Abstract
The bal defect in the Arabidopsis thaliana Columbia strain was spontaneously generated in an inbred ddm1 (decrease in DNA methylation 1) mutant background in which various genetic and epigenetic alterations accumulate. The bal variant displays short stature and curled leaves due to the constitutive activation of defense signaling. These bal phenotypes are metastable and phenotypic suppression is evident in more than one-third of ethyl methanesulfonate (EMS)-treated bal M1 plants. The semidominant bal allele maps to the RPP5 (recognition of Peronospora parasitica 5) locus, which includes a cluster of disease Resistance (R) genes, many of which show an increase in steady-state expression levels in the bal variant. Here, we report that activation of RPP5 locus R genes and dwarfing in the bal variant are caused by a 55-kb duplication within the RPP5 locus. Although many RPP5 locus R genes are duplicated in the bal variant, the duplication of SNC1 alone is necessary and sufficient for the phenotypic changes in the bal variant. Missense mutations in the SNC1 gene were identified in all three phenotypically suppressed EMS-treated bal lines investigated, indicating that the high-frequency phenotypic instability induced by EMS treatment is caused by a genetic mechanism. We propose that the high degree of variation in SNC1-related sequences among Arabidopsis natural accessions follows the two-step mechanism observed in the bal variant: gene duplication followed by hypermutation.
GENE duplication plays an important role in expanding the repertoire of the genome. Immediately after duplication the two gene copies are expected to be functionally equivalent, leading to reduced selection on one copy and an opportunity for subsequent specialization (i.e., sub- or neo-functionalization). This model is supported by Lynch and Conery's (2000) study of genomic sequences from different eukaryotes demonstrating that most duplicated protein-coding gene copies experience an initial period of relaxed selection during which time mutations accumulate consistent with the expectations of neutrality (Lynch and Conery 2000). However, some exceptions to this general trend in which nonsynonymous mutations within newly duplicated genes immediately predominate over silent mutations were observed. We describe here a molecular snapshot of a coupled gene duplication-hypermutation event involving plant disease Resistance or R genes that can generate such exceptional evolutionary genetic signatures.
R genes encode proteins that recognize, either directly or indirectly, plant pathogen components and modulate defense-signaling pathways (Belkhadir et al. 2004). Many Resistance proteins contain conserved domains, including Toll/Interleukin1 Receptor, nucleotide-binding site (NBS), and leucine-rich repeat (LRR) motifs, and paralogous genes encoding these proteins are often found in clusters within the genome resulting from local tandem duplications (Martin et al. 2003). The Arabidopsis RPP5 (for recognition of Peronospora parasitica 5) locus is an example of an R-gene cluster (Noël et al. 1999) that contains at least two functional genes (Parker et al. 1997; van der Biezen et al. 2002; Zhang et al. 2003) and several additional paralogs, many of which may also function in plant defense signaling (Meyers et al. 2003) (supporting information, Figure S1). RPP5 locus R genes can be coordinately regulated both positively and negatively. Positive transcriptional activation is mediated through the upregulation of SNC1 (SUPPRESSOR OF NPR1-1, CONSTITUTIVE 1) (Li et al. 2007; Yi and Richards 2007), while negative regulation by RNA silencing with small interfering RNA species targets multiple RPP5 locus R genes (Yi and Richards 2007).
Mutant screens in the Arabidopsis strain Columbia have recovered three mutations—cpr1, bal, and snc1—that map to the RPP5 locus and display similar yet distinct dwarfism and curled-leaf phenotypes (Bowling et al. 1994; Li et al. 2001; Stokes et al. 2002; Stokes and Richards 2002). These RPP5 locus mutants exhibit constitutive expression of downstream pathogen defense genes that lead to reduced fitness in both vegetative growth and reproduction (Bowling et al. 1994; Clarke et al. 2001; Li et al. 2001; Stokes et al. 2002; Zhang et al. 2003; Heidel et al. 2004). Several reports suggest that upregulation of SNC1 and possibly other RPP5 locus R genes is responsible for the phenotypic and gene expression changes in these mutants (Stokes et al. 2002; Yang and Hua 2004; Yi and Richards 2007, 2008). Unlike the snc1 mutation, a high incidence of phenotypic instability is induced for the bal and cpr1 mutations after mutagen treatment or genetic interaction in bal × cpr1 or cpr1 × snc1 F1 hybrids (Stokes et al. 2002; Stokes and Richards 2002; Zhang and Li 2005; Yi and Richards 2008). A gain-of-function missense mutation results in SNC1 activation in the snc1 mutant (Zhang et al. 2003). However, the molecular changes corresponding to the bal or cpr1 mutations and the mechanisms responsible for the phenotypic instability of these two alleles have not been elucidated.
