Abstract
Local availability of glycine near N-methyl-d-aspartate receptors (NMDARs) is partly regulated by neuronal glycine transporter 1 (GlyT1), which can therefore modulate NMDAR function because binding to the glycine site of the NMDAR is necessary for channel activation. Disrupting GlyT1 in forebrain neurons has been shown to enhance Pavlovian conditioning and object recognition memory. Here, we reported that the same genetic manipulation facilitated reversal learning in the water maze test of reference memory, but did not lead to any clear improvement in a working memory version of the water maze test. Facilitation in a non-spatial discrimination reversal task conducted on a T-maze was also observed, supporting the conclusion that forebrain neuronal GlyT1 may modulate the flexibility in (new) learning and relevant mnemonic functions. One possibility is that these phenotypes may reflect reduced susceptibility to certain forms of proactive interference. This may be relevant for the suggested clinical application of GlyT1 inhibitors in the treatment of cognitive deficits, including schizophrenia, which is characterized by cognitive inflexibility in addition to the positive symptoms of the disease.
Keywords: GlyT1, NMDA receptor, memory, schizophrenia, water maze
INTRODUCTION
Glycine is an endogenous modulator of glutamatergic neurotransmission via the N-methyl-d-aspartate receptor (NMDAR). The binding to NMDAR's glycine-B site by its endogenous ligands, glycine or d-serine, is required for NMDAR activation by glutamate (Berger, Dieudonne, & Ascher, 1998). Endogenous glycine is normally maintained at sub-saturation levels by glycine transporter 1 (GlyT1) (Aragon & Lopez-Corcuera, 2005; Borowsky & Hoffmann, 1998; Eulenburg, Armsen, Betz, & Gomeza, 2005), which is widely expressed in glial cells (Zafra et al., 1995), but also in glutamatergic synapses in the neocortex and hippocampus (Cubelos, Gimenez, & Zafra, 2005). In particular, its co-localization with NMDARs in the forebrain (Wood, 1995) allows GlyT1 to modulate NMDAR activity by controlling the local glycine availability. Enhanced NMDAR activity therefore can be achieved by systemic pharmacological blockade (Bergeron, Meyer, Coyle, & Greene, 1998; Depoortère et al., 2005; Martina Gorfinkel, Halman, & Lowe, 2004) or constitutive knockout of GlyT1 (Gabernet et al., 2005; Tsai et al., 2004a). A pivotal role of the neuronal GlyT1 component has been demonstrated by the selective GlyT1 deletion in forebrain neurons by CamKIIα-Cre-mediated recombination of a conditional loxP-flanked GlyT1 allele in mice (CamKIIαCre;GlyT1tm1.2fl/fl mice), which led to an increase in the ratio of NMDAR/AMPAR current and resistance to pharmacological blockade of NMDAR (Yee et al., 2006). It therefore represents an effective strategy to enhance NMDAR activity without directly interfering with the composition of the receptor complex (e.g., Tang et al., 1999) or the availability of associated downstream regulatory proteins (for a review, see Wang, Hu, & Tsien, 2006).
Functionally, forebrain neuronal GlyT1 disruption is sufficient to facilitate associative learning (Yee et al., 2006) and object recognition memory in a delay-dependent manner (Singer, Boison, Möhler, Feldon, & Yee, 2007). Furthermore, it also enhances selective learning in the form of latent inhibition: CamKIIαCre;GlyT1tm1.2fl/fl mice exhibited latent inhibition when control mice did not, suggesting that they are more sensitive to the non-reinforcement history of the conditioned stimulus (Yee et al., 2006). This implies that forebrain neuronal GlyT1 deletion does not indiscriminately strengthen the expression of learned behaviour at the expense of the selectivity in learning.
To further characterize this unique balance in cognitive function in these animals, we examined here the effects of forebrain neuronal GlyT1 deletion on spatial working memory function in the hidden platform version of the Morris water maze paradigm (Morris, 1983, 1984). Given that the platform was changed daily, effective performance required not only rapid learning within a day, but also demanded selective recall of the appropriate learned information. Next, the susceptibility to such negative proactive interference effects from previously learned platform positions was more explicitly tested using the reference memory version of the Morris water maze paradigm with new learning introduced repeatedly following the initial acquisition (Morris, 1984). Finally, the hypothesis that forebrain neuronal GlyT1 deletion might generally facilitate reversal learning was evaluated further in a simultaneous two-choice pattern discrimination paradigm (Sutherland & Mackintosh, 1971). The results are expected to be highly relevant to the proposed use of GlyT1 inhibitors in the pharmacotherapy of memory disorders (Coyle & Tsai, 2004; Danysz & Parsons, 1998; Javitt, 2008; Lechner, 2006).
MATERIALS AND METHODS
Generation of CamKIIαCre;GlyT1tm1.2fl/fI mice
In short, the neuron and forebrain specific deletion of the GlyT1 gene was accomplished by CamKIIα-Cre mediated recombination of a conditional loxP-flanked GlyT1 allele (Glyt1tm1.2fl/fl). A full description of the generation of the animals has been reported elsewhere (see Gabernet et al., 2005; Yee et al., 2006). The impacts of the specific deletion of the GlyT1 gene at the biochemical and electrophysiological levels have also been described elsewhere (Yee et al., 2006).
Subjects
The subjects were obtained by crossing CamKIIaCre:Glyt1tm1.2fl/fl mice with Glyt1tm1.2fl/fl mice (both on a pure C57BL/6J background). This allowed us to generate litters with a 1:1 mixture of CamKIIαCre:Glyt1tm1.2fl/fl (hereafter simply referred to as “mutant”) and Glyt1tm1.2fl/fl (“control”) mice and to maintain the Cre gene heterozygous in the mutants. Breeding took place in a specific-pathogen free (SPF) breeding facility. Genotyping was performed on postnatal days 21-30 by standard PCR (Yee et al., 2006). Litters were weaned at postnatal day 21, and male mice (7 mutant and 5 controls) employed in the present study. At the age of 11 weeks, the animals were transferred to a separate controlled animal vivarium (21±1°C, relative humidity at 55±5%) with a reversed light–dark cycle (lights off: 0800–2000). They were kept in groups of four in Makrolon® Type-III cages (Techniplast, Milan, Italy), and maintained under ad libitum water and food (Kliba 3430, Klibamuhlen, Kaiseraugst, Switzerland) unless otherwise stated. Prior to the last experiment described here, the animals were switched to single housing in Makrolon® Type-II cages (Techniplast), they were put on a 22h food-deprivation diet with their body weight reduced to not less than 85% of their ad lib weight.
Behavioural experimentation commenced when they were about 12 weeks old, and was always conducted in the dark phase of the cycle. The experimental manipulations and procedures described here had been previously approved by the Swiss Cantonal Veterinary Office; they conformed to the ethical standards required by the Swiss Act and Ordinance on Animal Protection and the European Council Directive 86/609/EEC.
Apparatus
■ Water maze
The water maze consisted of a white circular fibreglass tank, 102 cm in diameter and 36 cm deep. It was filled with a fresh mixture of hot and cold tap water on each test day to a depth of 19 cm and with a temperature of 24±1°C. A transparent Plexiglas cylinder (diameter 7cm, height 18.5 cm) was used as the escape platform, with its surface submerged 0.5 cm below the water surface and therefore invisible to the animals. Its location however could be flagged by a white circular disk cue (diameter 12 cm) mounted 12 cm directly above the platform. A digital camera was installed above the water maze, capturing and transmitting images at a rate of 5Hz to a PC running the Ethovision tracking system (Noldus, The Netherlands), to compute the escape latency and distance travelled on each trial, as well as the additional dependent measures on probe tests (see procedures below).
The water maze could be positioned in the centre of one of two possible testing rooms (referred as Room 1 and Room 2), each enriched with unique distal spatial cues under dim lighting condition (12 lux as measured from the maze centre). The working memory experiment was conducted in Room 1 throughout. The reference memory experiment with serial new learning was also conducted in Room 1, except for the final stage when the maze was moved to Room 2.
■T-maze
The T-maze (modified from an elevated plus maze) was elevated at a height of 50cm above a table-top 65 cm above floor level. It was made of transparent Plexiglas, comprising four arms (5 × 30 cm) joined at a central square (5 × 5 cm) and forming a cross. One open arm was blocked permanently to give a “T” configuration with the remaining open arm serving as the start stem and the two enclosed arms as choice stems. The maze floor was painted black throughout. The two choice arms (180° apart) were surrounded by two-ply transparent Plexiglas walls (15 cm high), except the side adjoining the central square. The start arm was surrounded by a 3-mm high perimeter rim to prevent slipping off.
Papers of either vertically or horizontally oriented black-white alternating stripes (1cm wide) printed on both sides could be inserted between the layers of the two-ply Plexiglas walls of the enclosed arms. The stripes were interchanged according to a pseudorandom sequence across trials to prevent a spatial solution to the discrimination task. The food reward (25mg Noyes sucrose pellet) was hidden behind a 5mm high barrier positioned 5 cm from the arm's distal end, so as to prevent its visual detection. Exit from the chosen arm was blocked by an opaque plastic barrier wall.
Behavioral Procedures
In the water maze the animals were tested first on working memory, and using the same apparatus, then tested on reference memory. Although both versions of water maze test followed a matching rule within-day and the reference memory task also followed a matching rule between-days (see procedural details below), the design of the working memory task demanded a win-shift rule between days. The latter difference might therefore imply some forms of negative transfer effect regardless of the test order selected. We elected to perform the working memory test in all animals first, because the primary interest here was on repeated new learning, and this test was initiated after performance between groups in the initial acquisition had converged. Hence, the potential negative impact of prior working memory training on new learning in the reference memory test should be minimal. It would have been difficult to counterbalance the test order between the two water maze tests because the duration of each test was not decided beforehand. Following the conclusion of the second water maze test, the animals were evaluated in two-choice discrimination reversal procedure conducted with a T-maze.
