardial oxygen demand (such as tachycardia and hypertension) or by decreasing the amount of oxygen delivered to the myocardium (such as heart failure, pulmonary disease, or anemia). The second step is to manage CAD risk factors as well as to prevent MI with lifestyle changes and pharmacological treatment, as shown in Table 6.40-74 In 2006, despite therapeutic advances, 9.8 million patients had angina in the United States,1 and thus the beneficial effect of aggressive secondary prevention cannot be overemphasized. The recognition of the importance of optimal medical therapy (OMT) is transforming patient management, both in patients undergoing coronary revascularization and in patients treated conservatively. Optimal medical therapy remains the cornerstone of management in all patients with CAD because it is logical, relatively inexpensive, and undeniably effective in improving long-term outcomes. The challenge is to implement these measures in all patients with CAD.
TABLE 6.
Cardiovascular Risk Reduction for Patients With Coronary Artery Diseasea,b
Indications for Coronary Revascularization
The indications for coronary revascularization continue to evolve as scientific and technological advances improve both the outcomes obtained with OMT and the techniques of revascularization. A critical issue is the extent to which all forms of therapy are appropriately used on the basis of the guidelines and appropriateness criteria, particularly with regard to the costs and affordability of health care.
The benefits of coronary revascularization in reducing cardiac events and death have been widely accepted in the context of acute coronary syndromes with ST-segment elevation MI75,76 and non—ST-segment elevation MI.76,77 However, the benefits of revascularization therapy for patients with chronic stable angina in regard to the “hard” end points of death and MI are much more controversial. In patients considered at higher risk, even in the setting of chronic stable angina, coronary revascularization is generally accepted as beneficial and indeed is acknowledged to have revolutionized the treatment of CAD during the past 30 years. In 2002, the American Heart Association/American College of Cardiology guidelines for management of chronic stable angina11 recommended coronary revascularization for symptom relief in patients with refractory symptoms despite OMT or for survival benefit in patients at high clinical risk of death based on noninvasive testing (moderate to large areas of reversible ischemia with or without LV dysfunction) or on angiography (left main stem, 3-vessel, or proximal left anterior descending artery [LAD] disease). Despite the recent furor over revascularization vs medical therapy (and particularly PCI) generated primarily by the COURAGE trial78 and 2 recent meta-analyses,79,80 these basic recommendations remain logical and reasonable.76
Moreover, there is a long history of neutral trials comparing coronary revascularization vs medical therapy in lower-risk patients with chronic stable angina. Possible reasons for these neutral results include inadequate sample sizes and low event rates in this low-risk population. The earliest trials of coronary revascularization, specifically coronary artery bypass grafting (CABG), vs medical therapy in patients with chronic stable angina were conducted in the 1970s and 1980s.81-83 Despite major advances in medical therapy (especially antiplatelet and lipid-lowering therapy) and surgical techniques (including the use of the internal mammary artery), the overall conclusions from these trials and associated registry studies remain valid today. Symptomatic relief was better with CABG; however, no overall difference was observed in survival or freedom from MI with CABG vs medical therapy, except in patients considered at higher risk on the basis of left main disease, multivessel disease plus LV dysfunction, severe angina, and probably proximal LAD disease in conjunction with multivessel disease.84-86 Revascularization also seems to offer a survival benefit to patients with postinfarction angina. The concept of the greater the risk, the greater the benefit is illustrated in Figure 3, which is based on data from the Duke University database, a large single-center registry study.32
The next series of trials performed in the 1990s and 2000s compared revascularization, specifically percutaneous balloon angioplasty, with medical therapy in patients with stable CAD. The most important information taken from these studies87-91 was that balloon angioplasty was associated with further symptomatic relief compared with medical therapy alone but had no significant effect on the hard end points of MI and death, even though cross-over from medical therapy to revascularization was frequent (up to 50%). Subsequent trials comparing medical therapy vs PCI with stenting were again neutral, and a recent meta-analysis summarizing 20 years of trials of PCI in patients with nonacute coronary artery disease found no evidence of any benefit on death or MI with PCI vs medical therapy.80 The only 2 studies showing benefit of revascularization on death or MI, the Asymptomatic Cardiac Ischemia Pilot study92 and the SWISSI-2 (Swiss Interventional Study on Silent Ischemia Type II) trial,93 included patients with “silent ischemia” who had a prior MI in addition to left main CAD and LV dysfunction. Another recent meta-analysis94 demonstrated a benefit for PCI on mortality, but this analysis had a number of flaws, the most important of which was inclusion of post-MI patients. For current practice, the 2 most relevant trials of whether patients with stable CAD will have better outcomes when treated with OMT and revascularization vs OMT alone are the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial78 and the BARI-2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes) trial.95
The COURAGE trial enrolled 2287 patients with greater than 70% coronary stenosis in at least 1 proximal epicardial coronary artery and evidence of myocardial ischemia on stress testing or resting electrocardiography or patients with at least 1 coronary artery stenosis of at least 80% and classic angina without provocative testing. For the primary outcome, a composite of death and nonfatal MI, no statistical difference was found between the 2 groups after a mean follow-up of 4.6 years. Rates of angina were consistently lower in the PCI group than in the medical therapy group during follow-up but were no longer statistically significant at 5 years. Rates of subsequent revascularization were likewise lower in the PCI group. The recently published BARI-2D trial,95 which evaluated 2368 patients with type 2 diabetes and CAD, 82% of whom had mild to moderate stable angina and 18% of whom had positive findings on a stress test, once again confirmed that there was no significant difference in the rate of survival between patients undergoing prompt revascularization (PCI or CABG) and those undergoing OMT. However, patients with diabetes who underwent CABG (but not PCI) vs OMT alone had significantly fewer major cardiac events, driven mainly by a reduction in nonfatal MI. Percutaneous coronary intervention may provide no benefit in reducing death and nonfatal MI because it is directed at culprit lesions that cause symptoms and/or ischemia. However, good evidence shows that progression of disease and subsequent coronary events may occur as a result of plaque rupture at sites that were considered non-obstructive stenoses on initial angiography, pointing to our current inability to predict “future” culprits.34 In contrast, it is becoming increasingly apparent that OMT, including aggressive control of risk factors and lifestyle modification, may greatly affect endothelial function and plaque stability and as such could reduce the incidence of future coronary events. Secondary prevention is currently an essential component of management, whether by revascularization or pharmacological treatment. These trials make the point that, in a selected subset of patients with stable CAD at low to moderate risk who underwent angiography before randomization, an initial trial of medical therapy is reasonable with the option of proceeding to revascularization if symptoms and quality of life do not improve with medical therapy alone. In general, these results support the guidelines and raise questions about the appropriate use of coronary revascularization, in particular PCI.