Here, we report that a 55-kb region, including all the R genes in the RPP5 locus except RPP4, is tandemly duplicated in the metastable bal variant. Our results demonstrate that a duplication of SNC1 in the bal variant is responsible for the development of morphological phenotypes and leads to activation of multiple RPP5 locus R genes. In addition, we show that the high level of phenotypic instability in the bal variant is caused not by an epigenetic mechanism but by hypermutation of SNC1, upregulation of which is necessary and sufficient for the development of bal phenotypes. Our study provides an example of a two-step mechanism by which polymorphic R-gene clusters evolve: R-gene cluster expansion via unequal crossing over, followed by hypermutation of duplicated R genes.
MATERIALS AND METHODS
Plants and growth conditions:
The bal (Stokes et al. 2002), cpr1 (Bowling et al. 1994), snc1 (Li et al. 2001; Zhang et al. 2003), and snc1-r1 (Zhang et al. 2003) mutants were previously described. Salk transfer DNA (T-DNA) insertional mutants were obtained from the Arabidopsis Biological Resource Center at The Ohio State University, and homozygous mutant lines for individual RPP5 locus R genes were identified with the PCR-based method as previously suggested (Alonso et al. 2003). All plants were grown in soil in a growth chamber under long-day conditions (16 hr light and 8 hr dark) as described previously (Stokes et al. 2002).
Nucleic acid isolation and analysis:
Genomic DNA was isolated by the urea lysis miniprep protocol (Cocciolone and Cone 1993). Total RNA and low-molecular-weight-enriched RNA were isolated from aerial parts of 2-week-old plants using the TRIzol reagent (Invitrogen) and mirVana miRNA isolation kit (Ambion), respectively. Protocols for DNA gel blot analysis and small RNA gel blot analysis were previously described (Jeddeloh et al. 1998; Yi and Richards 2007). Templates for probes used in gel blot analyses were either PCR products or oligonucleotides. Copy numbers and steady-state expression levels of RPP5 locus R genes were compared using quantitative real-time PCR, as described previously (Yi and Richards 2007). The Big Dye V1.1 Terminator Cycle Sequencing method (Applied Biosystems) was used to determine the nucleotide sequence of SNC1. Information on the oligonucleotide primers and TaqMan probes (Applied Biosystems) used in this study is included in Table S1.
RESULTS
Null alleles of SNC1 suppress the semidominant phenotypes of the bal allele:
Previously, we showed that SNC1 is upregulated in the bal variant and that transgenic overexpression of SNC1 is sufficient for the induction of bal-like phenotypes (dwarf stature and curled leaf) (Stokes et al. 2002). However, our subsequent study found that other RPP5 locus R genes, including RPP4, are also upregulated in the bal variant (Yi and Richards 2007). This finding made it unclear whether overexpression of SNC1, rather than another R gene or genes in the locus, is necessary for the development of phenotypes in the bal variant (van der Biezen et al. 2002; Zhang et al. 2003). To determine which R gene is required for the bal phenotypes, we took advantage of the dosage-dependent phenotypes of the bal allele (Kakutani et al. 1996; Stokes et al. 2002). Morphological phenotypes and R-gene expression levels are intermediate in heterozygous bal plants (BAL/bal) in the Columbia background compared to wild-type (BAL/BAL) and homozygous bal plants (bal/bal) (Stokes et al. 2002). However, Columbia/Landsberg interstrain F1 hybrids carrying one copy of the bal allele are phenotypically normal in body size and leaf morphology (Kakutani et al. 1996). Considering that R genes in the Landsberg RPP5 locus are quite diverged from those in the Columbia RPP5 locus (Noël et al. 1999), we reasoned that the expression level of a specific R gene or genes in the Columbia strain falls below a threshold level in the interstrain F1 hybrids, and consequently these plants show a wild-type morphology (Stokes et al. 2002). We predicted that a loss-of-function allele in the specific R gene required for the development of characteristic bal phenotypes would act as a dominant suppressor of the bal phenotypes, depending on the expression level of this gene.
We tested whether a specific RPP5 locus R gene is required for the bal phenotypes by analyzing the phenotypes of F1 plants containing a bal allele and different T-DNA insertional null alleles of individual RPP5 locus R genes in the Columbia background (Figure S1) (Alonso et al. 2003). We found that two independent T-DNA insertional null alleles of SNC1 (Yang and Hua 2004) acted as dominant suppressors of bal phenotypes in F1 plants (Figure 1). Suppression of bal phenotypes in F1 hybrids by snc1 null alleles was further confirmed by the use of a third SNC1 null allele, snc1-r1 (Zhang et al. 2003), that has a small deletion in the coding sequence of SNC1. In contrast, T-DNA lines with individual disruptions in each of the other R genes in the locus produced F1 hybrids with an intermediate phenotype indistinguishable from heterozygous bal mutants (BAL/bal) (Figure 1). These results indicate that SNC1 is necessary for the development of bal phenotypes while other R genes in the locus are dispensable. Taken together with our previous results showing that transgenic overexpression of SNC1 can induce bal-like phenotypes, we conclude that SNC1 is the only R gene in the RPP5 locus whose overexpression is necessary and sufficient for the phenotypic development of the bal variant (Stokes et al. 2002; Yi and Richards 2007).
