Abstract
In addition to addiction, the repeated use of (+)-methamphetamine [(+)-METH], (+)amphetamine [(+)-AMP], or (±)-methylenedioxymethamphetamine [(±)-MDMA, commonly called ecstasy] can lead to life-threatening medical problems including cardiovascular injury, severe depression, and psychosis. Currently, there are no specific pharmacotherapies to treat these medical problems. In this study, we report the design and synthesis of two haptens, (S)-(+)-3-(9-carboxynonyloxy)methamphetamine [3a, (+)-METHMO10] and (S)-(+)-3-(5-carboxypentyloxy)methamphetamine [3b, (+)-METH MO6], and their use in generating high affinity (low KD value) monoclonal antibodies (mAbs) against (+)-METH, (+)-AMP, and/or (+)-MDMA. Based on results from the determination of mAb KD values and ligand specificity, the mAbs generated from hapten 3a showed the greatest promise for generating active and passive immunotherapies for treating overdose or addiction from (+)-METH-like stimulants.
The abuse of (S)-(+)-methamphetamine [1a-(+)-METH]a and other amphetamine-like stimulants continues to be a major health problem worldwide.1–3 Indeed, a study by RAND Corporation estimates the economic cost of (+)-METH use in the United States in 2005 was $23.4 billion.4 This comprehensive estimate includes the economic burden of addiction, premature death, drug treatment, and many other aspects of the drugs impact on Americans. The 2008 National Survey on Drug Use and Health estimates that over 12 million individuals, aged 12 and older, had used (+)-METH in their lifetime, that 850,000 million had used (+)-METH during the past year, and that 314,000 individuals had used (+)-METH during the last month, which defines them as current users.5 According to the 2005 Drug Abuse Warning Network (DAWN) report, there were 108,905 methamphetamine-related emergency departments (ED) visits in 2005.6 If (+)-(S)-amphetamine [1b, (+)-AMP] was included, there were 138,950 ED visits.6
At present there are no specific pharmacotherapies for managing adverse (+)-METH-induced effects like acute overdose and chronic addiction. Preclinical studies in rats show that systemic administration of anti-(+)-METH monoclonal antibodies (mAbs) can rapidly remove the drug from its sites of action in critical tissues like the brain and heart suggesting that immunotherapy could provide an important new medical strategy for addressing (+)-METH-induced adverse health effects in humans, while others suggest antibody catalyzed inactivation of METH could be a possible therapeutic approach.7 (S)-(+)-Amphetamine [1b, (+)-AMP], which is a major metabolite of (+)-METH and a drug of abuse, and (+)-methylenedioxymethamphetamine, the stimulant-inducing chemical in the racemic mixture of (±)-methylenedioxymethamphetamine [2, (±)-MDMA, commonly referred to as ecstasy] are two other widely abused and dangerous stimulants. The potency and stimulant effects of these (+)-METH-like compounds are influenced by the drug’s stereochemistry, with the (+)- or (S)-isomers producing significantly more psychomimetic effects, stereotyped behavior and locomotor activity,8 and increased production of reactive oxygen species in mice.9 Given the medical importance of all three of these structurally related (+)- or (S)-isomers, it could be medically advantageous to have a single mAb that could be used in the treatment of medical problems resulting from (+)-METH, (+)-AMP, and/or (+)-MDMA. Indeed, we recently reported the immunological design strategy, discovery, and development of an mAb antibody named anti-(+)-METH mAb4G9 that exhibited a novel optimal combination of high affinity and drug-class specificity for (+)-METH, (+)-AMP, and (+)-MDMA without significant cross-reactivity against other METH-like ligands, over-the-counter medications, or endogenous neurotransmitters.10
In this study, we report the synthesis of the hapten (S)-(+)-3-(9-carboxynonyloxy)methamphetamine [3a, designated (+)-METH MO10] used to prepare the mAb4G9 along with comparisons with the synthesis of (S)-(+)-3-(5-carboxypentyloxy)methamphetamine [3b, designated (+)-METH MO6], an earlier prototypic hapten. We also present the development and characterization of new anti-(+)-METH mAbs resulting from immunizations with these haptens covalently bound to three different carrier proteins. The mAbs generated from immunization with (+)-METH MO10 hapten had significantly lower KD values than the mAbs generated from the (+)-METH MO6 hapten. Furthermore, the mAbs generated against the (+)-METH MO10 hapten coupled to ovalbumin showed the richest diversity of specificity and higher affinity for all three medically important (+)-METH-like stimulants, (+)-METH, (+)-AMP, and (+)-MDMA (see below).

Chemistry
The synthesis of the haptens 3a and 3b is shown in Scheme 1. (S,S)-3-Methoxyphenyl-2-propyl-α-methylbenzylamine (5) was synthesized by modification of a method reported for other similar compounds.11 Thus, reductive alkylation of m-methoxyphenylacetone (4) with (S)-(−)-α-methylbenzylamine using triacetoxyborohydride in ethylene dichloride gave a mixture of (R,S)- and (S,S)-5. Recrystallization of the hydrochloride salts of the mixture from an isopropanol and ethyl ether mixture gave pure (S,S)-5, which was converted to the N-formyl intermediate 6 using a formic acid/acetic anhydride mixture. O-Demethylation of 6 using bromine tribromide in methylene chloride afforded the phenol 7. The sodium salt of the phenol prepared using sodium hydride in dimethylformamide was alkylated with methyl 10-bromodecaneate or methyl 6-bromopentanate to give 8a and 8b, respectively. Reduction of 8a and 8b using diborane in tetrahydrofuran provided the N-methyl compounds 9a and 9b. Subjection of 9a and 9b to transfer hydrogenation using ammonium formate and 5% palladium on carbon catalyst in methanol yielded 10a and 10b. Hydrolysis of 10a and 10b using 6N hydrochloride acid afforded the desired haptens 3a and 3b.
Scheme 1.
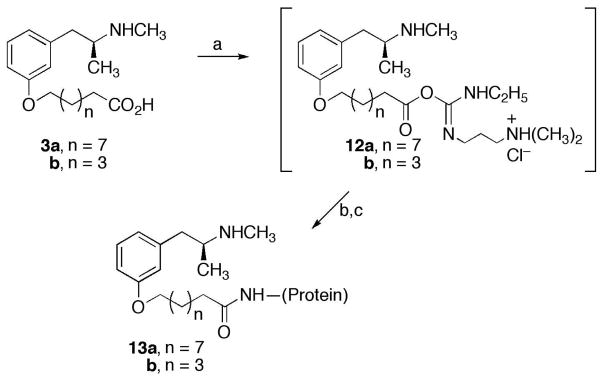
The hapten/protein antigens were synthesized as shown in Scheme 2 using a carbodiimide coupling procedure.12 Briefly, a solution of the hapten 3a or 3b was combined with a solution of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI) hydrochloride to give the activated intermediate 12a or 12b, which was not isolated. Addition of a solution of bovine serum albumin (BSA), cationized BSA (c-BSA), or ovalbumin (OVA) to the solution of 12a or 12b provided the desired hapten-protein antigen 13a or 13b, which was separated from unconjugated haptens and other starting materials by dialysis against multiple changes of distilled water followed by phosphate-buffered saline (pH 7.35). The degree of incorporation of the haptens onto the OVA and BSA carrier proteins was determined by Matrix-Assisted Laser Desorption/Ionization (MALDI) mass spectrometry (MS) analysis. The degree of incorporation of haptens onto the c-BSA carrier proteins was not determined since these antigens were generated before MS analysis of proteins was available to this project. Figures 1A and 1B show the MALDI mass spectra obtained from BSA and MO10-modified BSA, respectively. The observed mass for BSA by MALDI MS was in excellent agreement with the value derived from the known amino acid sequence. The mass difference between the two spectra divided by the mass added by each hapten (317 Da) indicated an average incorporation of 5.58 haptens. A similar MALDI MS analysis of OVA and the MO10-modified OVA indicated an incorporation of 4–5 haptens.