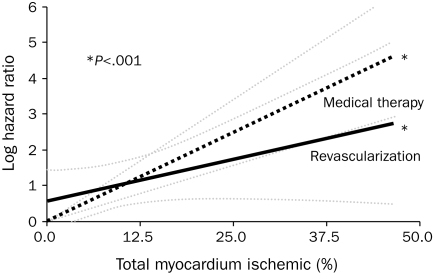
A relatively small nuclear perfusion substudy96 from COURAGE reinforces the logical precept that the extent of ischemia is an important determinant of long-term outcomes97-99 (Figure 4). This substudy showed that, in patients with ischemia on nuclear perfusion, the percent reduction in ischemia with PCI was superior to that with OMT and that patients with less ischemia had lower rates of death or MI. Ischemia may be used as an indicator for a more aggressive approach in such patients; however, this does not negate the overall results and conclusions from the COURAGE trial. Although data suggest that revascularization of viable myocardium in patients with LV dysfunction and symptoms of congestive heart failure may improve survival,100 this benefit has not yet been definitively proven and awaits the results of the ongoing STICH (Surgical Treatment for Ischemic Heart Failure) trial.101
FIGURE 4.
Revascularization vs medical therapy as a function of percentage of ischemic myocardium.
From Circulation,99 with permission from Wolters Kluwer Health.
Not all apparently significant stenoses on visual inspection are hemodynamically relevant, and a recent small trial showed the benefits of targeted revascularization on the basis of an objective measurement of hemodynamic severity.102 In this trial, fractional flow reserve (FFR)—guided (<0.80) PCI was superior to angiography-guided PCI for increasing survival free of MI or revascularization. Conversely, PCI in patients with stenotic lesions but a normal FFR (≥0.75) was associated with worse event-free survival.103 In summary, in the absence of symptoms or ischemia, revascularization is not indicated because lesions that may be future “culprits” in regard to subsequent MI or death cannot be identified at this time using current methodologies. The search for the location of future plaque ruptures or erosions leading to MI (so-called vulnerable plaques) is an important area of cardiovascular research and could potentially change drastically how CAD is diagnosed and treated.
A valid question arising from the COURAGE and other trials is whether current use of coronary revascularization, and in particular PCI, is appropriate or excessive. Only 44.5% of patients have noninvasive stress testing before PCI in the United States, with a substantial variation according to geography (22%-71%).17 These numbers are remarkably similar to those in the United Kingdom, where 43% of patients have stress testing before PCI.18 Furthermore, revascularization rates also vary widely, with an 83% higher rate in Florida than in Oregon. Revascularization rates depend on race (28% variation) and cardiac catheterization rates (68% variation), which in turn depend on hospital admission rates for CAD as well as the number of cardiac surgeons and interventionalists in the local population.104 Inappropriate use of PCI may be as high as 43% in stable CAD105; however, underuse of coronary angiography (57%-71%), PCI (34%), and CABG (26%) is also common in clinically indicated cases, and the rate of coronary events is higher in patients who have not undergone revascularization despite clinical indication. 106,107 In the United Kingdom, coronary angiography is underused in older people, women, south Asians, and people from deprived areas.108 Appropriateness of CABG in northern New England in 2008 was 87.7%.109
Preferred Method of Revascularization
Two methods of revascularization are well established for CAD: CABG, introduced in 1968, and PCI. Percutaneous coronary intervention includes percutaneous balloon angioplasty, introduced in 1977, and stenting with bare metal stents (BMSs), in use since 1995, or drug-eluting stents (DESs), in use since 2003. Of an estimated 1.3 million patients undergoing PCI procedures in the United States in 2006, 91% had a stent inserted (>70% of which were DES stents).1 In 2006, 253,000 patients underwent CABG in the United States. The mean cost was $48,000 for PCI and $100,000 for CABG.1 In a population-based observational study from Olmsted County, MN, revascularization use increased by 24% from 1990 to 2004, but the trends diverged by procedure type, with a sustained increase for PCI (69%) vs a stabilization and then a decline for CABG (−33%).110
During the past 30 years, multiple trials have attempted to find the preferred method of revascularization for patients with stable CAD. Trials have compared balloon angioplasty vs BMS, BMS vs DES, balloon angioplasty vs CABG, and BMS vs CABG. In all these trials, no difference in the incidence of death or nonfatal MI was observed between these methods of revascularization.80,111 Bare-metal stents decreased the rate of restenosis and the need for an additional PCI compared with balloon angioplasty.112 Drug-eluting stents further decreased the rate of in-stent restenosis and need for target lesion revascularization by 30% to 70% compared with BMS but did not improve survival or decrease the risk of MI up to 4 years after implantation.113 Rates of early (≤1 month) and late (>1 month to <1 year) stent thrombosis did not differ significantly between BMS and DES, but a slight increase in very late (>1 year) stent thrombosis (which is not associated with increased mortality or MI compared with BMS) was noted with DES.114