Figure 1.—
Null alleles of SNC1 can suppress the phenotypes of the bal allele in F1 hybrids. (A) Phenotypes of sibling plants with different numbers of bal alleles. BAL/BAL: wild-type plants with no bal allele. BAL/bal: heterozygous bal plant with one bal allele. bal/bal: homozygous bal plant with two bal alleles. (B) Phenotypes of F1 hybrid plants. Homozygous mutant alleles in female and male parents are indicated before and after the “X,” respectively. Null alleles of R genes in the RPP5 locus obtained from the Salk T-DNA insertional line collection are shown in Figure S1. rpp4: Salk_017569, snc1-11: Salk_047058, at4g16900: Salk_034491.
The copy number of SNC1 is increased in the bal variant:
Although overexpression of SNC1 can explain the development of bal phenotypes and locus-wide activation of RPP5 locus R genes in the bal variant, no nucleotide sequence changes are present in the 7-kb region covering the entire coding sequence and the putative promoter of SNC1 (Stokes et al. 2002; Yi and Richards 2007). On the basis of these results and the instability of the bal allele, we previously proposed that an epigenetic alteration at the RPP5 locus is responsible for the upregulation of SNC1 in the bal variant (Stokes et al. 2002; Stokes and Richards 2002). We examined the SNC1 gene in the bal variant for hallmarks of alternative epigenetic states, including differential cytosine methylation, small RNA accumulation, and histone modifications. We found that the promoter region of SNC1 is largely free from cytosine methylation in both wild-type and bal plants (data not shown), consistent with results from genomewide cytosine methylation profiling for wild-type Columbia strain plants (Zhang et al. 2006; Cokus et al. 2008). In addition, we did not find any significant decrease in the accumulation of small RNA species that could negatively regulate RPP5 locus R genes in the bal variant (Figure S2). Chromatin immunoprecipitation (ChIP) experiments demonstrated that the SNC1 coding region was associated with chromatin containing histone H3 lysine 4 trimethylation in both wild-type plants and the bal variant (data not shown), consistent with a transcriptionally active state in both genotypes.
Our search for the molecular basis of the bal defect shifted back to genetic alterations on the basis of the results from control samples in our ChIP analysis. Specifically, we noted a stronger amplification of the SNC1 coding sequence when the input DNA from the bal variant was compared to that from wild-type samples after normalization for amplification of sequences outside of the RPP5 locus. This finding prompted us to conduct DNA gel blot hybridization experiments to investigate whether SNC1 is present at an elevated copy number in the bal variant, leading to enhanced transcription of SNC1 mRNA. In the bal variant, more intense hybridization signals were observed for restriction fragments that cover the entire coding sequence of SNC1 (Figure S3). DNA gel blot results using SNC1 or an At4g16950 probe, which cross-hybridizes to other RPP5 locus R genes, suggested that many other R genes in the RPP5 locus are also duplicated along with SNC1 in the bal variant (Figure S3 and Figure S4). However, no significant change in copy number was observed for RPP4 or for At4g16970, a non-R gene in the RPP5 locus. Using a quantitative real-time PCR method and genomic DNA templates, we measured how many extra copies of SNC1 and At4g16950 are present in the bal variant. By comparing the copy number of SNC1 or At4g16950 to that of RPP4 in wild-type and bal plants, we found that one extra copy of SNC1 and At4g16950 is present in the bal haplotype (Figure 2).
Figure 2.—
Both SNC1 and At4g16950 are duplicated in the bal variant. Using quantitative real-time PCR, the copy numbers of SNC1 (A) and At4g16950 (B) were compared to that of RPP4, whose copy number is not altered in the bal variant. gSNC1/gRPP4 and gAt4g16950/gRPP4 are amplification ratios of SNC1 and At4g16950, respectively, relative to RPP4 when genomic DNA was used as template. BAL: wild-type plants. bal: bal/bal homozygotes.