■ Cued test in the water maze
On the first day, the animals were pre-trained using a cued (i.e., flagged) platform in order to familiarize them to the apparatus and to swimming in the water maze. This further served as a screen of any non-specific sensorimotor impairment. The platform was positioned in the centre of the maze and each animal underwent two consecutive trials, with the starting positions randomly selected from four release points (N, E, S, and W). In the first trial, the subject was gently released from the starting point with its head facing the platform location. In the second trial it was released from the starting point facing the wall of the maze. The animals were allowed 60s within which to locate the escape platform and on reaching the platform they spent an inter-trail interval (ITI) of 15s on it before the second trial commenced. If an animal failed to locate the platform within the 60s time limit, a maximal escape latency of 60s was recorded and it was guided to the platform by the experimenter and allowed to stay on it for 15s.
■ Working memory test
This was carried out over 12 days. The platform was now kept hidden (without the local cue), and placed in a new position each day, remaining in that position for both trial 1 and trial 2 on that day. Working memory was indexed by the improvement in trial 2, compared with trial 1 when the platform location was unknown. As previously, when an animal failed to locate the platform within 60s, it was guided to it. After 6 days of test with an ITI of 15s, the ITI was extended to 10 min in the next 6 tests days. Over the extended ITI, the animals were kept in an opaque waiting box in the testing room. Twelve platform positions were defined: six located at a distance of 35cm (in the N, S, NE, SE, SW, and NW directions), and six more at a distance of 15cm from the centre (in the N, E, S, W, NW and SE directions). The start position was always different for consecutive trials, and selected randomly from eight possible release points (N, E, S, W, NE, NW, SE and SW) for each mouse.
■ Reference memory & Serial new learning
This commenced five days after the working memory test, with the animals being trained first to locate the escape platform, which was now hidden at a constant location. There were 4 trials per daily session, conducted at an ITI of 15s, and carried out over 8 days. Testing was conducted in the same testing room as the working memory test had taken place (denoted as Room 1). Here, the position of the platform was fixed in the middle of quadrant SE, 35cm from the maze centre. The starting position was varied between eight possible release points (N, E, S, W, NE, NW, SE and SW), and determined for each animal separately as described above.
Probe tests were conducted prior to training on day 5, and again on day 9 (not followed by any training with platform), and are referred to as probe test 1a and 1b, respectively. The start positions of NW and SW were used on these two probe trials, respectively. During a probe test, the platform was removed and the animals were allowed to swim freely in the maze for 45s. The animals' spatial search pattern during the probe tests was analysed.
Next the animals were tested with 3 consecutive new spatial reference memory problems as described above, in blocks of 4 days. On days 10-13, the platform was hidden in quadrant NW (35cm from maze centre) in Room 1. On days 14-17, the platform was hidden in quadrant SW (35cm from maze centre) in Room 1. On days 18-21, the platform was hidden in quadrant NE (35cm from maze centre) but with the maze moved to Room 2 (a novel testing room). Prior to the beginning of the last day's training in each block of new learning (i.e., on days 13, 17 and 21), a probe test was performed (referred to as probe test 2, 3, and 4) as described above. The starting positions for these three additional probe tests were: SE, NE, and SW, respectively.
■ Acquisition and reversal of a simultaneous two-choice discrimination
One month after the conclusion of the water maze experiments, the animals were switched to single caging, and introduced gradually to a food deprivation diet, with a progressive reduction of feeding time across five days (12h, 6h, 4h, 2h, 2h). Discrimination learning commenced on the next day, with the animals maintained on 2-h feeding per day throughout the experimental period. Animal body weight was monitored daily and was not permitted to fall below 85% of the ad lib weight.
First, the animals were habituated to the T-maze over three consecutive days, by being individually placed in it, and allowed to explore freely for 5 min. On day 1, fifteen reward pellets were randomly scattered over the entire maze surface. On day 2, five pellets were scattered in each of the two choice arms. On day 3, five pellets were placed in the goal area at the end of each of the two choice arms. During habituation, plain white papers were inserted into the two-ply Plexiglas walls of both choice arms in order to make them identical.
Discrimination learning between vertical (V) and horizontal (H) visual patterns commenced on the next day. Half of the animals within each group were assigned the [V+ vs H−] contingency, and the other [V− vs H+] contingency, with ‘+’ and ‘−’ indicating whether or not the corresponding discrminandum was associated with food reward. The relative positions (i.e., left vs right choice arms) of the vertical and horizontal discriminanda were randomized across trials.
On days 1 and 2 of training, the animals were given 4 and 8 trials, respectively. Twelve trials were given on all subsequent days. The ITI was 20s in which the animals were placed inside a cardboard waiting box, and the maze was cleansed with 70% alcohol. To begin a trial, the animal was released at the end of the start arm, facing the central square, and allowed to travel down to the choice point to enter one of the two choice arms. A choice was deemed to have been made when the animal's head crossed halfway into a choice arm, upon which the exit was blocked. A correct choice was rewarded by 2 reward pellets, and an incorrect choice was followed by confinement to the chosen arm for 15s. The animals were allowed one minute to make a choice, after which the trial would be aborted. There were no aborted trials from day 2 onwards.
When an animal scored ≥10 correct choices per day on three consecutive days, it was considered to have reached the criterion for successful acquisition of the initial discrimination. It would then be subjected to reversal learning the next day with the reward contingency of the two discriminanda reversed. Training continued until the animals had achieved criterion performance in the reversal phase. The number of trials required to attain criterion performance in the acquisition and the reversal phase were taken as the dependent measures, with the presence of a reversal effect indicated by a higher number of trials to criterion in the reversal than in the acquisition phase.
Statistical Analysis
All data were subjected to parametric analysis of variance (ANOVA) with the between-subject factor genotype and the addition of any relevant within-subject factors (e.g., days, trials, quadrants and probes). The ANOVAs were performed using SPSS® for Windows (Release 13.0) on a PC running the Microsoft Windows XP Professional (SP2) operating system. Significant main effects and interaction terms were further investigated by appropriate restricted ANOVAs. A priori restricted ANOVAs were also conducted to assist interpretation of the data whenever necessary.
RESULTS
Cued test in the water maze
First, the animals were familiarized to the water maze procedure in a cued task with the location of the escape platform indicated by a local cue mounted directly above it. This pre-training phase also allowed the detection of possible non-specific differences in sensory and motor function.
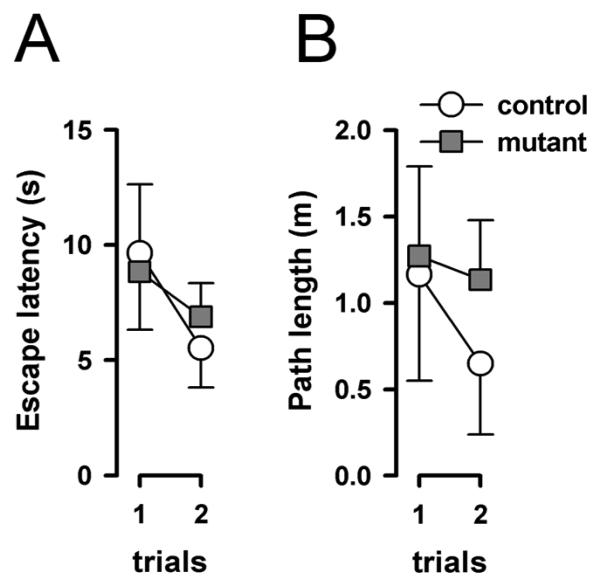
All animals rapidly acquired the escape response by locating and climbing onto the escape platform. Performance was already high on the first trial, because the animals were released in a direction facing the platform (Figure 1). There was a slight improvement from trial 1 to 2, but this did not attain statistical significance, based on separate 2 × 2 (genotype × trials) ANOVAs of escape latency and path length. Neither analyses yielded any indication of a genotype effect or its interaction [all F's<1]. The additional analysis of average swim speed also failed to show any significant effect [all F's<1]. The mean(±SE) swim speeds (in cm/s) of the two groups were: controls = 12.1±1.5, mutants = 15.5±1.1. The results indicated that escape performance between the mutant and control mice did not differ significantly at this stage of the task, when memory of the platform location was not explicitly required.
Figure 1.
Performance in the cued task in the water maze as indexed by escape latency (A) and path length (B). Error bars refer to ±SEM, mutant (n=7), control (n=5).
Working memory test in the water maze
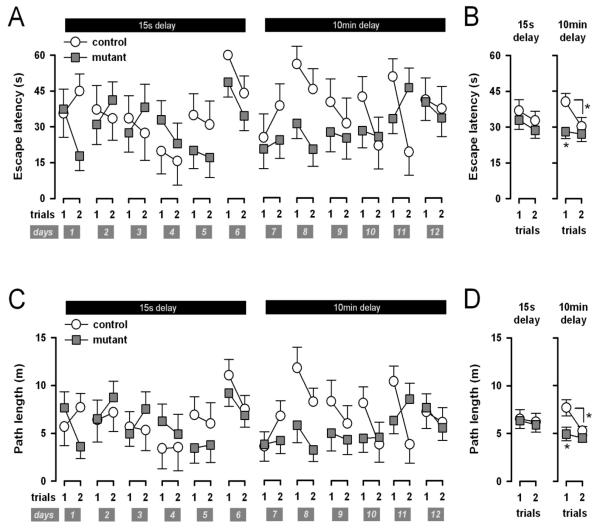
Next, working memory function was evaluated across twelve consecutive days. On each day, the animals were required to learn the new location of a hidden platform revealed to them on the first trial, and to quickly escape onto it on the second trial. The platform assumed a novel location on every day, thus demanding the use of working memory – in recalling the appropriate platform location learned on the day. During the first six days, the delay from trial 1 to 2 was limited to 15s (the minimal delay); this was extended to 10 min in the following six days in order to increase the retention demand of the task.