Patients with multivessel disease who undergo CABG have been shown to require less additional revascularization than those undergoing PCI111,115; however, no survival advantage has been demonstrated, except for diabetic patients in the BARI trial.116 Recent meta-analyses have had conflicting conclusions regarding this survival advantage in diabetic patients.111,115 Whether this survival difference in diabetic patients in favor of CABG still applies in the current era will depend to some extent on the long-term follow-up of the SYNTAX (Synergy Between PCI With TAXUS and Cardiac Surgery) trial and the results of the ongoing National Heart, Lung, and Blood Institute—sponsored FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial. Meta-analysis of the 4 most important trials of PCI with BMS vs CABG (ERACI-II [Argentine Randomized Trial of Percutaneous Transluminal Coronary Angioplasty Versus Coronary Artery Bypass Surgery in Multivessel Disease],117 Stent or Surgery trial,118 ARTS [Arterial Revascularization Therapy Study],119 and MASS-II [Medicine, Angioplasty, or Surgery Study-II] trial120) once again showed similar long-term safety profiles but increased need for revascularization in the BMS vs CABG group.121 The large New York state registry of 60,000 patients illustrated a selection bias toward CABG for patients at higher risk, with no difference in unadjusted survival between CABG and stenting, but a significant difference when the outcomes were adjusted for risk factors in patients with 2-vessel disease with proximal LAD stenosis.122 These findings highlight the role of clinical judgment in selecting optimal therapy for the individual patient. We must be cognizant that study trials may have entry and/or selection bias, resulting in skewing of results because of inherent differences between patients who are enrolled in studies and the general population of patients.123
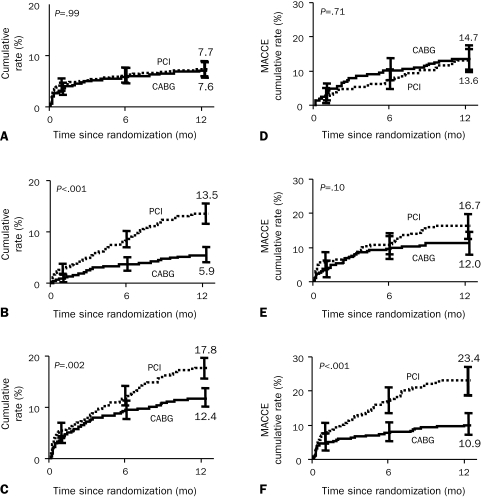
Results were recently published from the SYNTAX trial, in which patients with previously untreated left main stem or 3-vessel disease were randomly assigned to undergo state-of-the-art CABG or PCI with DES.124 At follow-up 12 months after the intervention, no difference in the composite end point of death, MI, or stroke was noted between the 2 groups, but the patients in the PCI group needed additional revascularization more often than did the CABG group (Figure 5, A-C). The rate of stroke was higher in the CABG group, perhaps because of the decreased use of antiplatelet agents. Furthermore, the investigators used an angiographic grading tool (the SYNTAX score) to determine the complexity of CAD. The SYNTAX score is the sum of the points assigned to each lesion identified in the 16 segments of the coronary tree with greater than 50% diameter narrowing in vessels with a diameter greater than 1.5 mm. The SYNTAX score may help identify patients at low or intermediate risk who may be appropriately treated with PCI with at least equivalent outcomes as CABG. In contrast, patients determined to be at high risk on the basis of their SYNTAX score were shown to have fewer major adverse cardiac and cerebrovascular events when assigned to CABG rather than PCI (Figure 5, D-F). The final verdict in regard to the SYNTAX trial awaits the long-term (5-year) follow-up data.
FIGURE 5.
Patient outcomes in the SYNTAX (Synergy Between PCI With TAXUS and Cardiac Surgery) trial according to treatment group and SYNTAX score. A, Death from any cause, stroke, or myocardial infarction; B, follow-up revascularization; C, major adverse cardiac or cerebrovascular event (MACCE); D, low SYNTAX score; E, intermediate SYNTAX score; and F, high SYNTAX score. CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention.
From N Engl J Med,124 with permission. Copyright ©2009 Massachusetts Medical Society. All rights reserved.
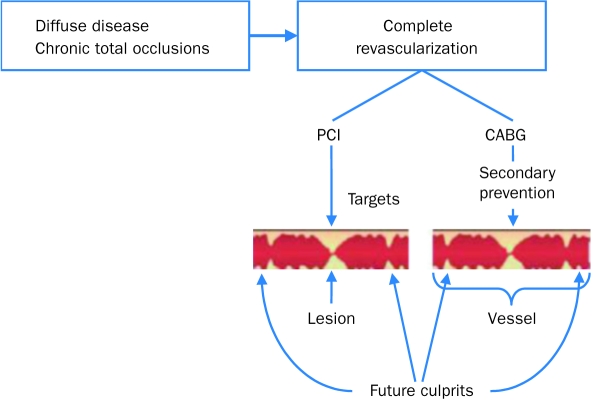
Thus, the method of choice for revascularization depends on the angiographic characteristics of the lesions causing ischemia, LV dysfunction, comorbid conditions and suitability of the patient for surgery, likelihood of technical success with PCI, quality-of-life expectations, and patient preference. The advantages of PCI include lesser invasiveness, no need for general anesthesia, less postprocedural morbidity, and a shorter hospital stay. Coronary artery bypass grafting has the advantages not only of bypassing chronic occlusions and complex stenoses but also of achieving complete revascularization by bypassing not only “culprit lesions” but “future culprits,” making fewer additional revascularizations necessary (Figure 6).125 However, CABG is associated with increased postoperative morbidity, including stroke, a longer period of hospitalization, and a slower return to normal activities (up to 6 weeks). In current practice, CABG is often the preferred method of revascularization in patients with high-risk left main, 3-vessel, or 2-vessel disease with substantial (especially proximal) LAD involvement and LV dysfunction, particularly in patients with diabetes.76,126 For some patients with left main disease, particularly osteal lesions, increasing data suggest that PCI may be an alternative to CABG.127 Coronary artery bypass grafting is often preferred in patients with chronic total occlusions and multiple complex (class C) lesions.126 Most patients requiring revascularization who have lesions amenable to PCI and do not fulfill the above criteria undergo PCI rather than CABG. When PCI is the chosen method of revascularization, DESs are usually preferred because of the decreased rate of in-stent restenosis and need for target lesion revascularization compared with BMS. However, in patients in whom long-term dual platelet therapy may be problematic (because of bleeding or financial issues), BMS may be the stent of choice. The relative indications for PCI vs CABG are part of a changing landscape, as technologies of PCI and CABG continue to evolve.
