Abstract
These electrophysiological experiments, in slices and intact animals, study the effects of in vivo chronic exposure to nicotine on functional α4β2* nAChRs in the nigrostriatal dopaminergic (DA) pathway. Recordings were made in wild-type and α4 nicotinic acetylcholine receptor (nAChR) subunit knock-out mice. Chronic nicotine enhanced methyllycaconitine citrate hydrate-resistant, dihydro-β-erythroidine hydrobromide-sensitive nicotinic currents elicited by 3–1000 μm ACh in GABAergic neurons of the substantia nigra pars reticulata (SNr), but not in DA neurons of the substantia nigra pars compacta (SNc). This enhancement leads to higher firing rates of SNr GABAergic neurons and consequently to increased GABAergic inhibition of the SNc DA neurons. In the dorsal striatum, functional α4* nAChRs were not found on the neuronal somata; however, nicotine acts via α4β2* nAChRs in the DA terminals to modulate glutamate release onto the medium spiny neurons. Chronic nicotine also increased the number and/or function of these α4β2* nAChRs. These data suggest that in nigrostriatal DA pathway, chronic nicotine enhancement of α4β2* nAChRs displays selectivity in cell type and in nAChR subtype as well as in cellular compartment. These selective events augment inhibition of SNc DA neurons by SNr GABAergic neurons and also temper the release of glutamate in the dorsal striatum. The effects may reduce the risk of excitotoxicity in SNc DA neurons and may also counteract the increased effectiveness of corticostriatal glutamatergic inputs during degeneration of the DA system. These processes may contribute to the inverse correlation between tobacco use and Parkinson's disease.
Introduction
Parkinson's disease (PD) is caused by degeneration of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc). Neuroprotective therapies are being sought to slow or delay the progression of PD (Wu and Frucht, 2005; Bonuccelli and Del Dotto, 2006).
A person's history of tobacco use is strongly anticorrelated to his/her risk of Parkinson's disease (Ritz et al., 2007; Thacker et al., 2007). Smoked or smoke-cured tobacco is not a medically acceptable therapy. Therefore, it is important to understand the apparent inadvertent neuroprotective and/or therapeutic mechanism of chronic exposure to nicotine. In animal models of PD, chronic exposure to nicotine protects DA neurons and terminals (Quik et al., 2007; Picciotto and Zoli, 2008) and also shows some therapeutic benefits, improving motor symptoms, ameliorating dyskinesia, and boosting l-DOPA efficacy (Quik et al., 2007, 2008). Hyperactivity of glutamatergic synapses may contribute to PD symptoms, because some glutamatergic receptor antagonists also control PD (Parsons et al., 1999; Steece-Collier et al., 2000; Hadj Tahar et al., 2004; Wu and Frucht, 2005).
The longest-known effect of chronic nicotine is upregulation of nicotinic receptors themselves. Among the several subtypes of nAChRs expressed in brain, chronic exposure to smoked doses of nicotine does not markedly upregulate α7* (Marks et al., 1986; Collins et al., 1994), α3β4*, or α6* nAChRs (Nguyen et al., 2003; McCallum et al., 2006a,b; Mugnaini et al., 2006; Perry et al., 2007; Walsh et al., 2008) but does effectively upregulate α4β2* nAChRs in some cell types and brain regions (the asterisk means that there may be other subunits in the pentameric nAChR). For instance, chronic nicotine upregulates α4β2* nAChRs in hippocampus, cortex, striatum, hypothalamus, and midbrain, but not in interpeduncular nucleus, medial habenula, or cerebellum (Flores et al., 1992; Marks et al., 1992; Perry et al., 1999; Nguyen et al., 2004; Nashmi et al., 2007). Importantly, the neuroprotective effect of chronic nicotine vanishes in α4 subunit knock-out (KO) mice (Ryan et al., 2001). In addition to (or possibly because of) α4β2* nAChR upregulation, chronic nicotine enhances synaptic plasticity in hippocampus (Fujii et al., 1999; Nashmi et al., 2007), increases nicotine-induced DA release in striatum (Marshall et al., 1997; Visanji et al., 2006), and increases firing in midbrain GABAergic neurons (Nashmi et al., 2007).
How might upregulation of α4β2* nAChRs protect DA neurons? Upregulation could modify activity in neural circuits in the basal ganglia (Nashmi et al., 2007). To understand how such changes could lead to neuroprotection, we chronically infused mice with nicotine or vehicle and electrophysiologically examined the function of α4β2* nAChRs in substantia nigra neurons and striatal DA terminals. The data reveal that by selectively upregulating α4β2* nAChRs, chronic nicotine exaggerates inhibition to SNc DA neurons and also tempers the release of glutamate in the dorsal striatum. These events could reduce the risk of excitotoxicity in SNc DA neurons and would counteract the hyperactivity of striatal glutamate synapses after DA denervation and therefore could confer the apparent neuroprotective effects of chronic nicotine on PD.
Materials and Methods
The care and use of animals and the experimental protocol of this study were approved by the Institutional Animal Care and Use Committee of the California Institute of Technology. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Patch-clamp recording.
The recordings were performed using brain slices which were prepared from 5–12-week-old C57BL/6 [wild-type (WT)] mice or α4 nAChR subunit knock-out mice (α4-KO mice, backcrossed >N10 to C57BL/6), by using the protocol described previously (Ye et al., 2006; Nashmi et al., 2007). In brief, the mice were deeply anesthetized by Nembutal (35 mg/kg) and decapitated. The brain was removed and sliced in the coronal plane (300 μm) with a microslicer (DTK-1000, Ted Pella Inc.), while immersed in ice-cold modified glycerol-based artificial CSF (ACSF) saturated with 95%O2/5%CO2 (carbogen) containing the following (in mm): 250 glycerol, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 26 NaHCO3, and 11 glucose. Brain slices (three to four per mouse) containing cortex and midbrain or dorsal striatum were allowed to recover at 31°C for at least 1 h in a holding chamber before they were placed in the recording chamber and superfused (1.5–2.0 ml/min) with carbogen-saturated ACSF. The recovering bath was filled with carbogenated ACSF containing the following (in mm): 125 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 26 NaHCO3, and 11 glucose.
The neurons were visualized with an upright microscope (BX50WI, Olympus) and near-infrared illumination. Whole-cell patch-clamp techniques were used to record electrical activity with MultiClamp 700B amplifiers (Axon Instruments, Molecular Devices), Digidata 1200 analog-to-digital converters (Axon Instruments), and pCLAMP 9.2 software (Axon Instruments). Data were sampled at 10 kHz and filtered at 2 kHz. The junction potential between the patch pipette and the bath solutions was nulled just before we formed the gigaseal.
The patch electrodes had resistances of 5–8 MΩ when filled with intrapipette solution 1, which contained the following (in mm): 135 potassium gluconate, 5 KCl, 5 EGTA, 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, and 0.1 GTP; or with solution 2, which contained the following (in mm, for IPSC recordings): 75 K gluconate, 65 KCl, 5 EGTA, 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, 0.1 GTP, and 2 mm QX-314. The pH of these solutions was adjusted to 7.2 with Tris base and the osmolarity to 300 mOsm with sucrose. Nernst potentials for Cl− in solutions 1 and 2 are −82.9 and −18 mV, respectively. Therefore, spontaneous inward currents recorded at the holding potential of −60 to −70 mV include, for solution 1, only EPSCs, and for solution 2, both IPSCs and EPSCs.
Series resistance was monitored and compensated by 70–80% throughout whole-cell patch-clamp recordings (Multiclamp 700B). The data were discarded if the series resistance (15–30 MΩ) changed by >20% during the whole-cell recording. Given the modest size of the sIPSCs, mIPSCs, sEPSCs, and eEPSPs in this study, these conditions produced minimal distortion of the waveforms (Takahashi et al., 1995). The bath was continuously perfused with ACSF. All recordings were done at a temperature of 32 ± 1°C.
In the midbrain slice, we identified the type of neurons in SNc and substantia nigra pars reticulata (SNr) according to the following criteria (Lacey et al., 1989; Nashmi et al., 2007): (1) spontaneous firing rate is higher in GABAergic neurons (≥5 Hz) than in DA neurons (<5 Hz); (2) the action potential is briefer (<2 ms) in GABAergic neurons than in DA neurons (>2 ms); (3) a prominent hyperpolarization-induced cationic current (Ih) appears in DA neurons but not in GABAergic neurons; (4) GABAergic but not DA neurons are inhibited by Tyr-d-Ala-Gly-MePhe-Gly-ol (DAMGO), a μ-opioid receptor agonist; (5) DA but not GABAergic neurons are inhibited by quinpirole, a dopamine D2/D3 receptor agonist.