Over the first six days, there was limited improvement in escape performance over trials when the ITI (or delay) between trial 1 and 2 was minimal – both in terms of escape latency and path length (Figures 2A~2D). Separate 2 × 6 × 2 (genotype × days × trials) ANOVA of escape latency and path length only yielded a significant effect of days [F(5,50)=3.90, p<0.005; F(5,50)=2.95, p<0.05, respectively]. Therefore there was no statistical support for an improvement of escape performance from trials 1 to 2 within these 6 days of training. No significant effect emerged from the analysis of swim speed. The mean (±SE) swim speeds (in cm/s) of the two groups on trials 1 and 2 were as follows: controls/trial 1 = 17.8±1.7, controls/trial 2 =20.1±1.6, mutants/trial 1 = 18.1±1.3, mutants/trial 2 = 19.8±1.2.
Figure 2.
Performance in the working memory task in the water maze as indexed by escape latency (A & B) and path length (C & D), across trials and days (A & C), or averaged across days as a function of trials (B & D). Error bars refer to ±SE, mutant (n=7), control (n=5). * p<0.05 based on restricted analyses, contrasting mutant and control performance on trial 1 (collapsed across days), and contrasting between trials 1 and 2 in the control mice.
Throughout the next six days (days 7–12) the ITI was increased to 10 min with the performance as follows: There was a notable improvement from trial 1 to 2 in the control but not the mutant mice, as illustrated in Figures 2B and 2D with performance averaged over days. However, it is also apparent that the two genotypes differed substantially in performance in trial 1, when the platform location was essentially unknown. On trial 2, on the other hand, the two groups were closely matched in both escape latency and path length. A 2 × 6 × 2 (genotype × days × trials) ANOVA of escape latency yielded a near-significant effect of trials [F(1,10)=4.72, p=0.05], and a suggestive tendency for a genotype effect [F(1,10)=3.84, p=0.08]. These statistical outcomes were paralleled by a main effect of trials [F(1,10)=5.98, p<0.05] and of genotype [F(1,10)=6.41, p<0.05] from the equivalent analysis of path length. To identify more precisely the source of the significant main effect of genotype, analysis restricted to each trial yielded a clear genotype effect only in trial 1 [latency: F(1,10)=6.16, p<0.05; path length: F(1,10)= 6.16, p<0.05], but not in trial 2 [corresponding F's<1]. However, the critical genotype × trials interaction term failed to reach significance in either performance measure [latency: F(1,10)=1.83, p=0.21; path length: F(1,10)=2.12, p=0.18]. Hence, it cannot be concluded that the saving from trial 1 to 2 differed statistically between mutant and control mice.
Parallel analyses of swim speed equivalent to all those described above never yielded any suggestion of a significant effect or interaction. The mean(±SEM) swim speed (in cm/s) of the two groups on trials 1 and 2 was as follows: controls/trial 1 = 19.4±1.7, controls/trial 2 =18.9±0.8, mutants/trial 1 = 16.3±1.3, mutants/trial 2 = 15.0±0.6.
The results of the working memory test indicated that mutant mice behaved differently in the 10min delay condition in comparison to the controls on trial 1. The data clearly do not support the hypothesis that forebrain neuronal GlyT1 deletion is associated with enhanced working memory, but the pattern of the results also does not suggest that mutant mice were impaired in working memory function. This divergence in trial 1 performance precludes a simple interpretation in terms of enhancement or impairment. As explained later (see Discussion), this might suggest that the mutant mice had resorted to a non-mnemonic search strategy, independent of extra maze cues, and thus had protected them against the negative impact of proactive interference, but such a strategy had at the same time minimized performance improvement from trial 1 to 2. In contrast, the control mice were relying on spatial mnemonic, and therefore under some degree of negative proactive interference, leading to lower trial 1 performance relative to controls, but allowing a degree of saving to be observed from trial 1 to 2. We therefore examined the tendency of the animals returning to the vicinity of the previous day's platform location on trial 1. This assesses the presences of possible influence of memories from the previous day's platform position.
On a given day N, the proportion (in percent) time or search path length (prior to the subject having located the new platform location) spent within a circular zone, measuring 14cm in diameter, centred on the previous day's (Day N-1) platform position were derived for trial 1. The two delay conditions were separately analysed by a 2 × 5 (genotype × days) ANOVA: Days 2 to 6 were included in the 15-sec delay condition; and Days 8 to 12 in the 10-min delay condition. As shown in Table 1, the mice exhibited a tendency in returning to the vicinity of previous day's platform above that expected by chance alone (1.9%) in both delay conditions, and this tendency was similarly observed in both mutant and control mice. This tendency somewhat varied amongst days, as evidenced by the presence of a significant days effect in the 10-min delay condition [percent path length in target zone: F(4,40)=3.27, p<0.05; percent time in target zone: F(4,40)=2.59, p=0.051]. Otherwise, there was no statistical indication for any significant genotype × days interaction in all analyses. Hence, mutants and controls appeared to be similarly influenced by memories of previous platform positions in their spatial search when the novel platform location had yet to be made known to the animals on trial 1.
Table 1. Tendency to return to the previous day's platform location on trial 1.
Percent time and percent path length (Trial 1, Day N) recorded in the target zone (14cm in diameter) centring on the previous day's (Day N-1) platform position. Data (mean±SE) presented were collapsed across days. Both mutant and control mice exhibited a tendency to return to the vicinity of the former platform position as would be expected by chance alone (i.e., >1.9%; p's<0.05 based on one-sample t-test).
| Trial 1-2 Delay | Days included | Variable | Mutant | Control |
|---|---|---|---|---|
| 15 sec | Days 2-6 | % time | 4.62±0.56 | 3.64±0.67 |
| % path length | 4.81±0.63 | 4.08±0.75 | ||
| 10 min | Days 8-12 | % time | 3.94±0.34 | 3.58±0.41 |
| % path length | 4.43±0.42 | 4.87±0.49 | ||
Although this additional analysis failed to show a differential sensitivity to past memories of platform location, it demonstrated that the present working memory test was under the influence of pro-active interference.
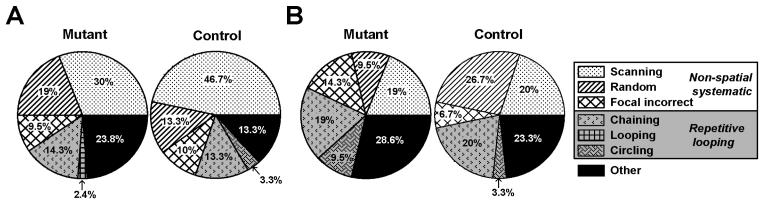
The possibility that the anomalous performance by mutant mice on trial 1 (emerged in the extended delay condition) might stem from the adoption of some systemic search strategy was evaluated by examining the swim paths recorded on trial 1 (see Figure 3). Following the classification scheme of search strategies described by Brody and Holtzman (2006), we attempted to identify some kind of non-mnemonic spatial scheme (not relying on memory of the spatial location of the platform as defined by the constant extra maze cues) such as “chaining” – swimming along a circular corridor at some specific distance from the wall that encompasses a higher probability of locating the platform than by a random search over the entire maze area. Such a strategy might conceivably give rise to the phenotype revealed here. The relative frequency of the different strategies summarized in Figure 3 indicates that the occurrence of chaining did not differ substantially between mutant and controls [χ2 test, ns]; and its frequency was only slightly higher in the 10-min delay condition in comparison to the 15-s delay condition. This analysis also showed that different forms of systematic search remained predominant (~50% collectively), whereas a looping-type strategy accounted for roughly 15% of the search patterns examined. In summary, this analysis suggested that (i) the animals did not favor one particular strategy in the exclusion of others on trial 1 of the working memory test, (ii) the mutant mice's aberrant performance on trial 1 (10-min delay condition) could not be entirely attributed to enhanced prevalence of a ‘chaining’ strategy, (iii) there was a lack of clear strategic difference between mutant and control mice within this particular classification system, although the possibility remains that a sufficiently complex system of analysis might reveal some subtle (yet significant) differences.
Figure 3.
Relative prevalence of different search strategies identified on trial 1 of the working memory experiment.
Search strategies were classified under blind conditions by two independent experimenters according to the qualitative system described by Brody and Holtzman (2006). Only six out of the nine strategies defined by these authors could be clearly identified here because short swim paths characterized by a direct trajectory to the platform were only meaningfully interpretable in a reference memory task or at least when the animals were expected to know the platform location. This was not the case here for swim paths derived only from trial 1 of each day's working memory test.
The three strategies under the category of Systematic search are: ‘scanning’ (searching randomly in the interior portion of the pool), ‘random’ (searching randomly in the entire pool), and ‘focal incorrect’ (searching intently in a quadrant that does not contain the platform before moving to another area of the pool). Under the category of Looping is: ‘chaining’ (circular swimming at a fixed distance from the wall) ‘peripheral looping’ (persistent swimming around the outer 15 cm of the pool), and ‘circling’ (swimming in tight circles with some net directional movement). Search paths that did not conform to any of the above descriptions were classified collectively as “other”.
Each pie chart illustrates the proportion (expressed in percent) of the different search strategies in mutants and controls, separately for the 15-s (A) and the 10-min (B) delay conditions. Separate χ2 test of independence conducted at each delay condition did not reveal any significant difference in the relative deployment of different strategies between mutant and control mice; and both did not yield a significant association. An additional χ2 test of independence was used to gauge the impact of delay on strategy use between the two delay conditions, which also failed to reveal a significant association.