Scheme 2a.
aReagents: (a) EDCI, DMF of 0.1M buffer; (b), BSA, OVA, or cationized BSA in buffer; (c) dialysis against water then PBS buffer.
Figure 1.
Figure 1A. MALDI mass spectrum of BSA. (The measured m/z is taken as the correct mass to minimize error in mass assignment.)
Figure 1B. MALDI mass spectrum of METH MO10-modified BSA. The number of modifying groups (5.6) was determined by dividing the mass increase upon modification by the mass added by each modifying group (317 Da).
Results and Discussion
The overall goal of this study was to design and develop a hapten that would stimulate immune cell production of mAbs with high affinity for (+)-METH as well as (+)-AMP and (+)-MDMA. Since all three structures have in common an (S)-aminopropyl substituent on an aromatic ring that helps make the molecules pharmacologically and immunologically distinct, it was decided to leave this part of the structure alone and to attach the linker moiety of the haptens to the aromatic ring at a site that was distal to the (S)-aminopropyl structures. The linker was connected to the aromatic ring through an oxygen atom at the meta position to improve the probability of MDMA recognition, which has oxygen atoms at both the meta and para positions.
Since (+)-AMP does not possess the N-methyl group present in (+)-METH and (+)-MDMA, we hypothesized that a longer linker group between the hapten (like MO10) and backbone structure would lead to improved immune recognition and higher affinity antibodies for (+)-METH, while maintaining cross-reactivity and affinity with (+)-MDMA as well as (+)-AMP. While our primary goal was to discover haptens that would generate mAbs with high affinity for (+)-METH, we also hoped to discover mAbs with a broader specificity for all three of these drugs of abuse. We hypothesized that this broader specificity for (+)-METH-like drugs would improve the likelihood of producing a single therapeutic antibody that could be used to treat medical problems (e.g., addiction, overdose) resulting from any of these drugs. By analogy, we attempted to produce a “designer antibody” to treat the medical problems caused by these “designer drugs.” We also reasoned that the future medical applications for a broader specificity antibody would be greater since hospital pharmacies would only have to stock one medication for the treatment of medical problems resulting from (+)-METH, (+)-MDMA, and (+)-AMP.
Our hypothesis was supported by the finding that immunizations with antigens containing an MO10 hapten epitope produced significantly greater affinities for (+)-METH (as judged by lower KD values for (+)-METH) than did immunization with the MO6-containing hapten epitope (p<0.05 using a Student’s t-test; Table 1 and Figures 2 and 3). It should be noted that we designed our immunization schedules to include the minimum antigen dose and long periods between boost (up to 2 months) to favor the likelihood that we would generate high affinity anti-(+)-METH mAbs. We also screened for anti-(+)-METH mAbs with a minimum amount of hapten protein conjugate to favor the discovery of high affinity antibodies. In practice, only antibodies of the highest affinity can stay bound when the hapten dose is minimal. However, on many occasions we also discovered low affinity antibodies but only kept the mAbs with KD values for (+)-METH of approximately 100 nM or less. We chose this cut-off point after considering the outcomes of a wide range of pharmacological and behavioral studies in rats from our laboratory using various anti-(+)-METH mAbs. From these observations, we hypothesize that mAbs with KD values of ≥00 nM will not be clinically useful, and KD values of at least ≤10–30 nM will be needed for the treatment of medical problems caused by addiction.13,14
Table 1.
Haptens, Antigens, and Immunochemical Specifications of anti-(+)-METH Monoclonal Antibodies.
| (+)-METH-like hapten and antigen | Monoclonal antibody | IgG isotype, light chain | (+)-METH KD (nM) | (+)-AMP KD (nM) or KI (nM)a | (+)-MDMA KI (nM)b |
|---|---|---|---|---|---|
| MO6 bound to c-BSA | mAb9B11 | IgG1, λ | 110 | >1,000 | 24 |
| mAb10E1 | IgG1, λ | 91 | >5,000 | 24 | |
| mAb7H11 | IgG1, κ | 52 | >5,000 | 30 | |
| mAb5C2 | IgG1, κ | 39 | >5,000 | 28 | |
| mAb9C4 | IgG2a, κ | 77 | >5,000 | 45 | |
| mAb1C1 | IgG1, κ | 58 | >5,000 | 22 | |
| MO10 bound to OVA | mAb4G9 | IgG2b, κ | 16 | 50 | 68 |
| mAb2F11 | IgG2a, κ | 13 | 49 | 65 | |
| mAb1A12 | IgG2a, κ | 13 | >1,000 | 5 | |
| mAb6C1 | IgG1, κ | 47 | 47 | 52 | |
| mAb10D1 | IgG1, κ | 34 | 51 | 69 | |
| MO10 bound to BSA | mAb7F9 | IgG1, κ | 9 | >1,000 | 14 |
| mAb9C10 | IgG1, κ | 38 | >1,000 | 27 | |
Only four mAbs (all from immunization with a MO10-OVA antigen) had high affinity for (+)-AMP, and these KD values were determine in a homologous immunoassay with [3H]-AMP as the radioligand and inhibition with unlabeled (+)-AMP. If we determined in screening assays that the mAb would have a very high KD value >1000–5,000 nM, we conducted a heterologous immunoassay with [3H]-METH as the radioligand and inhibition with a high range of unlabeled (+)-AMP doses to obtain an approximate KI value for (+)-AMP binding.
[3H]-(+)-MDMA was unavailable, so we determined a KI value for binding to each mAb using [3H]-METH as the radioligand and inhibition with unlabeled (+)-MDMA.
Figure 2.
Representative RIA plots for the determination of anti-(+)-METH mAb4G9 KD values for (+)-METH (upper) and (+)-AMP (middle), and KI values for (+)-MDMA (lower). Similar RIA inhibition curves were determined in duplicate or triplicate for all 13 mAbs listed in Table 1. After a mathematical correction for the contribution of [3H]-METH or [3H]-AMP binding, the final average KD and KI value was calculated.
Figure 3.

Individual (open circles) and average (solid bar) KD values for (+)-METH binding to all 13 monoclonal antibodies generated for these studies. Side-by-side circles indicate that two different antibodies had the same apparent KD value. The KD values for (+)-METH binding to antibodies generated against (+)-METH MO10 haptens (n = 7) were significantly lower (p <0.05; t-test) than the KD values for the antibodies generated against (+)-METH M06 haptens (n = 6).
Another critical factor in the discovery process was the number of haptens bound to the antigen. In our earlier studies with the MO6 haptens attached to c-BSA carrier proteins, we did not have the analytical capabilities to directly determine the number of haptens per c-BSA molecule. Furthermore, we later found MALDI MS analysis (or any other method) was not dependable with hapten-c-BSA, which is slightly larger in molecular size than BSA. More importantly, the c-BSA protein has less uniform properties than the other carrier proteins, with varying amounts of activation within lots or batches. However, by the time synthesis of the MO10 antigens was started, the mass spectrometry technology to directly determine the number of haptens per protein molecule became available to us. These mass spectrometry studies showed that about five haptens per protein molecule (either OVA or BSA) were sufficient to produce high affinity anti-(+)-METH antibodies. We also found that hapten incorporation rates on OVA and BSA of less than five per protein molecule led to inconsistent results or no antigenic response (results not shown). While the c-BSA proved a good antigenic protein, we chose to use OVA and BSA antigens because these proteins were small enough to allow direct mass spectrometric analysis and direct determination of hapten epitope densities. This proved valuable in optimizing various outcomes. In general, this allows the user to optimize the hapten to protein ratios [i.e., number of (+)-METH-like antigenic epitopes] in synthetic reactions and will also prove valuable in future studies of the relationship between epitope density and anti-drug response in vaccinated animals.
