Abstract
Exposure to alcohol during adolescence is predictive of adult alcohol abuse and dependence. The present experiment was designed to measure the impact of appetitive motivational engagement with ethanol during adolescence on adult ethanol consumption. To this end, one group of adolescent male Wistar rats was allowed to traverse an operant runway to obtain access to a sweetened 10% ethanol solution (w/v) over 18 sessions. An additional yoked – control group was allowed access to an identical solution, however, exposure to the solution was contingent on the experimental groups entry into the goal box of the runway. Once the adolescent exposure sessions were completed, the animals were allowed to mature into adults and then tested for differences in ethanol consumption during 30 min two – bottle limited access sessions. Following 14 ethanol consumption sessions during adulthood, a naltrexone dose – response challenge (0 – 0.4 mg/kg) was initiated for both groups. The results of the experiment showed that the animals allowed to traverse the runway during adolescence displayed increased ethanol consumption during adulthood when compared to the yoked – control group. In addition, both groups showed dose – dependent attenuation of ethanol consumption by naltrexone. Thus, appetitive motivational experience during adolescence can impact adult ethanol consummatory behavior – a process that appears to involve common reinforcement-related neural substrates. This model should prove useful in delineating appetitive motivation – related factors that contribute to excessive ethanol consumption.
Keywords: appetitive motivation, adolescent, ethanol, operant runway, self-administration
From an ontogenetic neurobehavioral perspective, a number of physiological and behavioral changes occur during adolescence that involve cognitive, emotional, hormonal and motivational systems (Chambers, Taylor, & Potenza, 2003; Yurgelun-Todd, 2007); components of which are susceptible to the effects of alcohol (for examples, see Witt, 1994; Spear, 2000; Smith, 2003). Current alcohol use has been estimated to occur in 17.7% of adolescents (age 12 – 17), with 6% designated as alcohol abusers or alcohol dependent (Substance Abuse and Mental Health Services Administration, 2005). Taking into consideration evidence suggesting that age of initial alcohol use is predictive of alcohol abuse and dependence in adult humans (Grant & Dawson, 1997; Ehlers, Slutske, Gilder, Lau, & Wilhelmsen, 2006), the identification of factors which occur during adolescence and alter adult alcohol use is an important objective.
The idea that behaviors related to the acquisition of biologically relevant stimuli can be divided into separate appetitive and consummatory processes was proposed early in the 20th century (Craig, 1918). In the last decade, as part of the attempt to elucidate the mechanisms that control drinking behavior, a novel approach has been applied to drinking data obtained from animal models of ethanol reinforcement (Samson, Slawecki, Sharpe, & Chappell, 1998) in which appetitive behaviors (i.e., lever-pressing for ethanol) were evaluated independently of alcohol consummatory behaviors (i.e., ethanol drinking).
As well as the operant self-administration model proposed by Samson (1998), another model that has utility in parsing the appetitive and consummatory aspects of reinforcer acquisition is the operant runway (see Ettenberg, 2004 for review). In this paradigm, the appetitive aspect consists of traversing the runway from the start box into the goal box, at which point a sweetened ethanol solution was available for consumption. One notable aspect of this procedure is that the appetitive motivational component is not confounded by the effects of alcohol on responding because the consumption session does not begin until the appetitive component has concluded. In order to vary the appetitive experience with sweetened alcohol for the adolescent animals in the present study, while keeping the consummatory aspects of the experiment constant, a yoked – control group was included. This allowed one group of adolescent animals to control access to the sweetened ethanol for both groups and therefore the only difference between the two was whether there was an appetitive component prior to the consummatory phase.
In addition to manipulating the appetitive aspect of ethanol self-administration during adolescence, pharmacological challenges of adult ethanol consummatory behaviors were also conducted in the present study. The opioid receptor antagonist naltrexone has been shown to reduce nondependent and ethanol-dependent self-administration of ethanol in animals (for examples, see Altshuler, Phillips, & Feinhandler, 1980; Walker & Koob, 2008) and based on its efficacy in reducing consumption of ethanol in humans (Volpicelli, Alterman, Hayashida, & O'Brien, 1992), naltrexone is one of the few FDA-approved medications indicated for the treatment of alcoholism. To test whether adult ethanol consummatory behavior could be modulated by opioid receptor antagonism, a naltrexone dose-response curve was conducted on adult limited – access two-bottle choice ethanol self-administration in the animals that differed in their appetitive experience with ethanol during adolescence.
Methods
Subjects
Twenty-four adolescent male Wistar rats (P23 on arrival) from Charles River (Wilmington, MA) were used in this study. The animals were pair-housed in standard home cages measuring 45 cm (l) × 25 cm (w) × 20.3 cm (h) in a temperature-controlled vivarium maintained on a 12 hour light/dark cycle (lights on at 6am). Ad Libitum food and water was available and the animal care was in accordance with the NIH Guide for the Care and Use of Laboratory Animals and was reviewed and approved by The Scripps Research Institute's Institutional Animal Care and Use Committee.
