Abstract
The race-specific resistance gene Pi-ta has been effectively used to control blast disease, one of the most destructive plant diseases worldwide. A single amino acid change at the 918 position of the Pi-ta protein was known to determine resistance specificity. To understand the evolutionary dynamics present, we examined sequences of the Pi-ta locus and its flanking regions in 159 accessions composed of seven AA genome Oryza species: O. sativa, O. rufipogon, O. nivara, O. meridionalis, O. glaberrima, O. barthii, and O. glumaepatula. A 3364-bp fragment encoding a predicted transposon was found in the proximity of the Pi-ta promoter region associated with the resistance phenotype. Haplotype network analysis with 33 newly identified Pi-ta haplotypes and 18 newly identified Pi-ta protein variants demonstrated the evolutionary relationships of Pi-ta haplotypes between O. sativa and O. rufipogon. In O. rufipogon, the recent directional selection was found in the Pi-ta region, while significant deviation from neutral evolution was not found in all O. sativa groups. Results of sequence variation in flanking regions around Pi-ta in O. sativa suggest that the size of the resistant Pi-ta introgressed block was at least 5.4 Mb in all elite resistant cultivars but not in the cultivars without Pi-ta. These findings demonstrate that the Pi-ta region with transposon and additional plant modifiers has evolved under an extensive selection pressure during crop breeding.
PLANT resistance (R) genes have evolved to fight against a wide range of pathogens in a race-specific manner where a particular R gene in a plant recognizes the corresponding avirulence (AVR) gene in a pathogen race (Flor 1971). Thus far, a number of R genes have been identified and characterized from diverse plant species. Most characterized R genes to date encode putative proteins with nucleotide binding sites (NBS) and leucine-rich repeats (LRR) (Hulbert et al. 2001). Most R genes are highly polymorphic and diversified, which is consistent with the ability to interact with diverse random molecules encoded by diverse pathogen AVR genes (Meyers et al. 2003; Bakker et al. 2006; Shen et al. 2006).
Blast disease, caused by the filamentous ascomycete Magnaporthe oryzae B.C. Couch [formerly M. grisea (T. T. Hebert) M. E. Barr] (Rossman et al. 1990; Couch and Kohn 2002), has been one of the major constraints to stable crop production. Currently, Oryza sativa and M. oryzae have been an excellent model pathosystem for uncovering the molecular coevolution mechanisms of host–pathogen (Valent et al. 1991; Talbot 2003). At least 80 race-specific R genes that confer resistance to specific pathogen races have been described in rice germplasm (Ballini et al. 2008). Eleven blast R genes (Pi-ta, Pib, Pi2/Piz-t, Pi5, Pi9, Pi21, Pi36, Pi37, Pi-d2, Pikm, and Pit) have been cloned, and most of them, except Pi21 and Pi-d2, were also predicted to encode receptor proteins with NBS (Chen et al. 2006; Fukuoka et al. 2009; Jia et al. 2009b). In most cases, blast R genes are members of small gene families with a single family member required for resistance. Pikm and Pi5 are exceptions that require two members of the same gene family for Pikm- and Pi5-mediated resistance, respectively (Ashikawa et al. 2008; Lee et al. 2009). Recently, a retrotransposon was predicted to be involved in the Pit resistance (Hayashi and Yoshida 2009).
The evolutionary dynamics and mechanisms of resistance mediated by Pi-ta is one of the best-studied R-genes. Pi-ta has been effectively deployed in the United States and around the globe for controlling blast disease (Bryan et al. 2000; Jia et al. 2000; Jia 2003; Jia et al. 2004a,b; Huang et al. 2008; Jia and Martin 2008; Wang et al. 2008; Jia et al. 2009a). Pi-ta encodes a predicted cytoplasmic protein with a centrally located NBS and a highly interrupted LRR domain (referred to as the LRD) at the carboxyl terminus that recognizes the corresponding avirulence gene AVR-Pita, triggering race-specific resistance. A single amino acid substitution, serine (Ser) to alanine (Ala) at the position of 918, in the LRD of the Pi-ta protein was demonstrated to determine the direct interaction with AVR-Pita and the resistance specificity to blast pathogen M. oryazae (Bryan et al. 2000; Jia et al. 2000). The resistant Pi-ta allele (Ala-918) was found in O. sativa and its ancestor O. rufipogon (Jia et al. 2004b; Huang et al. 2008). Surveys of Pi-ta nucleotide sequences with limited accessions of Oryza species have revealed that the degree of nucleotide diversity is higher at the intron of the Pi-ta gene (Jia et al. 2003; Huang et al. 2008; Wang et al. 2008; Yoshida and Miyashita 2009). Huang et al. (2008) further suggested that a selective sweep occurred recently at the Pi-ta gene in O. rufipogon, but the extent of selection around the Pi-ta genomic region has not been demonstrated in either O. rufipogon or O. sativa.
Knowledge of the historical introduction of the Pi-ta gene can help to understand the extent of selection at the Pi-ta locus. The landraces Tadukan and Tetep, containing Pi-ta and other blast R genes in chromosome 12, have been used as breeding parents for preventing blast disease worldwide. Tadukan was confirmed to be the Pi-ta donor for various Asian japonica cultivars (Rybka et al. 1997) whereas Tetep was the Pi-ta donor for the U. S. cultivars (Gravois et al. 1995; Moldenhauer et al. 1998; McClung et al. 1999; Gibbons et al. 2006; Moldenhauer et al. 2007). Recently, the large introgressed chromosomal segments surrounding the Pi-ta locus were identified in backcross BC5 progenies and elite rice cultivars (Jia 2009). This suggests that the broad spectrum of the Pi-ta resistance in the United States may include the effects of other loci in the Pi-ta region, inherited as a “superlocus.” Toward this end, Ptr(t), a nuclear gene that is required for the Pi-ta-mediated resistance, was also mapped at the Pi-ta region (Jia and Martin 2008). Further determination of DNA sequences around the Pi-ta gene should help to determine the minimal genomic region that is essential for Pi-ta-mediated resistance.
The two cultivated rice species, O. sativa and O. glaberrima, belong to the AA genome of Oryza species. O. rufipogon and O. nivara are wild progenitors of the Asian rice O. sativa, whereas O. barthii is a wild progenitor of the African cultivated rice O. glaberrima (Linares 2002; Yamanaka et al. 2003; Londo et al. 2006). The comparison of R-gene diversity between cultivated rice and its wild ancestors is important to understand the selection effects of crop domestication and breeding.
The objectives of this study were (1) to characterize distributions of the Pi-ta allele in O. sativa and to detect the potential presence/absence of polymorphism(s) associated with the resistance phenotype; (2) to examine the molecular evolution and patterns of selection in the Pi-ta gene in O. sativa and O. rufipogon; (3) to analyze molecular diversity around the Pi-ta locus in AA genome Oryza species; and (4) to understand the pattern and extent of selection for Pi-ta-mediated resistance in Oryza species during crop domestication.