The current studies provide a comprehensive analysis of the importance of hapten length and its relationship to affinity and specificity for very small haptens. Previous studies from our laboratory have shown that five unique mAbs generated against five different METH-like haptens (including MO6 and MO10) can produce mAbs against (+)-METH-like haptens that range in KD value from 250–10 nM.10 These five mAbs resulted from immunizing mice with (+)-METH coupled to protein antigens through progressively longer linker groups of 4, 6, and 10 atoms connected to the aromatic ring at the para or meta positions.
For the current studies, we re-determined the (+)-METH KD values for mAb9B11 and mAb4G9 (from the previous studies), along with 11 other never before reported anti-(+)-METH mAbs, using a significantly improved radioimmunoassay (RIA) for determination of KD and KI values. This improved RIA method does not require a second dilution or incubation step to separate the drug (+)-METH mAb complex from the unbound drug. These procedural steps in an RIA often result in a less than optimal estimation of the KD values for ligand binding. Indeed mAb9B11 and mAb4G9 KD values for (+)-METH in our previously reported RIA were 41 and 34 nM, 10 respectively, but in the current study they are 110 and 16 nM with improved reproducibility.
Importantly, four mAbs generated from MO10, bound to the OVA, have KD or Ki values of 13–47 nM, 47–51 nM, and 52–69 nM for (+)-METH, (+)-AMP, and (+)-MDMA, respectively. In contrast, six mAbs generated from (+)-METH MO6 bound to c-BSA antigen, and two mAbs generated from MO10, bound to BSA antigen, sometimes had very low KD values for (+)-METH and (+)-MDMA binding but always possessed KD values of >1,000 nM for (+)-AMP. One mAb (mAb1A12) generated from the MO10, bound to OVA antigen, also had a KD value of >1,000 nM for (+)-AMP. We are now in the process of testing the best of these antibodies as medications in preclinical studies in rodents. It will be of particular interest to determine if the mAb4G9, with its broad specificity for all three (+)-METH-like drugs, is actually therapeutically better than mAb7F9 (generated from the (+)-METH MO10-BSA antigen). In our experience, therapeutic efficacy of mAbs is best judged by the ability of the mAbs to (1) block and/or reduce brain concentration of (+)METH, (2) provide prolonged redistribution of (+)METH out of the brain and into the blood stream, and (3) the ability of the mAbs to block or significantly reduce (+)METH-induced behavioral effects in preclinical animal models like the rat. MAb7F9 had the highest affinity for (+)-METH of any of the antibodies (KD = 9 nM) in this report but no significant binding to (+)-AMP. Also worthy of noting is the very high affinity binding of mAb1A12 for (+)-MDMA (KI = 5 nM).
Over the course of more than ten years of studies of anti-(+)-METH mAbs, we have used a range of protein antigens including OVA, c-BSA, BSA, keyhole limpet hemocyanin, thymoglobulin, and immunoglobulin. We have also used five different adjuvants: Freund’s Complete, Freund’s Incomplete, RIBI, TiterMax, and Alum. The adjuvant does not appear to be the major factor in generating a broad specificity mAbs (i.e., cross-reactivity with (+)-METH, (+)-AMP, and (+)-MDMA), but it does significantly affect the ability to generate high affinity antibodies. Freund’s Complete adjuvant consistently proved to be the best of the five adjuvants. In addition, the use of haptens derived from (+)-isomers has never generated mAbs that significantly cross-react with (−)-isomers of METH-like ligands (results not shown). Our studies also suggest that attachment of the hapten spacer arm linkage at the meta-portion of the (+)-METH aromatic ring structure appears superior to a para position linkage since it is the only hapten to ever generate mAbs with significant cross reactivity with (+)-AMP (Table 1 and reference 7). Another important factor to emerge from this research program is the suggestion that the combination of MO10 linked to OVA is best for generating mAbs with simultaneously high affinity (i.e., low KD values) for (+)-METH, (+)-AMP, and (+)-MDMA (Table 1). Based on the results of these studies, the ability of a single (+)-METH-like hapten to generate high affinity for (+)-METH and (+)-MDMA appears less dependent on choosing the optimal combination of hapten and carrier protein.
In summary, haptens were designed and developed to maximize the potential for a single mAb to specifically recognize the common molecular features of (+)-METH, (+)-AMP, and (+)-MDMA. Four mAbs have been developed from the MO10 hapten bound to OVA, which have simultaneously low KD and KI values for (+)-METH, (+)-AMP, and (+)-MDMA. The high affinity Abs generated in this study have the potential to block or reduce the stimulant-induced medical effects in patients addicted to (or abusing) these drugs. In addition, the mAbs derived from hapten 3a have the potential to provide an acute treatment for life-threatening stimulant overdose resulting from (+)-AMP, (+)-METH, or (+)-MDMA by removing the drugs from their sites of action in the central nervous, heart and other critical organs, thereby reducing METH-induced psychosis, neurotoxicity and cardiovascular effects. Finally, these studies also suggest MO10 or a similar hapten will be the best for use in developing active vaccines. These studies are currently in progress in our laboratories.
Experimental Section
Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were recorded on a 300 MHz (Bruker AVANCE 300) spectrometer. Chemical shift data for the proton resonances were reported in parts per million (ppm) relative to internal standard. Optical rotations were measured on an AutoPol III polarimeter, purchased from Rudolf Research. Elemental analyses were performed by Atlantic Microlab, Norcross, GA. Purity of compounds (>95%) was established by elemental analyses. Analytical thin-layer chromatography (TLC) was carried out on plates precoated with silica gel GHLF (250 μM thickness). TLC visualization was accomplished with a UV lamp or in an iodine chamber. All moisture-sensitive reactions were performed under a positive pressure of nitrogen maintained by a direct line from a nitrogen source. Anhydrous solvents were purchased from Aldrich Chemical Co.
(S)-3-(9-Carboxynonyloxy)methamphetamine (3a) Hydrochloride
Compound 10a (420 mg, 1.1 mmol) was heated under reflux in 9 mL of 6N HCl for 8 h. The cooled solution was concentrated to a tan residue that was recrystallized 3× from a CH3OH and Et2O mixture to yield 260 mg (65%) of 3a as its white powdery HCl salt: mp 124–127 °C; [α]25D +0.30 (c 1.0, CH3OH). 1H NMR (CD3OD) δ 7.25 (t, J = 7.9 Hz, 1H), 6.75–6.90 (m, 3H), 3.97 (t, J = 6.4 Hz, 3H), 3.40–3.55 (m, 1H), 3.11 (dd, J = 13.2, 4.9 Hz, 1H), 2.73 (dd, J = 13.2, 9.0 Hz, 1H), 2.72 (s, 3H), 2.28 (t, J = 7.2 Hz, 2H), 1.77 (ap, J = 7.2 Hz, 2H), 1.60 (ap, J = 7.2 Hz, 2H), 1.42–1.54 (m, 2H), 1.28–1.43 (m, 9H), 1.25 (d, J = 6.8 Hz, 3H). 13C NMR (300 MHz, CD3OD) δ 161.5, 138.8, 131.4, 122.8, 117.2, 114.7, 69.3, 58.1, 40.3, 35.0, 30.9, 30.5, 30.4, 30.3, 30.2, 27.1, 26.1, 15.9; LCMS (APCI) m/z 336.7 (M+1)+. Anal. (C20H34ClNO3•0.25 H2O) C, H, N.