FIGURE 6.
Revascularization of future culprit lesions with coronary artery bypass grafting (CABG). PCI = percutaneous coronary intervention.
From Lancet,125 with permission from Elsevier. Copyright ©2006.
Alternative Therapies for Refractory Angina
Some patients have CAD with intractable angina despite maximum medical therapy and may not be candidates for revascularization. One option for these patients is spinal cord stimulation, in which an electrode is inserted into the epidural space at the C7-T1 level. This electrode stimulates axons in the spinal cord that do not transmit pain so as to reduce input to the brain of axons that do transmit pain (gate theory). Spinal cord stimulation has been shown to decrease angina frequency by up to 80%, decrease CCS score, and improve quality of life.128 One study showed spinal cord stimulation to be noninferior to CABG for quality of life and survival after 5 years of follow-up in patients with refractory angina.129 Additional trials are needed.
Another technique used to treat patients with refractory angina is enhanced external counterpulsation. This technique involves the use of a series of cuffs wrapped around the patient's legs, which are inflated with compressed air in sequence (distally to proximally) during diastole so as to propel blood back to the heart. Enhanced external counterpulsation is administered as 35 one-hour treatment sessions over the course of 7 weeks. The proposed mechanisms of action are reduced myocardial demand, improved myocardial perfusion, and improved endothelial function.130 This technique also reduces the frequency of angina and/or the CCS class in up to 80% of patients, extends time to exercise-induced ischemia, improves quality of life in patients with symptomatic CAD, and is generally well tolerated.131
Another technique that has been used for refractory angina is transmyocardial laser revascularization, which creates small channels from the epicardial to endocardial surfaces of the heart with a laser using a surgical approach. The mechanism of action of laser therapy is incompletely understood, and multiple randomized trials have failed to demonstrate an increase in survival. The lack of survival benefit for transmyocardial laser revascularization highlights the important role of the placebo effect in reducing angina with this now rarely used technique.132
Intramyocardial bone marrow stem cell injection is currently being investigated as a new therapeutic option for patients with chronic ischemia who are ineligible for revascularization. Bone marrow mononuclear CD34+ stem cells, harvested from the iliac crest or by leukapheresis after granulocyte colony—stimulating factor, are injected into the ischemic myocardium.133,134 In a small randomized placebo-controlled study, myocardial injection was found to be safe and to be associated with a modest but statistically significant improvement in myocardial perfusion, LVEF, exercise capacity, and CCS class. This technique is still in the experimental stages, and further studies are required to assess long-term results and efficacy for reducing mortality and morbidity.
CONCLUSION
All patients with stable CAD require comprehensive and aggressive control of risk factors. An initial trial of medical therapy alone is appropriate in most patients with chronic stable angina and is the cornerstone of treatment for chronic CAD. Persistent symptoms, the magnitude of the ischemic burden, or drug intolerance should drive decision making regarding subsequent revascularization. Ischemia should be established with a noninvasive stress test before angiography. In patients undergoing angiography without a previous noninvasive stress test, FFR may be used to make appropriate decisions regarding revascularization, but the technique requires experience and is not widely used in many catheterization laboratories. Revascularization with PCI or CABG is a very effective treatment for CAD, but only when performed on targeted culprit stenoses that are hemodynamically relevant or causing ischemia. The method of choice for revascularization depends on the angiographic characteristics of the lesions causing ischemia, LV dysfunction, comorbid conditions, suitability of the patient for surgery, and likelihood of technical success. The physician's ultimate decisions regarding patient care must incorporate current evidence-based medicine as well as the patient's preferences and quality-of-life expectations.
Supplementary Material
On completion of this article, you should be able to: (1) integrate the information obtained from a history, physical examination, and a stress test to diagnose and stratify the risk of patients with chronic coronary artery disease; (2) apply evidence-based management strategies to improve survival in patients with chronic coronary artery disease; and (3) determine when revascularization is indicated in a patient with chronic coronary artery disease, and, if indicated, choose the preferred method for each patient.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee [published correction appears in Circulation. 2009;119(3):e182] Circulation 2009;119(3):480-486 [DOI] [PubMed] [Google Scholar]
- 2.Omran AR. Changing patterns of health and disease during the process of national development. In: Albrecht GL, Higgins PC, eds. Health, Illness and Medicine: A Reader in Medical Sociology Chicago, IL: Rand McNally; 1979. [Google Scholar]
- 3.Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350(24):2438-2440 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Preventing chronic disease: a vital investment: WHO global report Geneva, Switzerland: World Health Organization; 2005. http://www.who.int/chp/chronic_disease_report/en/ Accessed September 17, 2009 [Google Scholar]
- 5.Leeder SR, Raymond S, Greenberg H. A Race Against Time: The Challenge of Cardiovascular Disease in Developing Economies Sidney, Australia: Earth Institute at Columbia University; 2004. http://www.earth.columbia.edu/news/2004/images/raceagainsttime_FINAL_051104.pdf Accessed September 17, 2009 [Google Scholar]