Apparatus
The runway apparatus used in the present study was constructed of opaque Plexiglas and consisted of a start box and a goal box (both with removable doors) and an alley (runway) connecting the two. The measurements of the start box and goal box were 26.7 cm (l) × 26.7 cm (w) × 30.5 cm (h) and 40.6 cm (l) × 25 cm (w) × 20.3 cm (h), respectively; whereas the alley component measured 152.4 (l) × 11.4 cm (w) × 30.5 cm (h). The goal box was identical to the animal's home cages. The doors of the start and goal box were manually operated and were the same width and height of the alley. The goal box had a wire mesh cage top (identical to the home cage top) and prior to each animal's session, fresh wood chips were evenly distributed on the floor. There was also an additional home cage with wood chips distributed on the floor with wire mesh top that was used for the yoked – control group. For adult drinking component of the study, cages identical to the home cage was used that had a Plexiglas divider (Ridout Plastics, San Diego, CA) to partition the cage (lengthwise) into equal compartments.
Adolescent Ethanol Exposure
All animals began the experiment with a three-day introductory period of home cage access to a 10% sucrose (10S; w/v) solution. A Plexiglas divider was used to separate the home cage into two equal, but separate compartments during the 3 hour introductory 10S consumption sessions. Subsequent to the introductory 10S consumption sessions, the animals were weight matched and separated into two groups: runway (n=12) and yoked – control (n=12). Each animal from the runway group was pair-housed housed with a control animal for the course of the experiment.
At age P29, the ethanol acquisition phase (see Table 1) began by allowing the adolescent runway and yoked – control groups limited – access to sweetened ethanol solutions (single bottle) for up to 15 minutes per day for a total of 20 sessions (5 consecutive days per week ending at age P54). The sessions began approximately 2 hrs into the light cycle, a time point that has been shown to be optimal for inducing adolescent ethanol consumption (Walker, Walker, & Ehlers, 2008). Two minutes prior to the start of the session, the runway animals were placed in the start box and the yoked – control animals were placed in a cage analogous to their home cage. The session was initiated by raising the start box door and the animals were allowed to traverse the alley. Once the animals had moved from the start box to the alley, the start box door was closed to prevent re-entry. Likewise, when the animals entered the goal box, the goal box door was closed. Once the animals reached the goal box and the door was closed, both the yoked – control and runway groups were presented with a single bottle of sweetened ethanol solution for the remainder of the 15 minutes session. Thus, the presentation of the solution for both animals was dependent on the runway animal entering the goal box. If a runway animal took longer than five minutes to leave the start box, they (and the yoked – control) were still allowed 10 minutes of solution access prior to the session ending. If an animal did not leave the start box for 15 minutes, the session for both groups was terminated. After the session and prior to the next group of animals, the runway and yoked – control drinking cage were cleaned with Quatricide PV® (Pharmacal, Naugatuck, CT) and the bedding was changed. The order of the solutions that were presented over the 20 acquisition sessions was as follows: 10S (10% sucrose (w/v) solution; 2 days), 10S + 1% ethanol (10S1E; 1 day), 10S2.5E (1 day), 10S5E (4 days), 10S7.5E (2 days) and 10S10E (10 days).
Table 1.
Timeline of the 15 min. consumption sessions during the adolescent acquisition phase. 10S = 10% Sucrose (w/v); 1E, 2.5E, 5E, 7.5E and 10E = 1%, 2.5%, 5%, 7.5% and 10% ethanol (w/v), respectively.
 |
Adult Ethanol Self-Administration
From age P55 until P71, the animals were not exposed to any limited – access sessions, but were handled 3 times per week. Following the two-week break, the animals (now adults) were allowed to consume sweetened ethanol solutions; however, the sweetener was gradually removed from the solution (adapted from Samson, 1986) until only 10% ethanol (10E) was being consumed. In contrast to the adolescent phase of the experiment, the adult animals were exposed to 30 minutes of two-bottle (ethanol and water) self-administration sessions in a cage identical to the home cage that was divided into equal compartments by a Plexiglas partition. Under the two-bottle conditions, the bottle position was alternated daily to avoid any position bias. The limited – access sessions continued until the animals were age P104 and were comprised of the following solutions: 10S10E (2 days), 5S10E (3 days), 2.5S10E (5 days), and 10E (14 days). Following the 14 sessions of 10E, a subcutaneous (SC) vehicle (i.e., saline) injection was administered to the animals. This was done to confirm that the injection procedure itself did not alter (e.g., reduce) the animal's consumption behavior. Once it was determined that the vehicle injection did not alter the behavior of the animals, pharmacological challenges with naltrexone were initiated.