A 55-kb region in the RPP5 locus, covering SNC1 and five additional R genes, is tandemly duplicated in the bal variant:
We next determined the genomic organization of the duplicated RPP5 locus R genes. On the basis of genomewide phylogenetic analyses of Arabidopsis R genes, it was proposed that many of NBS–LRR class R genes, including those in RPP5 locus R genes, were generated by tandem duplication (Baumgarten et al. 2003; Meyers et al. 2003; Cannon et al. 2004). Because our genetic mapping results for the bal allele showed that the duplicated copy of SNC1 is tightly linked to the RPP5 locus, we hypothesized that the duplicated segment of the RPP5 locus is tandemly located within the native RPP5 locus (Kakutani et al. 1996; Stokes et al. 2002). First, we delimited the centromere-proximal boundary of the duplicated segment of the RPP5 locus between RPP4 (not duplicated in the bal variant) and At4g16880 (duplicated in the bal variant) through the identification of a restriction fragment length polymorphism using DNA gel blot analysis (Figure 3A). Armed with information on the approximate location of the centromere-proximal duplication breakpoint, we used PCR to narrow down the position of the telomere-proximal breakpoint. We found that we could amplify a product from the bal variant, but not wild-type plants, using a primer (R) located in the promoter region of RPP4 facing toward the centromere and a second primer (F) located in the coding region of At4g16950 facing toward the telomere (Figure 3, B and C). Sequence analysis of the telomere-proximal duplication boundary revealed that the entire first exon of At4g16960 is fused to the promoter sequence of RPP4, a result of an apparent homologous recombination event within an 186-bp region with 100% identity shared between RPP4 and At4g16960 (position −14 to +171 relative to the translational start codon) (Figure S5). We confirmed the structure of the duplication border in the bal variant using DNA gel blot analysis with a hybridization probe that spans the putative breakpoint and detected the predicted 2-kb BclI restriction fragment specifically in bal but not in wild-type plants (Figure 3D). We ruled out the possibility of large DNA rearrangements inside the duplicated region because the sizes of the PacI and SwaI restriction fragments detected in our DNA gel blot analyses of the bal variant were consistent with a simple tandem duplication. These data indicate that a contiguous 55-kb segment extending from the promoter region of RPP4 to the first exon of At4g16960 is tandemly duplicated in the bal variant.
Figure 3.—
Many RPP5 locus R genes, including SNC1, are tandemly duplicated in the bal variant. (A) DNA gel blot analysis showed that At4g16880 is duplicated in the bal variant. The restriction fragment detected by the At4g16880 probe in a wild-type plant is marked by an asterisk. An extra band detected with the At4g16880 probe in the bal variant is marked with a plus symbol (+). The positions of restriction fragments in the bal haplotype are indicated with bars marked with the asterisk and plus labels in panel C. BAL: wild-type plant. bal: bal variant. (B) PCR amplification demonstrated that the sequence in the promoter region of At4g16860 (RPP4) is located upstream of the At4g16950 coding sequence in the bal variant. The positions of the primers used in the PCR reaction are shown with arrowheads and labeled “F” and “R” in panel C. The arrowheads are pointing to the 3′-ends of primers. (C) Organization of RPP5 locus R genes in wild-type plants and the bal variant. The regions duplicated in the bal variant are indicated by two thick bars above the RPP5 locus R genes in the bal variant. A bar labeled with double pluses (++) shows the position of the polymorphic band detected in D. Note that only the BclI sites that generated the polymorphic restriction fragment in D are shown. (D) DNA gel blot result that detected the predicted 2-kb band marked with “++” in the bal variant.
Expression levels of RPP5 locus R genes are positively correlated with SNC1 copy number:
We investigated whether the steady-state expression levels of RPP5 locus R genes in the bal variant are affected by SNC1 copy number, as is the case for the phenotypes in the bal variant. To this end, we used quantitative real-time–PCR to compare the expression levels of SNC1, RPP4, and At4g16950 in wild-type plants (BAL/BAL), heterozygous bal plants (BAL/bal), and hemizygous F1 plants (bal/−) generated by crossing a snc1 null mutant and the bal variant. The copy numbers of SNC1, RPP4, and At4g16950 differ among these genotypes (Figure 4), allowing us to assess the contributions of varying SNC1 copy numbers relative to that of other R genes in the locus. We found that both SNC1 and RPP4 were less strongly expressed in the F1 hemizygous compared to heterozygous bal plants (Figure 4, B and C). In contrast, comparable levels of expression were observed in wild-type and F1 plants, both of which contain two copies of SNC1. The higher steady-state expression level of RPP4 observed in the heterozygous bal plants, which contain three copies of SNC1, can be explained by transcriptional activation of RPP4 mediated through dosage-dependent SNC1 overexpression (Stokes et al. 2002; Yi and Richards 2007).
Figure 4.—
Expression levels of RPP5 locus R genes are different in plants with a different copy number of these R genes. (A) Copy numbers of RPP5 locus R genes in wild-type plants (BAL/BAL), heterozygous bal plants (BAL/bal), and hemizygous F1 hybrids (bal/−). F1 hybrids were generated by crossing the bal variant and a snc1 null plant, the Salk_047058 mutant. Transcript levels of SNC1 (B), RPP4 (C), and At4g16950 (D) relative to ACT2 were determined by real-time RT–PCR. Total RNA was extracted from 2-week-old plants.
The expression level of another R gene in the locus, At4g16950, was also dependent on SNC1 copy number. At4g16950 expression in the F1 hybrids was lower than that in heterozygous bal plants (Figure 4D). F1 hybrids have two copies of SNC1 and three copies of At4g16950 while heterozygous bal plants have three copies of both genes, indicating that the expression level of At4g16950 is influenced by the copy number of SNC1. The higher expression level of At4g16950 in F1 hybrids compared to wild-type plants revealed that the At4g16950 expression level is also correlated with the copy number of At4g16950. On the basis of these findings, we conclude that both gene copy number and transcriptional activation mediated via SNC1 are important factors influencing the expression levels of various RPP5 locus R genes.