(S)-3-(5-carboxypentyloxy)methamphetamine hydrochloride (3b)
Compound 3b was prepared by a procedure analogous to that described for 3a to afford 59% of 3b as the hydrochloride salt: mp 73–76 °C. [α]25D +0.64 (c 1.10, CH3OH). 1H NMR (CD3OD) δ 7.24 (t, J = 7.9 Hz, 1H), 6.76–6.91 (m, 3H), 3.97 (t, J = 6.4 Hz, 3H), 3.39–3.59 (m, 1H), 3.35 (s, 3H), 3.16 (dd, J = 13.2, 4.9 Hz, 1H), 2.75 (dd, J = 13.2, 9.0 Hz, 1H), 2.72 (s, 3H), 2.32 (t, J = 7.2 Hz, 2H), 1.78 (ap, J = 7.2 Hz, 2H), 1.67 (ap, J = 7.2 Hz, 2H), 1.42–1.58 (m, 2H), 1.25 (d, J = 6.8 Hz, 3H). 13C NMR (300 MHz, CD3OD) δ 131.4, 123.0, 117.1, 114.8, 69.2, 58.2, 40.6, 35.3, 31.4, 30.5, 27.2, 26.3, 16.2. LCMS (ESI) m/z 280.3 (M+1)+, m/z 278.6 (M-1)+. Anal. (C16H26ClNO3) C, H, N.
(S,S)-N-α-Methylbenzyl-3-methoxyamphetamine (5) Hydrochloride
To a solution of m-methoxyphenylacetone (5.0 g, 0.030 mol) and (S)-(−)- α-methylbenzylamine (3.81 g, 0.031 mol) in ethylene dichloride (200 mL) at room temperature was added sodium triacetoxyborohydride (10.0 g, 0.047 mol) in four equal portions over a 10-min interval. After stirring overnight at ambient temperature, the reaction mixture was quenched with 15% NH4OH (200 mL). The organic layer was separated, and the aqueous layer was extracted with CH2Cl2 (3 × 100 mL). The combined organic layers were washed with saturated brine solution (150 mL), dried (Na 2SO4), and concentrated to give 9.24 g (99%) of 5 as a colorless oil. 1H NMR (CDCl3) δ 7.27–7.44 (m, 4H), 7.20–7.27 (m, 1H), 7.16 (t, J = 7.9 Hz, 1H), 6.72 (dd, J = 7.9, 2.3 Hz, 1H), 6.68 (d, J = 7.5 Hz, 1H), 6.62 (at, J = 2.3 Hz, 1H), 3.92 (q, J = 6.4 Hz, 1H), 3.76 (s, 3H), 2.85 (dd, J = 12.8, 5.3 Hz, 1H), 2.78 (dq, J = 6.4, 5.3 Hz, 1H), 2.46 (dd, J = 12.8, 7.2 Hz, 1H), 1.42 (bs, 1H, NH), 1.30 (d, J = 6.4 Hz, 3H), 0.92 (d, J = 6.4 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 159.5, 146.2, 141.2, 129.1, 128.4, 126.8, 126.5, 121.8,115.0, 111.3, 55.3, 55.1, 51.8, 42.7, 24.5, 21.2.
The free base dissolved in Et2O (100 mL) was treated with HCl solution in Et2O (2.0 M, 16.0 mL, 10.0 eq) at ice-cold condition. Addition of Et 2O (200 mL) yielded 8.48 g (92%) of a white, amorphous solid. Recrystallization of the solid using hot isopropanol (725 mL) followed by the slow addition of Et2O (600 mL) at room temperature yielded 5.5 g (65%) of 5•HCl as a white crystalline solid: mp 219– 221 °C. 1H NMR (CDCl3) δ 10.32 (bs, 1H), 9.84 (bs, 1H), 7.69 (ad, J = 7.2 Hz, 2H), 7.48 (tt, J = 7.2, 1.9 Hz, 2H), 7.41 (tt, J = 7.2, 1.5 Hz, 1H), 7.15 (t, J = 7.9 Hz, 1H), 6.75 (dd, J = 7.9, 1.9 Hz, 1H), 6.59 (d, J = 7.5 Hz, 1H), 6.51 (at, J = 1.9 Hz, 1H), 4.39 (ap, J = 6.8 Hz, 1H), 3.73 (s, 3H), 3.40 (dd, J = 12.8, 4.1 Hz, 1H), 3.05 (bs, 1H), 2.85 (dd, J = 12.8, 9.8 Hz, 1H), 1.96 (d, J = 6.8 Hz, 3H), 1.43 (d, J = 6.8 Hz, 3H). 13C NMR (CDCl3) δ 159.8, 138.1, 136.5, 129.7, 129.5, 129.3, 127.9, 121.4, 114.6, 112.7, 55.9, 55.2, 53.9, 38.1, 21.2, 17.9. Anal. (C18H24ClNO) C, H.
(S,S)-N-Formyl-N-α-methylbenzyl-3-methoxyamphetamine (6)
To a stirred solution of 24 mL of formic acid (36.6 g, 0.795 mol) at 0 °C, 40 mL of acetic anhydride (58.3 g, 0.572 mol) in toluene (100 mL) was added dropwise. After 30 min, the amine 5 (10.2 g, 0.038 mol, obtained from the hydrochloride salt) in a minimum volume of formic acid was added. The reaction mixture was heated under reflux for 16 h and allowed to stir at RT for 8 h. The acidic solution was basified with 40 mL of 30% NH4OH (aq) solution and extracted with 3 × 100 mL of CH2Cl2. The extracts were washed with brine (100 mL) and dried (MgSO4). Concentration of the extracts gave an oil. The oil was purified by flash chromatography (silica gel, 200 g ISCO column) to give 10.3 g (92%) of 6 as a clear, colorless thick oil. 1H NMR (CDCl 3) δ 8.41 (s, 1H), 8.40 (s, 1H), 7.27– 7.50 (m, 5H), 7.07 (t, J = 7.9 Hz, 1H), 7.07 (t, J = 7.9 Hz, 1H), 6.69 (d, J = 1.9 Hz, 1H), 6.66 (d, J = 1.9 Hz, 1H), 6.41 (d, J = 7.5 Hz, 1H), 6.30 (at, J = 1.9 Hz, 1H), 6.26 (d, J = 7.5 Hz, 1H), 6.09 (at, J = 1.9 Hz, 1H), 5.94 (q, J = 7.2 Hz, 1H), 4.65 (q, J = 7.2 Hz, 1H), 3.70 (s, 3H), 3.68 (s, 3H), 3.34–3.53 (m, 1H), 3.19–3.36 (m, 1H), 3.00 (dd, J = 13.2, 9.8 Hz, 1H), 2.61 (dd, J = 13.2, 4.9 Hz, 1H), 2.38–2.54 (m, 2H), 1.58 (d, J =7.2 Hz, 3H), 1.54 (d, J = 7.2 Hz, 3H), 1.31 (d, J = 6.8 Hz, 3H), 1.25 (d, J = 6.8 Hz, 3H). l3C NMR (CDCl3) δ 162.5, 161.4, 159.55, 159.48, 140.9, 140.2, 139.8, 139.6, 129.3, 129.1, 128.6, 128.4, 128.1, 127.9, 127.7, 127.2, 121.5, 121.3, 114.3, 114.2, 112.1, 111.9, 56.9, 55.1, 53.3, 51.1, 49.7, 44.7, 40.4, 21.8, 19.7, 17.3, 16.4. LCMS (APCI) m/z 298 (M+1).