Naltrexone Challenge
Once the adult animal's 10E self-administration behavior was stable, naltrexone (0.0, 0.025, 0.1 and 0.4 mg/kg) was administered according to a Latin square-design. Naltrexone was administered (SC) 30 minutes prior to the ethanol (10E) self-administration sessions and following the injections, the animals were placed in the drinking cages with partitions until the 30 minute self-administration session began. Following the session, the animals were returned to the vivarium. In all cases, there were at least two days in between each naltrexone challenge and the animals were allowed to self-administer ethanol on those intervening days.
Drugs
95 % ethanol (Gold Shield Chemicals, Hayward, CA) was diluted with tap water to the appropriate concentration (w/v). Naltrexone HCl (Sigma Chemicals, St. Louis, MO) was soluble in 0.9% physiological saline and injected in a volume of 1ml/kg.
Data Analysis
Ethanol consumption controlled for by weight (g/kg) during the adolescent ethanol acquisition phase, adult sucrose fade-out and baseline 10E self-administration phase was analyzed using a mixed-model two-way analysis of variance (ANOVA). Water consumption (g/kg) during the adult baseline 10E consumption session was also analyzed using a mixed-model two-way ANOVA. In these analyses, the between-subjects factor was adolescent condition (i.e., runway or yoked – control) with ethanol intake (g/kg) or water intake (g/kg) over the various sessions as the repeated measure. If assumptions of sphericity were not met, the Greenhouse-Geisser correction was used to adjust the degrees of freedom in order to establish more conservative F-test critical values for the analysis.
Ethanol and water intake (g/kg) following administration of different naltrexone doses was analyzed using a two-way mixed-model ANOVA with adolescent condition as the between-subjects factor and naltrexone dose as the within-subjects factor. To ascertain differences within groups if there were significant main effects or interactions, a repeated measures one-way ANOVA was conducted on ethanol intake (g/kg) for either the runway or yoked – control group following different doses of naltrexone. If significant main effects were found, post-hoc Fisher's LSD tests were used to determine differences between doses within the runway and yoked – control groups. The effects of naltrexone on ethanol intake (g/kg) were also evaluated by comparing the percent decrease from baseline (i.e., vehicle-administration) following administration of the three naltrexone doses using a two-way mixed-model ANOVA with adolescent condition as the between-subjects factor and naltrexone dose as the within-subjects factor. Lastly, ethanol preference ratios following naltrexone administration (ethanol intake / ethanol intake + water intake) were also evaluated using a two-way mixed-model ANOVA with adolescent condition and naltrexone dose as the between-subject and within-subject factors, respectively.
Results
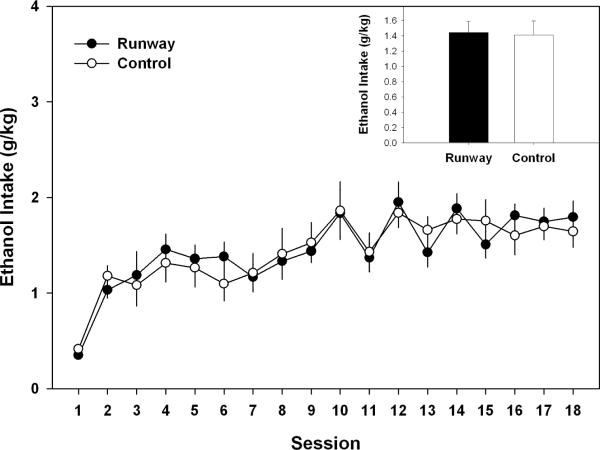
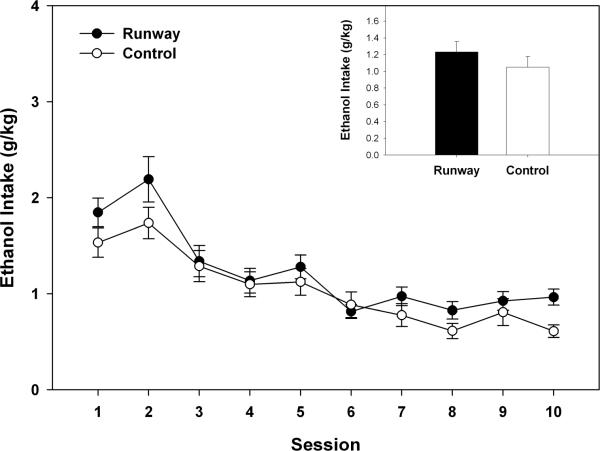
As seen in Figure 1, the two-way ANOVA indicated that there were no group differences in adolescents during the acquisition of ethanol self-administration phase of the experiment (F (1, 22) = 0.089, p > 0.05), although ethanol intake (g/kg) levels did change over the 18 sessions (F (17, 374) = 11.344, p < 0.001). Likewise, ethanol intake in adulthood during the fade-out component of the experiment (see Figure 2) showed no differences between groups ( F(1, 22) = 0.048, p > 0.05), with a significant change in ethanol intake levels (g/kg) over the 10 sessions as the sweetener was faded out (F (9, 198) = 13.681, p < 0.001).