High-frequency suppression of the bal allele is associated with SNC1 hypermutation:
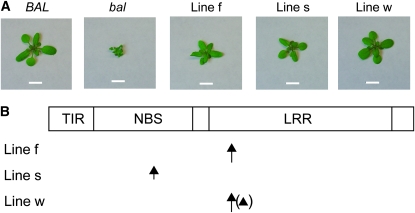
Previously, we reported that high-frequency (>10%) phenotypic suppression is observed in bal M2 populations (i.e., progeny of M1 plants grown from mutagenized seed) and that the alterations responsible for the high-frequency phenotypic suppression are tightly linked to the RPP5 locus, arguing against an extragenic suppressor mutation mechanism (Stokes et al. 2002). Our subsequent study found that heritable genetic or epigenetic changes involved in the high-frequency phenotypic suppression occur in more than one-third of EMS-treated bal M1 plants (Yi and Richards 2008). We investigated whether genetic mutations, such as reversions or intragenic suppressor mutations, or epigenetic alterations (e.g., RNA silencing in the RPP5 locus) are responsible for the high-frequency phenotypic suppression of the bal variant. We focused on possible epigenetic or genetic changes affecting the SNC1 gene because overexpression of this gene is necessary and sufficient for the development of bal phenotypes. For these studies, we established three stable lines, which exhibited significantly milder phenotypes and did not segregate any plants with bal morphology over several generations from a new EMS M2 population (Figure 5A). We first examined the abundance of small RNA targeting SNC1 but failed to find a significant change in our preliminary studies. We next investigated a genetic mechanism: reversion by a reduction in SNC1 copy number. The organization of duplicated segments in the bal variant, as well as the RPP5 locus R genes themselves, resemble tandem repeats, and we reasoned that stress conditions, such as those induced by DNA damage, might increase the frequency of homologous recombination, as was noted for a tandemly repeated reporter construct in Arabidopsis (Lucht et al. 2002; Molinier et al. 2006). However, no significant SNC1 copy number variation was detected in any of the three stable phenotypically suppressed lines that we examined (Figure S6). Therefore, we rejected the hypothesis that phenotypic suppression in EMS-treated bal plants is mediated by a reversion mechanism.
Figure 5.—
Phenotypic suppression of the bal variant by SNC1 mutation. (A) Phenotypes of bal → BAL suppressed lines. BAL: wild-type plant. bal: bal variant. Lines f, s, and w are three suppressed lines. Bar, 1 cm. (B) The positions of amino acid changes found in the three suppressed lines. The SNC1 protein with TIR (Toll/Interleukin-1 Receptor), NBS (nucleotide binding-site), and LRR (leucine-rich repeat) domains is depicted as a rectangle. Long and short arrows indicate the position of Glu to Lys at position 639 and Gly to Glu at position 313 in SNC1, respectively. The arrowhead in parentheses indicates a silent mutation detected in the coding sequence. It was not determined whether the two point mutations in line w are in the same copy or in different copies of SNC1.
Next, we determined whether nucleotide sequence changes in SNC1 are responsible for the high-frequency phenotypic suppression. To obtain unbiased nucleotide sequence reads from both copies of SNC1 in the genome of phenotypically suppressed lines, we determined the sequence of uncloned PCR products amplified from genomic DNA. A 4950-bp region extending from the start codon to the stop codon of SNC1 was examined in three phenotypically suppressed lines, and a total of four double peaks of A and G sequence were detected where single G peaks were expected (Figure S7). Three of these nucleotide sequence changes cause amino acid sequence changes in the NBS or LRR regions of SNC1 (Figure 5B). These missense mutations in SNC1 change amino acid residues conserved among RPP4, SNC1, and RPP5 (Parker et al. 1997; van der Biezen et al. 2002; Zhang et al. 2003). One missense mutation changes a Gly to Glu at the first position in the RNBS-B motif in the NBS domain. Extensive structure and function analysis revealed that a Gly-to-Glu mutation at the same position in RPM1, an R protein with NBS and LRR domains, destroys protein function (Tornero et al. 2002). The other missense mutation, which we recovered in two independent lines, is located in the fourth LRR domain within the predicted solvent-exposed β-sheet that is important for ligand recognition of R proteins (Mondragón-Palomino et al. 2002). The steady-state expression levels of SNC1 in the three suppressed lines examined are significantly lower than those in the bal variant (data not shown), suggesting that the missense mutations cause defects in SNC1 protein function and thereby affect the positive feedback amplification of SNC1 transcription (Yang and Hua 2004). Our discovery that all three stable phenotypically suppressed lines characterized in this study carry missense mutations in the SNC1 gene indicates that the unusual phenotypic instability of bal plants is caused mainly by hypermutation of the SNC1 gene.
DISCUSSION
Here, we define the molecular events responsible for the unusual behavior of the bal allele, providing a molecular snapshot of a two-step mechanism that can drive disease Resistance gene evolution. The first step involves an increase in gene copy number within paralogous gene clusters via homologous recombination. The second and more remarkable step involves the rapid inactivation or divergence of paralogs that relieves the fitness penalty exacted by the dosage effects of R-gene duplication.