(S,S)-N-Formyl-N-α-methylbenzyl-3-hydroxyamphetamine (7)
To a stirred cold (0 °C) solution of 6 (2.10 g, 0.007 mol) in 60 mL of CH2Cl2 under N2 atmosphere was added dropwise 1.33 mL (3.54 g, 0.014 mol) of BBr3. The reaction mixture was allowed to stir at 25 °C for 8 h, 100 mL of water was carefully added to quench excess BBr3, and the resulting mixture was extracted with 3 × 100 mL of CH2Cl2 and dried (Na2SO4). The resulting oil was subjected to silica gel chromatography using hexanes/CH2Cl2/MeOH (4:8:1) as the eluent to give 1.96 g (98%) of pure 7 as a white powder: mp 195–198 °C; Rf 0.43 (1:1, hexanes/EtOAc). 1H NMR (CDCl3) δ 8.36 (s, 1H), 8.32 (s, 1H), 7.29–7.46 (m, 4H), 7.26 (at, J = 6.8 Hz, 1H), 7.01 (t, J = 7.2 Hz, 1H), 6.99 (t, J = 7.2 Hz, 1H), 6.72 (d, J = 1.9 Hz, 1H), 6.69 (d, J = 1.9 Hz, 1H), 6.55 (at, J = 1.9 Hz, 1H), 6.26 (at, J = 1.9 Hz, 1H), 6.24 (d, J = 7.5 Hz, 1H), 6.11 (d, J = 6.8 Hz, 1H), 5.89 (q, J = 7.2 Hz, 1H), 4.56 (q, J = 7.2 Hz, 1H), 3.42–3.63 (m, 1H), 3.23–3.42 (m, 1H), 2.93 (dd, J = 13.2, 9.4 Hz, 1H), 2.61 (dd, J = 13.2, 5.3 Hz, 1H), 2.25–2.46 (m, 2H), 1.54 (d, J = 6.4 Hz, 3H), 1.52 (d, J = 6.4 Hz, 3H), 1.30 (d, J = 6.8 Hz, 1H), 1.21 (d, J = 6.8 Hz, 1H). 13C NMR (300 MHz, CDCl3) δ 162.9, 162.1, 156.61, 156.58, 140.5, 139.8, 139.3, 139.2, 129.4, 129.3, 128.65, 128.57, 128.5, 128.0, 127.91, 127.86, 127.0, 120.4, 116.2, 116.1, 113.7, 113.4, 57.5, 53.8, 51.2, 50.2, 44.2, 40.1, 21.6, 19.8, 17.2, 16.3. Anal. (C18H21NO2•0.25 H2O) C, H, N.
(S,S)-N-Formyl-N-α-methylbenzyl-3-(9′-carbomethoxynonyloxy)amphetamine (8a)
A solution of 1.97 (0.007 mol) of phenol 7 in 3 mL of DMF was carefully added to a suspension of 300 mg of 60% NaH (washed with hexanes in 5 mL of DMF) followed by the addition of 2 g of methyl-10-bromodecanoate in 4 mL of DMF. After 16 h stirring, the reaction mixture was quenched with water and extracted with 4 × 200 mL of CH2Cl2 and dried (Na2SO4). The oil obtained on concentration was purified using an 80 g silica gel ISCO column with gradient 0–10% of polar B: hexanes/CH2Cl2/MeOH (4:8:1) in a polar CH2Cl2 collecting 25 mL fraction. Concentration of the product fraction gave 1.98 g (63%) of pure 8a as an oil. 1H NMR (CDCl3) δ 8.40 (s, 1H), 8.37 (s, 1H), 7.25–7.47 (m, 5H), 7.054 (t, J = 7.9 Hz, 1H), 7.045 (t, J = 7.9 Hz, 1H), 6.68 (d, J = 1.9 Hz, 1H), 6.65 (d, J = 1.9 Hz, 1H), 6.39 (d, J = 7.5 Hz, 1H), 6.29 (at, J = 1.9 Hz, 1H), 6.23 (d, J = 7.5 Hz, 1H), 6.08 (at, J = 1.9 Hz, 1H), 5.93 (q, J = 7.2 Hz, 1H), 4.64 (q, J = 7.2 Hz, 1H), 3.80 (qd, J = 13.2, 6.4 Hz, 2H), 3.66 (s, 3H), 3.36–3.55 (m, 1H), 3.18–3.36 (m, 1H), 2.98 (dd, J = 13.2, 9.8 Hz, 1H), 2.61 (dd, J = 13.2, 5.3 Hz, 1H), 2.36–2.53 (m, 2H), 2.31 (t, J = 7.5 Hz, 2H), 1.68–1.82 (m, 2H), 1.50–1.68 (m, 2H), 1.57 (d, J = 6.8 Hz, 3H), 1.54 (d, J = 7.2 Hz, 3H), 1.39–1.50 (m, 2H), 1.28–1.39 (m, 13H), 1.25 (d, J = 7.2 Hz, 3H). 13C NMR (CDCl3) δ 174.1, 162.4, 161.4, 159.05, 158.98, 140.8, 140.1, 139.7, 139.5, 129.2, 129.0, 128.5, 128.4, 128.0, 127.9, 127.8, 127.7, 127.6, 127.15, 127.07, 121.2, 121.0, 114.9, 114.7, 112.7, 112.5, 67.73, 67.7, 56.9, 53.3, 51.3, 51.1, 49.7, 44.7, 40.4, 34.0, 29.23, 29.21, 29.18, 29.16, 29.1, 29.0, 25.9, 24.8, 21.7, 19.7, 17.2, 16.3. Anal. (C29H41NO4•H2O) C, H, N.
(S,S)-N-Methyl-N-α-methylbenzyl-3-(9′-carbomethoxynonyloxy)amphetamine (9a)
To 1.90 g (0.004 mol) of 8a in 5 mL of anhydrous THF was added 10 mL of 1 M BH3 in THF solution. The reaction mixture was stirred for 1 h, carefully quenched with 1 mL MeOH, 10 mL of 1 M HCl, and basified with 20 mL of 15% NH4OH (aq) solution. The aqueous layer was extracted with 3 × 50 mL of CH2Cl2. The oil obtained on concentration was subjected to chromatography using a 120-g silica gel ISCO column with hexanes/Et2O/TEA (10:9:1) as the eluent collecting 25 mL fractions. The fractions containing the first major product were concentrated to give 489 mg (26%) of 9a as an oil. 1H NMR (CDCl3) δ 7.18–7.34 (m, 4H), 7.09–7.18 (m, 1H), 7.03 (t, J = 7.9 Hz, 1H), 6.60 (dd, J = 7.5, 1.9 Hz, 1H), 6.52 (d, J = 7.5 Hz, 1H), 6.45 (s, 1H), 3.69–3.87 (m, 2H), 3.56 (s, 3H), 3.50–3.68 (m, 1H), 2.89–3.07 (m, 1H), 2.82 (dd, J = 12.8, 4.5 Hz, 1H), 2.17 (s, 3H), 2.15–2.30 (m, 2H), 1.44–1.74 (m, 5H), 1.09–1.44 (m, 13H), 0.82 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3) δ 174.3, 159.0, 146.4, 142.6, 128.9, 128.3, 127.3, 126.6, 121.4, 115.1, 111.8, 67.8, 62.7, 62.1, 55.8, 51.4, 38.0, 34.1, 32.3, 29.8, 29.3, 29.14, 29.08, 26.0, 24.9, 21.9, 15.5.