Reference memory: From initial acquisition to multiple new learning
To further examine the importance of proactive interference, we next employed the water maze reference memory procedure with additional manipulations designed to maximize the influence of proactive interference. To this end, we changed the demands of the water maze task to evaluate reference memory function, with the platform location no longer changing from one day to the next, but remaining in the same position across an extended period of days. The solution by reference memory (in contrast to working memory) was therefore encouraged, because information acquired on a day would be relevant to the solution between as well as within days. However, we also challenged the animals by changing the position of the platform without warning once performance had indicated successful acquisition. This was designed to evaluate the impact of proactive interference on new acquisition, and was repeated in discrete phases, until the last phase when the entire maze was moved to a new testing room. It was expected that the mutant mice might show facilitation only when the position of the escape platform was suddenly changed, and that this effect would be eliminated when new learning took place in a novel testing room where there would be no proactive interference to negatively influence the control mice.
During the initial acquisition phase, one male control mouse exhibited very weak swimming accompanied by severe floating from day 4 onwards and was therefore excluded from this experiment. The sample size of the control group was thus reduced to 4. The four phases of reference memory learning are separately analysed and detailed below as problems 1 to 4.
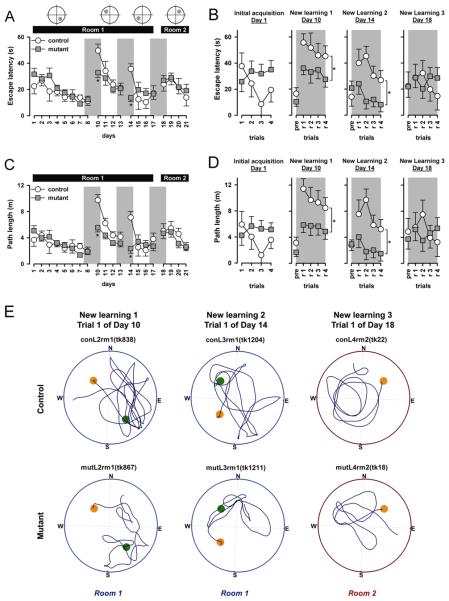
Problem 1
First, the initial acquisition over the first eight days was examined. Performance showed a progressive improvement over test days as evidenced by both escape latency (Figure 4A) and path length (Figure 4C), and this was equivalently observed in both mutant and control mice. Consistent with this impression, separate 2 × 8 × 4 (genotype × days × trials) ANOVAs of escape latency and path length yielded a main effect of days [latency: F(7,63)=5.15, p<0.0005; path length: F(7,63)=3.7, p<0.005]; but neither the main effect of genotype nor its interactions attained statistical significance. To facilitate comparison with later analyses that focused on the first day of new learning, we also performed such a 2 × 4 (genotype × trials) ANOVA of the first day of acquisition. This revealed no statistically significant effects. The results on the first day of the initial learning phase (and subsequent phases of new learning) are depicted as a function of trials separately in Figures 4B and 4D.
Figure 4.
Performance in the initial reference memory task and subsequent new learning in the water maze as indexed by escape latency (A & B) and path length (C & D). An overview of performance is expressed as a function of days (A and C). To allow a closer inspection of both performance measures [escape latency (B) and path length (D)], performance as a function of trials on day 1, day 10, day 14 and day 18, are illustrated separately. The critical transition from one phase to the next (new) learning is highlighted by the shaded background in A and C (across days); and in B and D (across trials). Performance on the first day in each phase of new learning, is further depicted in a trial-by-trial manner, in comparison to the final trial in the preceding phase of learning (indicated as ’pre’) in the corresponding plot presented with a shaded background (B and D). Error bars refer to ±standard error, mutant (n=7), control (n=4). * p<0.05, based on restricted analyses contrasting performance on the first day of new learning (New learning 1 and 2) between mutant and control mice. The different locations of the platform used in each phase of the experiment are illustrated in the sketches on top of A. Representative swim paths are illustrated (E) for the first trial at new-learning phases of 2, 3, and 4 (from left to right). The top row refers to control performance, and the bottom mutant mice. Current platform location is indicated by the orange circle, and the previous phase's platform location the green circle. Tests conducted in Room 1 and Room 2 are indicated by the blue and red perimeter borders, respectively. N, E, S and W refer to the cardinal reference coordinates used in the experiment.
Problem 2
Over days 10 to 13, the animals were trained using a new platform location. As expected, performance reduced drastically on the initial day. It took the control mice much longer to locate the platform in comparison to the initial day of the first acquisition, which constitutes the reversal effect. In contrast, although the mutant mice also experienced an increase in search time and path length, they re-bounded to a level comparable to their performance on the first day of the first acquisition. This led to the divergence of performance on day 1 of new learning, although the two groups eventually converged by the fourth day of new learning. These impressions were confirmed by separate 2 × 4 × 4 (genotype × days × trials) ANOVAs of escape latency and path length. Both analyses revealed a significant genotype by days interaction [latency: F(3,27)=2.98, p<0.05; path length: F(3,27)=3.85, p<0.05] which was accompanied by a significant days effect [latency: F(3,27)=21.27, p<0.0001; path length: F(3,27)=24.51, p<0.0001]. The main effect of genotype also attained significance in the analysis of path length [F(1,9)=10.67, p<0.01]. Separate analyses of each of the four days confirmed that the mutant and control only differed on the first day of new learning, yielding a main effect of genotype [latency: F(1,9)=7.28, p<0.05; path length: F(1,9)=14.68, p<0.005]. And, this effect was apparent across the 4 trials on the first day (see Figures 4B and 4D).
Problem 3
Over the next four days, the animals were trained with yet another platform location. A very similar pattern of results emerged (Figure 4). The control mice exhibited a pronounced reversal effect on the first day of new learning, whereas the mutant mice hardly showed such an effect. Again the two groups largely converged in the subsequent three days, such that their performances were indistinguishable by the fourth day. Separate 2 × 4 × 4 (genotype × days × trials) ANOVAs of escape latency and path length over these four days again yielded a highly significant genotype by days interaction [F(3,27)=5.83, p<0.005 and F(3,27)=6.03, p<0.005, respectively], which was accompanied by a significant days effect in the escape latency analysis [F(3,27)=3.16, p<0.05] and a near significant days effect in the path length analysis [F(3,27)=2.82, p=0.06]. Additional analyses restricted to each day also indicated that the mutant and control mice differed significantly from each other only on the first day of new learning [latency: F(1,9)=19.41, p<0.005; path length: F(1,9)=16.78, p<0.005], but not on any subsequent days. Again, the difference between mutant and control mice persisted throughout the four trials on the first day (see Figures 4B and 4D).
Problem 4
On the following four days, new learning was conducted in a novel testing room (Room 2) distinct from the one that was used thus far. This change led to the disappearance of the mutant mice's phenotype revealed in the previous two new spatial learning problems (Figure 4). On the first day of this phase of new learning, both groups returned to a level of performance similar to that seen on the first day of the first acquisition phase. Throughout the four days, the behavior of both mutant and control mice was highly similar. ANOVAs of escape latency and path length only yielded a significant effect of days [F(3,27)=3.29, p<0.05 and F(3,27)=5.29, p=0.005, respectively]. No other effects even approached statistical significance [all F's<1] (see Figures 4B and 4D).
Swim Speed
Analyses of swim speed at each phase of spatial learning failed to reveal any significant effect, except in the last new learning phase (Problem 4) conducted in Room 2, which yielded a significant genotype effect [F(1,9)=9.09, p<0.05], as the mutant mice were swimming at a slower speed than controls [mean(±SEM): controls = 19.2±1.1 cm/s and mutants = 15.0±0.8 cm/s]. This however should not compromise the interpretation of the results in this stage, because the impressions obtained from escape latency (which might be expected to be confounded by swim speed) and path length were essentially identical. For comparison, the mean speeds (cm/s) of the two groups at the different stages of learning in Room 1 were as follows. First learning: controls = 16.2±0.8, mutants = 14.8±0.6; second learning: controls = 19.0±1.3, mutants = 15.7±1.0; third learning: controls = 19.1±1.2, mutants = 16.1±0.9. These indicated a stable non-significant tendency that mutant mice were swimming consistently at a marginally slower speed. This would not be expected to contribute to their apparent superior performance on day 1 in successive new learning.
In summary, the results had confirmed our expectation that mutant mice would show an advantage over the controls when the platform assumed a novel position, when control mice would evidently suffer from some form of proactive interference. It was apparent that information acquired from the preceding phase(s) had impeded acquisition of the new location in the control group, whilst the mutant mice by contrast were much less affected by this negative proactive interference. When the procedure prevented such interference from affecting the control mice, the difference between groups essentially disappeared.
Reference memory: Probe trials
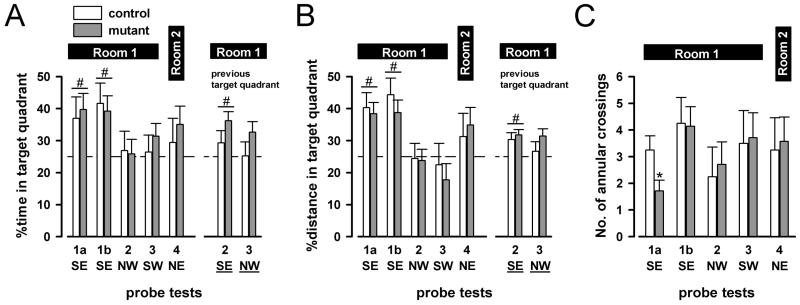
During the course of the reference memory test, probe tests (in which the escape platform was removed from the maze) were performed. Two probe trials were conducted during the first acquisition phase (1a and 1b). Subsequently, one probe trial was conducted prior to training on the 4th (and the last) day in each successive new learning phase (probe tests 2, 3 and 4). Three main measures were taken from each probe test to index search accuracy: percent time spent in the target quadrant, percent path length (distance) made in the target quadrant and the number of annular crossings made to the relevant platform location at each learning phase.