Copy number variation by homologous recombination:
The diverse phenotypes of the bal variant are caused by the duplication of a 55-kb region that includes six RPP5 locus R genes: SNC1, At4g16900, At4g16920, At4g16940, At4g16950, and At4g16960 (Figure 3). Although several genes are duplicated, our dominant genetic suppressor screen indicated that SNC1 is the only R gene in the locus whose expression over a certain threshold is sufficient and necessary for the development of bal phenotypes (Figure 1). Moreover, the increased copy number of SNC1 is critical for the upregulation of paralogous R genes in the locus (Figure 4). These conclusions are supported by the report that introduction of extra genomic copies of the RPP5 locus R genes other than SNC1 does not induce bal-like phenotypes, while the addition of one or two extra copies of SNC1 is enough to induce bal-like phenotypes (Li et al. 2007).
The bal allele arose by a homologous recombination event between two paralogous R genes in the RPP5 locus that fused the At4g16960 coding sequence with the RPP4 upstream region to create a novel gene (Figure S5). Sequence comparison of the various RPP5 locus R genes previously suggested that similar sequence exchanges among neighboring paralogous genes contributed to generation of the extant R genes in the locus (Noël et al. 1999). The recovery of a chimeric R gene at the duplication breakpoint in the bal variant is a real-time example of a novel R-gene formation event, supporting inferences gained from comparative genomic studies (Song et al. 1997; Ramakrishna et al. 2002; Kuang et al. 2004; Friedman and Baker 2007).
The bal variant was originally isolated in an inbred ddm1 mutant background, and it is possible that a loss of heterochromatic marks and transposon silencing in the RPP5 locus contributed to the formation of the bal duplication. An alternative hypothesis is that loss of DDM1 might have played a more direct role in stimulating homologous recombination events. Support for this hypothesis comes from a recent report that deletion of the budding yeast ortholog of DDM1 (YFR038W, IRC5) increases the rate of recombination between homologous chromosomes (Alvaro et al. 2007). A third possibility is that the RPP5 locus is inherently dynamic and the isolation of the bal allele in a ddm1 mutant background was a coincidence. This view is reinforced by our observation that SNC1-related sequences show a high frequency of restriction fragment length polymorphisms as well as copy number variation among different wild-type A. thaliana accessions (Figure S8) (Noël et al. 1999; Yang and Hua 2004).
Rapid divergence of duplicated paralogs:
Our results demonstrate an unexpected mechanism by which such R-gene diversity might be facilitated: hypermutation of duplicated paralogs. Although phenotypes conditioned by the bal allele are stable in unmutagenized populations, we recovered phenotypically suppressed individuals in mutagenized M1 and M2 populations at an extremely high frequency (Stokes et al. 2002; Yi and Richards 2008). For example, more than one-third of EMS-treated bal M1 plants are phenotypically suppressed or contain sectors with milder phenotypes (Yi and Richards 2008). Moreover, nearly all M1 plants examined gave rise to phenotypically suppressed M2 progeny. The frequencies with which phenotypically suppressed M1 and M2 plants are recovered are orders of magnitude higher than expected by traditional mutagenesis, a finding that implicates an epigenetic mechanism. We show here, however, that the principal cause of this unusual phenotypic instability is mutation in one of the duplicated copies of SNC1. We characterized three independent, stable, phenotypically suppressed lines derived from an EMS-treated M2 population and found that each line carries a different missense mutation in the coding region of SNC1 (Figure 5B). Although four bp substitutions were identified within the 4950-bp SNC1 coding region among the three phenotypically suppressed lines, no nucleotide sequence changes were detected in these lines in another genic region on chromosome 4 that was sequenced as a control [i.e., an ∼3260-bp region encoding part of the FWA (Flowering Wageningen) gene; data not shown]. In addition, progeny of EMS-treated bal plants only rarely exhibited aberrant gross morphological phenotypes such as albinism, floral defects, or sterility. These findings suggest that the high frequency of mutation observed at SNC1 did not occur broadly across the genome.