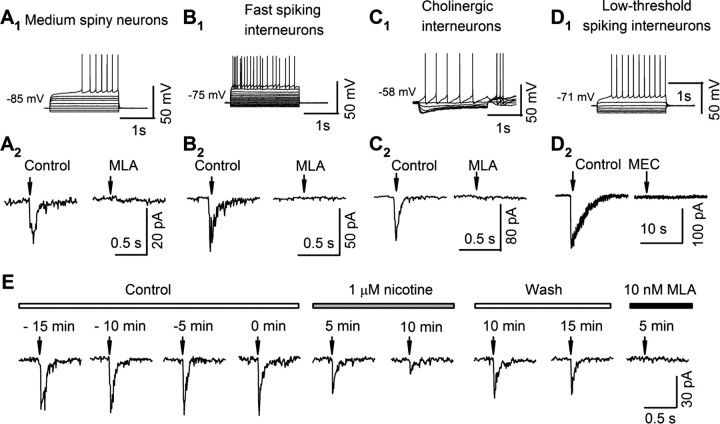
In the dorsal striatum slice recordings, we identified striatal neurons according to their distinct electrophysiological properties (Jiang and North, 1991; Nisenbaum and Wilson, 1995; Koós and Tepper, 1999). (1) Medium spiny neurons (MSNs) have comparatively hyperpolarized membrane potentials (−75 to −85 mV), a comparatively low input resistance (<100 MΩ), inward rectification (smaller response to negative than to positive current pulses), and, for rheobase stimuli, a gradually developing depolarization and comparatively long latency to the first spike (see Fig. 4A1). (2) Fast-spiking (FS) interneurons have comparatively hyperpolarized membrane potentials (−75 to −85 mV), transient postspike hyperpolarizations, and comparatively brief interspike intervals (see Fig. 4B1). (3) Cholinergic interneurons have membrane potentials of −55 to −65 mV and voltage sag due to Ih in response to hyperpolarizing current injection (see Fig. 4C1). (4) Low-threshold-spike (LTS) interneurons have membrane potentials of −60 to −70 mV, a comparatively high input resistance (>200 MΩ), and no Ih (see Fig. 4D1).
Figure 4.
Current–voltage relations and nicotinic currents in striatal neurons. A–D, Top, Representative records from an MSN (A1), an FS interneuron (B1), a cholinergic interneuron (C1), and an LTS interneuron (D1), identified as described in Materials and Methods by their distinct responses to 2 s current injections (for MSN and FS: initiated from −160 pA with an increment of 40 pA; for cholinergic and LTS: initiated from −80 pA with an increment of 20 pA). Middle, Nicotinic currents recorded from voltage-clamped neurons (VH = −70 mV) in response to a puff of ACh (100–300 μm) with 0.5 μm atropine and 20 μm CNQX. The nicotinic currents in MSN (A2), FS (B2), and cholinergic neurons (C2) were blocked by 10 nm MLA. The nicotinic current in one LTS was blocked by 5 μm MEC. E, In a typical MSN, nicotinic current was recorded at 5 min intervals before (Control), during (1 μm nicotine), and after (Wash) the perfusion of 1 μm nicotine, and also in the presence of 10 nm MLA. Upper bars show each condition; negative minutes denote the time before the application of nicotine. Arrows in A2–D2 and E indicate ACh puff (100 ms, 20 PSI).
We optimized several conditions for subsecond assessments of nAChR sensitivity. We measured responses to ACh rather than to nicotine, avoiding distortions due to passive nicotine accumulation and release by neurons. We also added atropine to block muscarinic acetylcholine receptors. Responses were tested using a focal, relatively rapid drug application system designed to minimize desensitization (Tapper et al., 2004). Briefly, a 2-μm-tip glass pipette filled with ACSF containing ACh (3 μm–1 mm), atropine (0.5 μm), and 20 μm 6,7-dinitroquinoxaline-2,3-dione (CNQX) was mounted on a piezoelectric manipulator and connected to a controlled source of pressure. The pipette was moved from a starting position (>100 μm from the recorded neuron) to a “puffing position” (<20 μm from the recorded neuron), acetylcholine was applied by a pressure pulse (20 psi, 100 ms), and then, the pipette was again retracted to the starting position. We used 100 ms puffs because longer puffs did not increase the peak current. We applied ACh at 3 min intervals to minimize desensitization. Although repetitive applications of 1 mm ACh did not cause desensitization of nicotinic responses, repeated application of 3 and 10 mm ACh did produce >15% decrement in successive responses. Therefore, the dose–response studies used concentrations of ≤1 mm ACh.
Chronic nicotine treatment.
Nicotine or vehicle (saline) was administered to mice (4–11 weeks old) by subcutaneously implanted mini-osmotic pumps (model 2002, Alzet, Cupertino, CA) for 10–14 d (Nashmi et al., 2007). For midbrain slice recordings, we studied 4–6-week-old mice. For other recordings, we studied 8–12-week-old mice. On the day of minipump implantation, vehicle or (−) nicotine hydrogen tartrate salt was prepared freshly and loaded into the pump to deliver vehicle or nicotine (2 mg/kg/h at 0.5 μl/h). This produces a blood concentration of 590 nm (Marks et al., 2004), near the peak concentration of nicotine in the blood of smokers. This method provides consistent nicotine dosage and effectively induces cellular, molecular, and systemic changes related to tobacco use (Fung and Lau, 1988; Collins et al., 1994; Nguyen et al., 2003, 2004; Marks et al., 2004; Nashmi et al., 2007) but limits the influence of stress from repeated nicotine injection.
In each set of experiments, we implanted minipumps in mice (three for each treatment) almost at the same age (±2 d). The electrophysiologist recorded from one mouse per day and alternated between mice from the vehicle- and nicotine-treated groups (but without knowing the treatment), thus maintaining the same average mouse age and treatment period for data from the two groups. Surgical treatments and electrophysiological experiments were performed by separate individuals who did not share records (S.M. and C.X., respectively) until the data had been summarized, thus achieving “blind” experiments.
Single-unit extracellular recording in vivo.
After 10–14 d of chronic administration of vehicle or nicotine, mice were anesthetized with chloral hydrate (400 mg/kg, i.p.), and the minipumps were removed. When the mice awakened, ketoprofen (2 mg/kg), an analgesic, was subcutaneously injected for pain relief. One day later, the mice were anesthetized again and immobilized in a stereotaxic frame (Stoelting). Body temperature was monitored and maintained at 37.0 ± 0.1°C by a heated plate (WPI). A hole (1 mm in diameter) for the placement of recording electrode was drilled, centered at 3.2 mm posterior to bregma and 1.0 mm lateral to the midline on the skull. The dura was opened over the recording sites. To maintain deep anesthesia, chloral hydrate was intraperitoneally injected at 100 mg/kg/30 min.
Extracellular potentials were recorded by a single recording micropipette filled with 3 m NaCl mounted on a Narishige hydraulic microdrive. Signals were led to an Axoclamp-2A amplifier, then to a Brownlee Precision Model 440 amplifier, and then to a Digidata 1200 digitizer controlled by pCLAMP 9.2 (Axon Instruments) software. The recording electrode was positioned via stereotaxic coordinates into the substantia nigra (from bregma: 3.0–3.4 mm posterior, 0.5–1.5 mm lateral, and 4.0–5.0 mm ventral) (Paxinos and Franklin, 2004). The signals were sampled at 20 kHz and bandpass filtered at 1 Hz–10 kHz. In analyses of in vivo single-unit recordings, the spikes of SNr GABAergic neurons were identified by high frequencies (>5 Hz), brief duration (<2 ms), and insensitivity to quinpirole (supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
Immunohistochemistry.
The α4-YFP mice (Nashmi et al., 2007) were anesthetized with halothane (2-bromo-2-chloro-1,1,1-trifluorothane) and subjected to cardiac perfusion with 7 ml of PBS with heparin and then with 30 ml of 4% paraformaldehyde in PBS. The mouse brain was removed, fixed in 4% paraformaldehyde for 2 h, and immersed in 30% sucrose in PBS for 24–48 h till sink. After being rapidly frozen in 2-methylbutane (−40°C), the mouse brain was sectioned into 30 μm coronal slices by using a sliding microtome (Leica SM 2010R).
The slices were washed twice (10 min each) with cold PBS (4°C), permeabilized for 1 h at room temperature in PBS/0.5% saponin, blocked for 1 h in 10% donkey serum in PBS, incubated in primary antibodies at 4°C overnight (18 h): sheep anti-tyrosine hydroxylase (TH) (1:500, Alzheimer Research Forum) and rabbit anti-GFP (1:300, Abcam) in 4% donkey serum in PBS, washed three times (15 min each) in PBS, incubated in secondary antibody at room temperature for 1.5 h: Cy3-conjugated donkey anti-sheep IgG (1:500), and Cy5-conjugated donkey anti-rabbit IgG (1:500) in PBS/4% donkey serum, washed three times (10 min each) in PBS, and then mounted onto the slide and coverslipped with mounting medium (Vector Laboratories). We imaged the slices with a spectrally resolved confocal microscope (Nikon C1si), equipped with a 60×, 1.4 numerical aperture, plan apochromat oil-immersion objective. Cy3 or Cy5 was excited with a 514 or 637 nm laser and detected in the range of 520–680 nm or 640–720 nm. The acquired images were linearly unmixed with reference spectra using EZ-C1 software (Nikon). We obtained the reference spectra of Cy3 from midbrain dopaminergic neurons in brain slices processed for TH staining with or without added TH antibody, and those of Cy5 from midbrain neurons in slices of α4-YFP mice or their wild-type littermates, processed for GFP staining. The specificity of the anti-GFP staining is shown in supplemental Fig. S2, available at www.jneurosci.org as supplemental material, which contrasts the images for α4-YFP and WT mice.
Chemicals and applications.
Sigma-Aldrich furnished most of the chemicals including acetylcholine chloride (ACh), CNQX, dihydro-β-erythroidine hydrobromide (DHβE), mecamylamine hydrochloride (MEC), methyllycaconitine citrate hydrate (MLA), (−)-nicotine hydrogen tartrate salt, picrotoxin, SR-95531 (GABAzine), (±)-sulpiride, and tetrodotoxin (TTX). The chemicals were added in known concentrations to the superfusate.
Data analysis.