Figure 1.
Mean (± S.E.M.) daily sweetened ethanol intake (g/kg) over 18 sessions during 15 min limited – access sessions that occurred in adolescence. Daily intake did not differ between animals allowed to traverse a runway for ethanol and the yoked-control group. Inset of figure corresponds to the mean (+S.E.M.) ethanol intake (g/kg) of the 18 sessions.
Figure 2.
Mean (± S.E.M.) daily sweetened ethanol intake (g/kg) over 10 sessions during the gradual fade-out of sweetener in 30 min limited – access sessions that occurred in adulthood. Daily intake did not differ between animals allowed to traverse a runway for ethanol and the yoked-control group. Inset of figure corresponds to the mean (+S.E.M.) ethanol intake (g/kg) of the 10 sessions.
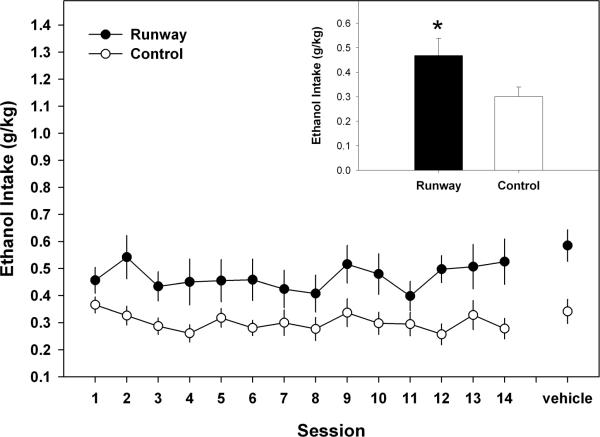
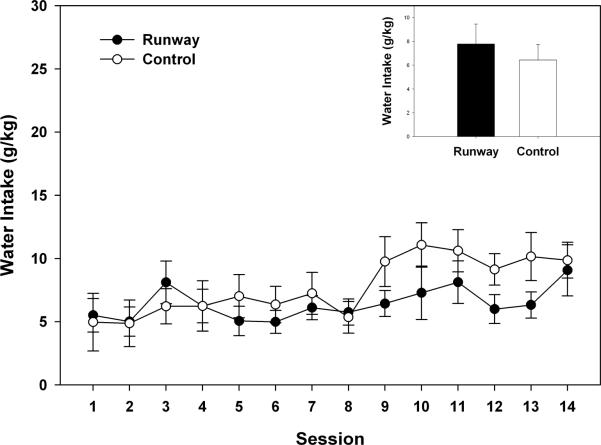
During the baseline adult ethanol self-administration component of the experiment (see Figure 3), the ANOVA showed that ethanol intake (g/kg) across the 14 sessions was stable (F (13, 286) = 1.654, p > 0.05), but significantly different for the runway and yoked – control groups (F (1, 22) = 6.15, p < 0.05) with the runway animals consuming significantly more ethanol. However, during the 14 baseline sessions, there were no group differences in water consumption (see Figure 4; F (1, 22) = 0.593, p > 0.05), although water consumption did change over time (F(13, 286) = 5.227, p < 0.001).
Figure 3.
Mean (± S.E.M.) daily 10% ethanol (w/v) intake (g/kg) over 14 sessions during 30 min limited – access sessions that occurred in adulthood. Daily intake was significantly elevated for animals that were allowed to traverse a runway for ethanol during adolescence when compared to the yoked-control group. Inset of figure corresponds to the mean (+S.E.M.) ethanol intake (g/kg) of the 14 sessions (* = p < 0.05).
Figure 4.
Mean (± S.E.M.) daily water intake (g/kg) over 14 sessions during 30 min limited – access sessions that occurred in adulthood. No differences in group water intake were observed. Inset of figure corresponds to the mean (+S.E.M.) ethanol intake (g/kg) of the 14 sessions
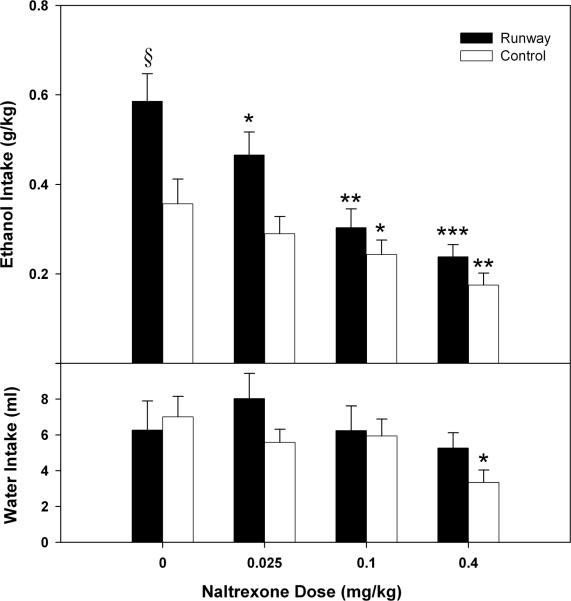
The results of the naltrexone challenge are presented in Figure 5. The ANOVA showed a main effect of condition (F(1, 22) = 7.875, p ≤ 0.01), a main effect of dose (F (3, 66) = 27.577, p < 0.001) and a Condition x Dose interaction (F (3, 66) = 3.454, p < 0.05), which reflected that the runway and control animals responded differently to the naltrexone doses with naltrexone having a more pronounced effect on the runway animals. The one-way ANOVA showed a main effect of naltrexone for runway (F (3, 33) = 19.571, p < 0.001) and control (F (3, 33) = 8.379, p < 0.001) animals. Post-hoc LSD tests identified that the 0.025 (p < 0.05), 0.1 (p ≤ 0.001) and 0.4 mg/kg (p < 0.001) doses were different from vehicle for the runway group, while only the 0.1 (p < 0.05) and 0.4 mg/kg (p < 0.01) doses were different from vehicle for the control group.