The unusually high incidence of phenotypic suppression in EMS-treated bal plants might be explained by at least two different mechanisms, which are not mutually exclusive. One possibility is that the high-frequency phenotypic suppression in EMS-treated bal plants results from the selection within the meristematic tissues that favors cells carrying a mutation in SNC1 (Comai and Cartwright 2005; Henikoff 2005). Constitutive activation of RPP5 locus R genes exacts a significant fitness cost during vegetative growth (Heidel et al. 2004). Cells in which the expression levels of RPP5 locus R genes are reduced by mutations in the SNC1 gene might have a selective advantage in the stem cell niche within the meristem. Consequently, cells containing SNC1 mutations in the context of the bal duplication would be expected to out-compete cells without suppressor mutations as development progresses. Consistent with the meristem selection hypothesis, all of the recovered mutations are G/C-to-A/T transitions, matching the mutagenic specificity of EMS (Greene et al. 2003). Additional support for this hypothesis comes from the prevalence of EMS-treated M1 bal plants that show phenotypically suppressed sectors. An alternative hypothesis for the apparent hypermutation of SNC1 in phenotypically suppressed plants is stress-induced mutagenesis (Galhardo et al. 2007)—the stress in this case being initiated by DNA damage. The recovery of two SNC1 mutations in one of the phenotypically suppressed lines is consistent with stress-induced mutagenesis mediated by error-prone DNA polymerases. This scenario, however, necessitates an additional postulate that the mutation rate of the SNC1 gene in the bal variant is elevated preferentially. The constitutively active state of SNC1 in the bal variant might play a role, as transcription-coupled mutagenesis has been documented in both yeast and bacteria (Datta and Jinks-Robertson 1995; Wright et al. 1999). It is also possible that the large duplication in the bal variant of the already repetitious RPP5 locus provides a platform for increased homologous recombination, which has been linked to stress-induced mutagenesis (Galhardo et al. 2007). We note that high-frequency phenotypic instability is induced by EMS treatment of the cpr1 mutant, which carries an unknown defect in the RPP5 locus (Bowling et al. 1994; Yi and Richards 2008). An increased mutation frequency has been observed under special circumstances in diverse organisms, for example, repeat-induced point mutation in fungi during the sexual phase of their life cycle (Galagan and Selker 2004), and somatic hypermutation of vertebrate immunoglobulin during B-cell proliferation (Teng and Papavasiliou 2007). The recovery of SNC1 mutations in the bal variant provides a unique system for studying the mechanistic basis of high-frequency mutation in plants.
Acknowledgments
We thank Hye Ryun Woo and Travis Dittmer for helpful comments on the manuscript, Sanjida Rangwala for bisulfite sequencing results, and Michael Dyer and the Washington University Biology Department greenhouse staff for plant care. The work was supported by grants from the National Science Foundation to E.J.R. (MCB-0321990 and MCB-0548597). Additional support was provided by the Danforth Foundation.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.105569/DC1.
References
- Alonso, J. M., A. N. Stepanova, T. J. Leisse, C. J. Kim, H. Chen et al., 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Alvaro, D., M. Lisby and R. Rothstein, 2007. Genome-wide analysis of Rad52 foci reveals diverse mechanisms impacting recombination. PLoS Genet. 3 e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten, A., S. Cannon, R. Spangler and G. May, 2003. Genome-level evolution of resistance genes in Arabidopsis thaliana. Genetics 165 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir, Y., R. Subramaniam and J. L. Dangl, 2004. Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr. Opin. Plant Biol. 7 391–399. [DOI] [PubMed] [Google Scholar]
- Bowling, S. A., A. Guo, H. Cao, A. S. Gordon, D. F. Klessig et al., 1994. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, S. B., A. Mitra, A. Baumgarten, N. D. Young and G. May, 2004. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 4 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, J. D., N. Aarts, B. J. Feys, X. Dong and J. E. Parker, 2001. Constitutive disease resistance requires EDS1 in the Arabidopsis mutants cpr1 and cpr6 and is partially EDS1-dependent in cpr5. Plant J. 26 409–420. [DOI] [PubMed] [Google Scholar]
- Cocciolone, S. M., and K. C. Cone, 1993. Pl-Bh, an anthocyanin regulatory gene of maize that leads to variegated pigmentation. Genetics 135 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokus, S. J., S. Feng, X. Zhang, Z. Chen, B. Merriman, C. D. Haudenschild et al., 2008. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai, L., and R. A. Cartwright, 2005. A toxic mutator and selection alternative to the non-Mendelian RNA cache hypothesis for hothead reversion. Plant Cell 17 2856–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, A., and S. Jinks-Robertson, 1995. Association of increased spontaneous mutation rates with high levels of transcription in yeast. Science 268 1616–1619. [DOI] [PubMed] [Google Scholar]
- Friedman, A. R., and B. J. Baker, 2007. The evolution of resistance genes in multi-protein plant resistance systems. Curr. Opin. Genet. Dev. 17 493–499. [DOI] [PubMed] [Google Scholar]
- Galagan, J. E., and E. U. Selker, 2004. RIP: the evolutionary cost of genome defense. Trends Genet. 20 417–423. [DOI] [PubMed] [Google Scholar]