Using Clampfit 9.2, we measured the peak amplitude of nicotinic currents and counted and analyzed spontaneous/miniature inhibitory/EPSCs (sIPSCs/mIPSCs, sEPSCs) and firing. The sIPSCs, mIPSCs, sEPSCs, and action potentials were selected by “template search,” in which the template was first selected visually according to its rise and decay phase, and amplitude threshold was set to 5 pA for sIPSCs/mIPSCs/sEPSCs, and 10 mV for action potentials. The frequencies of sIPSCs, sEPSCs, mIPSCs, and firings during and after drug applications were normalized to their mean values observed during the initial control period (>2 min). These data were used to depict summarized time courses (10 s/bin). The baseline mean values were obtained during the initial control period, while the mean values during drug application were obtained over a >2 min period at the peak of a drug response. Drug effects were expressed as percentage change (mean ± SEM) from baseline. The statistical significance of drug effects was assessed by a paired two-tailed t test. A two-tailed t test was also used to evaluate the statistical significance of differences in drug effects (percentage change from baseline) in two different situations, e.g., the absence and presence of blockers, or chronic nicotine and vehicle treatments. p values of <0.05 were considered significant.
The sIPSCs and sEPSCs displayed a wide range of amplitudes. To show all the events in the figures, we used an amplification that clipped a few large responses. For amplitude determinations, unclipped data were measured.
The in vivo single-unit recording data were analyzed using Clampfit 9.2 after high-pass (10 Hz) and low-pass (3 kHz) filtering. A template was made by averaging 20 spikes with apparently similar waveforms and amplitudes and used for automatic spike detection. Sometimes, the recorded trace displayed several classes of spikes with different waveforms, amplitudes, or both. We defined several spike templates (≤3) and simultaneously analyzed them. For each neuron, the firing rate was calculated from 3–5 min stable recording. The spike duration was determined by measuring the time between half-peak amplitude for the falling and rising edges. The significance of the difference in firing frequencies of SNr GABAergic neurons between chronic nicotine- and vehicle-treated mice was analyzed with a t test.
Results
Chronic nicotine selectively enhances α4β2* nAChRs in SNr GABAergic neurons
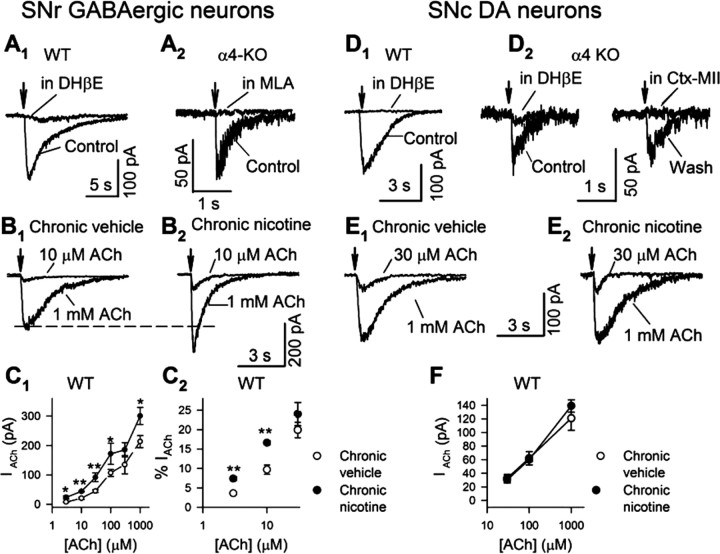
Midbrain GABAergic neurons express multiple subtypes of nAChRs, including α7 and α4β2* (Pidoplichko et al., 1997; Azam et al., 2002; Wooltorton et al., 2003; Dani and Bertrand, 2007; Nashmi et al., 2007) but not α6β2* (Drenan et al., 2008). Therefore, to record nicotinic currents mediated by α4β2* nAChRs, we puffed ACh to SNr GABAergic neurons in the presence of MLA (10 nm), a specific antagonist for α7 nAChRs, and atropine (0.5 μm), an antagonist of muscarinic ACh receptors. In this recording condition, the nicotinic current evoked by 1 mm ACh was eliminated by DHβE (300 nm), an antagonist for β2* nAChRs (Fig. 1A1). We also conducted experiments with α4-KO mice. In the absence of nicotinic blockers, we detected fast ACh-induced currents in 12 of 14 SNr GABAergic neurons. These currents were completely blocked by MLA (10 nm) (Fig. 1A2). These data suggest that MLA-resistant nicotinic currents in WT SNr GABAergic neurons are primarily mediated by α4β2* nAChRs.
Figure 1.
Chronic nicotine modifies nicotinic currents in substantia nigra neurons. Nicotinic currents in response to puff application of 1 mm ACh were recorded from voltage-clamped substantia nigra neurons (VH = −60 mV) in midbrain slices of WT mice (A1, B1, B2, D1, E1, and E2 in the presence of 10 nm MLA) and α4-KO mice (A2 and D2). A1, DHβE (0.3 μm) blocked MLA-resistant nicotinic currents in SNr GABAergic neurons of WT mice. A2, Nicotinic currents in SNr GABAergic neurons of α4-KO mice were blocked by MLA (10 nm). B1, B2, Typical nicotinic responses, elicited by 10 μm and 1 mm ACh, recorded from SNr GABAergic neurons in chronic vehicle-treated (B1) and nicotine-treated (B2) mice. C1, Dose–response relations of MLA-resistant nicotinic currents in SNr GABAergic neurons of chronic nicotine (solid circle)- and vehicle (open circle)-treated WT mice. C2, MLA-resistant currents elicited by lower ACh concentrations as a percentage of 1 mm ACh responses. D1, DHβE (0.3 μm) blocked MLA-resistant nicotinic currents in SNc DA neurons of WT mice. D2, In seven of nine SNc DA neurons of α4-KO mice, MLA-resistant nicotinic currents were blocked by either DHβE (0.3 μm) or α-conotoxin MII (Ctx MII, 0.1 μm). The data in the two panels were recorded from the same neuron. E1, E2, Typical nicotinic responses, elicited by 30 μm and 1 mm ACh, recorded from SNc DA neurons in chronic vehicle-treated (E1) and nicotine-treated (E2) mice. F, Dose–response relations of MLA-resistant nicotinic currents in SNc DA neurons of chronic nicotine (solid circle)- and vehicle (open circle)-treated WT mice. Arrows indicate the application of ACh. Data in summary are shown as mean ± SEM. *p < 0.05, **p < 0.01, chronic nicotine- versus vehicle-treated mice.
Chronic nicotine increases the number of fluorescent α4* nAChRs in SNr GABAergic neurons (Nashmi et al., 2007). To directly assess the function of these nAChRs, we measured MLA-resistant nicotinic currents in SNr GABAergic neurons (Fig. 1A1,B1,B2). As illustrated in Figure 1, B1, B2, and C1, and Table 1, ACh induced larger currents in chronic nicotine-treated mice than chronic vehicle-treated mice, and this increase occurred at all concentrations from 3 μm to 1 mm. Because desensitization limited the precision of the measurements for [ACh] >1 mm, while the ACh bolus was spreading to distant parts of the neuron, we did not obtain a true “saturating” dose–response relation. Dose–response relations for α4β2 nAChRs expressed in vitro are typically comprised of two or more components. One mm ACh exceeds the EC50 of the higher- and lower-sensitivity components by ∼1000-fold and ∼10-fold, respectively (Buisson and Bertrand, 2001; Rodrigues-Pinguet et al., 2003; Moroni et al., 2006), so that the 1 mm responses presumably reflect nearly full activation of nAChRs that were reached by the ACh bolus. At 1 mm ACh, the responses from chronic nicotine-treated animals were 41% higher than from chronic vehicle-treated animals. This value agrees well with the 46% increase of α4* nAChR fluorescence measured under equivalent conditions by Nashmi et al. (2007), consistent with the idea that the increased ACh responses measured in this study correspond to the numerically upregulated α4* nAChRs measured by Nashmi et al. (2007). We compared the waveform of MLA-resistant nicotinic currents induced by 1 mm ACh in SNr GABAergic neurons. The decay time constant showed little or no difference between chronic vehicle- and nicotine-treated mice (1011 ± 115 ms, n = 14; and 853 ± 45 ms, n = 20, respectively).
Table 1.
Nicotinic currents in SNr GABAergic neurons
| ACh(μm) | Chronic vehicle |
Chronic nicotine |
||||
|---|---|---|---|---|---|---|
| IACh (pA) | % IACh (1 mm) | n | IACh (pA) | % IACh (1 mm) | n | |
| 3 | 7.8 ± 1.6 | 3.6 ± 0.5 | 3 | 23.7 ± 5.4* | 7.4 ± 0.5** | 5 |
| 10 | 20.5 ± 3.8 | 9.7 ± 1.2 | 6 | 43.9 ± 1.9** | 16.6 ± 0.5** | 5 |
| 30 | 45.0 ± 4.9 | 19.9 ± 2.0 | 8 | 91.9 ± 15.0* | 24.0 ± 3.0 | 9 |
| 100 | 107.0 ± 12.0 | 39.9 ± 8.0 | 8 | 172.0 ± 36.0* | 43.4 ± 4.0 | 9 |
| 300 | 135.0 ± 31.0 | 68.0 ± 14.0 | 4 | 185.5 ± 24.3 | 75.4 ± 11.5 | 3 |
| 1000 | 213.5 ± 20.5 | 1 | 19 | 300.8 ± 28.8* | 1 | 19 |
*p < 0.05,
**p < 0.01, compared with chronic vehicle-treated mice.
To compare the effects of chronic nicotine on lower- and higher-sensitivity α4β2* nAChRs, we also normalized the nicotinic currents to those induced by 1 mm ACh in the same neuron. Interestingly, the normalized responses at 3 and 10 μm, but not higher concentrations, were significantly increased by chronic nicotine (Fig. 1C2, Table 1). Apparently higher-sensitivity α4β2* nAChR(s) is preferentially upregulated by chronic nicotine. Our data lack the precision to state whether the increased high-sensitivity component alone can explain the enhancement by chronic nicotine at all ACh concentrations.