- 6.Kannel WB, Feinleib M. Natural history of angina pectoris in the Framingham study: prognosis and survival. Am J Cardiol. 1972;29(2):154-163 [DOI] [PubMed] [Google Scholar]
- 7.Diamond GA. A clinically relevant classification of chest discomfort [letter]. J Am Coll Cardiol. 1983;1(2, pt 1):574-575 [DOI] [PubMed] [Google Scholar]
- 8.Campeau L. Grading of angina pectoris [letter]. Circulation 1976;54(3):522-523 [PubMed] [Google Scholar]
- 9.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350-1358 [DOI] [PubMed] [Google Scholar]
- 10.Chaitman BR, Bourassa MG, Davis K, et al. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation 1981;64(2):360-367 [DOI] [PubMed] [Google Scholar]
- 11.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 Guideline update for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for the Management of Patients With Chronic Stable Angina) http://www.acc.org/qualityandscience/clinical/guidelines/stable/stable_clean.pdf. http://www.acc.org/qualityandscience/clinical/guidelines/stable/stable_clean.pdf Accessed September 17,2009. [DOI] [PubMed]
- 12.Pryor DB, Shaw L, Harrell FE, Jr, et al. Estimating the likelihood of severe coronary artery disease. Am J Med. 1991;90(5):553-562 [PubMed] [Google Scholar]
- 13.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score [published correction appears in JAMA. 2007;297(13):1433] JAMA 2007;297(6):611-619 [DOI] [PubMed] [Google Scholar]
- 14.Connolly DC, Elveback LR, Oxman HA. Coronary heart disease in residents of Rochester, Minnesota: IV, Prognostic value of the resting electrocardiogram at the time of initial diagnosis of angina pectoris. Mayo Clin Proc. 1984;59(4):247-250 [DOI] [PubMed] [Google Scholar]
- 15.Rihal CS, Davis KB, Kennedy JW, Gersh BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75(4):220-223 [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation 1994;90(4):1786-1793 [DOI] [PubMed] [Google Scholar]
- 17.Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA 2008;300(15):1765-1773 [DOI] [PubMed] [Google Scholar]
- 18.Fox KA. COURAGE to change practice? Revascularisation in patients with stable coronary artery disease [letter]. Heart 2009;95(9):689-692 Epub 2009 Feb 23 [DOI] [PubMed] [Google Scholar]
- 19.Gibbons RJ, Miller TD, Hodge D, et al. Application of appropriateness criteria to stress single-photon emission computed tomography sestamibi studies and stress echocardiograms in an academic medical center. J Am Coll Cardiol. 2008;51(13):1283-1289 [DOI] [PubMed] [Google Scholar]
- 20.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol. 2003;42(7):1318-1333 [DOI] [PubMed] [Google Scholar]
- 21.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003;108(9):1146-1162 [DOI] [PubMed] [Google Scholar]
- 22.Schuijf JD, Poldermans D, Shaw LJ, et al. Diagnostic and prognostic value of non-invasive imaging in known or suspected coronary artery disease. Eur J Nucl Med Mol Imaging 2006;33(1):93-104 [DOI] [PubMed] [Google Scholar]
- 23.Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301(15):1547-1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard BL, Gibbons RJ, Lapeyre AC, III, Zinsmeister AR, Clements IP. Identification of severe coronary artery disease using simple clinical parameters. Arch Intern Med. 1992;152(2):309-312 [PubMed] [Google Scholar]
- 25.Frank CW, Weinblatt E, Shapiro S. Angina pectoris in men: prognostic significance of selected medical factors. Circulation 1973;47(3):509-517 [DOI] [PubMed] [Google Scholar]
- 26.Ruberman W, Weinblatt E, Goldberg JD, Frank CW, Shapiro S, Chaudhary BS. Ventricular premature complexes in prognosis of angina. Circulation 1980;61(6):1172-1182 [DOI] [PubMed] [Google Scholar]
- 27.Emond M, Mock MB, Davis KB, et al. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation 1994;90(6):2645-2657 [DOI] [PubMed] [Google Scholar]
- 28.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987;76(1):44-51 [DOI] [PubMed] [Google Scholar]
- 29.Mark DB, Hlatky MA, Harrell FE, Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793-800 [DOI] [PubMed] [Google Scholar]
- 30.Ladenheim ML, Kotler TS, Pollock BH, Berman DS, Diamond GA. Incremental prognostic power of clinical history, exercise electrocardiography and myocardial perfusion scintigraphy in suspected coronary artery disease. Am J Cardiol. 1987;59(4):270-277 [DOI] [PubMed] [Google Scholar]
- 31.Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography). Circulation 1999;99(17):2345-2357 [DOI] [PubMed] [Google Scholar]
- 32.Jones RH, Kesler K, Phillips HR, III, et al. Long-term survival benefits of coronary artery bypass grafting and percutaneous transluminal angioplasty in patients with coronary artery disease. J Thorac Cardiovasc Surg. 1996;111(5):1013-1025 [DOI] [PubMed] [Google Scholar]
- 33.Gersh BJ, Califf RM, Loop FD, Akins CW, Pryor DB, Takaro TC. Coronary bypass surgery in chronic stable angina. Circulation 1989;79(6, pt 2):I46-I59 [PubMed] [Google Scholar]
- 34.Bigi R, Cortigiani L, Colombo P, Desideri A, Bax JJ, Parodi O. Prognostic and clinical correlates of angiographically diffuse non-obstructive coronary lesions. Heart 2003;89(9):1009-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006;114(16):1761-1791 Epub 2006 Oct 2 [DOI] [PubMed] [Google Scholar]
- 36.Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009;119(7):1056-1065 Epub 2009 Feb 2 [DOI] [PubMed] [Google Scholar]
- 37.Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(9):849-857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuster V, Kim RJ. Frontiers in cardiovascular magnetic resonance. Circulation 2005;112(1):135-144 [DOI] [PubMed] [Google Scholar]
- 39.Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention. Circulation 2007;116(24):2878-2891 Epub 2007 Nov 19 [DOI] [PubMed] [Google Scholar]
- 40.Thompson PD, Buchner D, Piña IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003;107(24):3109-3116 [DOI] [PubMed] [Google Scholar]