MATERIALS AND METHODS
Plant materials and DNA preparation:
A total of 159 geographically diverse accessions of O. sativa, O. rufipogon, and five other closely related AA genome Oryza species were selected for this study. These included 43 Asian landraces, 18 U. S. domesticated cultivars, and 58 U. S.weedy rice strains in O. sativa; 28 geographically diverse accessions of O. rufipogon; 4 accessions of O. glaberrima; and 2 accessions each of O. nivara, O. barthii, O. meridionalis, and O. glumaepatula (Table S1). U. S. cultivars and weedy rice seeds were obtained from the USDA-ARS Dale Bumpers National Rice Research Center, and all Asian landrace accessions consisting of 15 indica, 7 aus, 3 aromatic, 12 tropical japonica, and 4 temperate japonica were obtained from Susan McCouch at Cornell University and the International Rice Research Institute. Plants were grown in greenhouses at Washington University and the University of Massachusetts. DNA extracted from 2- to 4-week-old seedlings was diluted to 2 ng/μl for further analysis.
Primer design and DNA sequencing:
Primer pairs were designed using the Primer3 program (Rozen and Skaletsky 2000) to amplify overlapping fragments (∼700 bp each) for Pi-ta, including 5′ upstream, 3′ downstream, and a coding region with an intron (Table S2). All primers were verified by BLAST against both 93-11 (indica) and Nipponbare (japonica) genome sequences. Primers were also designed to amplify 400- to 700-bp fragments of six flanking genes in the regions from 9.6 to 11.6 Mb on chromosome 12. The six flanking loci around the Pi-ta gene were LOC_OS12G16690 (9.6 Mb), LOC_OS12G17080 (9.8 Mb), and LOC_OS12G17830 (10.2 Mb) and LOC_OS12G18690 (10.8 Mb), LOC_OS12G19290 (11.2 Mb), and LOC_OS12G20260 (11.8 Mb) (http://rice.plantbiology.msu.edu/). For 11 resistant cultivars carrying Pi-ta (Tadukan, Tetep, Te Qing, Yashiro-mochi, Pi4, Reiho, IR64, Katy, Banks, Drew, and Madison), fragments from six additional flanking loci were sequenced: LOC_OS12G12370 (6.8 Mb), LOC_OS12G13570 (7.6 Mb), LOC_OS12G14330 (8.2 Mb), LOC_OS12G22360 (12.6 Mb), LOC_OS12G24020 (13.7 Mb), and LOC_OS12G25630 (14.8 Mb) (http://rice.plantbiology.msu.edu/) (Figure 1).
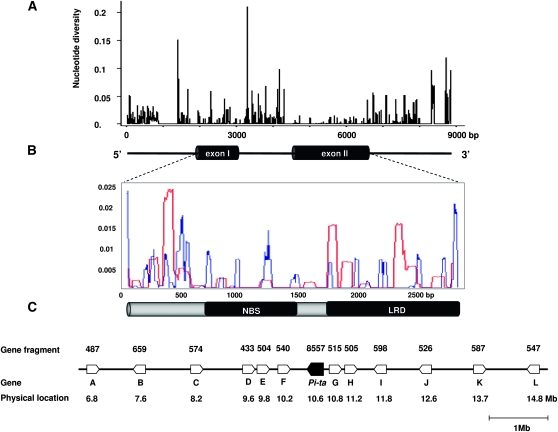
Figure 1.—
Patterns of DNA sequence variation at and around the Pi-ta locus in the AA genome of Oryza species. (A) Sliding-window analysis of the Pi-ta locus in 159 accessions (top). The gene structure of Pi-ta is shown at the bottom. (B) Sliding-window analysis at the Pi-ta coding region (top). The structure of the Pi-ta coding region is shown at the bottom. Values were assigned to the nucleotide at the midpoint of 5 bp for A and 25 bp for B, respectively. The parameter of difference per site (y-axis) is plotted against the nucleotide position (x-axis). Each line indicates synonymous (red) or nonsynonymous variation (blue). (C) Graphic presentation of the genomic region of the Pi-ta locus and 12 flanking loci. Sequenced fragments and physical locations on the chromosome are indicated, and the names of loci are represented as A–L. A: outer envelope protein (LOC_OS12G12370); B: Myb-like protein (LOC_OS12G13570); C: NBS–LRR disease resistance protein (LOC_OS12G14330); D: ubiquitin–protein ligase/zinc ion-binding protein (LOC_OS12G16690); E: pentatricopeptide repeat-containing protein (LOC_OS12G17080); F: unknown (LOC_OS12G17830); G: unknown (LOC_OS12G18690); H: serine/threonine-protein kinase (LOC_OS12G19290); I: unknown (LOC_OS12G20260); J: unknown (LOC_OS12G22360); K: senescence-associated protein DIN1 (LOC_OS12G24020); L: sulfite oxidase (LOC_OS12G25630).
Sequence data analysis:
All DNA sequences from Pi-ta and 12 flanking genes were aligned using Vector NTI 10 (Invitrogen) and MEGA 4 (Tamura et al. 2007). The genomic sequence from Nipponbare, a temperate japonica cultivar, was included as the reference sequence (http://rice.plantbiology.msu.edu/). Additional sequences of the Pi-ta gene of 50 accessions of O. rufipogon, 3 accessions of O. nivara, 2 accessions of O. meridionalis, 6 accessions of O. glaberrima, and 6 accessions of O. barthii were obtained from the GenBank database (Table S1), yielding a total of 226 accessions. For the sequence analysis, accessions of temperate japonica, tropical japonica, and aromatics collectively formed the japonica subspecies, and aus and indica together formed the indica subspecies. Nucleotide polymorphisms at and around the Pi-ta region were analyzed using the software DnaSP 4.9 (Rozas et al. 2003). The level of nucleotide diversity at silent sites (πsilent) and the population mutation parameter θw (Watterson estimator) of Pi-ta and the flanking gene fragments were estimated for each group of O. sativa and compared with that of other Oryza species. Average rates of nonsynonymous (Ka) and synonymous (Ks) substitutions were calculated to examine selections at the Pi-ta coding region in all accessions of O. sativa and O. rufipogon. Joint analyses of interspecific comparisons using O. barthii as an outgroup species were used for estimating the ratio of Ka/Ks and for determining deviations from neutral evolution (Akashi 1999). Sliding-window analysis was performed to examine nucleotide polymorphism across the Pi-ta gene in all Oryza species. Statistical tests of neutrality such as Tajima's D, Fu and Li's D* and F*, and Fay and Wu's normalized H were calculated to examine the selection present at and around Pi-ta. Extended haplotype homozygosity (EHH) (Sabeti et al. 2002) was calculated to visualize the effect of selection on the alleles containing Ala-918 or Ser-918. A haplotype network was also constructed for comparisons of genealogical relationships among Pi-ta haplotypes using TCS 1.21 (Clement et al. 2000).