Figure 5.
Upper panel - mean (+S.E.M.) ethanol consumption following naltrexone (0.0 – 0.4 mg/kg) administration in runway – exposed and yoked – control animals during 30 min limited – access self – administration sessions. Naltrexone dose – dependently attenuated ethanol consumption (* = p < 0.05, ** = p < 0.01 and *** = p < 0.001 compared to vehicle dose; § = p < 0.05 compared to yoked – control vehicle dose). Lower panel - mean (+S.E.M.) water consumption following naltrexone (0.0 – 0.4 mg/kg) administration in runway – exposed and yoked – control animals (* = p < 0.05 compared to vehicle dose).
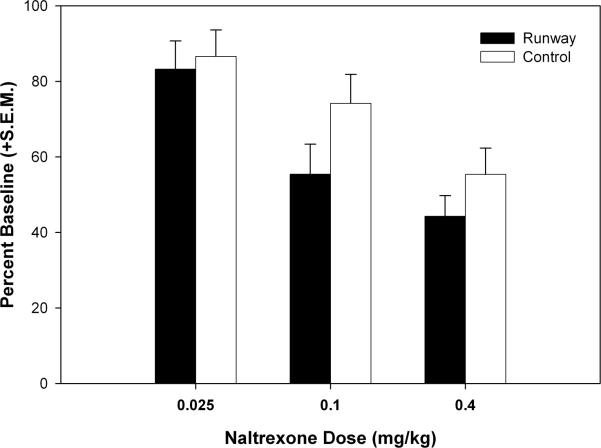
The percentage of ethanol intake compared to baseline following varied naltrexone doses is presented Figure 6. The two-way ANOVA showed that a main effect of dose (F (2, 44) = 28.22, p < 0.001). In contrast to the analysis of ethanol intake (g.kg), there was no main effect of condition (F (1, 22) = 1.341, p > 0.05) or a significant interaction (F (2, 44) = 1.372, p > 0.05).
Figure 6.
Mean (+S.E.M.) percent of baseline ethanol consumption following naltrexone (0.025 – 0.4 mg/kg) administration in runway – exposed and yoked – control animals during 30 min limited – access self – administration sessions. Naltrexone dose – dependently attenuated ethanol consumption for both groups of animals (p < 0.001), but no between – groups differences in naltrexone-induced reductions were observed.
The two-way ANOVA conducted on water responses during the naltrexone challenge (see Figure 5) showed a main effect of dose (F (3, 66) = 4.101, p < 0.05), but no effect of condition (F (1, 22) = .573, p > 0.05) nor a Condition x Dose interaction (F (3, 66) = 0.536, p > 0.05). A one-way ANOVA showed a main effect of dose for the control animals (F (3, 33) = 5.513, p < 0.05), with LSD tests showing that the 0.4 mg/kg dose of naltrexone significantly reduced water responding in these animals when compared to vehicle. Conversely, the one-way ANOVA conducted on the water responding data from the runway animals showed no main effect of dose (F (3, 33) = 1.562, p > 0.05).
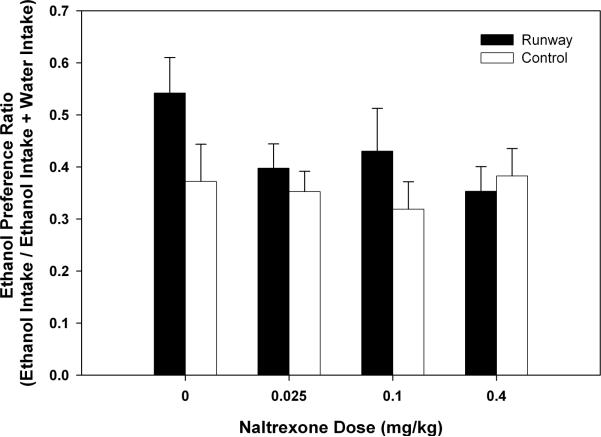
Preference ratios for ethanol following naltrexone administration were also evaluated (see Figure 7). The two-way ANOVA indicated that there were no between-subjects (F (1, 22) = 1.307, p > 0.05), within-subject (F (3, 66) = 1.947, p > 0.05) or interaction (F (3, 66) = 1.984, p > 0.05) effects.