Chronic nicotine appears to selectively upregulate fluorescent α4* nAChRs in SNr GABAergic neurons but not in SNc DA neurons (Nashmi et al., 2007). To test the cell type selectivity of chronic nicotine effects on α4* nAChR function, we also examined MLA-resistant nicotinic currents from SNc DA neurons (Fig. 1D1,E1,E2). In contrast to its effect in SNr GABAergic neurons, chronic nicotine did not change the currents induced by puffing 30, 100, and 1000 μm ACh onto SNc DA neurons (Fig. 1F, Table 2).
Table 2.
Nicotinic currents in SNc DA neurons
| ACh (μm) | Chronic vehicle |
Chronic nicotine |
||
|---|---|---|---|---|
| IACh (pA) | n | IACh (pA) | n | |
| 30 | 32.8 ± 5.5 | 5 | 30.5 ± 4.5 | 6 |
| 100 | 61.9 ± 9.8 | 9 | 60.0 ± 8.4 | 5 |
| 1000 | 121.0 ± 17.6 | 10 | 139.0 ± 9.0 | 8 |
We next tested whether α4* nAChRs principally mediate MLA-resistant nicotinic currents in SNc DA neurons. We elicited nicotinic currents with 1 mm ACh in SNc DA neurons of α4-KO mice. Three pharmacological classes were observed in the nicotinic currents tested in nine neurons: complete blockade by 10 nm MLA alone (in two neurons) (data not shown), by 0.3 μm DHβE alone (in five neurons) (Fig. 1D2), and by the combination of MLA and DHβE (two neurons) (data not shown). The currents sensitive to 0.3 μm DHβE (33.7 ± 2.2 pA, n = 7) were also sensitive to 0.1 μm α-conotoxin MII, a selective blocker of α6* nAChRs (Fig. 1D2). This indicates that MLA-resistant nicotinic currents in WT SNc DA neurons are mediated primarily by α4β2* nAChRs, with a detectable contribution from (non-α4) α6β2* nAChRs. The data are consistent with previous findings that midbrain DA neurons express functional α6* nAChRs (Klink et al., 2001; Champtiaux et al., 2003; Drenan et al., 2008). Interestingly, the experiments on α4-KO mice showed that (non-α4) α6β2* nAChRs were significantly downregulated by chronic nicotine (1 mm ACh-induced nicotinic currents, chronic vehicle: 33.7 ± 2.2 pA, n = 7; chronic nicotine: 17.0 ± 0.6 pA, n = 4; p = 0.001) (data not shown). This apparent nicotine-induced downregulation of α6* receptors is consistent with some (McCallum et al., 2006a,b; Mugnaini et al., 2006) but not other (Visanji et al., 2006; Perez et al., 2008) previous studies. Because the (non-α4) α6β2* nAChR response is a small fraction of the total non-α7 nicotinic response in WT DA neurons, any up- or downregulation of (non-α4) α6β2* nAChRs would escape detection in the response of DA neurons to chronic nicotine.
In a test of the hypothesis that only α4* receptors are upregulated by chronic nicotine, we measured MLA-sensitive nicotinic currents in SNr GABAergic neurons of α4-KO mice (Fig. 1A2). Chronic nicotine did not change 1 mm ACh-evoked currents (chronic vehicle: 34.0 ± 14.0, n = 4; chronic nicotine: 39.0 ± 8.1 pA, n = 8; p = 0.38). The data agree with previous findings that chronic exposure to nicotine with the protocol we were using selectively upregulates α4β2* but not α7 nAChRs (Marks et al., 1986; Collins et al., 1994).
Chronic nicotine acting via SNr GABAergic neurons inhibits SNc DA neurons
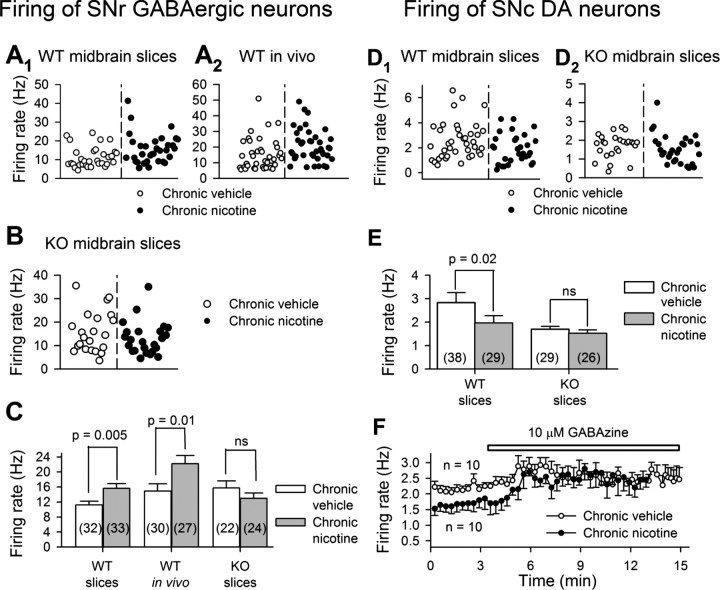
Some α4β2 nAChRs are activated by comparatively low concentrations of ACh or nicotine (Buisson and Bertrand, 2001; Nelson et al., 2003; Moroni et al., 2006). As shown in Figure 1, C1 and C2, and Table 1, chronic nicotine enhanced MLA-resistant nicotinic currents and the ratio of low ACh (≤10 μm) to 1 mm ACh-induced currents in SNr GABAergic neurons. This enhancement of sensitivity may be important in cholinergic modulation of neuronal activity by the relatively low concentrations (∼100 nm measured by in vivo microdialysis) of ambient ACh (Descarries et al., 1997; Dani and Bertrand, 2007). To address this issue, we recorded the firing of these neurons using both in vitro brain slice patch-clamp recordings and in vivo single-unit recordings. In chronic nicotine-treated mice, both in vitro and in vivo SNr GABAergic neurons fire at higher frequencies than in chronic vehicle-treated mice (Fig. 2A1,A2,C). In contrast, SNc DA neurons in midbrain slices fire more slowly in chronic nicotine-treated mice (1.97 ± 0.31 Hz, n = 29) than in vehicle-treated mice (2.84 ± 0.42 Hz, n = 38) (p = 0.003) (Fig. 2D1,E). These results on firing rates agree with a previous report (Nashmi et al., 2007).
Figure 2.
Chronic nicotine modifies firing rates in substantia nigra neurons: the role of α4* nAChRs. The spikes were recorded, using whole-cell patch-clamp technique, from substantia nigra neurons in midbrain slices of WT (A1, D1, and F) and α4-KO (B and D2) mice, and also with SNr GABAergic neurons in vivo by using single-unit extracellular recording (A2). A1, A2, B, Chronic nicotine increases the firing rate of SNr GABAergic neurons in midbrain slices (A1) and in vivo (A2), but not in midbrain slices of α4-KO mice (B). C, Summary of data. D1, D2, E, In contrast, chronic nicotine decreases the activity of SNc DA neurons in WT mice (D1, pooled data; E, summary), but not in α4-KO mice (D2, pooled data; E, summary). F, Summarized time course showing that GABAzine (10 μm, a GABAA receptor blocker) eliminated the difference in the baseline firing rate of SNc DA neurons between chronic nicotine- and vehicle-treated WT mice. Data in summary are shown as mean ± SEM. ns, Not significant, chronic nicotine- versus vehicle-treated mice.
Nicotine modulates sIPSCs in DA neurons by activating α4β2* nAChRs in GABAergic neurons (Mansvelder et al., 2002). The hypothesis that the major effect of chronic nicotine on GABAergic neurons is mediated by the upregulation of α4* nAChRs predicts that the effects of chronic nicotine will be absent from α4-KO mice. We therefore measured the firing of SNr GABAergic and SNc DA neurons in in vitro midbrain slices of α4-KO mice. We detected little difference between the basal firing rates of SNr GABAergic neurons from chronic vehicle-treated α4-KO versus WT mice (Fig. 2A1,A2,B,C). SNc DA neurons from chronic vehicle-treated α4-KO mice fired at approximately half the frequency of their counterparts from WT mice (Fig. 2D1,D2,E), consistent with the high-level expression of α4* nAChRs in DA neurons (Nashmi et al., 2007). In α4-KO mice, chronic exposure to nicotine changed neither the firing frequency of SNr GABAergic neurons (chronic nicotine: 13 ± 1.4 Hz, n = 24; chronic vehicle: 15.7 ± 1.8 Hz, n = 22, p = 0.26) (Fig. 2B,C) nor that of SNc DA neurons (chronic nicotine: 1.53 ± 0.14 Hz, n = 29; chronic vehicle: 1.58 ± 0.16 Hz, n = 26; p = 0.41) (Fig. 2D2,E). These data suggest that, in WT mice, the effect of chronic nicotine on the activity of both SNr GABAergic neurons and SNc DA neurons is mediated by its effect on α4* nAChRs. That is, chronic nicotine selectively upregulates α4β2* nAChRs and enhances the firing rate in SNr GABAergic neurons, which may cause stronger inhibition to SNc DA neurons (Nashmi et al., 2007). The blockade of GABAA receptors by 10 μm GABAzine excited SNc DA neurons (Fig. 2F). In the presence of GABAzine, the firing rates of SNc DA neurons were equal in chronic nicotine- and vehicle-treated mice (Fig. 2F). These data indicate that chronic nicotine enhancement of firing frequency in GABAergic neurons may contribute to the depression of firing in SNc DA neurons.