RESULTS
Nucleotide diversity at the Pi-ta region:
High levels of nucleotide variation were observed in the intron, 5′-UTR, and 3′-UTR regions of Pi-ta in 159 accessions (Figure 1A). Insertions and deletions (indels) ranging from 10 to 540 bp in the noncoding regions were distinguished among the Pi-ta haplotypes. A 242-bp deletion in an intron of Pi-ta was found only in O. glaberrima, O. barthii, and O. glumaepatula. Within the coding region, levels of nucleotide and amino acid polymorphism were substantially higher in the first exon. Comparisons of amino acid mutations among partitions of the coding region showed that nonsynonymous were more common than synonymous changes in the NBS region (Figure 1B). Nucleotide diversity in O. sativa was lower than that in O. rufipogon. A total of 175 polymorphic sites, excluding indels, were found in the coding region, including an intron; of these polymorphic sites, 29 occurred in O. sativa, 121 in O. rufipogon, and 25 in other Oryza species. Average pairwise nucleotide diversity (πsilent) and silent Watterson's nucleotide diversity estimator (θw) over the Pi-ta gene was lowest in O. sativa (πsilent = 0.00292, θw = 0.00180) compared to other Oryza species, including O. rufipogon (πsilent = 0.00522–0.01366, θw = 0.00888–0.01520) (Table 1). The levels of diversity in African cultivated rice O. glaberrima and its wild progenitor O. barthii were similar to O. rufipogon and O. nivara (Table 1). Analyses for O. glumaepatula and O. meridionalis were not included because of sample limitation.
TABLE 1.
Molecular evolutionary parameters of the Pi-ta gene in Oryza species analyzed in this study
| Species | Sample no. | Nucleotide | θw | πsilent | D | D* | F* | Hn |
|---|---|---|---|---|---|---|---|---|
| O. sativa | 55 | 4250 | 0.00206 | 0.00287 | 1.36790 | 0.43956 | 0.92805 | 0.16426 |
| O. sativa indica | 23 | 4250 | 0.00235 | 0.00257 | −0.02930 | −0.18210 | −0.15867 | −0.29793 |
| O. sativa japonica | 32 | 4250 | 0.00143 | 0.00230 | 1.63577 | 1.25707 | 1.62162 | 0.41988 |
| O. sativa japonica Asian cultivar | 16 | 4250 | 0.00174 | 0.00244 | 0.78421 | 0.47668 | 0.64840 | 0.01420 |
| O. sativa japonica U. S. cultivar | 16 | 4250 | 0.00174 | 0.00226 | 1.41409 | 1.53348** | 1.72883* | 0.64640 |
| O. rufipogon | 91 | 3988 | 0.00888 | 0.00522 | −2.14289* | −2.09795 | −2.54113* | −3.65945 |
| O. nivara | 5 | 4003 | 0.01520 | 0.01322 | −1.06420 | −1.06420 | −1.15583 | −3.39370 |
| O. glaberrima | 10 | 4002 | 0.00966 | 0.01366 | 1.88503 | 1.03161 | 1.41069 | 0.19870 |
|
O. barthii |
9 |
4002 |
0.01066 |
0.01336 |
1.21069 |
1.07971 |
1.24886 |
−1.63819 |
θw, Watterson's nucleotide diversity estimator (1975) based on silent site; π, Nei's nucleotide diversity (1987) based on silent site; D, Tajima's D statistics (1989) based on the differences between the number of segregating sites and the average number of nucleotide differences; D* and F*, the neutral test proposed by Fu and Li (1993); and Hn, normalized Fay and Wu's H test statistics. Statistical significance: **P < 0.02 and *P < 0.05.
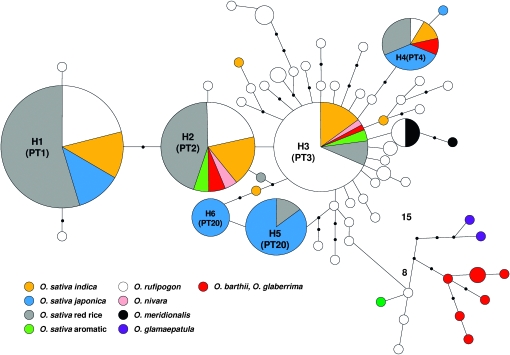
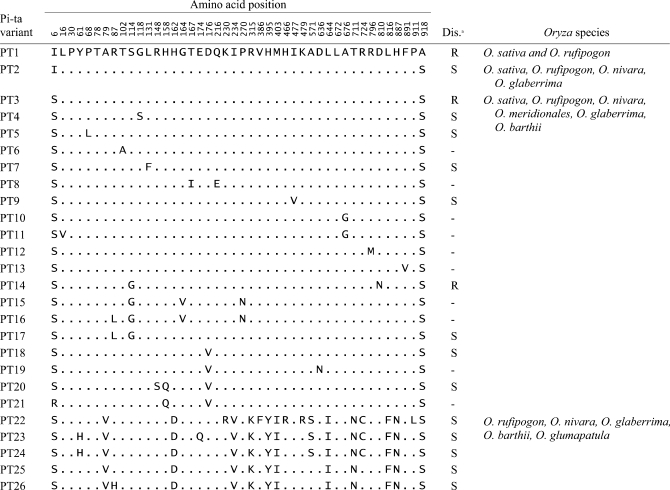
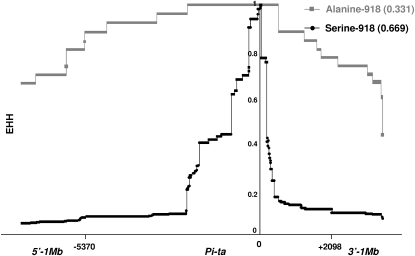
A total of 53 Pi-ta haplotypes were identified (Table S3) from seven AA genome Oryza species, including the previously reported 20 haplotypes (Huang et al. 2008; Wang et al. 2008; Yoshida and Miyashita 2009). Among them, 32 Pi-ta haplogroups were identified from different Oryza species in the haplotype network, suggesting that the diversification of Pi-ta haplotypes occurred before the divergence of these Oryza species (Figure 2). Nineteen haplotypes were from O. sativa and 25 haplotypes were from O. rufipogon (Table S3). A total of 26 Pi-ta variants from PT1 to PT26 were identified on the basis of the amino acid sequence of the Pi-ta protein in Oryza species (Table 2); these include 8 Pi-ta variants previously identified (Wang et al. 2008). Five Pi-ta variants—PT1, PT2, PT3, PT4, and PT20—were the most prevalent type of the variants in O. sativa (Figure 2 and Table S1). PT1 containing the functional amino acid alanine at 918 was found only in accessions of O. sativa and O. rufipogon. PT22, PT23, PT24, PT25, and PT26, were the major types of Pi-ta variants found in O. glaberrima, O. barthii, and O. glumaepatula (Table 1). The EHH test in O. sativa revealed that the level of regional recombination around the Pi-ta allele (Ala-918) was lower (EHH = 0.331) than that in the allele (Ser-918) (EHH = 0.669). This result suggests that the alanine-918 allele was recently derived from the ancestral Pi-ta variants that carry serine at 918 (Figure 3).