- 41.Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004;109(11):1371-1378 Epub 2004 Mar 8 [DOI] [PubMed] [Google Scholar]
- 42.O'Connor GT, Buring JE, Yusuf S, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989;80(2):234-244 [DOI] [PubMed] [Google Scholar]
- 43.Kennedy LM, Dickstein K, Anker SD, et al. Weight-change as a prognostic marker in 12 550 patients following acute myocardial infarction or with stable coronary artery disease. Eur Heart J. 2006;27(23):2755-2762 Epub 2006 Aug 4 [DOI] [PubMed] [Google Scholar]
- 44.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003;290(1):86-97 [DOI] [PubMed] [Google Scholar]
- 45.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42(6):1206-1252 Epub 2003 Dec 1 [DOI] [PubMed] [Google Scholar]
- 46.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 2003;289(19):2534-2544 [DOI] [PubMed] [Google Scholar]
- 47.Grundy SM, Cleeman JI, Merz CN, et al. Coordinating Committee of the National Cholesterol Education Program Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines [published correction appears in 2004;110(6):763] Circulation 2004;110(2):227-239 [DOI] [PubMed] [Google Scholar]
- 48.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):7-22 [DOI] [PubMed] [Google Scholar]
- 49.LaRosa JC, Grundy SM, Waters DD, et al. Treating to New Targets (TNT) Investigators Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-1435 [DOI] [PubMed] [Google Scholar]
- 50.Nissen SE, Nicholls SJ, Sipahi I, et al. ASTEROID Investigators Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 2006;295(13):1556-1565 Epub 2006 Mar 13 [DOI] [PubMed] [Google Scholar]
- 51.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207 Epub 2008 Nov 9 [DOI] [PubMed] [Google Scholar]
- 52.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive Investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366(9493):1279-1289 [DOI] [PubMed] [Google Scholar]
- 54.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348(9038):1329-1339 [DOI] [PubMed] [Google Scholar]
- 55.Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation [published corrections appear in N Engl J Med. 2001;345(23):1716 and 2001;345(20):1506] N Engl J Med. 2001;345(7):494-502 [DOI] [PubMed] [Google Scholar]
- 56.2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee 2007 Focused update of the ACC/AHA/SCAI 2005 Guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2008;117(6):e161] Circulation 2008;117(2):261-295 Epub 2007 Dec 13 [DOI] [PubMed] [Google Scholar]
- 57.Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients [published correction appears in BMJ. 2002;324(7330):141] BMJ 2002;324(7329):71-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.β-Blocker Heart Attack Trial Research Group A randomized trial of propranolol in patients with acute myocardial infarction: I, mortality results. JAMA 1982;247(12):1707-1714 [DOI] [PubMed] [Google Scholar]
- 59.β-Blocker Heart Attack Trial Research Group A randomized trial of propranolol in patients with acute myocardial infarction: II, morbidity results. JAMA 1983;250(20):2814-2819 [DOI] [PubMed] [Google Scholar]
- 60.Quyyumi AA, Crake T, Wright CM, Mockus LJ, Fox KM. Medical treatment of patients with severe exertional and rest angina: double blind comparison of β blocker, calcium antagonist, and nitrate. Br Heart J. 1987;57(6):505-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solomon SD, Rice MM, K AJ, et al. Prevention of Events with ACE inhibition (PEACE) Investigators Renal function and effectiveness of angiotensin-converting enzyme inhibitor therapy in patients with chronic stable coronary disease in the Prevention of Events with ACE inhibition (PEACE) trial. Circulation 2006;114(1):26-31 Epub 2006 Jun 26 [DOI] [PubMed] [Google Scholar]
- 62.Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients [published corrections appear in N Engl J Med. 2000;342(18):1376 and 2000;9;342(10):748] N Engl J Med. 2000;342(3):145-153 [DOI] [PubMed] [Google Scholar]
- 63.EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362(9386):782-788 [DOI] [PubMed] [Google Scholar]
- 64.ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547-1559 Epub 2008 Mar 31 [DOI] [PubMed] [Google Scholar]
- 65.Tadamura E, Mamede M, Kubo S, et al. The effect of nitroglycerin on myocardial blood flow in various segments characterized by rest-redistribution thallium SPECT. J Nucl Med. 2003;44(5):745-751 [PubMed] [Google Scholar]
- 66.Akhras F, Jackson G. Efficacy of nifedipine and isosorbide mononitrate in combination with atenolol in stable angina. Lancet 1991;338(8774):1036-1039 [DOI] [PubMed] [Google Scholar]
- 67.Chahine RA, Feldman RL, Giles TD, et al. Amlodipine Study 160 Group Randomized placebo-controlled trial of amlodipine in vasospastic angina. J Am Coll Cardiol. 1993;21(6):1365-1370 [DOI] [PubMed] [Google Scholar]
- 68.Savonitto S, Ardissiono D, Egstrup K, et al. Combination therapy with metoprolol and nifedipine versus monotherapy in patients with stable angina pectoris: results of the International Multicenter Angina Exercise (IMAGE) Study. J Am Coll Cardiol. 1996;27(2):311-316 [DOI] [PubMed] [Google Scholar]
- 69.Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005;95(3):311-316 [DOI] [PubMed] [Google Scholar]
- 70.Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291(3):309-316 [DOI] [PubMed] [Google Scholar]
- 71.Wilson SR, Scirica BM, Braunwald E, et al. Efficacy of ranolazine in patients with chronic angina observations from the randomized, double-blind, placebo-controlled MERLIN-TIMI (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Segment Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol. 2009;53(17):1510-1516 [DOI] [PubMed] [Google Scholar]
- 72.Morrow DA, Scirica BM, Chaitman BR, et al. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 2009April21;119(15):2032-2039Epub 2009 Apr 6 [DOI] [PubMed] [Google Scholar]