(S)-3-(9-Carbomethoxynonyloxy)methamphetamine (10a)
A mixture of 489 mg (1.08 mmol) of 9a in 40 mL of CH3OH containing 2.7 g of HCO2NH4 and 350 mg of 5% Pd/C was refluxed for 2 h. The cooled reaction solution was filtered through a celite pad and concentrated. The resulting residue was basified in 100 mL of Et2O using 1 mL of TEA. This mixture was gravity filtered (to remove excess formate salts), and the filtrate was concentrated to an oil. The oil was dissolved in a minimum amount of CH2Cl2 (about 1–2 mL) and was subjected to column chromatography using a 120-g silica gel ISCO column using 5% B/A to 100% B/A gradient elution over 30 min (A = hexanes/Et 2O/TEA (10:9:1); B = 10% MeOH/CH2Cl2). Product fractions were concentrated to give 378 mg (91%) of 10a as an oil. 1H NMR (CDCl3) δ 7.16 (dt, J = 7.2, 1.5 Hz, 1H), 6.62–6.83 (m, 3H), 3.90 (t, J = 6.4 Hz, 2H), 3.63 (s, 3H), 2.76 (sixtet, J = 6.4 Hz, 1H), 2.60 (dq, J = 13.2, 6.4 Hz, 2H), 2.35 (s, 3H), 2.27 (t, J = 7.2 Hz, 2H), 1.74 (p, J = 6.4 Hz, 2H), 1.59 (p, J = 7.2 Hz, 2H), 1.18–1.50 (m, 10H), 1.03 (d, J = 6.4 Hz, 3H). 13C NMR (CDCl3) δ 174.1, 159.1, 140.9, 129.2, 121.3, 121.4, 115.5, 112.0, 67.7, 56.2, 51.3, 43.4, 34.0, 33.8, 29.21, 29.20, 29.04, 28.99, 25.9, 25.8, 19.6.
(S,S)-N-Formyl-N-α-methylbenzyl-3-(5′-carbomethoxypentyloxy)amphetamine (8b)
Compound 8b was prepared by a procedure analogous to that described for 8a to afford 76% of 8b. 1H NMR (300 MHz, CDCl3) δ 8.41 (s, 1H), 8.40 (s, 1H), 7.22–7.50 (m, 5H), 7.06 (t, J = 7.9 Hz, 1H), 7.05 (t, J = 7.9 Hz, 1H), 6.67 (d, J = 1.9 Hz, 1H), 6.64 (d, J = 1.9 Hz, 1H), 6.39 (d, J = 7.5 Hz, 1H), 6.28 (bs, 1H), 6.24 (d, J = 7.5 Hz, 1H), 6.08 (bs, 1H), 5.93 (q, J = 7.2 Hz, 1H), 4.64 (q, J = 7.2 Hz, 1H), 3.82 (q, J = 6.4 Hz, 1H), 3.68 (s, 3H), 3.35–3.54 (m, 1H), 3.19–3.35 (m, 1H), 2.98 (dd, J = 12.8, 9.8 Hz, 1H), 2.60 (dd, J = 12.8, 4.9 Hz, 1H), 2.42–2.50 (m, 2H), 2.33 (t, J = 7.2 Hz, 3H), 1.64–1.83 (m, 4H), 1.58 (d, J = 6.8 Hz, 3H), 1.54 (d, J = 6.8 Hz, 3H), 1.41–1.52 (m, 2H), 1.31 (d, J = 6.8 Hz, 3H), 1.25 (d, J = 6.8 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 173.5, 162.4, 161.4, 158.94, 158.88, 140.8, 140.2, 139.7, 139.5, 129.2, 129.0, 128.5, 128.4, 128.0, 127.8, 127.6, 127.1, 121.3, 121.0, 114.8, 114.7, 112.6, 112.4, 67.9, 60.1, 56.8, 53.2, 51.1, 49.7, 44.6, 40.4, 34.1, 28.9, 28.8, 25.5, 24.6, 21.7, 19.7, 17.2, 16.3.
(S,S)-N-Methyl-N-α-methylbenzyl-3-(5′-carbomethoxypentyloxy)amphetamine (9b)
Compound 9b was prepared by a procedure analogous to that described for 9a to afford 58% of 9b. 1H NMR (300 MHz, CDCl3) δ 7.24–7.44 (m, 4H), 7.15 (m, 1H), 7.10 (t, J = 7.9 Hz, 1H), 6.67 (d, J = 1.9 Hz, 1H), 6.60 (d, J = 7.9 Hz, 1H), 6.52 (at, J = 1.9 Hz, 1H), 3.78–3.96 (m, 2H), 3.55–3.75 (m, 3H), 2.96–3.12 (m, 1H), 2.58 (dd, J = 12.8, 4.5 Hz, 1H), 2.31 (dd, J = 12.8, 9.8 Hz, 1H), 2.24 (s, 3H), 1.69–1.84 (m, 2H), 1.54–1.69 (m, 2H), 1.37–1.54 (m, 4H), 1.32 (d, J = 6.8 Hz, 3H), 0.90 (d, J = 6.8 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 129.35, 128.72, 127.68, 127.07, 121.95, 115.52, 112.22, 67.83, 62.56, 56.22, 51.90, 38.36, 34.41, 32.77, 29.42, 26.11, 25.14, 22.41, 16.02.
(S)-3-(5-Carbomethoxypentyloxy)methamphetamine Hydrochloride (10b)
Compound 10b was prepared by a procedure analogous to that described for 10a to afford 67% of 10b. 1H NMR (CDCl3) δ 7.20 (t, J = 7.5 Hz, 1H), 6.67–6.81 (m, 3H), 3.94 (t, J = 6.4 Hz, 3H), 3.67 (s, 3H), 2.73–2.87 (m, 1H), 2.68 (dd, J = 13.2, 6.8 Hz, 1H), 2.58 (dd, J = 13.2, 6.4 Hz, 1H), 2.39 (s, 3H), 2.35 (t, J = 7.2 Hz, 2H), 1.80 (ap, J = 7.2 Hz, 2H), 1.71 (ap, J = 7.2 Hz, 2H), 1.40–1.62 (m, 3H), 1.06 (d, J = 6.0 Hz, 3H). 13C NMR (300 MHz, CDCl3) δ 174.4, 159.7, 138.0, 129.7, 121.4, 115.4, 113.0, 67.6, 56.8, 51.4, 39.9, 33.9, 30.5, 28.9, 25.6, 24.6, 16.1.
General Synthesis of Hapten-protein Antigens
In preliminary studies, each hapten, (+)-METH MO6 or (+)-METH MO10, was covalently bound to at least 2–3 different protein antigens: cationized bovine serum albumin (c-BSA), ovalbumin (OVA), or bovine serum albumin (BSA) and then used to immunize groups of mice (see below) to test for the serum anti-(+)-METH immune response. The individual mouse and hapten-protein antigen combination that yielded the highest anti-(+)-METH IgG titers from each group of immunizations was chosen for production of mAbs.
A. From c-BSA
To generate the antigens for immunization with the c-BSA carrier protein, each hapten was conjugated to Imject® SuperCarrier® Immune Modulator (c-BSA; Thermo Fisher, Rockford, IL), following the manufacturer’s protocol. To separate the hapten/c-BSA conjugates from uncoupled hapten and other synthesis reagents, the antigens were passed through a PD-10 Sephadex G-25 column (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) equilibrated with phosphate-buffered saline. Absorbance of the protein eluent was monitored at 280 nM to determine when to collect the conjugated product as it eluted from the column. Once collected, the final antigen concentrations were determined by adding 8.3 μL of antigen solution to 250 μL of Coomassie® Plus Protein Assay Reagent (Pierce Chemical Company, Rockford, IL) and comparing the color development against a BSA standard curve. A microtiter plate reader with detection at 590 nM was used to detect the color change. The final products were stored at −20 °C until needed.