Figure 2.—
A haplotype network based on nucleotide polymorphisms of the Pi-ta coding region of 226 accessions of seven AA genome Oryza species: O. sativa, O. rufipogon, O. nivara, O. meridionallis, O. glaberrima, O. barthii, and O. glumaepatula. Each group of haplotypes is shown as a solid circle, and seven major haplotypes are marked in larger circles. The Pi-ta variants are in parentheses. Each branch represents a single mutational step. Branches with small solid circles indicate that there is more than a single mutational step between haplotypes. A number next to a branch represents the length of the mutational steps. Different sizes of circles represent the different numbers of each haplotype.
TABLE 2.
Description of Pi-ta variants based on a 928-amino-acid sequence in 159 accessions of seven Oryza species: O. sativa, O. rufipogon, O. nivara, O. meridionalis, O. glumaepatula, O. barthii, and O. glaberrima
a The disease reactions for Pi-ta variants PT1, PT2, PT3, PT4, PT9, PT20, PT22, PT23, PT24, PT25, and PT26 were obtained from two U. S. races, IB17 and IB49 of M. oryzae (Wang et al. 2008 and this study). Disease reactions for PT5, PT7, PT14, PT17, and PT18 were marked according to the previous report of Huang et al. (2008). R, resistance; S, susceptibility.
Figure 3.—
Comparison of the EHH of two core haplotypes (alanine-918 and serine-918) in the Pi-ta region in O. sativa. The core was defined by a single amino acid change at the position of 918 (serine: TCT or alanine: GCT) that determines the resistance specificity of Pi-ta. The starting EHH value for alanine-918 is 0.331 while the EHH value is 0.669 for serine-918.
Selection at the Pi-ta locus:
Tests of neutrality were performed for the Pi-ta gene (coding region and intron) using the statistics of Tajima's D, Fu and Li's D* and F*, and Fay and Wu's Hn (Table 1). The value of Tajima's D was positive and deviated from neutrality in O. sativa (D = 1.32357); however, other values for Fu and Li's D* and F* and for Fay and Wu's Hn (D* = 0.21562, F* = 0.77317, Fay and Wu's Hn = 0.16426) were not significantly different from the neutral model. To determine if the statistical differences were from the population structure of the Pi-ta gene in O. sativa, accessions separated into four subpopulations—indica, japonica, japonica Asian cultivars, and japonica U. S. cultivars—were analyzed for neutral tests. As shown in Table 1, all statistical values for neutral tests did not significantly deviate from neutrality except in U. S. cultivars (D = 1.41409, D* = 1.53348, and F* = 1.72883, P < 0.05), consistent with the fact that Pi-ta has been substantially selected for preventing blast disease in the United States. On the contrary, significant negative values of neutrality tests in O. rufipogon suggest an excess of rare alleles, consistent with a recent selective sweep (Table 1) and with the report using different accessions of O. rufipogon (Huang et al. 2008). Positive values of Tajima's D and Fu and Li's D* and F* were found in O. glaberrima and its wild ancestor O. barthii, suggesting a balancing selection (Table 1). However, it was not determined if it was due to selection or population structure in both species because of sample limitation.
The level of synonymous divergence (Ks) exceeded that of nonsynonymous divergence (Ka) in all partitions of the coding region of the Pi-ta protein except the NBS region in O. sativa and O. rufipogon, indicating purifying selection against amino acid substitutions in most portions of the gene (Table 3). These findings were also confirmed in comparisons between synonymous nucleotide polymorphism (πsyn) and nonsynonymous nucleotide polymorphism (πnon) in O. rufipogon (Table 3). However, the πsyn:πnon ratio was smaller than one (πsyn:πnon < 1) in the NBS in O. sativa due to the very low polymorphism present in the species. The NBS of the Pi-ta protein in both O. sativa and O. rufipogon showed a greater number of interspecies nonsynonymous-to-synonymous substitutions (Ka/Ks > 1), indicating that positive directional selection has favored amino acid substitutions in this domain (Table 3).
TABLE 3.
Molecular variation and selection at the Pi-ta gene in O. sativa (indica, japonica, and weedy rice) and O. rufipogon
| Gene segment | S | πsyna | πnona | πnon/πsyn | Ks(JC)b | Ka(JC)b | Ka/Ksb |
|---|---|---|---|---|---|---|---|
| O. sativa (n = 113) | |||||||
| Coding | 12 | 0.0015 | 0.00098 | 0.654 | 0.00871 | 0.00560 | 0.643 |
| 5′ coding to NBS | 6 | 0.00305 | 0.00337 | 1.105 | 0.01513 | 0.01034 | 0.683 |
| NBS | 2 | 0.00009 | 0.00003 | 0.295 | 0.00004 | 0.00451 | 102.5 |
| NBS to LRD | 0 | 0 | 0 | 0 | 0.03457 | 0.00371 | 0.107 |
| LRD | 4 | 0.00196 | 0.00063 | 0.319 | 0.00774 | 0.00536 | 0.692 |
| O. rufipogon (n = 91) | |||||||
| Coding | 62 | 0.00249 | 0.00166 | 0.668 | 0.00849 | 0.00530 | 0.625 |
| 5′ coding to NBS | 24 | 0.00491 | 0.00278 | 0.564 | 0.01211 | 0.00698 | 0.577 |
| NBS | 15 | 0.00101 | 0.00155 | 1.543 | 0.00052 | 0.00484 | 9.341 |
| NBS to LRD | 4 | 0.00401 | 0.00049 | 0.121 | 0.03384 | 0.00359 | 0.106 |
| LRD |
19 |
0.00174 |
0.00139 |
0.801 |
0.00694 |
0.00514 |
0.741 |
πsyn, nucleotide diversity at synonymous site; πnon, nucleotide diversity at nonsynonymous site.
Jukes–Cantor (JC) corrected synonymous differences per synonymous site (Ks) and nonsynonymous differences per nonsynonymous site (Ka) using intraspecific and interspecific comparisons using O. barthii.