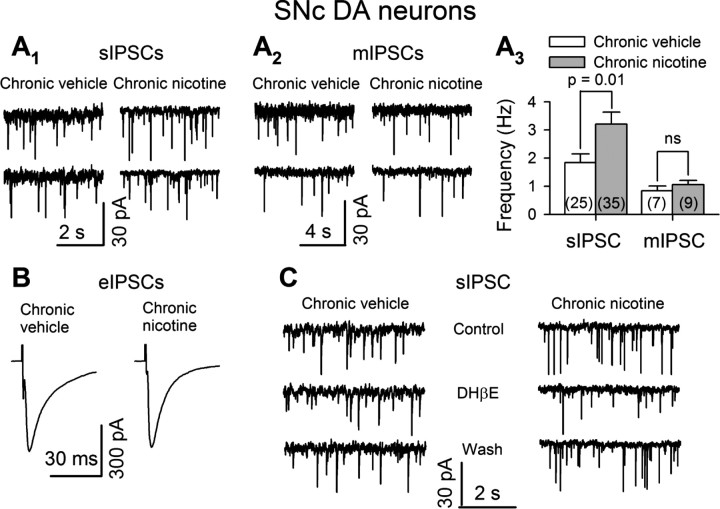
Further experiments support the idea that a presynaptic mechanism underlies chronic nicotine enhancement of GABAergic inhibition to SNc DA neurons. The sIPSCs were recorded from voltage-clamped SNc DA neurons (VH = −60 mV) in midbrain slices, in the presence of 20 μm CNQX. In the same medium, the eIPSCs were stimulated by pulses from a glass pipette electrode filled with 2 m NaCl and placed in the SNr 100 μm away from the recorded neuron. The mIPSCs were recorded in the presence of 0.5 μm TTX to block action potential-dependent sIPSCs. The sIPSCs, eIPSCs, and mIPSCs were blocked by 10 μm bicuculline (data not shown). First, chronic nicotine increased the baseline frequency of sIPSCs (chronic nicotine: 3.25 ± 0.43 Hz, n = 35; chronic vehicle: 1.9 ± 0.31 Hz, n = 25) (p = 0.01) (Fig. 3A1,A3) but not the amplitude of sIPSCs (chronic nicotine: 14.9 ± 1.6 Hz, n = 35; chronic vehicle: 14.9 ± 1.3 Hz, n = 25) (p = 0.5) (data not shown). Second, chronic nicotine did not change the frequency of mIPSCs (chronic nicotine: 1.06 ± 0.15 Hz, n = 9; chronic vehicle: 0.84 ± 0.17, n = 7) (Fig. 3A2,A3). Third, the maximal evoked IPSCs were not significantly different between chronic nicotine-treated (480 ± 70 pA, n = 9) and vehicle-treated (471 ± 71 pA, n = 9) mice (p = 0.48) (Fig. 3B). Fourth, we found that DHβE (0.3 μm) significantly inhibited sIPSC frequency in SNc DA neurons (by 22 ± 3%, n = 6, p = 0.003) (Fig. 3C). Interestingly, chronic nicotine enhanced this inhibition (to 36 ± 4%, n = 7, p = 0.0001) (p = 0.02, chronic nicotine vs vehicle) (Fig. 3C).
Figure 3.
Chronic nicotine augments GABAergic inhibition to SNc DA neurons via presynaptic mechanisms. The sIPSCs (A1, A3), eIPSCs (B), and mIPSCs (A2, A3) were recorded from SNc DA neurons. A1–A3, Representative traces of sIPSCs (A1), mIPSCs (A2), and summary of IPSC frequency (A3) in chronic vehicle- and nicotine-treated mice. B, Averaged traces of maximal eIPSCs in all recorded neurons in chronic vehicle- or nicotine-treated mice. C, Representative traces of sIPSCs, showing that chronic nicotine enhanced the inhibition of sIPSC frequency by DHβE (0.3 μm). Summarized data are shown as mean ± SEM. ns, Not significant, chronic nicotine- versus vehicle-treated mice.
The data reported in this section are all consistent with the ideas (1) that chronic nicotine suppresses the firing of WT SNc DA neurons by upregulating the number and/or sensitivity of α4β2* nAChRs in WT GABAergic neurons, (2) that this upregulation enhances SNr GABAergic inhibitory input (see Fig. 8A,B), and (3) that chronic nicotine produces no direct change in somatodendritic nicotinic responses in SNc DA neurons.
Figure 8.
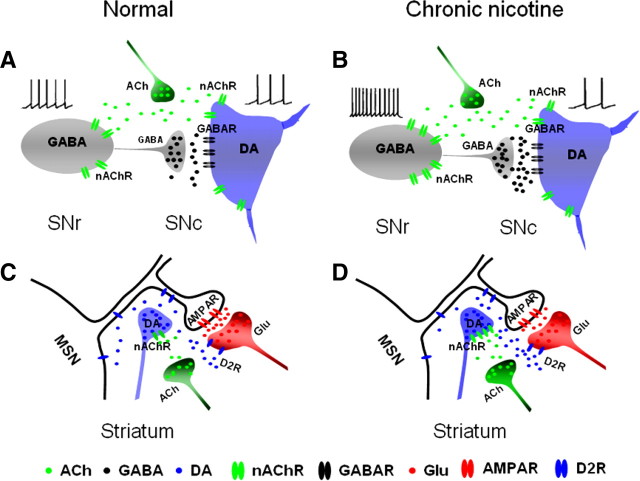
Diagram of chronic nicotine modulation of nigrostriatal pathway. A, In untreated substantia nigra, ACh activates nAChRs on both SNr GABAergic neurons and SNc DA neurons. B, After chronic nicotine treatment, the nAChRs in the SNr GABAergic neuron somata, but not those in the SNc DA neuron somata, are upregulated. The activation of nAChRs by endogenous ACh causes hyperactivity of SNr GABAergic neurons, which inhibits SNc DA neurons. C, In untreated striatum, ACh activates nAChRs on DA terminals and facilitates DA release. DA activates D2/D3 receptors (D2R) on glutamatergic terminals and inhibits glutamate (Glu) release. D, Chronic nicotine upregulates nAChRs on DA terminals, enhances DA release, and causes a further inhibition of Glu release.
Functional α4β2* nAChRs in the dorsal striatum
Low but detectable levels of α4β2* nAChR fluorescence are reported in the dorsal striatum (Nashmi et al., 2007). Numerous studies focus on the α4β2* nAChRs in DA terminals in the striatum. However, the localization of functional α4β2* nAChRs in the striatal neurons has not been systematically studied.
To address this issue, we examined the nicotinic currents in electrophysiologically identified striatal neurons (Fig. 4A1,B1, C1,D1) (see Materials and Methods). We detected nicotinic currents in 32% (12/37) of MSNs (27.6 ± 5.2 pA), 44% (7/16) of cholinergic interneurons (54.3 ± 10.7 pA), 100% (7/7) of FS interneurons (58.5 ± 8.2 pA), and 33% (2/6) of LTS interneurons (219 pA and 19 pA). All of the currents in MSNs, cholinergic neurons, and FS interneurons were blocked by MLA (Fig. 4A2,B2,C2,D2) and were desensitized by 10 min perfusion of 1 μm nicotine (38 ± 6% of control, n = 4, p < 0.001) (Fig. 4E) but not by 0.1 μm nicotine (97 ± 9% of control, n = 3, p = 0.45) (data not shown). In recordings from LTS interneurons, one response (219 pA) was blocked by MEC (Fig. 4D2), while the other one was blocked by MLA (data not shown). These properties (sensitivity to MLA and lack of desensitization by 0.1 μm nicotine) suggest that the somatodendritic nAChRs in the striatal neurons are primarily composed of α7 subunits.
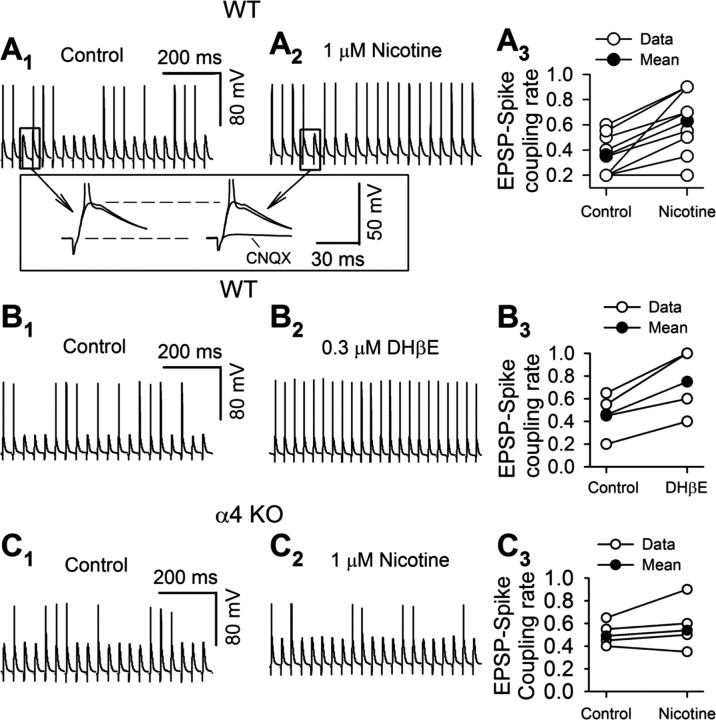
We next evoked EPSPs in MSNs with local electric stimuli. The stimuli were adjusted to evoke near-threshold EPSPs, so that 20–60% of the stimuli produced postsynaptic spikes (Fig. 5A1,B1,C1). CNQX (20 μm) blocked both EPSPs and spikes (Fig. 5A1,A2, inset), indicating that glutamate receptors mediated the EPSPs, which triggered spikes. Interestingly, both 1 μm nicotine and 0.3 μm DHβE increased the EPSP–spike coupling ratio (nicotine: from 0.36 ± 0.05 to 0.63 ± 0.08, n = 8, p = 0.001) (Fig. 5A1–A3) (DHβE: from 0.46 ± 0.10 to 0.75 ± 0.15, n = 4, p = 0.01) (Fig. 5B1–B3). In contrast, 1 μm nicotine failed to enhance the EPSP–spike coupling ratio in α4-KO mice (from 0.49 ± 0.05 to 0.54 ± 0.10, n = 5, p = 0.21) (Fig. 5C1–C3). These data indicate that functional nAChRs do exist in the dorsal striatum, contain α4 and β2 subunits, and modulate the excitability of MSNs. Because (1) we detected no functional α4β2* nAChRs in MSN somata and (2) desensitization of functioning nAChRs would cause repolarization, decreasing the excitability, we hypothesize that the contributing nAChRs are directly or indirectly presynaptic to the MSNs.