Figures 5A and 5B illustrate the first two measures across the five successive probe tests. Probe tests 1a and 1b were conducted in the middle and at the end of the first acquisition phase, respectively. Both mutant and control mice showed a clear preference to the target (SE) quadrant. By contrast, performance in probe tests 2 and 3 was relatively low, and close to chance level; and mutant and control mice again behaved similarly. In probe test 4, which was conducted in Room 2 just prior to the last day's training, performance was somewhat intermediate between probes 1a~1b and probes 2~3. These impressions obtained from these two measures are highly congruent with each other.
Figure 5.
Probe trial performance is indexed by percent time in target quadrant (A), percent distance in target quadrant (B) and the number of annular crossings (C). Two probe trials were conducted during the first acquisition phase (1a and 1b). Subsequently, one probe trial was conducted prior to training on the 4th (and the last) day in each successive new learning phase (probe tests 2, 3 and 4). The corresponding target quadrant is also listed below the x-axis. In addition, percent time (A) and percent distance (B) spent in the (underlined) quadrants in which the platform was located in the previous learning phase are also illustrated for probe tests 2 and 3. Error bars refer to ±SEM, mutant (n=7), control (n=4). # indicates that mutants and controls performed significantly above chance level (25%) based one-sample t-tests separately conducted for the five probe tests (p<0.05). * denotes a significant difference (p<0.05) between mutants and controls according to a one-way ANOVA of annular crossings restricted to probe trial 1a.
Separate 2 × 5 (genotype × probes) ANOVAs yielded a near-significant effect of probes in percent time spent [F(4,39)=2.59, p=0.05], and in percent distance in target quadrant [F(4,39)=6.23, p<0.001]. There was no indication of any genotype effect or its interaction [all F's<1]. To assess whether preference for the target quadrant differed from chance level performance, we conducted separate one-sample t-tests for each of the five probe tests. To this end, mutant and control mice were pooled for the purpose of analysis because there was no statistical indication of any significant genotype difference in the overall ANOVAs. In agreement with the above impressions: significant above-chance preference (in percent time or percent distance) for the current target quadrant was detected in probe tests 1a and 1b only [p<0.005], although a non-significant tendency seemed also to be present in probe test 4 [p<0.1].
Next, we also examined whether the two genotypes differed in terms of their search in the quadrants containing the escape platform in the preceding learning phase – on probe tests 2 and 3 (see Figures 5A and 5B). The relatively poor performance on probe tests 2 and 3 might stem from a tendency to search in the target quadrant of the previous learning phase. To this end, we conducted 2 × 2 (genotype × quadrants) ANOVA contrasting the two relevant (current vs previous target) quadrants for probe tests 2 and 3 separately. However, none of these analyses (based either on percent time or percent distance per quadrant) yielded any significant effects. Likewise, we also evaluated whether preference for the previous target quadrant might differ from chance level using one-sample t-tests as described before. These suggested a highly significant above-chance preference for the previous target quadrant in probe test 2 [p<0.005 and p<0.001 for percent time and percent distance, respectively], but not in probe test 3.
The overall lack of any genotype effects described above was consistent with additional one-way ANOVAs conducted for each probe test (either current or previous target quadrant), and two-way ANOVAs that included all four quadrants within a given probe test.
Finally, the measure of annular crossings was examined as an additional dependent variable of search accuracy. As illustrated in Figure 5C, mutant and control mice performed similarly on this measure, although a transient impairment in the mutant was detected in probe test 1a. A 2 × 5 (genotype × probes) ANOVA however failed to yield any significant effect, including the critical interaction term [F<1]. Nonetheless, we conducted separate one-way ANOVAs for each successive probe test. These yielded a significant main effect of genotype only in probe test 1a [F(1,9)=5.31, p<0.05], indicating that search accuracy was lower in the mutant mice on this particular probe trial, although they performed similarly to control mice from probe test 1b onwards.
Overall, the various probe tests confirmed that at the end of the first acquisition phase, and by the end of each additional phase of new learning, the mutant and control mice were largely similar in performance, exhibiting similar spatial search in the water maze. This suggests that both groups were relying on extra maze to a large extent in guidance of their spatial search.
Discrimination reversal
As an explicit and alternative test of the proactive interference effect on learning, we next compared the mutant and control mice on an appetitive (motivated by food reward) non-spatial two-choice discrimination task on a T-maze. Proactive interference was demonstrated by reversing the reinforcement contingency of the two visual discriminanda upon successful discrimination. In the initial acquisition, two mutant mice failed to acquire the running response, and they were dropped from the experiment at the end of day 2. The number of subjects in the mutant group was thus reduced to 5. All controls from the reference memory test (n=4) were included here.
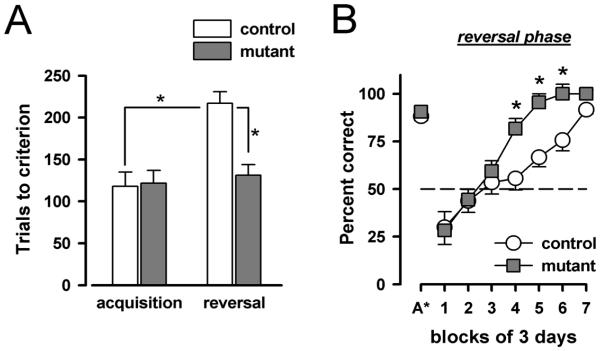
As depicted in Figure 6A, mutant and control mice did not differ in the acquisition of a pattern discrimination task in the T-maze. They took a similar number of trials before reaching criterion performance. However, their performance markedly differed in the reversal phase. The control mice displayed a clear reversal effect: they took longer to achieve criterion performance in the reversal phase in comparison to the initial acquisition. By contrast, the reversal effect was absent in the mutant mice. These impressions were confirmed by a 2 × 5 (genotype × phases) ANOVA of the dependent measure, which yielded a significant interaction [F(1,7)=6.99, p<0.05], accompanied also by the emergence of a significant main effect of genotype [F(1,7)=11.41, p<0.05]. Analyses of performance restricted to each phase indicated the presence of a highly significant genotype effect in the reversal phase [F(1,7)=21.00, p<0.005], but not in the acquisition phase [F<1]. Analyses restricted to each genotype confirmed the presence of a reversal effect in the control [F(1,3)=48.76, p<0.01] but not in the mutant mice [F<1].
Figure 6.
Performance in the acquisition and reversal phase of the discrimination reversal experiment is indexed by the number of trials required to achieve criterion performance (A). Error bars refer to ±SEM, mutant (n=5), control (n=4). * p<0.05, indicates the presence of a reversal effect in the control mice by a restricted analysis contrasting their acquisition and reversal performance; and the attenuation of the impact of reversal in the mutant mice by a contrast between mutant and control mice in their reversal performance. Choice accuracy achieved at the end of acquisition, and in the course of the succeeding reversal phase is expressed in percent correct every 3-day blocks in (B). The horizontal (dotted) reference line indicates chance level. A* refers to the final 3-day block of acquisition phase (of individual mouse) in which criterion performance was achieved. The initial drop below chance level is evident in both mutant and control mice. However, the two groups diverge at about chance level: while the control group shows a characteristic inflexion at around 50% chance levels, the mutant shows a straight linear progression through the 50% barrier. * denotes between-groups difference at p<0.05, based on the pooled variance taken from the error term associated with the significant genotype by blocks interaction.
To allow an examination of the progression of learning in the reversal phase, we performed a separate analysis of choice accuracy as a function of 3-day blocks throughout the course of reversal training (Figure 6B). Animals having achieved criterion performance before the 7th block were assigned a criterion score of 100% on subsequent blocks. There was a clear drop of performance at the onset of reversal compared to the end of acquisition (denoted as “A*” in the x-axis of Figure 6B) in both mutant and control mice. However, the two groups diverged at about chance level: while the control group showed a characteristic inflexion at around 50% correct trials (when the competition over behavioural control by the old and new reward contingency was expected to be maximal), the mutant showed a straight linear progression through the 50% barrier. A 2 × 7 (genotype × blocks) ANOVA of percent correct over the seven blocks of reversal learning yielded a significant main effect of genotype [F(1,7)=6.21, p<0.05], of blocks [F(6,42)=61.16, p<0.001], and critically of their interaction [F(6,42)=4.26, p<0.005]. Consistent with the shape of this interaction, with the two groups matching at the end and beginning of the reversal phase, orthogonal trend analysis indicated that the interaction term was significant specifically in the quadratic trend [F(1,7)=30.17, p<0.0001].
The results of this experiment clearly showed that the reversal effect was attenuated in the mutant mice, which is consistent with the hypothesis based on previous experiments that they were less prone to the negative effect of proactive interference. The phenotype on new learning therefore appeared to be only apparent when previously acquired behaviour is potentially in conflict with current behaviour.