Nucleotide polymorphisms in genomic regions around Pi-ta:
We sequenced all fragments of targeted flanking loci around Pi-ta except one locus encoding a NBS–LRR disease resistance protein (LOC_OS12G14330), the RPM-1 homolog located at 8.2 Mb. The presence and absence of the RPM-1 homolog was found in both O. sativa and O. rufipogon accessions. The absence of the RPM-1 homolog was found in two Asian cultivars, Yashiro-mochi (japonica) and Te Qing (indica), and in all U. S. weedy rice carrying resistant Pi-ta (Table S4).
Nucleotide data sets shown in Figure 1 were aligned for 433–659 bp of six loci in 2 Mb around Pi-ta in all 159 accessions. The estimated values of nucleotide diversity for these loci were 0–0.00391 in O. sativa and 0.0015–0.00508 in O. rufipogon. The levels of sequence variation in flanking loci around Pi-ta were similar to the levels in the Pi-ta locus found in both species (Table 4). The test of Tajima's D in the region around Pi-ta in O. sativa and O. rufipogon revealed that no significant pattern of selection presents around the Pi-ta locus in O. sativa. However, a significant negative value of Tajima's D was detected around the Pi-ta locus in O. rufipogon, similar to the result found in the Pi-ta gene (Table 4).
TABLE 4.
Molecular diversity of genomic regions around Pi-ta in O. sativa (indica, japonica, and weedy rice) and O. rufipogon
| Nucleotide polymorphism (π) | Tajima's D | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical location (Mb) | 9.6 | 9.8 | 10.2 | Pi-taa | 10.8 | 11.2 | 11.8 | 9.6 | 9.8 | 10.2 | Pi-taa | 10.8 | 11.2 | 11.8 |
| O. sativa | 0.00178 | 0.00115 | 0.00108 | 0.0018 | 0.0015 | 0 | 0.00391 | 0.66938 | 0.69958 | 0.82595 | 0.17149 | 0.92414 | NA | 0.91739 |
| O. indica | 0.0019 | 0.00109 | 0.00204 | 0.00218 | 0.00213 | 0 | 0.00479 | −0.96803 | −1.51481 | −0.10605 | −0.70826 | 1.08052 | NA | 1.02022 |
| O. japonica | 0.00118 | 0.00148 | 0.00047 | 0.00253 | 0.00048 | 0 | 0.00416 | 0.97327 | 0.27501 | 0.44003 | 1.05235 | 0.6426 | NA | 0.13462 |
| Weedy rice | 0.001 | 0.00129 | 0.0004 | 0.00166 | 0.00168 | 0 | 0.00365 | −0.93379 | −0.45271 | −0.51132 | −0.44628 | −0.42536 | NA | 1.13812 |
|
O. rufipogon |
0.00508 |
0.0015 |
0.0057 |
0.00477 |
0.00301 |
0.0015 |
0.00388 |
−0.15872 |
0.23998 |
0.16801 |
−2.57275 |
0.22133 |
−1.36029 |
−0.3066 |
The sequence of Pi-ta including flanking region (2 kb upstream and downstream of Pi-ta) and coding region with intron was used for nucleotide polymorphism and Tajima's D.
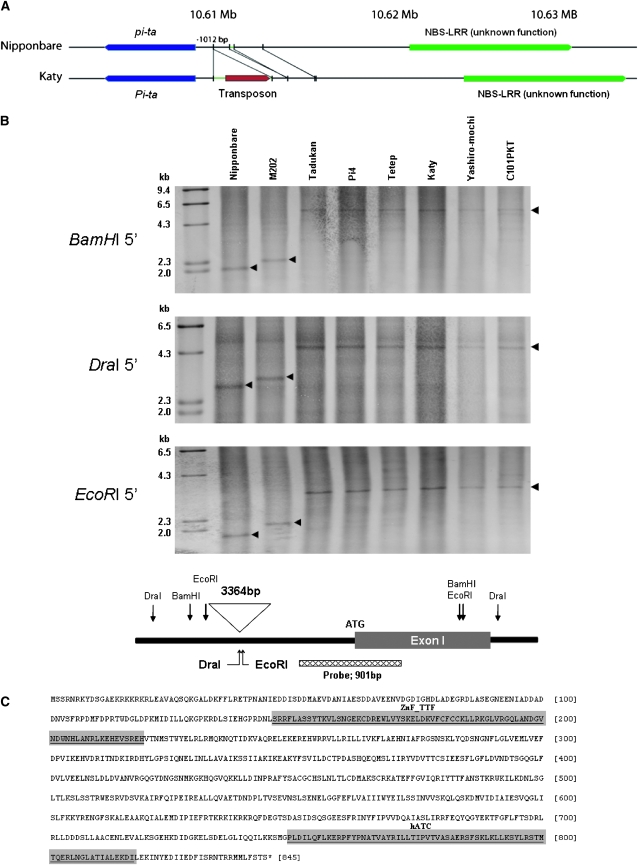
Interestingly, a 3364-bp insertion located 1012 bp upstream of the start codon (ATG) was found only in all accessions carrying the resistance Pi-ta allele (Figure 4A). The presence of the insertion in resistant accessions was verified by Southern blot analysis using a probe derived from the 5′ region of Pi-ta (Figure 4B). The inserted fragment was cloned and sequenced from the U. S. cultivar, Katy (GenBank accession no. GQ984160). Sequences of the 3364-bp fragment were predicted to encode a protein with 844 amino acids with domains commonly found in zinc fingers and transcription factors and with domains commonly found in hAT family dimerization (hATC) of a transposable element (Figure 4C). Using the rice sequence database of Nipponbare (japonica) and 93-11 (indica), a highly homologous sequence with the insertion was found on chromosome 2 of 93-11 while no homologous sequence was found in the Nipponbare. From a Southern blot using the probe in the insertion and PCR analysis with primers amplifying the flanking region of the insertion, the 3364-bp insertion was determined on chromosome 2 in susceptible indica cultivars; however, the insertion was on both chromosomes 2 and 12 in resistant indica cultivars or japonica cultivars possessing indica-derived resistant Pi-ta (data not shown).
Figure 4.—
Genomic organization around indica (resistant Pi-ta) and japonica (susceptible Pi-ta) cultivars. (A) Comparisons of genomic regions around the Pi-ta locus between Nipponbare and Katy. (B) An insertion in the proximate Pi-ta promoter region differentiates the size of hybridized bands between two susceptible cultivars (Nipponbare and M202) and six resistant cultivars (Pi-ta) (top). Schematic of the Pi-ta genomic region with indicated restriction enzymes (bottom). (C) The two domains (shaded)—zinc finger in transposases and transcription factors (ZnF_TTF) and hAT family dimerization (hATC)—were identified by searching the conserved domain of proteins from NCBI database.