- 73.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: the FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation 2002;105(18):2143-2147 [DOI] [PubMed] [Google Scholar]
- 74.Fraker TD, Jr, Fihn SD, 2002 Chronic Stable Angina Writing Committee 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation 2007;116(23):2762-2772 Epub 2007 Nov 12 [DOI] [PubMed] [Google Scholar]
- 75.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361(9351):13-20 [DOI] [PubMed] [Google Scholar]
- 76.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 appropriateness criteria for coronary revascularization. J Am Coll Cardiol. 2009;53(6):530-553 [DOI] [PubMed] [Google Scholar]
- 77.FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet 1999;354(9180):708-715 [PubMed] [Google Scholar]
- 78.Boden WE, O'Rourke RA, Teo KK, et al. COURAGE Trial Research Group Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503-1516 Epub 2007 Mar 26 [DOI] [PubMed] [Google Scholar]
- 79.Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation 2005;111(22):2906-2912 Epub 2005 May 31 [DOI] [PubMed] [Google Scholar]
- 80.Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis [published correction appears in Lancet. 2009;374(9687)378] Lancet 2009;373(9667):911-918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.VA Coronary Artery Bypass Surgery Cooperative Study Group Eighteen-year follow-up in the Veterans Affairs cooperative study of coronary artery bypass surgery for stable angina. Circulation 1992;86(1):121-130 [DOI] [PubMed] [Google Scholar]
- 82.Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988;319(6):332-337 [DOI] [PubMed] [Google Scholar]
- 83.Passamani E, Davis KB, Gillespie MJ, Killip T. A randomized trial of coronary artery bypass surgery: survival of patients with a low ejection fraction. N Engl J Med. 1985;312(26):1665-1671 [DOI] [PubMed] [Google Scholar]
- 84.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration [published correction appears in Lancet. 1994;344(8934):1446] Lancet 1994;344(8922):563-570 [DOI] [PubMed] [Google Scholar]
- 85.Myers WO, Schaff HV, Gersh BJ, et al. Improved survival of surgically treated patients with triple vessel coronary artery disease and severe angina pectoris: a report from the Coronary Artery Surgery Study (CASS) registry. J Thorac Cardiovasc Surg. 1989;97(4):487-495 [PubMed] [Google Scholar]
- 86.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) [published correction appears in Circulation. 2005;111(15):2014] Circulation 2004;110(14):e340-e437 [PubMed] [Google Scholar]
- 87.Parisi AF, Folland ED, Hartigan P, Veterans Affairs ACME Investigators A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. N Engl J Med. 1992;326(1):10-16 [DOI] [PubMed] [Google Scholar]
- 88.Pitt B, Waters D, Brown WV, et al. Atorvastatin versus Revascularization Treatment Investigators Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med. 1999;341(2):70-76 [DOI] [PubMed] [Google Scholar]
- 89.RITA-2 trial participants Coronary angioplasty versus medical therapy for angina: the second Randomised Intervention Treatment of Angina (RITA-2) trial. Lancet 1997;350(9076):461-468 [PubMed] [Google Scholar]
- 90.TIME Investigators Trial of invasive versus medical therapy in elderly patients with chronic symptomatic coronary-artery disease (TIME): a randomised trial. Lancet 2001;358(9286):951-957 [DOI] [PubMed] [Google Scholar]
- 91.Hueb W, Soares PR, Gersh BJ, et al. The medicine, angioplasty, or surgery study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results. J Am Coll Cardiol. 2004;43(10):1743-1751 [DOI] [PubMed] [Google Scholar]
- 92.Davies RF, Goldberg AD, Forman S, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation 1997;95(8):2037-2043 [DOI] [PubMed] [Google Scholar]
- 93.Erne P, Schoenenberger AW, Burckhardt D, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA 2007;297(18):1985-1991 [DOI] [PubMed] [Google Scholar]
- 94.Schömig A, Mehilli J, de Waha A, Seyfarth M, Pache J, Kastrati A. A meta-analysis of 17 randomized trials of a percutaneous coronary intervention-based strategy in patients with stable coronary artery disease. J Am Coll Cardiol. 2008;52(11):894-904 [DOI] [PubMed] [Google Scholar]
- 95.BARI 2D Study Group A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503-2515 Epub 2009 Jun 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117(10):1283-1291 Epub 2008 Feb 11 [DOI] [PubMed] [Google Scholar]
- 97.Jones RH, Floyd RD, Austin EH, Sabiston DC., Jr The role of radionuclide angiocardiography in the preoperative prediction of pain relief and prolonged survival following coronary artery bypass grafting. Ann Surg. 1983;197(6):743-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ladenheim ML, Pollock BH, Rozanski A, et al. Extent and severity of myocardial hypoperfusion as predictors of prognosis in patients with suspected coronary artery disease. J Am Coll Cardiol. 1986;7(3):464-471 [DOI] [PubMed] [Google Scholar]
- 99.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107(23):2900-2907 Epub 2003 May 27 [DOI] [PubMed] [Google Scholar]
- 100.Tarakji KG, Brunken R, McCarthy PM, et al. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation 2006;113(2):230-237 Epub 2006 Jan 3 [DOI] [PubMed] [Google Scholar]
- 101.Velazquez EJ, Lee KL, O'Connor CM, et al. STICH Investigators The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134(6):1540-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224 [DOI] [PubMed] [Google Scholar]
- 103.Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105-2111 Epub 2007 May 17 [DOI] [PubMed] [Google Scholar]
- 104.Hannan EL, Wu C, Chassin MR. Differences in per capita rates of revascularization and in choice of revascularization procedure for eleven states. BMC Health Serv Res. 2006;6:35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hemingway H, Crook AM, Dawson JR, et al. Rating the appropriateness of coronary angiography, coronary angioplasty and coronary artery bypass grafting: the ACRE study. J Public Health Med. 1999;21(4):421-429 [DOI] [PubMed] [Google Scholar]