Figure 5.
α4β2* nAChRs modulate MSN neuronal excitability. A, B, In MSN, the EPSP–spike coupling ratio was increased by 1 μm nicotine (A1, A2, typical traces, A3, pooled data), and 0.3 μm DHβE (B1, B2, typical traces, B3, pooled data). Insets of A1, A2 are expanded traces as indicated. CNQX (20 μm) blocked EPSPs. C, Nicotine (1 μm) did not change the EPSP–spike coupling ratio in MSNs of α4-KO mice (C1, C2, typical traces; C3, pooled data).
α4β2* nAChRs modulate glutamate release onto MSNs
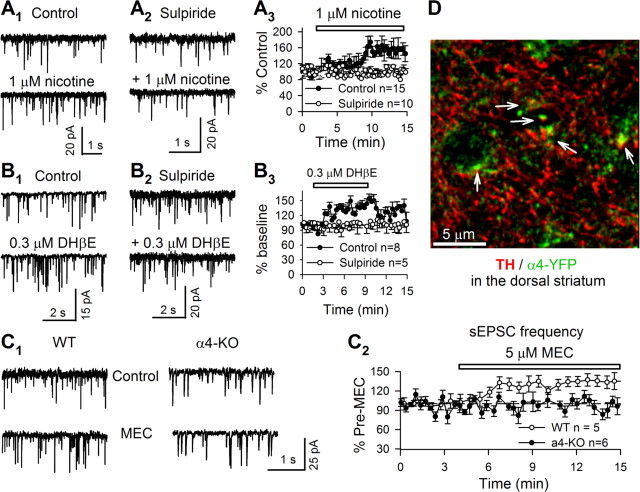
The excitability of MSNs is strongly controlled by glutamatergic excitatory synaptic transmission (Jiang and North, 1991). We next tested whether nicotine enhanced this glutamatergic transmission. We recorded evoked EPSCs from voltage-clamped (VH = −70 mV) MSNs. Nicotine (1 μm) enhanced EPSC amplitudes by 34 ± 2% (n = 3, p = 0.002, paired t test) (data not shown). To discriminate presynaptic versus postsynaptic mechanisms of nicotine action, we recorded spontaneous EPSCs (sEPSCs). Both 30 nm and 1 μm nicotine perfusion (10 min) increased sEPSC frequency, and by a similar extent (1 μm: by 44 ± 6%, n = 13, p = 0.0001) (Fig. 6A1,A3) (30 nm: 35 ± 6%, n = 9, p = 0.004) (data not shown), without changing the amplitude of sEPSCs (data not shown). The presence of MEC at 5 μm (a broad spectrum nicotinic antagonist) throughout the experiment blocked (or occluded) nicotine-induced enhancement of sEPSC frequencies (change of −6 ± 4%, n = 4, p = 0.2) (data not shown). Surprisingly, either of two nAChR antagonists, DHβE (0.3 μm) or MEC (5 μm), but not MLA (10 nm), significantly enhanced sEPSC frequency (DHβE: by 40 ± 3%, n = 8, p = 0.003) (Fig. 6B1,B3) (MEC: by 35 ± 11%, n = 5, p = 0.018) (Fig. 6C1,C2) (MLA: by 4 ± 4%, n = 9, p = 0.17) (data not shown). Moreover, MEC (5 μm) did not alter sEPSC frequency in α4-KO mice (change of 4 ± 11%, n = 6, p = 0.21) (Fig. 6C1,C2). These data suggest that nicotine facilitated glutamate release via desensitizing α4β2* nAChRs that are directly or indirectly presynaptic to MSNs.
Figure 6.
nAChRs and DA D2/D3 receptors modulate sEPSCs in MSNs. A, Typical traces (5 s, A1, A2) and time course (A3) of nicotine enhancement of sEPSCs in the absence (A1) or presence (A2) of sulpiride (10 μm). B, DHβE (0.3 μm) increases sEPSC frequency (B1, 5 s typical traces; B3, time course). The effect was also blocked by sulpiride (10 μm, B2, typical traces; B3, time course). C, MEC enhances sEPSC frequency in WT but not in α4-KO mice (C1, representative traces; C2, time course). D, Immunohistochemistry of TH (red), α4-YFP* nAChRs (green), and their colocalization (arrow) in the dorsal striatum. Data in time course and summary are shown as mean ± SEM.
The activation of α4β2* nAChRs on dopaminergic terminals facilitates DA release in the dorsal striatum (Zhou et al., 2001; Salminen et al., 2004; Grady et al., 2007; Nashmi et al., 2007; Drenan et al., 2008); in contrast, desensitization of these receptors reduces DA release (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004; Exley et al., 2008). We previously observed low but detectable expression of α4-YFP* nAChRs in the dorsal striatum (Nashmi et al., 2007); immunostaining for GFP now confirms that these signals mostly occurred in TH-immunostained structures (Fig. 6D). These data support the localization of α4β2* nAChRs in dopaminergic fibers/terminals. In addition, DA acting via D2/D3 receptors inhibits glutamatergic terminals (Bamford et al., 2004). We hypothesized that nicotine (≤1 μm) may modulate glutamate release through this pathway. That is, nicotine desensitizes α4β2* nAChRs in DA terminals, reduces DA release, and therefore indirectly disinhibits glutamatergic terminals. We observed that 10 μm sulpiride, a D2/D3 receptor antagonist, robustly enhanced sEPSC frequency (by 34 ± 3%, n = 9, p = 0.0001) (data not shown) without changing the amplitude of sEPSCs (data not shown). This suggests that the baseline DA level in the dorsal striatum is high enough to inhibit glutamate release by activating presynaptic D2/D3 receptors (Bamford et al., 2004; David et al., 2005). Meanwhile, in the presence of 10 μm sulpiride, neither nicotine (1 μm) (Fig. 6A2,A3) nor DHβE (0.3 μm) (Fig. 6B2,B3) enhanced sEPSC frequency. These data suggest that α4β2* nAChRs on striatal DA terminals are the primary contributors to nicotine (≤1 μm) modulation of glutamate release (see Fig. 8C) and that their function can be indirectly evaluated by this nicotine (1 μm) or DHβE (0.3 μm) enhancement of glutamate release.
Chronic nicotine upregulates α4β2* nAChRs on dopaminergic terminals in striatum
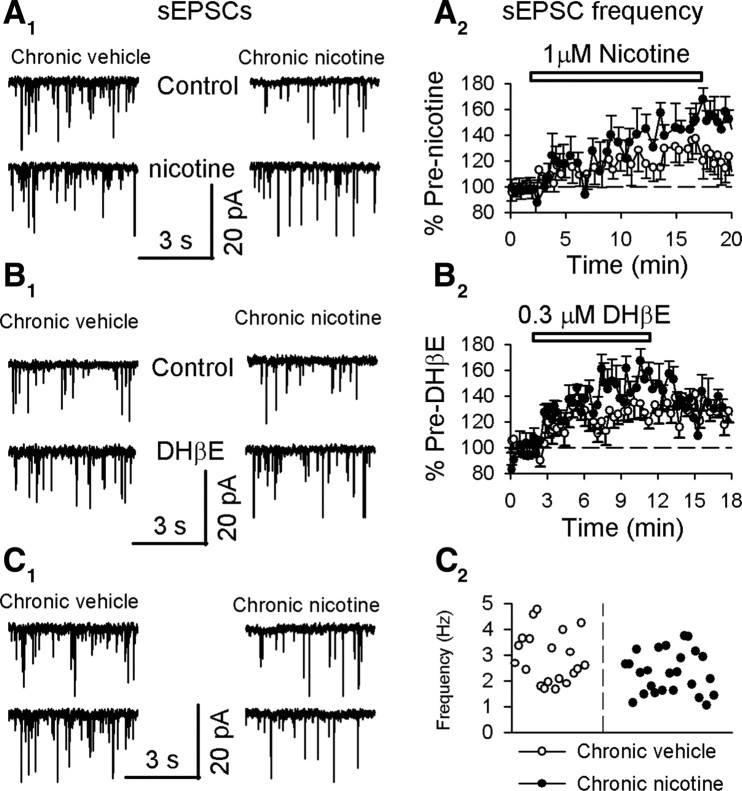
We used the effect described here, indirect modulation of glutamate release by α4β2* nAChRs, to assess the effects of chronic nicotine exposure on α4β2* nAChRs. Interestingly, we observed that chronic nicotine significantly enhanced the facilitation of sEPSC frequency by either 1 μm nicotine (chronic nicotine: by 50 ± 7%, n = 6, p = 0.0003; chronic vehicle: by 32 ± 3%, n = 8, p = 0.00001) (p = 0.01) (Fig. 7A1,A2) or DHβE (0.3 μm) (chronic nicotine: by 52 ± 7%, n = 7, p = 0.0007; chronic vehicle: by 35 ± 4%, n = 8, p = 0.0002) (p = 0.03) (Fig. 7B1,B2). These data agree with previous observations that chronic nicotine exposure upregulates α4β2* nAChRs in the striatum (Fig. 8C,D) (Marshall et al., 1997; Visanji et al., 2006; Nashmi et al., 2007).