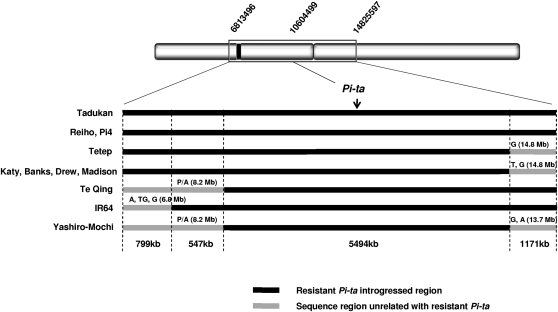
After surveying in the 2-Mb region around Pi-ta in 118 accessions of O. sativa, no polymorphism was detected in all resistant O. sativa accessions. Six additional flanking gene fragments were sequenced in the 8-Mb region to identify polymorphisms in those accessions (4 Mb upstream and 4 Mb downstream of Pi-ta). The different sizes of the Pi-ta introgressed block in resistant cultivated rice were estimated by detecting the initial breaking point of recombination surrounding the Pi-ta locus. A range from 5 to 8 Mb of the Pi-ta introgression block (the average being 7 Mb) was identified in 11 resistant cultivars (Jia et al. 2004b; Wang et al. 2007). Among them, the smallest block (5.4 Mb) was identified in Yashiro-mochi and the largest Pi-ta introgression (>8 Mb) was found in the two Japanese cultivars Pi4 and Reiho whose Pi-ta region was derived from Tadukan. A 6.8-Mb portion of the Pi-ta region in Tetep was identified in the U. S. cultivars Katy, Drew, Banks, and Madison (Figure 5).
Figure 5.—
Sizes of Pi-ta introgressions in O. sativa Asian and U. S. cultivated rice through breeding selection during domestication. The Pi-ta region of Tadukan, which is the major donor for Pi-ta in Asian cultivars, was used to compare the size of the introgression block with other Pi-ta-containing cultivars. The solid bar represents the identical sequence of Pi-ta introgressed into resistant cultivars. The shaded bar represents sequence polymorphisms unrelated to the Pi-ta introgression that resulted from recombination events at the genomic region of Pi-ta. Sequence polymorphisms are marked on the breakpoint of the Pi-ta introgression block. P/A indicates the presence and absence of polymorphism.
DISCUSSION
In this study, we analyzed DNA sequence polymorphisms in and around the genomic region of Pi-ta in 159 geographically diverse Oryza accessions composed of several Oryza species to gain insight into the origin and evolution of Pi-ta. We discovered that the extended genomic region (>5 Mb) surrounding resistant Pi-ta was consistently maintained in resistant accessions to M. oryzae containing AVR-Pita. Significantly, one of the largest linkage blocks of resistant Pi-ta was identified in backcrossing and elite rice cultivars (Jia 2009). The identification of a large linkage block around Pi-ta raised at least two possibilities. First, other blast R genes in the Pi-ta region also introgressed into diverse elite rice cultivars. Other R genes such as Pi-ta2, Pi39, and Pi20(t) (Rybka et al. 1997; Liu et al. 2007; Li et al. 2008) were also mapped at the Pi-ta region, but it was unknown if these and/or other unknown R genes were clustered in the Pi-ta region that have been introgressed as a large linkage block. Second, other components for the Pi-ta-mediated resistance reside within the 5-Mb region to form a superlocus. R-gene-mediated resistance may involve additional R genes that may be physically linked to provide a complete resistance to a plant pathogen. In tomato, Prf, a NBS–LRR protein, was identified to be involved in the Pto-mediated resistance (Mucyna et al. 2006). In rice, at least two NBS–LRR proteins at the Pikm and Pi5 loci have been identified as providing complete resistance to blast (Ashikawa et al. 2008; Lee et al. 2009). At the Pikm locus, Pikm1-TS and Pikm2-TS within 2.5 kb are required for Pikm-mediated disease resistance (Ashikawa et al. 2008). Similarly, two NBS–LRR proteins within 50 kb, Pi5-1 and Pi5-2, were required for complete resistance (Lee et al. 2009). At the Pi-ta locus, another gene Ptr(t) was found to be essential for Pi-ta-mediated resistance (Jia and Martin 2008). The possible artificial selection of the large Pi-ta genomic region has been reported for maintaining the broad spectrum of Pi-ta-mediated blast resistance (Jia 2009). Taken together with other studies, this study suggests that other components such as Ptr(t) or R genes for the Pi-ta-mediated resistance may occur within at least 5 Mb of the Pi-ta region.
Simple insertion/deletion or transposon may play an important role in R-gene evolution. It has been reported that 18.8% of total R genes in Arabidopsis and 22.2% in rice are under presence/absence polymorphism (Meyers et al. 2003; Shen et al. 2006). An example of transposon and R-gene activation was found in the Pit gene. The insertion of a long-terminal-repeat retrotransposon in the promoter of Pit was predicted to regulate Pit transcription and its function for resistance (Hayashi and Yoshida 2009). In our study, we found a transposon in the proximity of the Pi-ta promoter in resistant cultivars carrying Pi-ta, which was absent in accessions without Pi-ta. This finding suggests that the transposon may activate the Pi-ta-mediated resistance. Further study may lead to a better understanding of any associations of the transposon with Pi-ta-mediated resistance.
The divergence of indica and japonica subgroups in O. sativa was predicted to be caused by two independent domestications from geographically divergent O. rufipogon populations (Londo and Schaal 2007). The Pi-ta haplotypes of indica or japonica origin were identified in this study (Figure 2). Resistant Pi-ta was found only in indica, weedy rice, japonica cultivars carrying the indica-derived Pi-ta region and O. rufipogon, suggesting that resistant Pi-ta did not originate from japonica. The Pi-ta variants in H5 and H6 were found only in japonica accessions, while H2 and H3 were found only in indica (Figure 2), consistent with a previous study (Londo and Schaal 2007). The Pi-ta variant containing Ala-918 (PT1) separates the resistant Pi-ta variant from other variants in both O. sativa and O. rufipogon. This suggests that PT1 existed before the divergence of the two subspecies indica and japonica. The recent divergence of resistant Pi-ta from susceptible Pi-ta has also been proposed from the previous studies (Huang et al. 2008; Yoshida and Miyashita 2009). Most of the Pi-ta variants possess serine at the position of 918. There was no amino acid sequence polymorphism in the group with PT1; however, significant amino acid polymorphism was identified in groups containing Ser-918, consistent with previous reports (Huang et al. 2008; Wang et al. 2008; Yoshida and Miyashita 2009). These findings further suggest that there was recently a strong selection constraint on the resistant Pi-ta protein (PT1), and such pressures were not observed on other Pi-ta protein variants.