DISCUSSION
The present study has provided clear evidence that GlyT1 disruption restricted to forebrain neurons is sufficient to induce anomalous behaviour suggestive of enhanced cognitive flexibility across a variety of learning situations, when negative proactive interference is substantially limiting performance in the controls. These novel findings represent significant advances in the cognitive characterisation of CamKIIαCre;GlyT1tm1.2fl/fl mice, far beyond our previous demonstrations of their phenotypes in enhancing Pavlovian associative learning and instrumental active avoidance learning (Yee et al., 2006), and in improving memory retention in an object recognition test (Singer et al., 2007). Hence, forebrain neuronal knockout of GlyT1 does not simply strengthen memory trace or increase the rate of learning. The constellation of behavioural phenotypes identified thus far in CamKIIαCre;GlyT1tm1.2fl/fl mice is suggestive of a general enhancement in behavioural adaptability presumably due to enhanced NMDAR function in multiple forebrain structures. As in our previous reports, some of the critical findings were not derived from completely naïve animals, and there may be concern over the contribution of their previous learning experience to some of the observed phenotypes. This centres on the critical issue of generality of the findings, which indeed needs to be addressed regardless of whether the data were derived from behaviourally naïve animals or not. Here, we have attempted to deal with this interpretative issue in a within-subject manner: From the tentative phenotype seen in the working memory experiment, to the clear replicable phenotype in the reference memory experiment, and extension subsequently to the non-spatial discrimination reversal experiment. Collectively, our findings are highly instructive for the potential use of GlyT1 inhibitors as therapeutic agents in different forms of cognitive inflexibility present in a number of neuropsychiatric conditions, and their potential application as cognitive enhancing agents in non-pathological situations.
The first phenotype here emerged in the working memory test. Over the first six days with minimal delay, learning as indexed by improvement from trial 1 to 2 was weak in all animals. With further training, a statistically significant effect of trials was detected in the next six days with a longer delay. This is likely attributable to the general effect of training rather than an effect of a change in delay intervals. It was against this background that a difference in performance was observed: the mutant mice were performing somewhat better than controls on trial 1, and showed very limited change in performance from trial 1 to 2. The latter does not seem to reflect a floor effect because performance was still at ~30s and ~5m in escape latency and path length, respectively. Should one therefore interpret this as an indication of poor working memory? Such a conclusion would be premature given that it did not conform to the typical pattern of working memory deficit with comparable trial 1 performance and poor trial 2 performance. A satisfactory explanation would need to accommodate both anomalies observed here in the mutant mice, namely, improved trial 1 performance and lack of trial 1 to 2 saving.
The apparent lack of trial 1 to 2 saving may indicate that the mutant mice were not resorting to memory of the platform location acquired in trial 1 to guide their search in trial 2. This as we discovered later in the reference memory experiment was not due to their inability to use extra maze cues. The mutant mice might have developed some non-mnemonic strategies, the nature of which remains to be delineated. However, if such strategies are not dependent on memory of spatial location of the platform as defined by the extra maze cues (e.g., using strategy such as ‘chaining’), they would be expected to out-perform the controls provided that the trial 1 performance in controls was limited by any forms of proactive interference. Although we failed to identify any difference in the occurrence of chaining strategy, the possibility that strategies of a similar non-mnemonic nature might be detectable with more detailed and quantitative analyses cannot be excluded. The scheme we adopted from Brody and Holtzman (2006) was qualitative in nature, and it was originally developed for analysis of reference water maze learning, and its application here for analysis of working memory swim track, and on trial 1 specifically when the platform location was not known to the animals, might limit our interpretation.
Moreover, even without specializing in one unique form of non-mnemonic strategy, but with a mixture of such strategies, the mutant mice would be less susceptible to potential proactive interference by the previous day's platform location, which, particularly in trial 1 each day, affected the performance of the control mice. If so, the present two-trials-per-day paradigm was not sufficiently demanding to tax working memory in the mutant mice. They were able to resort to a non-mnemonic strategy without losing out to the controls in terms of overall escape performance: the controls' gain in performance from trial 1 to 2 failed to compensate for the gain achieved by the mutant's non-mnemonic strategy in trial 1. It would therefore be very interesting to assess the impact of additional trials, when continual use of such a strategy by the mutant should eventually lose out to the controls who would be expected to make further gains in performance if there were more trials to follow on the same day. Would the mutant mice maintain such a strategy, or would they begin to rely on recollection of the platform location learned from previous trials? Although we cannot address this in our working memory test since only two trials were performed per day, the first day of the reference memory test provided such an opportunity (even with the ITI back at 15s) as the constancy of location of the platform across days had yet to be made known to the animals.
As illustrated in Figures 4B and 4D, there was hardly any indication in the mutant mice of an improvement in performance in both dependent measures across trials on the first day of initial acquisition in the reference memory test. At the same time, the controls displayed a non-significant trend towards improvement across trials. Although this contrast failed to achieve statistical significance here, it certainly deserves further evaluation. As training on the reference memory task progressed, however, the mutant mice apparently gained in performance, displaying both within-day and between-days improvement that was highly comparable to the control mice. Thus, the overall performance of the two groups of mice was closely matched across the eight days of the first reference memory test (Figures 4A and 4C) in spite of the anomaly on the first day described above. The results of the two probe tests (conducted in the middle and at the end of the initial acquisition) are in full agreement with this impression (see results of probe tests 1a and 1b depicted in Figures 5A and 5B), as is the observation of poor spatial search in the first probe test suggested by the measure of annular crossings. The latter probably implies that the mutant mice were still following a non-mnemonic strategy (adopted since the working memory test) in the early phase of acquisition. Nonetheless, our results clearly indicated that in the water maze test, the mutant mice could acquire a reference memory solution in a manner that was essentially indistinguishable from the controls by the end of acquisition training. It was following this that the second clear phenotype emerged.
Next, when the platform was moved to a new location, performance reduced in all subjects as expected. However, whilst the mutant mice were substantially less affected by this manipulation, the control mice exhibited a clear reversal effect – performance at the beginning of new learning was poorer than at the beginning of the first acquisition. This reflected a negative proactive interference effect; memory of the first platform location interfered with the learning of the second location. The mutant mice on the other hand reverted to a level of performance not dissimilar to the beginning of their first learning; they thus essentially did not show a reversal effect. The absence of a reversal effect (or reduced proactive interference) in the mutant mice observed here cannot be attributed to poorer learning in comparison to the control in the first phase, because the performance of the two groups remained highly comparable throughout the first phase of learning, in particular with respect to their probe test performance at the end.
Following another 4 days of training with the second platform location, the escape platform was once again moved, and a very similar pattern of results emerged. The initial negative impact of platform re-location was again notably weaker in the mutant mice. To test the idea that this advantage enjoyed by the mutant over the control mice was dependent on a proactive interference effect operating in the controls, we introduced the next new learning not only by moving the platform to a new location as defined by the maze itself, but the entire maze was moved to a novel and distinct testing room (Room 2). By re-setting the extra maze cues, and thereby removing the source of proactive interference, it was predicted that the two groups should no longer respond differently to the initiation of new learning. This prediction was confirmed by the elimination of the reversal effect previously seen in the control mice by moving the maze to Room 2. The initial performance of the control mice in this phase matched their initial performance in the first acquisition, but the change in rooms had little impact on the mutant mice. The difference in initial new learning between mutant and control mice seen previously in Room 1 was thereby eliminated in Room 2. This also represents a replication of the result in the acquisition the very first reference memory problem. This provides some indication that the animals had not developed a learning set (Harlow, 1949) in spite of the repeated presentations of differing reference memory problems (four in totals). Even if the change in control performance in Room 2 was a mixture of learning set development and the resetting of extra maze cues, our conclusion that overcoming proactive interference remains a parsimonious and consistent account of the mutant's phenotype in the water maze studies.
Further examination of the probe tests conducted in Room 1 on the last day of each new learning phase indicated that preference for the most recent target platform was weak in both mutant and control mice (see Figures 5A and 5B). This likely suggests that when the platform was removed from the water maze, making escape impossible, neither mutants nor controls focused their search solely on the more recent target quadrant. It can be seen that they expended significantly more time (and swam further) than would be expected by chance searching in the preceding target quadrant. The fact that this was equivalently seen in both groups again suggests that the anomalous response exhibited by the mutant mice at the initiation of new learning did not stem from a memory retention deficit per se. This leads to the conclusion that the phenotype observed in the mutant was directly related to the presence of the escape platform in a new location. They detected the new location more effectively, perhaps because they were less persistent in searching where the platform used to be, and were able to use this newly acquired knowledge quickly in subsequent trials. This may also suggest that they were more responsive to the absence of the platform in the predicted location, and were thus less hindered than the controls. This facilitation should therefore be understood strictly in contrast to the presence of persistent search exhibited by the control mice on the first day of new learning. The mutant mice were thereby able to detect the new location of the platform earlier than controls; and as a consequence perhaps, more readily shifted their search preference to the vicinity of the new location. On the first day of new learning, the mutant mice consistently outperformed the control animals across all four trials, leading to a significant genotype effect on such days. However, the advantage of the mutants was temporary, as the control mice were able to catch up in subsequent training days.
In summary, we were able to show that forebrain neuronal deletion of GlyT1 can lead to some forms of learning enhancement, but the emergence of such effects is highly specific to the test situation. Its presence is linked to conditions in which the control mice are negatively affected by proactive interference. Although further evaluation is needed to clarify if this interpretation fits the results of the working memory test, its application to the reference memory (and repeated new learning) results represents a simple and parsimonious explanation. We therefore adopted a suitable paradigm as a direct test of this hypothesis. To this end, we conducted a two-choice simultaneous discrimination reversal task, to maximize the proactive interference effect: not only were the animals required to stop visiting the previously rewarded arm, they needed to choose the previously non-rewarded arm in order to obtain food reward. In addition, we implemented this test in a non-spatial setting to further assess the generality of the findings obtained in the water maze. The results of the discrimination reversal experiment (see Figure 6A) are strongly supportive of the hypothesis that the CamKIIαCre;GlyT1tm1.2fl/fl mice are resistant to the negative impact of proactive interference on learning, especially when maximal competition between the old and new reward contingency on behaviour was expected (see Figure 6B). This is indicative that the phenotype observed was affecting (the effect of proactive interference on) response selection or attentional function, rather than response perserveration – i.e., in persisting responding to the former response-reward contingency. This offers a possible explanation of, and is consistent with, the fact that we failed to observe any clear suggestion that mutant mice returned less to the previous day's platform location in the working memory test.