- Galhardo, R. S., P. J. Hastings and S. M. Rosenberg, 2007. Mutation as a stress response and the regulation of evolvability. Crit. Rev. Biochem. Mol. Biol. 42 399–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, E. A., C. A. Codomo, N. E. Taylor, J. G. Henikoff et al., 2003. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics 164 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel, A. J., J. D. Clarke, J. Antonovics and X. Dong, 2004. Fitness costs of mutations affecting the systemic acquired resistance pathway in Arabidopsis thaliana. Genetics 168 2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff, S., 2005. Rapid changes in plant genomes. Plant Cell 17 2852–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddeloh, J. A., J. Bender and E. J. Richards, 1998. The DNA methylation locus DDM1 is required for maintenance of gene silencing in Arabidopsis. Genes Dev. 12 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakutani, T., J. A. Jeddeloh, S. K. Flowers, K. Munakata and E. J. Richards, 1996. Developmental abnormalities and epimutations associated with DNA hypomethylation mutations. Proc. Natl. Acad. Sci. USA 93 12406–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang, H., S. S. Woo, B. C. Meyers, E. Nevo and R. W. Michelmore, 2004. Multiple genetic processes result in heterogeneous rates of evolution within the major cluster disease resistance genes in lettuce. Plant Cell 16 2870–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., J. D. Clarke, Y. Zhang and X. Dong, 2001. Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol. Plant Microbe Interact. 14 1131–1139. [DOI] [PubMed] [Google Scholar]
- Li, Y., S. Yang, H. Yang and J. Hua, 2007. The TIR-NB-LRR gene SNC1 is regulated at the transcript level by multiple factors. Mol. Plant Microbe Interact. 20 1449–1456. [DOI] [PubMed] [Google Scholar]
- Lucht, J. M., B. Mauch-Mani, H. Y. Steiner, J. P. Metraux, J. Ryals et al., 2002. Pathogen stress increases somatic recombination frequency in Arabidopsis. Nat. Genet. 30 311–314. [DOI] [PubMed] [Google Scholar]
- Lynch, M., and J. S. Conery, 2000. The evolutionary fate and consequences of duplicate genes. Science 290 1151–1155. [DOI] [PubMed] [Google Scholar]
- Martin, G. B., A. J. Bogdanove and G. Sessa, 2003. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 54 23–61. [DOI] [PubMed] [Google Scholar]
- Meyers, B. C., A. Kozik, A. Griego, H. Kuang and R. W. Michelmore, 2003. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinier, J., G. Ries, C. Zipfel and B. Hohn, 2006. Transgeneration memory of stress in plants. Nature 442 1046–1049. [DOI] [PubMed] [Google Scholar]
- Mondragón-Palomino, M., B. C. Meyers, R. W. Michelmore and B. S. Gaut, 2002. Patterns of positive selection in the complete NBS-LRR gene family of Arabidopsis thaliana. Genome Res. 12 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël, L., T. L. Moores, E. A. van der Biezen, M. Parniske, M. J. Daniels et al., 1999. Pronounced intraspecific haplotype divergence at the RPP5 complex disease resistance locus of Arabidopsis. Plant Cell 11 2099–2112. [PMC free article] [PubMed] [Google Scholar]
- Parker, J. E., M. J. Coleman, V. Szabò, L. N. Frost, R. Schmidt et al., 1997. The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell 9 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna, W., J. Emberton, M. Ogden, P. SanMiguel and J. L. Bennetzen, 2002. Structural analysis of the maize rp1 complex reveals numerous sites and unexpected mechanisms of local rearrangement. Plant Cell 14 3213–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W. Y., L. Y. Pi, G. L. Wang, J. Gardner, T. Holsten et al., 1997. Evolution of the rice Xa21 disease resistance gene family. Plant Cell 9 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes, T. L., and E. J. Richards, 2002. Induced instability of two Arabidopsis constitutive pathogen-response alleles. Proc. Natl. Acad. Sci. USA 99 7792–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes, T. L., B. N. Kunkel and E. J. Richards, 2002. Epigenetic variation in Arabidopsis disease resistance. Genes Dev. 16 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, G., and F. N. Papavasiliou, 2007. Immunoglobulin somatic hypermutation. Annu. Rev. Genet. 41 107–120. [DOI] [PubMed] [Google Scholar]
- Tornero, P., R. A. Chao, W. N. Luthin, S. A. Goff and J. L. Dangl, 2002. Large-scale structure-function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 14 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Biezen, E. A., C. T. Freddie, K. Kahn, J. E. Parker and J. D. Jones, 2002. Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB-LRR genes and confers downy mildew resistance through multiple signalling components. Plant J. 29 439–451. [DOI] [PubMed] [Google Scholar]
- Wright, B. E., A. Longacre and J. M. Reimers, 1999. Hypermutation in derepressed operons of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 96 5089–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., and J. Hua, 2004. A haplotype-specific Resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 16 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H., and E. J. Richards, 2007. A cluster of disease resistance genes in Arabidopsis is coordinately regulated by transcriptional activation and RNA silencing. Plant Cell 19 2929–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H., and E. J. Richards, 2008. Phenotypic instability of Arabidopsis alleles affecting a disease resistance gene cluster. BMC Plant Biol. 8 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., J. Yazaki, A. Sundaresan, S. Cokus, S. W. Chan et al., 2006. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126 1189–1201. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., and X. Li, 2005. A putative nucleoporin 96 is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1–1,constitutive 1. Plant Cell 17 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., S. Goritschnig, X. Dong and X. Li, 2003. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1–1, constitutive 1. Plant Cell 15 2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]