An excess of amino acid substitutions over neutral expectations were observed in the NBS region in both O. sativa and O. rufipogon, indicating that positive directional selection favored amino acid substitutions in the domain. The NBS domain in diverse proteins with ATP or GTP binding activity is involved in activating the NBS–LRR protein in resistance. It has been documented that the Toll-interleukin 1 receptor region of the L class of flax rust R genes (Ellis et al. 1999) and the N-terminal domain with the NBS region of tomato MI protein (Hwang et al. 2000) are key regulators of signal transduction of disease resistance. Our findings suggest that the highly diversified NBS region may be important for maintaining the integrity of the Pi-ta protein with the LRD domain. In the LRD of the Pi-ta protein, the level of synonymous diversity was found to exceed the level of nonsynonymous diversity, which is suggestive of possible purifying selection acting on this domain. The Ka:Ks ratio for the LRD of Pi-ta (Ka:Ks = 0.692–0.741) is relatively low compared to that observed in other LRRs (Ellis et al. 1999; Mauricio et al. 2003; Rose et al. 2004; Bakker et al. 2006; Orgil et al. 2007). It is possible that conservation of LRD in the Pi-ta protein may be necessary for recognizing AVR-Pita for the signal transduction (Jia et al. 2000). High nucleotide diversity and a large number of AVR-Pita haplotypes were recently identified, suggesting that AVR-Pita is under diversifying selection (Y. Dai and Y. Jia, unpublished data). Diversified selection at NBS and purifying selection against amino acid variants in the conserved functional LRD region may have played a major role in shaping the molecular evolution of Pi-ta.
In conclusion, this study revealed that (1) a transposon may be a part of the evolution with resistant Pi-ta, (2) all components needed for the Pi-ta-mediated resistance may be embedded within 5 Mb, and (3) strong artificial selection has acted at and around resistant Pi-ta in the modern cultivated rice O. sativa, while such selection is absent in cultivars without resistant Pi-ta. These findings suggest that the evolution of Pi-ta is much more complicated than previously documented. Further studies will be necessary for a better understanding of the molecular mechanism of Pi-ta-mediated signal recognition and transduction pathway.
Acknowledgments
The authors thank Briana Gross (Washington University), Michael Reagon (University of Massachusetts), David Gealy, and Anna McClung for extensive support throughout the research. We are also grateful to all members of the Molecular Plant Pathology lab and other staff members at the U. S. Department of Agriculture–Agricultural Research Service Dale Bumpers National Rice Research Center for their technical assistance (http://ars.usda.gov/spa/dbnrrc/mpp). This work was supported by the National Science Foundation under grant no. 0638820.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.108266/DC1.
References
- Akashi, H., 1999. Inferring the fitness effects of DNA mutations from polymorphism and divergence data: statistical power to detect directional selection under stationary and free recombination. Genetics 151 221–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikawa, I., N. Hayashi, H. Yamane, H. Kanamori, J. Wu et al., 2008. Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180 2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, E. G., C. Toomajian, M. Kreitman and J. Bergelson, 2006. A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 18 1803–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballini, E., J.-B. Morel, G. Droc, A. Price, B. Coutois et al., 2008. A genome-wide meta-analysis of rice blast resistance genes and quantitative trait loci provides new insights into partial and complete resistance. Mol. Plant-Microbe Interact. 21 859–868. [DOI] [PubMed] [Google Scholar]
- Bryan, G. T., K. S. Wu, L. Farrall, Y. Jia, H. P. Hershey et al., 2000. A single amino-acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. W., J. Shang, D. Chen, C. Lei, Y. Zou et al., 2006. A B-lectin receptor kinase gene conferring rice blast resistance. Plant J. 46 794–804. [DOI] [PubMed] [Google Scholar]
- Clement, M., D. Posada and K. A. Crandall, 2000. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9 1657–1660. [DOI] [PubMed] [Google Scholar]
- Couch, B. C., and L. M. Kohn, 2002. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94 683–693. [DOI] [PubMed] [Google Scholar]
- Ellis, J. G., G. J. Lawrence, J. E. Luck and P. N. Dodds, 1999. Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor, H. H., 1971. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 9 275–296. [Google Scholar]
- Fu, Y. X., and W. H. Li, 1993. Statistical tests of neutrality of mutations. Genetics 133 693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka, S., N. Saka, H. Koga, K. Ono, T. Shimizu et al., 2009. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325 998–1001. [DOI] [PubMed] [Google Scholar]
- Gibbons, J. W., K. A. K. Moldenhauer, K. Gravois, F. N. Lee, J. L. Bernhardt et al., 2006. Registration of ‘Cybonnet’ rice. Crop Sci. 46 2317–2318. [Google Scholar]
- Gravois, K. A., K. A. K. Moldenhauer, F. N. Lee, R. J. Norman, R. S. Helms et al., 1995. Registration of ‘Kaybonnet’ rice. Crop Sci. 35 586–587. [Google Scholar]
- Hayashi, K., and H. Yoshida, 2009. Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. Plant J. 57 413–425. [DOI] [PubMed] [Google Scholar]
- Huang, C., S. Hwang, Y. Chiang and T. Lin, 2008. Molecular evolution of the Pi-ta gene resistant to rice blast in wild rice (Oryza rufipogon). Genetics 179 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert, S. H., C. A. Webb, S. M. Smith and Q. Sun, 2001. Resistance gene complexes: evolution and utilization. Annu. Rev. Phytopathol. 39 285–312. [DOI] [PubMed] [Google Scholar]
- Hwang, C. F., A. V. Bhakta, G. M. Truesdell, W. M. Pudlo and V. M. Williamson, 2000. Evidence for a role of the N terminus and leucine-rich repeat region of the Mi gene product in regulation of localized cell death. Plant Cell 12 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Y., 2003. Marker assisted selection for the control of rice blast disease. Pesticide Outlook 14 150–152. [Google Scholar]
- Jia, Y., 2009. Artificial introgression of a large chromosome fragment around the rice blast resistance gene Pi-ta in backcross progeny and several elite rice cultivars. Heredity 103 333–339. [DOI] [PubMed] [Google Scholar]
- Jia, Y., and R. Martin, 2008. Identification of a new locus, Ptr(t), required for rice blast resistance gene Pi-ta-mediated resistance. Mol. Plant-Microbe Interact. 21 396–403. [DOI] [PubMed] [Google Scholar]
- Jia, Y., S. A. McAdams, G. T. Bryan, H. P. Hershey and B. Valent, 2000. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Y., G. T. Bryan, L. Farrall and B. Valent, 2003. Natural variation at the Pi-ta rice blast resistance locus. Phytopathology 93 1452–1459. [DOI] [PubMed] [Google Scholar]
- Jia, Y., M. Redus, Z. Wang and J. N. Rutger, 2004. a Development of a SNLP marker from the Pi-ta blast resistance gene by tri-primer PCR. Euphytica 138 97–105. [Google Scholar]
- Jia, Y., Z. Wang, R. G. Fjellstrom, K. A. K. Moldenhauer, M. A. Azam et al., 2004. b Rice Pi-ta gene confers resistance to the major pathotypes of the rice blast fungus in the US. Phytopathology 94 296–301. [DOI] [PubMed] [Google Scholar]
- Jia, Y., F. N. Lee and A. McClung, 2009. a Determination of resistance spectra of the Pi-ta and Pi-k genes to U.S. races of Magnaporthe oryzae causing rice blast in a recombinant inbred line population. Plant Dis. 93 639–644. [DOI] [PubMed] [Google Scholar]
- Jia, Y., X. Wang, S. Costanzo and S. Lee, 2009. b Understanding the coevolution of rice blast resistance gene Pi-ta and Magnaporthe oryzae avirulence gene AVR-Pita, pp. 137–147 in Advances in Genetics, Genomics and Control of Rice Blast Disease, edited by G. L. Wang and B. Valent. Springer Science, New York.