Further experimentation on related paradigms in set-shifting would be highly relevant in identifying the critical psychological impact by which GlyT1 forebrain neuronal deletion may enhance behavioural flexibility. In this respect, suggestion that GlyT1 inhibition also exerts a functional impact on prefrontal and accumbal dopaminergic activity (Deportèere et al., 2005; Leonetti et al., 2006; Singer, Feldon, & Yee, 2009) may be relevant. This is consistent with the known interaction between NMDAR and dopaminergic mechanisms in relevant cortical–subcortical circuits underlying behavioral flexibility such as set shifting and related motivational and attentional mechanisms (e.g., Egerton et al., 2008; Floresco, Zhang, & Enomoto, in press; Rodefer, Murphy, & Baxter, 2005; Stefani, & Moghaddam, 2003, 2005; for a review also see Boulougouris, & Tsaltas, 2008). Hence, the possibility that the mutant mice's phenotypes observed in reversal and new learning may stem from changes in the balance of NMDAR-dopamine interactions certainly warrant considerations.
Here, although the present study yielded little support for a cognitive enhancing effect in these animals on the acquisition and retention of spatial reference memory as reported by Tsai et al. (2004a) in constitutive GlyT1 +/− mice, we have provided specifications (of the cognitive demands) as to when inhibition of GlyT1 activity may prove particularly effective in facilitating learning. The nature of this specificity suggests that the cognitive effects of our genetic manipulation on GlyT1 expression have particular impact on the executive functions of memory – i.e., the selective expression of learned information, and the readiness to adopt and entertain possible alternative strategies in problem solving (e.g., escaping from water) in a given test situation. This specification is highly relevant to the possible clinical application of GlyT1 inhibitors in the treatment of cognitive deficits. Indeed, this profile fits the suggested therapeutic efficacy of GlyT1 inhibitors against the negative and cognitive symptoms of schizophrenia, which are characterized by overt preservative behaviour and cognitive inflexibility (e.g., Chang, Liu, Chiu, & Tsai, 2005; Lane; Shim, Hammonds, & Kee, 2008; Tsai, Lane, Yang, Chong, & Lang, 2004b).
The GlyT1 deletion achieved in our mutant mice is expected to enhance the availability of glycine in the vicinity of the NMDAR and thereby to affect the activity of this receptor. A recent report has shown that systemic d-serine treatment (600mg/kg/day) also enhances reversal learning in mice (Duffy, Labrie, & Roder, 2008). However, it should be pointed out that the effect of d-serine reported by Duffy et al. (2008) is qualitatively rather different from the enhancement of the new learning demonstrated here in the CamKIIαCre;GlyT1tm1.2fl/fl mice. d-serine pre-treatment did not facilitate learning in the initial phase of new learning (in a reference memory task design; see Figure 3a of Duffy et al. (2008) as our mutant mice did, when proactive interference was expected to be at its strongest. Instead, d-serine was only effective when new learning was already into its third day. Moreover, d-serine treated mice demonstrated a clear preference for the new platform location in the probe test conducted following reversal learning, against a lack of such preference in the vehicle-treated animals (Duffy et al., 2008). In spite of this finer difference, it may be tempting to speculate that modulation of NMDAR function via the glycine-B site is particularly implicated in reversal learning and similar situations when proactive interference is pronounced.
In the same study, Duffy et al. (2008) also reported an enhancement effect of d-serine on working memory as evaluated in the water maze, similar in design to the first experiment here. The effect however appeared weak and transient: (a) it was only apparent on days 5-8 in the test, but not the four days before or after that, (b) this effect coincided with a time when the control failed to improve from trial 1 to 2, and (c) it was only reported in the measure of path length but not escape latency. One may rather conclude that d-serine enhances the consistency of working memory performance rather than mnemonic power in terms of increased memory load and resistance to temporal decay. However, even when d-serine's effect on working memory is far from convincing, its impact on behaviour is still markedly dissimilar to that of forebrain neuronal GlyT1 deletion revealed here. As discussed above, we cannot be certain that the mutant mice employed a matching-to-position (demanding spatial working memory) strategy in the test. This strategy is prone to proactive interference and thus an alternative non-mnemonic strategy can nonetheless enable effective performance, especially when only two trials were administered. Indeed, one can envisage that although a matching-to-position strategy would allow improvement from trial 1 to 2, this may not be sufficient to outpace the overall performance produced by a non-mnemonic strategy. In this respect, it should be noted that the test design employed by Duffy et al. (2008) employed four consecutive trials, and this might promote the competitive edge associated with a matching-to-position strategy over any non-mnemonic strategy.
What seems to be a recurring feature is that regardless of whether it was d-serine treatment or the genetic disruption of GlyT1 studied here, the enhancing effect of either manipulation was most clearly seen when the control groups were performing particularly poorly. The latter may be by design, as in the discrimination reversal experiment here, or perhaps accidental as the results by Duffy et al. (2008) seem to suggest. This is noteworthy because our previous demonstration of enhanced object recognition memory in the CamKIIαCre;GlyT1tm1.2fl/fl mice was also against the background of a complete lack of novelty preference in the control mice (Singer et al., 2007). This was achieved at a delay retention interval of two hours, which should still yield appreciable novelty preference in wild type C57BL/6 mice according to some studies (e.g., Hale & Good, 2005). Similarly, we reported that CamKIIαCre;GlyT1tm1.2fl/fl mice exhibited the latent inhibition effect when control mice failed to do so (Yee et al., 2006). The number of stimulus pre-exposures employed was typically sufficient to generate latent inhibition in wild type C57BL/6 mice. Although the results here did not suggest that our control mice behaved differently from wild type mice of the same genetic background, yet the emergence of apparent cognitive enhancing effects tended to coincide with relatively weak performance in the controls. Weak performance may not only be the result of task difficulty, but it may also arise when the solution to the task is inconsistent with previous experience – leading to some form of cognitive dissonance or conflict, as in the case of reversal learning. Thus, although proactive interference is a prominent source of cognitive conflict in the experiments reported here, the possibility remains that GlyT1 inhibition or glycine-B site activation may promote the resolution of such conflict whenever a similar cognitive demand is elicited. An obvious step forward is to evaluate the situation under retroactive interference. One argument that reduction in proactive interference may not be the key task demand to solicit the memory/learning enhancing effect of GlyT1 disruption is the finding of enhanced latent inhibition in CamKIIαCre;GlyT1tm1.2fl/fl mice (Yee et al., 2006).
One theoretical account of the latent inhibition effect is precisely in terms of proactive interference (e.g., Bouton, 1993; Kraemer & Spear, 1991; Weiner, 1990), such that non-reinforced exposures to a neutral conditioned stimulus (CS) leads to formation of an [CS-nothing] association, which competes or interferes with the expression of a conditioned response to the CS following the pairing between the CS and an unconditioned stimulus (US). If GlyT1 disruption reduces proactive interference per se, it should attenuate rather than enhance the latent inhibition effect. One alternative account we recently suggested (Singer, Feldon, & Yee, 2008) is that CamKIIαCre;GlyT1tm1.2fl/fl mice learned both [CS→nothing] (during pre-exposure) and [CS→US] (during conditioning) more effectively, and hence allowing the former association to reveal its impact on the expression of conditioned response resulting from the latter association. In contrast, the test parameters were insufficient to generate latent inhibition in the control mice because the impact of both [CS→nothing] and [CS→US] on subsequent non-responding and responding to the CS, respectively, was weaker than in the mutant mice (Yee et al., 2006). In this manner, the enhancement of latent inhibition in CamKIIαCre;GlyT1tm1.2fl/fl mice reported earlier (Yee et al., 2006) can be explained solely in terms of enhanced associative learning as a result of enhanced NMDAR activity. Indeed, it has been shown that NMDA blockade by MK-801 (0.1-0.2mg/kg i.p. in rats) produces the exact symmetrical pattern of results: MK-801 abolished the latent inhibition effect by reducing conditioning in the non-pre-exposure subjects (see Gaisler-Salomon & Weiner, 2003; their Figure 1).
Hence, although the diverse phenotypes emerged in this mutant mouse line may be, to varying degrees, understood as a form of cognitive enhancement across different experimental preparations, they do not necessarily conform to a single psychological description. This may not be too surprising given that the mutation affected the whole forebrain and thus would exert an effect on multiple psychological functions. Given that the NMDAR is generally involved in synaptic plasticity, the phenotypic profile of our mutant mice is likely to stem from altered NMDAR function across multiple forebrain structures. The current knockout system does not allow one to differentiate the relative contributions of different forebrain brain regions. To satisfactorily address this, either region-specific molecular intervention or local pharmacological blockade would be necessary.
Here, we have emphasized the importance of fine behavioural analysis to pinpoint more precisely the nature of potential cognitive enhancing effects associated with our specific genetic manipulation targeting GlyT1. A similar approach would also benefit evaluation of other manipulations with similar pro-cognitive potential. In this respect, one should not overlook the possible caveats in the present study. The fact that we performed the reference memory test after the working memory test in the same cohort of subjects may introduce possible transfer effects, and thus might confound the interpretation of the findings emerging from the latter test. This potential shortcoming led us to perform the two-choice simultaneous discrimination reversal experiment as a further confirmation of our major finding. Despite the reduced number of subjects, the effect was very clear, suggesting that when the condition is conducive the associated statistical effect size can be considerable. The observed phenotype therefore seems very robust.