Figure 7.
Chronic nicotine augments nicotinic modulation of spontaneous EPSCs in MSNs. A, chronic nicotine augments nicotine (1 μm) enhancement of spontaneous EPSC frequency (A1, 5 s typical traces; A2, summarized time course). B, Chronic nicotine strengthens DHβE (0.3 μm) enhancement of spontaneous EPSC frequency (B1, 5 s typical traces; B2, summarized time course). C, Chronic nicotine reduces baseline sEPSC frequency (C1, 5 s typical traces; C2, pooled data for sEPSC frequency). Data in summarized time courses are shown as mean ± SEM.
We also noted that the baseline frequency of sEPSCs in MSNs was significantly reduced in chronic nicotine-treated mice (2.32 ± 0.16, n = 25) when compared with chronic vehicle-treated mice (2.91 ± 0.22, n = 18) (p = 0.02) (Fig. 7C1,C2). This suggests that endogenous ACh, acting via the upregulated α4β2* nAChRs on DA terminals, leads to a higher baseline release of DA, exerting stronger inhibition at glutamatergic terminals (Fig. 8C,D). This idea is further supported by the result that chronic nicotine strengthened sulpiride (10 μm) enhancement of sEPSC frequency (chronic nicotine by 44 ± 2%, n = 11, p = 0.0001; chronic vehicle by 34 ± 3%, n = 9, p = 0.0001) (p = 0.01) (data not shown).
Discussion
Upregulated α4β2* nAChRs after chronic nicotine are functional
Upregulation of α4β2* nAChR numbers is often reported in both smokers and chronic nicotine-treated animals. It has been uncertain whether the upregulated receptors are functional or desensitized (Ochoa et al., 1990; Buisson and Bertrand, 2001, 2002; Gentry and Lukas, 2002; Nashmi et al., 2003, 2007; Moroni et al., 2006; Quik et al., 2007; Picciotto and Zoli, 2008; Lester et al., 2009).
The present data support the idea that the upregulated nAChRs are functional: chronic nicotine directly enhanced MLA-resistant (presumably non-α7) nicotinic currents in SNr GABAergic neurons (Table 1). These non-α7 currents were mainly mediated by α4β2* nAChRs, since the currents were blocked by DHβE in WT mice and absent from α4-KO mice (Fig. 1A1,A2). Also of note is that chronic nicotine increased the proportion of α4β2* nAChRs responding to low concentrations (≤10 μm) of ACh (Fig. 1C2, Table 1); importantly, these receptors can be activated by ambient ACh in SNr. Indeed, the 50% increase in the firing rate of SNr GABAergic neurons chronically exposed to nicotine, but tested in the absence of nicotine, was measured both in slices and in vivo (Fig. 2A1,A2,C), showing that the slice preparation preserves at least some influences on GABAergic neurons via nAChRs.
In the dorsal striatum, all observed nicotinic currents in striatal neurons were MLA sensitive (Fig. 4), implying that the responses arise from α7 nAChRs. Although puffs of 1 μm nicotine could not activate these receptors directly (data not shown), bath-applied 1 μm nicotine enhanced EPSP–spike coupling ratio in MSNs (Fig. 5A1–A3). This effect was mimicked by DHβE (Fig. 5B1–B3) but was absent from α4-KO mice (Fig. 5C1,C2). These facts indicate the existence of functional α4β2* nAChRs in WT mice. Interestingly, the desensitization or blockade of these receptors respectively by 1 μm nicotine or DHβE facilitated glutamate release (Fig. 6A1,A3,B1,B3). Sulpiride prevented these effects (Fig. 6A2,A3,B2,B3). Invoking well established functional evidence that α4β2* nAChRs are localized in DA terminals, we deduce that the contributing α4β2* nAChRs reside in DA terminals. These contributing presynaptic nAChRs are probably not localized in glutamatergic terminals in the striatum, because (1) higher concentrations of nicotine (≥10 μm) are needed to activate or desensitize α7 nAChRs, the principal nAChRs in glutamatergic terminals of the striatum (Kaiser and Wonnacott, 2000; Wonnacott et al., 2000; Marchi et al., 2002); and (2) like previous workers, we found no evidence for α4-YFP* receptors on the corticostriatal glutamatergic afferents (unpublished data).
Chronic nicotine increased the frequency of sIPSCs (Fig. 3A1,A3) and enhanced the facilitation of glutamate release by nicotine (Fig. 7A) and DHβE (Fig. 7B). In combination with previous findings that chronic nicotine elevates α4β2* nAChRs in the striatum (Marks et al., 2004; Nashmi et al., 2007; Perry et al., 2007), we conclude that chronic nicotine upregulates α4β2* nAChRs in DA terminals, and again that these receptors are functional. This is consistent with other studies showing that chronic nicotine increases DA levels (Fung and Lau, 1988; Court et al., 1998), α4β2* nAChRs (Marks et al., 2004; Nashmi et al., 2007; Perry et al., 2007), and α4β2* nAChR-mediated DA release (McCallum et al., 2006a,b) in the striatum.
Tiers of selectivity in chronic nicotine upregulation of nAChRs
We observed that chronic nicotine enhanced MLA-resistant (Fig. 1) but not MLA-sensitive nicotinic currents in SNr GABAergic neurons, consistent with the concept that chronic exposure to low concentrations of nicotine can upregulate α4β2* but not α7 nAChRs (Marks et al., 1986; Collins et al., 1994). High-affinity nAChR binding assays previously revealed that chronic nicotine upregulates α4β2* nAChRs in some but not other brain regions (Flores et al., 1992; Marks et al., 1992; Perry et al., 1999; Nguyen et al., 2004). A previous study demonstrated that chronic nicotine upregulates fluorescent α4* nAChRs in midbrain GABAergic neuron somata but not in midbrain DA neuron somata (Nashmi et al., 2007). The present study confirms that these numerically upregulated receptors are functional. These data indicate that chronic nicotine upregulation of nAChRs displays selectivity in nAChR subtype, brain region, and cell type.
SNc DA neurons project to the dorsal striatum. Although chronic nicotine did not change the function of α4β2* nAChRs in SNc DA neuron somata (Table 2), it did enhance the modulation of glutamate release by α4β2* nAChRs in the dorsal striatum (Fig. 7). These data extend a previous study showing that, although chronic nicotine does not increase fluorescent α4* nAChRs in the somata of SNc DA neurons, such treatment does increase fluorescence in the dorsal striatum (Nashmi et al., 2007). The previous study did not provide information about the localization of α4* nAChRs in the several types of intrinsic neurons and axon terminals in the dorsal striatum. We have now concluded that there are no detectable nicotinic responses mediated by α4* nAChRs in the somata of MSNs and interneurons (Fig. 4). We have further inspected α4* nAChRs by using anti-GFP antibodies to amplify the YFP signal in α4-YFP mice, which clearly visualized α4* nAChRs in neuronal somata of the substantia nigra (supplemental Fig. S3, available at www.jneurosci.org as supplemental material) (Nashmi et al., 2007) and DA fibers of the dorsal striatum (Fig. 6D), but not in the somata of striatal neurons (Fig. 6D). Given that in the dorsal striatum, α4β2* nAChRs modulating glutamate release are located in DA terminals, chronic nicotine upregulation of α4β2* nAChRs most likely occurs in DA terminals. This dataset suggests that chronic nicotine upregulation of α4β2* nAChRs displays an additional selectivity in cellular compartment (soma vs axon terminal).
Chronic nicotine not only enhanced the MLA-resistant nicotinic currents elicited by 3–1000 μm ACh but also increased the ratio of rather low-dose (≤10 μm) ACh-induced currents to 1 mm ACh-induced currents (Table 1). This suggests that chronic nicotine may upregulate the higher-sensitivity (α4)2(β2)3 stoichiometry to a greater extent than the lower-sensitivity (α4)3(β2)3 nAChRs, as found in several types of in vitro experiments (Buisson and Bertrand, 2001; López-Hernández et al., 2004; Vallejo et al., 2005; Moroni et al., 2006; Lester et al., 2009).
Chronic nicotine upregulates nAChRs through various mechanisms, including increased subunit assembly via pharmacological chaperoning (Nashmi et al., 2003; Kuryatov et al., 2005; Lester et al., 2009), increased receptor trafficking to the plasma membrane (Harkness and Millar, 2002; Darsow et al., 2005), reduced receptor turnover (Peng et al., 1994), and improved maturation of the receptors (Darsow et al., 2005; Sallette et al., 2005). Tiers of selectivity in chronic nicotine upregulation of α4β2* nAChRs could result from the selectivity of pharmacological chaperoning, interaction with accessory subunits of nAChRs, interactions with auxiliary proteins such as lynx (Miwa et al., 2006), and other unknown factors (Lester et al., 2009).
Chronic nicotine modifies synaptic circuits in nigrostriatal DA pathways
We show that the upregulation of α4β2* nAChRs by chronic nicotine influences the activity of neural circuits in the nigrostriatal DA pathway. In the substantia nigra (Fig. 8A,B), the enhanced function of α4β2* nAChRs by chronic nicotine increased the activity of SNr GABAergic neurons and thus strengthened GABAergic inhibition in SNc DA neurons. This may reduce the risk of excitotoxicity in SNc DA neurons, because GABAA receptor agonists or GABA mimetic agents ameliorate some brain injuries by attenuating excitotoxicity (Green et al., 2000).