Figure 7.
Mean (+S.E.M.) ethanol preference ratios following varied doses of naltrexone (0.0 – 0.4 mg/kg). No between – groups or within – subjects differences were observed.
Discussion
The primary objective of the present study was to ascertain whether the inclusion of an appetitive motivational component with limited – access sweetened ethanol consumption sessions during adolescence could influence adult limited – access unsweetened ethanol consumption over a protracted period of time. To accomplish this goal, a subset of animals was subjected to an operant runway paradigm during adolescence and the impact of such experience was evaluated on adult ethanol consumption. As a control measure, a second group of adolescent animals served as a yoked – control group and was provided equivalent access to sweetened alcohol once the runway group had completed the appetitive operant requirement. Thus, both groups had identical access time to sweetened ethanol with the primary contrast between the two groups being the ability to control such access.
During adolescence, the runway and yoked – control groups consumed comparable amounts of sweetened ethanol (g/kg) solutions during 15 min limited – access sessions that initiated with 1E ethanol (w/v) and concluded with eight sessions of 10E ethanol (w/v). Therefore, on a consummatory basis, there were no differences between the two groups. Once the animals matured into adults, sweetened 10E ethanol limited – access consumption sessions were initiated for both groups of animals and over 10 sessions, the sweetener was faded out. During this fade procedure, both the experimental and yoked – control groups consumed comparable amounts of sweetened ethanol (g/kg). In contrast, once the sweetener had been removed from the 10E solution, the experimental group of animals consistently self-administered more 10E, at levels that were significantly different from the control group over 14 limited – access sessions. The fact that the sweetened ethanol intake during the fade – out period was not different between the experimental and yoked – control groups could be attributable to consumption patterns that were driven more by the sweetened solution than the ethanol itself. Thus, those animals that had control over their ethanol consumption during adolescence showed a selective enhancement of ethanol consumption as adults when compared to the yoked – control group that consumed comparable amounts of ethanol during adolescence.
The average levels of unsweetened 10E ethanol consumption observed in adults over the fourteen limited-access sessions and naltrexone dose-response components of the study ranged from 0.31 – 0.36 g/kg (control group) and 0.47 - 0.59 g/kg (experimental group). Following 30 minute operant sessions or 30 minutes post-gavage administration, lower doses of ethanol are highly correlated with blood alcohol levels (BAL) in Wistar rats (Richardson, Lee, O'Dell, Koob, & Rivier, 2008; Walker & Ehlers, 2009), with levels of 0.5 g/kg and 0.75 g/kg reliably resulting in BALs of ~0 .05 and 0.075 g%. Therefore, in the present study, one could infer that the control 0.31 – 0.36 and experimental 0.47 – 0.59 g/kg levels of intake would have resulted in BALs of approximately 0.031 – 0.036 g% and 0.047 – 0.059 g%, respectively. In addition, these levels of intake within 30 minute sessions have been shown to consistently support operant ethanol self-administration in Wistar rats (Walker & Koob, 2007; Ji, Gilpin, Richardson, Rivier, & Koob, 2008; Richardson et al., 2008; Richardson, Zhao, Fekete, Funk, Wirsching, Janda, Zorrilla, & Koob, 2008). Thus, the evidence suggests that the levels of and any observed differences in alcohol consumption in the present experiment for the control and experimental groups are pharmacologically relevant. However, because BALs were not directly measured in the present experiment, the possibility exists that there could be a pharmacokinetic explanation for the differential intake between the runway-exposed and yoked-control group animals.
When focusing on the appetitive motivational aspects of reinforcer acquisition, it has been shown that rats given the concurrent option of lever-pressing for food reinforcement or having free access to food prefer to operantly respond (i.e., work) for the food (Jensen, 1963). This phenomenon is termed `contrafreeloading' and although it appears to contradict certain basic motivational theories such as the `principle of least effort' (i.e., when organisms are given a choice, behavior will be in the direction of a minimum expenditure of physical energy; Tolman, 1949), the `contrafreeloading' effect has been observed in a variety of vertebrates (e.g., chimpanzees, fish, humans, pigeons and rats; for review, see Inglis, Forkman, & Lazarus, 1997). This concept has also been demonstrated in animals receiving electrical brain stimulation by showing that rats will choose self-administered (compared to experimenter-delivered) electrical brain stimulation (Ettenberg, Laferrioere, Milner, & White, 1981).