B. From BSA and OVA
To generate the antigens for immunization with OVA or BSA carrier proteins, we used a carbodiimide procedure similar to the method of Owens et al.15 All reagents used in hapten-protein conjugation were obtained from Sigma Chemical Company (St. Louis, MO) unless otherwise specified. For the synthesis of MO6 antigens using c-BSA, the starting hapten/carrier protein molar ratio was 90:1. For the synthesis of MO10 antigens using OVA or BSA, the starting hapten/carrier protein molar ratio was 60:1. These ratios of hapten to carrier protein were optimized in preliminary experiments. Each hapten was dissolved in 1 mL of DMF or 0.1M 2-(N-morphalino)ethanesulfonic acid buffer (pH 4.5) to conduct the synthesis. The reaction mixture was allowed to stir overnight at 25 °C protected from light. The final product was dialyzed against 4 L of deionized water (with at least 2–3 changes of water) for 24 h at 4 °C then against 4 L of pH 7.35 phosphate buffered saline (with at least 2–3 changes) for 24 h at 4 °C to yield the hapten/protein antigens. Due to the protein modification resulting from the addition of haptens to the protein surface, the antigens often partially precipitated. After dialysis, the soluble and insoluble fractions of the antigenic preparations were stored at −20 °C until needed for immunization.
Mass Spectrometry Analysis of (+)-MO6-BSA, (+)-MO6-OVA, (+)-METH MO10-BSA, and (+)- METH MO10-OVA
Since the analysis of (+)-MO6 and (+)-MO10 antigens were similar, we will only discuss (+)-MO10 antigens. Hapten to carrier protein incorporation rates for the (+)-MO6-cBSA or (+)-M010-cBSA antigens were not determined, as discussed earlier. Mass spectra were obtained of (+)-METH MO10-OVA and (+)-METH MO10-BSA using a Bruker Reflex III MALDI TOF mass spectrometer. The mass spectrometer was operated in the linear mode with an acceleration voltage of approximately 25 kV. Pulsed ion extraction with a delay of 700 ns was used. The instrument was calibrated using a commercial reference material from the manufacturer (Bruker Protein Standard II), for high mass (10–100 kDa) range calibration. Ion suppression was used up to m/z 10 KDa to enhance the high mass ions. After synthesis of the (+)-METH MO10-OVA or (+)-METH MO10-BSA conjugate, a small aliquot of the soluble antigen in the supernatant fraction, along with the native OVA or BSA protein, was analyzed using dihydroxy benzoic acid (approximately 10 mg/mL in 0.1% TFA/ACN 2:1) as the MALDI matrix. Small volumes of dialyzed samples were mixed 1:1 with the matrix solution, and 1μL was spotted on the target. For samples that had not previously been dialyzed, a standard desalting Zip Tip procedure was used to clean-up and concentrate the samples. However, the protein dialysis procedure against deionized H2O consistently produced better results.
Immunization, Screening, and Hybridoma Generation
Female BALB/c mice (Charles River Laboratories, Wilmington, MA) were used for all immunizations, as previously described by Peterson et al.10 For production of the (+)-METH MO6 and (+)-METH MO10 mAbs, all mice were initially immunized subcutaneously in the hindquarters with 10–100 μg of antigen emulsified in either Hunter’s TiterMax adjuvant (for mAb9B11 and mAb10E1) or Freund’s Complete Adjuvant (for all others). This initial immunization was followed by a boost with 10–50 μg of antigen emulsified in TiterMax or Freund’s Incomplete Adjuvant (or Freund’s Complete Adjuvant for the mice used to produce mAb1A2, mAb1C1, mAb10D1, mAb7F9, and mAb9C10; see Table 1) three to six weeks later. Subsequent boost followed at six week intervals until a high anti-(+)-METH IgG titer was reached. Serum samples were taken via tail bleed periodically to measure anti-(+)-METH IgG response by a enzyme-linked immunosorbant assay (ELISA) similar to the method described by Peterson et al.10
After sufficient anti-(+)-METH IgG titers were achieved (typically after 2–4 months of immunization), hybridomas were produced using previously reported methods.16 The hybridoma fusion partner for mouse B cells was cell line P3X63Ag8.653 (American Type Culture Collection, Manassas, VA). Once hybridomas were produced, initial screening for potential anti-(+)-METH mAbs was conducted by an ELISA using 96-well microtiter plates coated with the original hapten (either MO6 or MO10) conjugated to a different protein carrier (thyroglobulin, Sigma Chemical Company). This procedure avoided selecting carrier protein-reactive antibodies (e.g., anti-OVA antibodies for mice immunized with a MO10-OVA antigen). To assure that only mouse IgG isotypes were selected during these processes, anti-IgG constant region antibodies were used for the ELISA. The IgG isotype and light chain identity were determined using a mouse antibody isotyping kit (Boehringer Mannheim, Indianapolis, IN). Once potential anti-(+)-METH secreting hybridoma cell lines were discovered in a specific well of a micro titer plate, the cells from this location were repeatedly subcloned to assure the monoclonality of the cell line. The supernatant from these cell lines were also rescreened by a [ 3H]-(+)-METH radioimmunoassay (RIA) with (+)-METH, (+)-AMP, and (+)-MDMA as the inhibitors to confirm that the cell line was secreting IgG antibodies against (+)-METH-like compounds. This RIA method is described in an upcoming section.
Production and Purification
Monoclonal antibodies were produced in either a Cell-Pharm System 2500 hollow fiber bioreactor16,17 (Unisyn Technologies, Inc., Hopkinton, MA) or in a Biostat B 10-L bioreactor (Sartorius Corp., Edgewood, NY).10 All antibodies were harvested and stored at −80 °C until purification. mAb were purified either by affinity chromatography using Protein-G Sepharose (Amersham Biosciences, Piscataway, NJ) as described by Peterson et al.,10 ion exchange chromatography using SP Sepharose (Amersham Biosciences, Piscataway, NJ) as described in Hardin et al.,18 or a combination of the two methods. Following purification, mAbs were concentrated and buffer exchanged into 15 mM sodium phosphate containing 150 mM sodium chloride (pH 6.5–7.5), as described in McMillan et al.19
Determination of Monoclonal Antibody KD and KI Values for (+)-METH, (+)-AMP, and (+)-MDMA
A 100-μL aliquot of (+)-[2′,6′-3H(n)]methamphetamine ([3H]-(+)-METH; specific activity = 18 Ci/mmol; ~50,000 decays/min) in RIA buffer (0.05 M Tris; 0.9% NaCl; 2% BSA; 0.2% NaN3; 0.05% Tween 20, adjusted to pH 7.6) was added to 50 × 14 mm polypropylene test tubes. The [3H]-(+)-METH radioligand was synthesized at the Research Triangle Institute (Research Triangle Park, NC) and was a gift from the National Institute on Drug Abuse (Bethesda, MD). The mAbs (in 100 μL) were diluted in RIA buffer to a concentration that would bind ~15–22% of the ~50,000 decays/min of the [3H]-(+)-METH in each tube. Each tube also received 10 μL of unlabeled (+)-METH [or (+)-MDMA for determining the KI value for (+)-MDMA] at an appropriate range of (+)-METH concentrations above and below the expected KD (or KI) value for each mAb. [3H]-(+)-MDMA was unavailable, so we determined a KI value for binding to each mAb using [3H]-METH as the radioligand and inhibition with unlabeled (+)-MDMA. Next, 20 μL of Pierce MagnaBind™ Protein G Magnetic Beads was added to each tube to allow easy separation of bound (to mAb) and free [3H]-(+)-METH at the end of the assay. The nonspecific binding tubes had all of the same reagents except that a saturating dose (10 μM) of (+)-METH was added to inhibit all specific antibody binding. The tubes were vortexed, centrifuged at 1000 rpm for 30 sec, and incubated overnight with gentle shaking at 4 °C. The next day tubes were centrifuged at 1,000 rpm for 30 sec and placed in a magnetic separating rack for 6 min. The supernatant fluid was slowly aspirated, and the magnetic bead pellet containing mAb was resuspended in the same test tube with 2 mL scintillation fluid (Ecoscint A, National Diagnostics, Atlanta, GA). The tubes were placed into an empty 20-mL scintillation vial carrier and the mAb bound concentration of [3H]-METH in each tube was determined by liquid scintillation spectrometry. An IC50 value for inhibition of [3H]-(+)-METH for each mAb was determined after fitting a logistic curve to the data points using Origin graphing and data analysis software (OriginLab Corporation, Northampton, MA). KD [for (+)-METH or KI (+)-MDMA values for (+)-METH binding to each mAb] were determined after correction for the binding of [3H]-(+)-METH by the method of Akera and Cheng.20
A similar homogenous assay was conducted using [3H]-(+)-AMP with inhibition using (+)-AMP for determination of KD values for (+)-AMP binding to the mAbs. If we determined in initial screening assays that the mAb would have a very high KD value (>1000–5,000 nM) for (+)-AMP, we conducted a heterologous immunoassay with [3H]-METH as the radioligand and inhibition with a high range of unlabeled (+)-AMP doses to obtain an approximate KI value for (+)-AMP binding.