- Lee, S.-K., M.-Y. Song, Y.-S. Seo, H.-K. Kim, S. Ko et al., 2009. Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two CC-NB-LRR genes. Genetics 181 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., C. L. Lei, Z. J. Cheng, Y. L. Jia, D. Y. Huang et al., 2008. Identification of SSR markers for a broad-spectrum blast resistance gene Pi20(t) for marker-assisted breeding. Mol. Breed. 22 141–149. [Google Scholar]
- Linares, O. F., 2002. African rice (Oryza glaberrima): history and future potential. Proc. Natl. Acad. Sci. USA 99 16360–16365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. Q., Q. Z. Yang, F. Lin, L. X. Hua, C. T. Wang et al., 2007. Identification and fine mapping of Pi39(t), a major gene conferring the broad-spectrum resistance to Magnaporthe oryzae. Mol. Genet. Genomics 278 403–410. [DOI] [PubMed] [Google Scholar]
- Londo, J. P., and B. A. Schaal, 2007. Origins and population genetics of US weedy red rice in the USA. Mol. Ecol. 16 4523–4535. [DOI] [PubMed] [Google Scholar]
- Londo, J. P., Y. Chiang, K. Hung, T. Chiang and B. A. Schaal, 2006. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 103 9578–9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio, R., E. A. Stahl, T. Korves, D. Tian, M. Kreitman et al., 2003. Natural selection for polymorphism in the disease resistance gene Rps2 of Arabidopsis thaliana. Genetics 163 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung, A. M., M. A. Marchetti, B. D. Webb and C. N. Bollich, 1999. Registration of ‘Madison’ rice. Crop Sci. 39 1256. [Google Scholar]
- Meyers, B. C., A. Kozik, A. Griego, H. Kuang and R. W. Michelmore, 2003. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldenhauer, K. A. K., K. A. Gravois, F. N. Lee, R. J. Norman, J. L. Bernhardt et al., 1998. Registration of ‘Drew’ rice. Crop Sci. 38 896–897. [Google Scholar]
- Moldenhauer, K. A. K., F. N. Lee, J. W. Gibbons, J. L. Bernhardt, R. J. Norman et al., 2007. Registration of ‘Ahrent’ rice. Crop Sci. 47 446–447. [Google Scholar]
- Mucyna, T. S., A. Clementea, V. M. E. Andriotisa, A. L. Balmutha, G. E. D. Oldroydb et al., 2006. The tomato NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell 18 2792–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgil, U., H. Arakit, S. Tangchaiburana, R. Herkey and S. Xiao, 2007. Intraspecific genetic variations, fitness cost and benefit of RPW8, a disease resistance locus in Arabidopsis thaliana. Genetics 176 2317–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, L. E., P. D. Bittner-Eddy, C. H. Langley, E. B. Holub, R. W. Michelmore et al., 2004. The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 166 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman, A. Y., R. J. Howard and B. Valent, 1990. Pyricularia oryzae, the correct name for the rice blast fungus. Mycologia 82 509–512. [Google Scholar]
- Rozas, J., J. C. Sánchez-DelBarrio, X. Messegyer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19 2496–2497. [DOI] [PubMed] [Google Scholar]
- Rozen, S., and H. Skaletsky, 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132 365–386. [DOI] [PubMed] [Google Scholar]
- Rybka, K., M. Miyamoto, I. Ando, A. Saito and S. Kawasaki, 1997. High resolution mapping of indica-derived rice blast resistance genes II. Pi-ta2 and Pi-ta and a consideration of their origin. Mol. Plant- Microbe Interact. 10 517–524. [Google Scholar]
- Sabeti, P. C., D. E. Reich, J. M. Higgins, H. Z. P. Levine, D. J. Richter et al., 2002. Detecting recent positive selection in the human genome from haplotype structure. Nature 419 832–837. [DOI] [PubMed] [Google Scholar]
- Shen, J., H. Araki, L. Chen, J.-Q. Chen and D. Tian, 2006. Unique evolutionary mechanism in R-genes under the presence/absence polymorphism in Arabidopsis thaliana. Genetics 172 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot, N. J., 2003. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 57 177–202. [DOI] [PubMed] [Google Scholar]
- Tamura, K., J. Dudley, M. Nei and S. Kumar, 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24 1596–1599. [DOI] [PubMed] [Google Scholar]
- Valent, B., L. Farrall and F. G. Chumley, 1991. Magnaporthe grisea genes for pathogenicity and virulence identified through a series of backcrosses. Genetics 127 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Y. Jia, Q. Y. Shu and D. Wu, 2008. Haplotype diversity at the Pi-ta locus in cultivated rice and its wild relatives. Phytopathology 98 1305–1311. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Y. Jia, J. N. Rutger and Y. Xia, 2007. Rapid survey for presence of a blast resistance gene Pi-ta in rice cultivars using the dominant DNA markers derived from portions of the Pi-ta gene. Plant Breed. 126 36–42. [Google Scholar]
- Yamanaka, S., I. Nakamura, H. Nakai and Y. Sato, 2003. Dual origin of the cultivated rice based on molecular markers of newly collected annual and perennial strains of wild rice species, Oryza nivara and O. rufipogon. Genet. Resour. Crop Evol. 50 529–538. [Google Scholar]
- Yoshida, K., and N. T. Miyashita, 2009. DNA polymorphism in the blast disease resistance gene Pita of the wild rice Oryza rufipogon and its related species. Genes Genet. Syst. 84 121–136. [DOI] [PubMed] [Google Scholar]