In terms of the nature of this cognitive enhancement, our results may bring about the following afterthought: Just as cognitive disability is context-specific (Durkin et al., 2006), cognitive enhancement should be similarly considered (e.g., Sandberg & Bostrom, 2006). Hence, any given therapeutic manipulation that can bring about biases or shifts in a given cognitive function may arguably lead to both benefits as well as harms, depending on the precise circumstances. Learning (as an exemplary cognitive function) has evolved as it bestows the ability to exploit predictive cues in one's environment so as to enhance adaptability through appropriate behavioural adjustment or modification. Thus, flexibility is a pre-requisite to effective learning, and any extreme or rigid form of cognitive bias is likely to be detrimental and counterproductive. The behavioural profile of the CamKIIαCre;GlyT1tm1.2fl/fl mouse line does not seem to fall into this trapping, and it may suggest that pharmacological interference of GlyT1-mediated glycine re-uptake may represent a promising strategy to enhance adaptive cognitive behaviour, and to correct maladaptive cognitive deficits in a number of neuropsychiatric conditions including schizophrenia (Coyle & Tsai, 2004: Danysz & Parsons, 1998; Javitt, 2008; Lechner, 2006).
ACKNOWLEDGEMENTS
The present study was supported by the NCCR (National Centre for Competence in Research) in Neural Plasticity and Repair, with funding from the Swiss National Science Foundation (SNSF), the SNSF grant 3100–066855, and NIH grant MH083973. Additional support from the Swiss Federal Institute of Technology and the Good Samaritan Hospital foundation are also duly acknowledged. We would also like to express our gratitude to Peter Schmid for technical support, Liz Weber for her assistance in PCR genotyping, Dr. Frank Bootz for his veterinary expertise, and the animal technicians for their assistance in animal husbandry.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
REFERENCES
- Aragon C, Lopez-Corcuera B. Glycine transporters: crucial roles of pharmacological interest revealed by gene deletion. Trends in Pharmacological Sciences. 2005;6:283–286. doi: 10.1016/j.tips.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Dieudonne S, Ascher P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. Journal of Neurophysiology. 1998;80:3336–3340. doi: 10.1152/jn.1998.80.6.3336. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Hoffmann BJ. Analysis of a gene encoding two glycine transporter variants reveals alternative promoter usage and a novel gene structure. Journal of Biological Chemistry. 1998;273:29077–29085. doi: 10.1074/jbc.273.44.29077. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Progress in Brain Research. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- Bouton M. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Experimental Neurology. 2006;197:330–340. doi: 10.1016/j.expneurol.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia. Psychopharmacology (Berl) 2004;174:32–38. doi: 10.1007/s00213-003-1709-2. [DOI] [PubMed] [Google Scholar]
- Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cerebral Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons AC. Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacological Reviews. 1998;50:597–664. [PubMed] [Google Scholar]
- Depoortère R, Dargazanli G, Estenne-Bouhtou G, Coste A, Lanneau C, Desvignes C, Poncelet M, Heaulme M, Santucci V, Decobert M, Cudennec A, Voltz C, Boulay D, Terranova JP, Stemmelin J, Roger P, Marabout B, Sevrin M, Vigé X, Biton B, Steinberg R, Françon D, Alonso R, Avenet P, Oury-Donat F, Perrault G, Griebel G, George P, Soubrié P, Scatton B. Neurochemical, electrophysiological and pharmalogical profiles of the selective inhibitor of the glycine transporter-1 SSR504734, a potential new type of antipsychotic. Neuropsychopharmacology. 2005;30:1963–1985. doi: 10.1038/sj.npp.1300772. [DOI] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC. D-Serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Schneider H, Pathania VS, Nelson KB, Solarsh GC, Bellows N, Scheffler RM, Hofman KJ. Learning and Developmental Disabilities. In: Jamison DT, editor. Disease Control Priorities in Developing Countries. Second Edition Oxford University Press; New York: 2006. pp. 933–951. [Google Scholar]
- Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt JA. Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology. 2008;198:37–49. doi: 10.1007/s00213-008-1071-5. [DOI] [PubMed] [Google Scholar]
- Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends in Biochemical Sciences. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural Brain Research. 2009 doi: 10.1016/j.bbr.2008.12.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gabernet L, Pauly-Evers M, Schwerdel C, Lentz M, Bluethmann H, Vogt K, Alberati D, Möhler H, Boison D. Enhancement of the NMDA receptor function by reduction of glycine transporter-1 expression. Neuroscience Letters. 2005;373:79–84. doi: 10.1016/j.neulet.2004.09.064. [DOI] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Weiner I. Systemic administration of MK-801 produces an abnormally persistent latent inhibition which is reversed by clozapine but not haloperidol. Psychopharmacology. 2003;116:333–342. doi: 10.1007/s00213-002-1311-z. [DOI] [PubMed] [Google Scholar]
- Hale G, Good M. Impaired visuospatial recognition memory but normal object novelty detection and relative familiarity judgements in adult mice expressing the APPswe Alzheimer's disease mutation. Behavioral Neuroscience. 2005;119:884–891. doi: 10.1037/0735-7044.119.4.884. [DOI] [PubMed] [Google Scholar]
- Harlow HF. The formation of learning sets. Psychol Rev. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glycine transport inhibitors and the treatment of schizophrenia. Biological Psychiatry. 2008;63:6–8. doi: 10.1016/j.biopsych.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Kraemer PJ, Spear NE. The effect of nonreinforced stimulus exposure on the strength of a conditioned taste aversion as a function of retention interval: Do latent inhibition and extinction involve a shared process. Animal Learning & Behavior. 1991;20:1–7. [Google Scholar]
- Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Archives of General Psychiatry. 2005;62:1196–1204. doi: 10.1001/archpsyc.62.11.1196. [DOI] [PubMed] [Google Scholar]
- Lechner SM. Glutamate-based therapeutic approaches: inhibitors of glycine transport. Current Opinion in Pharmacology. 2006;6:75–81. doi: 10.1016/j.coph.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Leonetti M, Desvignes C, Bougault I, Souilhac J, Oury-Donat F, Steinberg R. 2-Chloro-N-[(S)-phenyl [(2S)-piperidin-2-yl] methyl]-3-trifluoromethyl benzamide, monohydrochloride, an inhibitor of the glycine transporter type 1, increases evoked-dopamine release in the rat nucleus accumbens in vivo via an enhanced glutamatergic neurotransmission. Neuroscience. 2006;137:555–564. doi: 10.1016/j.neuroscience.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Martina M, Gorfinkel Y, Halman S, Lowe JA. Glycine transporter type 1 blockage changes NMDA receptor responses and LRP in hippocampal CA pyramidal cells by altering extracellular glycine levels. Journal of Physiology. 2004;557:489–500. doi: 10.1113/jphysiol.2004.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. An attempt to dissociate ‘spatial mapping’ and ‘working-memory’ theories of hippocampal function. In: Seifert W, editor. Neurobiology of the hippocampus. Academic Press; New York: 1983. pp. 405–432. [Google Scholar]
- Morris RGM. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Murphy ER, Baxter MG. PDE10A inhibition reverses subchronic PCP-induced deficits in attentional set-shifting in rats. European Journal of Neuroscience. 2005;21:1070–1076. doi: 10.1111/j.1460-9568.2005.03937.x. [DOI] [PubMed] [Google Scholar]
- Sandberg A, Bostrom N. Converging cognitive enhancements. Annals of the New York Academy of Sciences. 2006;1093:201–227. doi: 10.1196/annals.1382.015. [DOI] [PubMed] [Google Scholar]
- Shim SS, Hammonds MD, Kee BS. Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. European Archives of Psychiatry and Clinical Neuroscience. 2008;258:16–27. doi: 10.1007/s00406-007-0757-8. [DOI] [PubMed] [Google Scholar]
- Singer P, Boison D, Möhler H, Feldon J, Yee BK. Enhanced recognition memory following glycine transporter 1 deletion in forebrain neurons. Behavioral Neuroscience. 2007;121:815–825. doi: 10.1037/0735-7044.121.5.815. [DOI] [PubMed] [Google Scholar]
- Singer P, Feldon J, Yee BK. Interference of glycine transporter 1: Modulation of cognitive functions via activation of glycine-B site of the NMDA receptor. CNS Agents in Medicinal Chemistry. 2008;7:259–268. [Google Scholar]
- Singer P, Feldon J, Yee BK. Interactions between the glycine transporter 1(GlyT1) inhibitor SSR504734 and psychoactive drugs in mouse motor behaviour. European Neuropsychopharmacology. 2009 doi: 10.1016/j.euroneuro.2009.02.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Distinct contributions of glutamate receptor subtypes to cognitive set-shifting abilities in the rat. Annals of the New York Academy of Sciences. 2003;1003:464–467. doi: 10.1196/annals.1300.064. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behavioral Neuroscience. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Sutherland NS, Mackintosh NJ. Mechanisms of Animal Discrimination Learning. Academic Press; New York: 1971. [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Jiang Z, Caine SB, Coyle JT. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2004a;2101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Lane HY, Yang P, Chong MY, Lange N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biological Psychiatry. 2004b;55:452–456. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Wang H, Hu Y, Tsien JZ. Molecular and systems mechanisms of memory consolidation & storage. Progress in Neurobiology. 2006;79:123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Weiner I. Neural substrates of latent inhibition: the switching model. Psychological Bulletin. 1990;108:442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- Wood PL. The co-agonist concept. Is the NMDA-associated glycine receptor saturated in vivo? Life Sciences. 1995;57:301–310. doi: 10.1016/0024-3205(95)00288-h. [DOI] [PubMed] [Google Scholar]
- Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Möhler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. Journal of Neuroscience. 2006;26:3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. Journal of Neuroscience. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]