- 106.Hemingway H, Crook AM, Feder G, et al. Underuse of coronary revascularization procedures in patients considered appropriate candidates for revascularization. N Engl J Med. 2001;344(9):645-654 [DOI] [PubMed] [Google Scholar]
- 107.Hemingway H, Chen R, Junghans C, et al. Appropriateness criteria for coronary angiography in angina: reliability and validity. Ann Intern Med. 2008;149(4):221-231 [DOI] [PubMed] [Google Scholar]
- 108.Sekhri N, Timmis A, Chen R, et al. Inequity of access to investigation and effect on clinical outcomes: prognostic study of coronary angiography for suspected stable angina pectoris. BMJ 2008;336(7652):1058-1061 Epub 2008 Apr 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Connor GT, Olmstead EM, Nugent WC, et al. Appropriateness of coronary artery bypass graft surgery performed in northern New England. J Am Coll Cardiol. 2008;51(24):2323-2328 [DOI] [PubMed] [Google Scholar]
- 110.Gerber Y, Rihal CS, Sundt TM, III, et al. Coronary revascularization in the community: a population-based study, 1990 to 2004. J Am Coll Cardiol. 2007;50(13):1223-1229 Epub 2007 Sep 10 [DOI] [PubMed] [Google Scholar]
- 111.Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009;373(9670):1190-1197 Epub 2009 Mar 19 [DOI] [PubMed] [Google Scholar]
- 112.Brophy JM, Belisle P, Joseph L. Evidence for use of coronary stents: a hierarchical bayesian meta-analysis. Ann Intern Med. 2003;138(10):777-786 [DOI] [PubMed] [Google Scholar]
- 113.Kastrati A, Mehilli J, Pache J, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):1030-1039 Epub 2007 Feb 12 [DOI] [PubMed] [Google Scholar]
- 114.Morice MC, Serruys PW, Sousa JE, et al. RAVEL Study Group A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773-1780 [DOI] [PubMed] [Google Scholar]
- 115.Bravata DM, Gienger AL, McDonald KM, et al. Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Ann Intern Med. 2007;147(10):703-716 Epub 2007 Oct 15 [DOI] [PubMed] [Google Scholar]
- 116.The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol. 2007;49(15):1600-1606 Epub 2007 Apr 2 [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez A, Rodríguez Alemparte M, Baldi J, et al. Coronary stenting versus coronary bypass surgery in patients with multiple vessel disease and significant proximal LAD stenosis: results from the ERACI II study. Heart 2003;89(2):184-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.SoS Investigators Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet 2002;360(9338):965-970 [DOI] [PubMed] [Google Scholar]
- 119.Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46(4):575-581 [DOI] [PubMed] [Google Scholar]
- 120.Hueb W, Lopes NH, Gersh BJ, et al. Five-year follow-up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation 2007;115(9):1082-1089 [DOI] [PubMed] [Google Scholar]
- 121.Daemen J, Boersma E, Flather M, et al. Long-term safety and efficacy of percutaneous coronary intervention with stenting and coronary artery bypass surgery for multivessel coronary artery disease: a meta-analysis with 5-year patient-level data from the ARTS, ERACI-II, MASS-II, and SoS trials. Circulation 2008;118(11):1146-1154 Epub 2008 Aug 25 [DOI] [PubMed] [Google Scholar]
- 122.Hannan EL, Racz MJ, Walford G, et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med. 2005;352(21):2174-2183 [DOI] [PubMed] [Google Scholar]
- 123.Brown ML, Gersh BJ, Holmes DR, Bailey KR, Sundt TM., III From randomized trials to registry studies: translating data into clinical information. Nat Clin Pract Cardiovasc Med. 2008;5(10):613-620 Epub 2008 Aug 5 [DOI] [PubMed] [Google Scholar]
- 124.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous Coronary Intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009March5;360(10):961-972 Epub 2009 Feb 18 [DOI] [PubMed] [Google Scholar]
- 125.Opie LH, Commerford PJ, Gersh BJ. Controversies in stable coronary artery disease. Lancet 2006;367(9504):69-78 [DOI] [PubMed] [Google Scholar]
- 126.Kim LJ, King SB, III, Kent K, et al. BARI 2D (Bypass Angioplasty Revascularization Investigation Type 2 Diabetes) Study Group Factors related to the selection of surgical versus percutaneous revascularization in diabetic patients with multivessel coronary artery disease in the BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes) trial. JACC Cardiovasc Interv. 2009;2(5):384-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodés-Cabau J, Deblois J, Bertrand OF, et al. Nonrandomized comparison of coronary artery bypass surgery and percutaneous coronary intervention for the treatment of unprotected left main coronary artery disease in octogenarians. Circulation 2008;118(23):2374-2381 Epub 2008 Nov 25 [DOI] [PubMed] [Google Scholar]
- 128.Hautvast RW, DeJongste MJ, Staal MJ, van Gilst WH, Lie KI. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J. 1998;136(6):1114-1120 [DOI] [PubMed] [Google Scholar]
- 129.Ekre O, Eliasson T, Norrsell H, Währborg P, Mannheimer C. Long-term effects of spinal cord stimulation and coronary artery bypass grafting on quality of life and survival in the ESBY study. Eur Heart J. 2002;23(24):1938-1945 [DOI] [PubMed] [Google Scholar]
- 130.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41(10):1761-1768 [DOI] [PubMed] [Google Scholar]
- 131.Barsness G, Feldman AM, Holmes DR, Jr, Holubkov R, Kelsey SF, Kennard ED, International EECP Patient Registry Investigators The International EECP Patient Registry (IEPR): design, methods, baseline characteristics, and acute results. Clin Cardiol. 2001;24(6):435-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saririan M, Eisenberg MJ. Myocardial laser revascularization for the treatment of end-stage coronary artery disease. J Am Coll Cardiol. 2003;41(2):173-183 [DOI] [PubMed] [Google Scholar]
- 133.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation 2007;115(25):3165-3172 Epub 2007 Jun 11 [DOI] [PubMed] [Google Scholar]
- 134.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA 2009;301(19):1997-2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.