Representative binding curves for mAb4G9 for (+)-METH, (+)-AMP, and (+)-MDMA are shown in Figure 2. Each of the determinations was made with duplicate [for (+)-METH and (+)-AMP] or triplicate [for (+)-MDMA] RIA dose-response curves, and the average value was reported in Table 1.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Drug Abuse under projects DA05477, DA-1-881, DA11560, DA14361, and U01 DA23900.
Footnotes
METH-like Hapten Synthesis and Therapeutic mAbs
ABBREVIATIONS. (+)-METH, (+)-methamphetamine; mAb, monoclonal antibody(ies); (+)-AMP, (+)-amphetamine; (+)-MDMA, (+)-3,4-methylenedioxymethamphetamine; (−)-METH, (−)-methamphetamine; (−)-AMP, (−)-amphetamine; (−)-MDMA, (−)-3,4-methylenedioxymethamphetamine; (±)-MDMA, (±)-3,4-methylenedioxymethamphetamine; BSA, bovine serum albumin; c-BSA, cationized bovine serum albumin; RIA, radioimmunoassay; DAWN, Drug Abuse Warning Network; ED, emergency department; OVA, ovalbumin; ELISA, enzyme-linked immunosorbent assay; MALDI, Matrix-Assisted Laser Desorption/Ionization; MS, mass spectrometry.
Supporting Information Available: Elemental analysis. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.National Association of Counties The Meth Epidemic in America, 2005.
- 2.Murray JB. Psychophysiological aspects of amphetamine-methamphetamine abuse. J Psychol. 1998;132:227–237. doi: 10.1080/00223989809599162. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Office of Drug Control and Crime Prevention. World Drug Report 2000. Oxford University Press; New York: 2000. Methamphetamine; pp. 99–121. [Google Scholar]
- 4.Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States. RAND Corporation; 2005. 2009. [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Office of Applied Studies Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2009. [Google Scholar]
- 6.2005: National Estimates of Drug-Related Emergency Departments Visits. U.S. Department of Health and Human Services, Office of Applied Studies; Washington, DC: 2005. Substance Abuse and Mental Health Services Administration Drug Abuse Warning Network. [Google Scholar]
- 7.Xu Y, Hixon MS, Yamamoto N, McAllister LA, Wentworth AD, Wentworth P, Jr, Janda KD. Antibody-catalyzed anaerobic destruction of methamphetamine. Proc Natl Acad Sci U S A. 2007;104:3681–3686. doi: 10.1073/pnas.0611094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho AK. Ice: A new doage form of an old drug. Science. 2009;249:631–634. doi: 10.1126/science.249.4969.631. [DOI] [PubMed] [Google Scholar]
- 9.Thrash B, Karuppagounder SS, Uthayathas S, Suppiramaniam V, Dhanasekaran M. Neurotoxic effects of methamphetamine. Neurochem Res. 2009 doi: 10.1007/s11064-009-0042-5. http://dx.doi.org/10.1007/s11064-11009-10042-11065. [DOI] [PubMed]
- 10.Peterson EC, Gunnell M, Che Y, Goforth RL, Carroll FI, Henry R, Liu H, Owens SM. Using Hapten Design to Discover Therapeutic Monoclonal Antibodies for Treating Methamphetamine Abuse. J Pharmacol Exp Ther. 2007;322:30–39. doi: 10.1124/jpet.106.117150. [DOI] [PubMed] [Google Scholar]
- 11.Nichols DE, Barfknecht CF, Rusterholz DB, Benington F, Morin RD. Asymmetric synthesis of psychotomimetic phenylisopropylamines. J Med Chem. 1973;16:480–483. doi: 10.1021/jm00263a013. [DOI] [PubMed] [Google Scholar]
- 12.Owens SM, Zorbas M, Lattin DL, Gunnell M, Polk M. Antibodies against arylcyclohexylamines and their similarities in binding specificity with the phencyclidine receptor. J Pharmacol Exp Ther. 1988;246:472–478. [PubMed] [Google Scholar]
- 13.Peterson EC, Laurenzana EM, Atchley WT, Hendrickson HP, Owens SM. Development and preclinical testing of a high-affinity single-chain antibody against (+)-methamphetamine. J Pharmacol Exp Ther. 2008;325:124–133. doi: 10.1124/jpet.107.134395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentry WB, Ruedi-Bettschen D, Owens SM. Development of active and passive human vaccines to treat methamphetamine addiction. Hum Vaccin. 2009;5:206–213. doi: 10.4161/hv.5.4.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MT, Preston JF. A simple modified carbodiimide method for conjugation of small-molecular-weight compounds to immunoglobulin G with minimal protein crosslinking. Anal Biochem. 1981;116:402–407. doi: 10.1016/0003-2697(81)90380-8. [DOI] [PubMed] [Google Scholar]
- 16.Valentine JL, Arnold LW, Owens SM. Anti-phencyclidine monoclonal Fab fragments markedly alter phencyclidine pharmacokinetics in rats. J Pharmacol Exp Ther. 1994;269:1079–1085. [PubMed] [Google Scholar]
- 17.Valentine JL, Owens SM. Antiphencyclidine monoclonal antibody therapy significantly changes phencyclidine concentrations in brain and other tissues in rats. J Pharmacol Exp Ther. 1996;278:717–724. [PubMed] [Google Scholar]
- 18.Hardin JS, Wessinger WD, Proksch JW, Owens SM. Pharmacodynamics of a monoclonal antiphencyclidine Fab with broad selectivity for phencyclidine-like drugs. J Pharmacol Exp Ther. 1998;285:1113–1122. [PubMed] [Google Scholar]
- 19.McMillan DE, Hardwick WC, Li M, Gunnell MG, Carroll FI, Abraham P, Owens SM. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+) methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309:1248–1255. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- 20.Akera T, Cheng VK. A simple method for the determination of affinity and binding site concentration in receptor binding studies. Biochim Biophys Acta. 1977;470:412–423. doi: 10.1016/0005-2736(77)90132-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.