The fact that animals prefer to earn food when they could have free access to it, suggests that there is a preference for situations in which appetitive response engagement occurs even though the consummatory aspects are comparable. Based on this fact, the present experiment evaluated different levels of appetitive response opportunity during adolescence on ethanol consummatory behaviors in adulthood. It is interesting to note that the inclusion of the appetitive component did not influence sweetened ethanol consumption during adolescence or sweetened ethanol consumption during adulthood – only adult unsweetened ethanol consumption was affected. Escalating consumption of ethanol has been posited to be an indicator of “loss of control” drinking in rodents (Samson & Czachowski, 2003), although one should be cautious in considering the present results to be indicative of “loss of control” behavior. The present data confirm in an animal model what has been reported in humans that ethanol consumption during adolescence can be a predictor of adult alcohol abuse and dependence (Grant & Dawson, 1997; Ehlers et al., 2006) . It is currently unknown whether this effect is restricted to adolescent animals or whether appetitive response engagement during adulthood would also impact subsequent adult ethanol consumption. Further studies will need to be conducted to address that question.
It is of interest to note that numerous studies have evaluated the impact of self – administered and experimenter – administered drugs of abuse on the brain and behavior and have shown distinct differences in their effects (Dworkin, Mirkis, & Smith, 1995; Mark, Hajnal, Kinney, & Keys, 1999; Stefanski, Ladenheim, Lee, Cadet, & Goldberg, 1999; Robinson, Gorny, Savage, & Kolb, 2002). However, the operant components of those studies utilized a traditional operant approach (i.e., lever-pressing) that did not distinguish between appetitive and consummatory aspects of reinforcer acquisition. Based on the present results showing that the availability of appetitive motivation influences later behaviors, it could be that the differences that have been observed between self – and experimenter – delivered paradigms are in fact differences in engaging in appetitive behaviors. Further research would need to be conducted to address such a hypothesis.
A possible alternative explanation for the enhanced ethanol consummatory behavior that was observed during adulthood in the present study involves data showing that stress can induce elevated ethanol consumption in rodents that initiates 2–3 weeks after exposure to the stressor (for examples, see Chester, Blose, Zweifel, & Froehlich, 2004; Croft, Brooks, Cole, & Little, 2005; Lowery, Sparrow, Breese, Knapp, & Thiele, 2008). Therefore, any differences between the experiential histories of the experimental and yoked – control groups that involve potential stressors should be considered and evaluated. For example, it could be that being placed in the runway environment was stressful and that stress could contribute to enhanced ethanol consumption via a negative reinforcement mechanism. However, if that were the case, one might expect an increase in ethanol consumption during the adolescent phase of the experiment or during the fade – out of the sweetener during the initial adult phase of the experiment. However, there were no differences in ethanol consumption between the experimental and yoked – control groups during either phase of the experiment, even though the 2–3 week time frame overlaps with them. Thus, it is unlikely that stress is contributing to the increased consumption in adulthood.
The secondary objective in the present study was to evaluate the ability of naltrexone, an opioid receptor antagonist, to reduce ethanol self-administration during adulthood for the experimental and yoked – control groups. Following the 14 limited – access 10E self-administration sessions in adulthood and confirmation that vehicle administration did not alter the ethanol consummatory behavior of the experimental and yoked – control groups, a naltrexone dose-response challenge was initiated. Naltrexone (0 – 0.4 mg/kg) was shown to dose – dependently reduce ethanol self-administration for both the experimental and yoked – control groups (see Fig. 5). However, the doses of naltrexone that showed efficacy differed for the experimental and yoked – control groups. Namely, the lowest dose of naltrexone tested (0.025 mg/kg) was efficacious in the experimental group, whereas 0.1 mg/kg naltrexone was necessary to reduce consumption in the yoked – control group. However, when the effect of naltrexone was evaluated as a percentage of baseline, the increase in potency did not persist (see Fig. 6). If naltrexone had been unable to affect the increased ethanol consumption observed in the experimental group, then one might infer that circuitry distinct from opioid-related systems was contributing to the altered ethanol consummatory behavior induced by engaging in appetitive motivational processes. However, because naltrexone impacted ethanol consumption in the experimental and control group comparably, common ethanol reinforcement–related substrates are implicated in the appetitive motivation-induced alterations in consummatory behavior, although the specific neural circuitry that is involved remains to be established.