In the dorsal striatum the function of α4β2* nAChRs on the DA terminals is also augmented by chronic nicotine, leading to the inhibition of glutamate release (Fig. 8C,D). Increased glutamate release occurs in the striatum both of PD patients and of PD animal models (Calabresi et al., 2000; Koutsilieri and Riederer, 2007; Boulet et al., 2008) and might contribute to dyskinesia following long-term l-DOPA treatment. Memantine and amantadine, two NMDA receptor antagonists, relieve dyskinesia (Parsons et al., 1999; Steece-Collier et al., 2000; Hadj Tahar et al., 2004). Coincidently, chronic nicotine effectively controls the dyskinesia when coapplied with l-DOPA (Quik et al., 2008). We now report that nicotine increases the proportion of corticostriatal presynaptic action potentials leading to MSN action potentials (Fig. 5), providing a possible synaptic basis for this apparent therapeutic effect. The proposed mechanism (Fig. 8) is indirect, and other possible, albeit more complicated, explanations might also be consistent with the data.
The subcellular selectivity of α4* nAChR upregulation by chronic nicotine exposure may alter the overall function of basal ganglia circuitry. The two major cholinergic cell types that influence DA release are the mesopontine neurons, which project to substantia nigra, and the giant intrinsic cholinergic interneurons of striatum. Because chronic nicotine does not upregulate α4* nAChRs on DA neuron somata and dendrites in SNc, but does so on DA neuron axon terminals in striatum, one expects chronic nicotine to cause a relatively stronger influence by the latter cholinergic cell types. Despite the lower DA neuron firing rate caused by events in substantia nigra, the direct presynaptic excitation may maintain striatal DA release at near-normal levels. Because the DA terminals are rather distant from the soma, one does not expect the nicotinic depolarization to become excitotoxic by extensively perturbing somatodendritic metabolism, and it would not counteract the excitotoxic-sparing mechanism.
Chronic exposure to nicotine itself may have advantages over GABA mimetic agents and glutamate antagonists as neuroprotective agents in PD: (1) nicotine effects are relatively mild in general, (2) chronic nicotine leads to long-term modification of synaptic circuits by upregulating α4β2* nAChRs, and (3) chronic nicotine upregulation of α4β2* nAChRs presents tiers of selectivity: brain regions, neuronal cell types, cellular compartments, and stoichiometry, fortuitously restricting the targets and reducing the side effects. However, nicotine analogs that are highly selective for α4β2* nAChRs might be better therapeutic choices, especially if they efficiently upregulate these receptors in SNr GABAergic neurons and in dorsal striatum DA terminals.
In conclusion, α4β2* nAChRs can be upregulated by chronic nicotine and remain functional. The upregulation shows selectivity in nAChR subtype, neuronal cell type, cellular compartment, and possibly stoichiometry. These tiers of selectivity may reshape the activity of neural circuits in the nigrostriatal DA pathway, protecting DA neurons from excitotoxic damage by moderating burst firing initiated at the soma, while also preserving normal levels of DA release at the striatal DA terminals.
Footnotes
This work was supported by grants from the U.S. National Institutes of Health (DA17279, AG033954, MH53631), from Targacept Inc., and from Louis and Janet Fletcher. We also acknowledge support from the Natural Sciences and Engineering Research Council Canada (R.N.), the NARSAD Young Investigator Program (R.N.), and the California Tobacco-Related Disease Research Program (16FT-0066 to C.X.). We thank J. Drago for α4 knock-out mice, P. Deshpande and C. D. Son for much assistance, and R. Srinivasan and B. N. Cohen for comments.
References
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Del Dotto P. New pharmacologic horizons in the treatment of Parkinson disease. Neurology. 2006;67:S30–S38. doi: 10.1212/wnl.67.7_suppl_2.s30. [DOI] [PubMed] [Google Scholar]
- Boulet S, Mounayar S, Poupard A, Bertrand A, Jan C, Pessiglione M, Hirsch EC, Feuerstein C, François C, Féger J, Savasta M, Tremblay L. Behavioral recovery in MPTP-treated monkeys: neurochemical mechanisms studied by intrastriatal microdialysis. J Neurosci. 2008;28:9575–9584. doi: 10.1523/JNEUROSCI.3465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Bernardi G. Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci. 2000;23:S57–S63. doi: 10.1016/s1471-1931(00)00017-3. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Luo Y, Selvaag S, Marks MJ. Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. J Pharmacol Exp Ther. 1994;271:125–133. [PubMed] [Google Scholar]
- Court JA, Lloyd S, Thomas N, Piggott MA, Marshall EF, Morris CM, Lamb H, Perry RH, Johnson M, Perry EK. Dopamine and nicotinic receptor binding and the levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience. 1998;87:63–78. doi: 10.1016/s0306-4522(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Darsow T, Booker TK, Piña-Crespo JC, Heinemann SF. Exocytic trafficking is required for nicotine-induced up-regulation of α4β2 nicotinic acetylcholine receptors. J Biol Chem. 2005;280:18311–18320. doi: 10.1074/jbc.M501157200. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, Lester HA. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity α6* nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. α6-Containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Fung YK, Lau YS. Receptor mechanisms of nicotine-induced locomotor hyperactivity in chronic nicotine-treated rats (striatum) Eur J Pharmacol. 1988;152:263–271. doi: 10.1016/0014-2999(88)90721-2. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Hainsworth AH, Jackson DM. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacology. 2000;39:1483–1494. doi: 10.1016/s0028-3908(99)00233-6. [DOI] [PubMed] [Google Scholar]
- Hadj Tahar A, Grégoire L, Darré A, Bélanger N, Meltzer L, Bédard PJ. Effect of a selective glutamate antagonist on L-dopa-induced dyskinesias in drug-naive parkinsonian monkeys. Neurobiol Dis. 2004;15:171–176. doi: 10.1016/j.nbd.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Harkness PC, Millar NS. Changes in conformation and subcellular distribution of α4β2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras. J Neurosci. 2002;22:10172–10181. doi: 10.1523/JNEUROSCI.22-23-10172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. α-bungarotoxin-sensitive nicotinic receptors indirectly modulate [3H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Riederer P. Excitotoxicity and new antiglutamatergic strategies in Parkinson's disease and Alzheimer's disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S329–S331. doi: 10.1016/S1353-8020(08)70025-7. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J. 2009;11:167–177. doi: 10.1208/s12248-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Hernández GY, Sánchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz J, Salas-Vincenty J, Rojas LV, Lasalde-Dominicci JA. Nicotine-induced up-regulation and desensitization of α4β2 neuronal nicotinic receptors depend on subunit ratio. J Biol Chem. 2004;279:38007–38015. doi: 10.1074/jbc.M403537200. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. J Pharmacol Exp Ther. 1986;239:358–364. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86[Rb]+ efflux and 125[I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology. 2004;46:1141–1157. doi: 10.1016/j.neuropharm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic α3*/α6*β2 and α4*β2 nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J Pharmacol Exp Ther. 2006a;318:381–388. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, Tyndale RF, Langston JW, McIntosh JM, Quik M. Increases in α4* but not α3*/α6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem. 2006b;96:1028–1041. doi: 10.1111/j.1471-4159.2005.03646.x. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, Xiao C, Fitzsimonds RM, Pavlides C, Lester HA, Picciotto MR, Heintz N. The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron. 2006;51:587–600. doi: 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. α4β2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Garzotti M, Sartori I, Pilla M, Repeto P, Heidbreder CA, Tessari M. Selective down-regulation of [125I]Y0-α-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137:565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, Lester HA. Assembly of α4β2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci. 2003;23:11554–11567. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–49. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci. 1995;15:4449–4463. doi: 10.1523/JNEUROSCI.15-06-04449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa EL, Li L, McNamee MG. Desensitization of central cholinergic mechanisms and neuroadaptation to nicotine. Mol Neurobiol. 1990;4:251–287. doi: 10.1007/BF02780343. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotactic coordinates. Ed 4. San Diego: Elsevier; 2004. [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994;46:523–530. [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. Long-term nicotine treatment differentially regulates striatal α6α4β2* and α6(Nonα4)β2* nAChR expression and function. Mol Pharmacol. 2008;74:844–853. doi: 10.1124/mol.108.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates α6- and β3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Quik M, Bordia T, O'Leary K. Nicotinic receptors as CNS targets for Parkinson's disease. Biochem Pharmacol. 2007;74:1224–1234. doi: 10.1016/j.bcp.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, O'Leary K, Tanner CM. Nicotine and Parkinson's disease: implications for therapy. Mov Disord. 2008;23:1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, Ross GW, Strickland D, Van Den Eeden SK, Gorell J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Pinguet N, Jia L, Li M, Figl A, Klaassen A, Truong A, Lester HA, Cohen BN. Five ADNFLE mutations reduce the Ca2+ dependence of the α4β2 acetylcholine response. J Physiol. 2003;550:11–26. doi: 10.1113/jphysiol.2003.036681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–1656. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp Neurol. 2000;163:239–243. doi: 10.1006/exnr.2000.7374. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kovalchuk Y, Attwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. J Neurosci. 1995;15:5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Thacker EL, O'Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visanji NP, Mitchell SN, O'Neill MJ, Duty S. Chronic pre-treatment with nicotine enhances nicotine-evoked striatal dopamine release and α6 and β3 nicotinic acetylcholine receptor subunit mRNA in the substantia nigra pars compacta of the rat. Neuropharmacology. 2006;50:36–46. doi: 10.1016/j.neuropharm.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. Upregulation of nicotinic receptors by nicotine varies with receptor subtype. J Biol Chem. 2008;283:6022–6032. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Frucht SJ. Treatment of Parkinson's disease: what's on the horizon? CNS Drugs. 2005;19:723–743. doi: 10.2165/00023210-200519090-00001. [DOI] [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods. 2006;158:251–259. doi: 10.1016/j.jneumeth.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]