Ethanol produces its effects on the central nervous system via a variety of pharmacological mechanisms. In the case of the endogenous opioid system and its receptor subtypes (μ, δ, κ - selective for the three main classes of endogenous opioids: β-endorphin, enkephalins and dynorphins, respectively), acute ethanol has been shown to stimulate the release of β-endorphin, enkephalins and dynorphin in humans and rats (Gianoulakis, Krishnan, & Thavundayil, 1996; Marinelli, Quirion, & Gianoulakis, 2003; Marinelli, Quirion, & Gianoulakis, 2004; Dai, Thavundayil, & Gianoulakis, 2005; Marinelli, Bai, Quirion, & Gianoulakis, 2005; Marinelli, Lam, Bai, Quirion, & Gianoulakis, 2006). The reduction of ethanol self-administration by naltrexone in the present study is consistent with established research (Altshuler et al., 1980; Coonfield, Hill, Kaczmarek, Ferraro, III, & Kiefer, 2002; Gonzales & Weiss, 1998; Ji et al., 2008; Stromberg, Mackler, Volpicelli, & O'Brien, 2001; Walker & Koob, 2008) and because of naltrexone's classification as a general opioid receptor antagonist, opioid receptor subtype specificity could be difficult to establish. However, research has shown that naltrexone, at low doses, has higher affinity for the μ – rather than the δ – or κ – opioid receptors (Abbott, Franklin, & Libman, 1986; Millan, 1989; Millan, Czlonkowski, Lipkowski, & Herz, 1989; Walker, Makhay, House, & Young, 1994; Stromberg, Casale, Volpicelli, Volpicelli, & O'Brien, 1998). Therefore, the fact that ethanol consumption in the present experiment is sensitive to such low doses of naltrexone putatively suggests the involvement of the μ – opioid receptor, as opposed to the δ – or κ – opioid receptor subtypes. Further research with selective opioid receptor antagonists would be needed to confirm such a hypothesis.
In sum, the present study identified that animals with differential appetitive motivational experience, but equivalent consummatory experience with ethanol during adolescence showed consistent increases in ethanol self-administration during adulthood. The fact that working for ethanol during adolescence can impact adult ethanol consumption and that such consumption is susceptible opioidergic manipulation shows that this model should be useful as an animal model of adolescent ethanol exposure with the ability to induce long – term changes in adult consummatory behavior.
Acknowledgements
Support for this research was provided by National Institute on Alcohol Abuse and Alcoholism grants awarded to CLE (AA006059 & AA014339). The authors would like to thank Jennifer Walker and Derek Wills for their technical assistance.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
Reference List
- Abbott FV, Franklin KB, Libman RB. A dose-ratio comparison of mu and kappa agonists in formalin and thermal pain. Life Sciences. 1986;39:2017–2024. doi: 10.1016/0024-3205(86)90325-5. [DOI] [PubMed] [Google Scholar]
- Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sciences. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcoholism: Clinical and Experimental Research. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Hill KG, Kaczmarek HJ, Ferraro FM, III, Kiefer SW. Low doses of naltrexone reduce palatability and consumption of ethanol in outbred rats. Alcohol. 2002;26:43–47. doi: 10.1016/s0741-8329(01)00180-x. [DOI] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biological Bulletin. 1918;34:91–107. [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2005;29:1965–1975. doi: 10.1097/01.alc.0000187599.17786.4a. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology (Berl) 1995;117:262–266. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcoholism: Clinical and Experimental Research. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neuroscience and Biobehavioral Reviews. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Laferrioere A, Milner PM, White N. Response involvement in brain stimulation reward. Physiology and Behavior. 1981;27:641–647. doi: 10.1016/0031-9384(81)90236-5. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Archives of General Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. Journal of Neuroscience. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J.Subst.Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Inglis IR, Forkman B, Lazarus J. Free food or earned food? A review and fuzzy model of contrafreeloading. Anim Behav. 1997;53:1171–1191. doi: 10.1006/anbe.1996.0320. [DOI] [PubMed] [Google Scholar]
- JENSEN GD. Preference for bar pressing over “freeloading” as a function of number of rewarded presses. J.Exp.Psychol. 1963;65:451–454. doi: 10.1037/h0049174. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural Pharmacology. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcoholism: Clinical and Experimental Research. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcoholism: Clinical and Experimental Research. 2005;29:1821–1828. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcoholism: Clinical and Experimental Research. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Kappa-opioid receptor-mediated antinociception in the rat. I. Comparative actions of mu- and kappa-opioids against noxious thermal, pressure and electrical stimuli. Journal of Pharmacology and Experimental Therapeutics. 1989;251:334–341. [PubMed] [Google Scholar]
- Millan MJ, Czlonkowski A, Lipkowski A, Herz A. Kappa-opioid receptor-mediated antinociception in the rat. II. Supraspinal in addition to spinal sites of action. Journal of Pharmacology and Experimental Therapeutics. 1989;251:342–350. [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. European Journal of Neuroscience. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, et al. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacology, Biochemistry and Behavior. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism: Clinical and Experimental Research. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. International Review of Neurobiology. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcoholism: Clinical and Experimental Research. 1998;22:1783–1787. [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol.Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. Modeling Adolescent Development and Alcohol Use in Animals. Alcohol Research and Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. European Journal of Pharmacology. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O'Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA, Volpicelli JR, O'Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol. 2001;23:109–116. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Office of Applied Studies, NSDUH Series H-28, DHHS Publication No.SMA 05-4062. Rockville, MD: 2005. Results from the 2004 National Survey on Drug Use and Health. [Google Scholar]
- Tolman EC. Purposive behavior in animals and men. University of California Press; Berkeley: 1949. [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacology, Biochemistry and Behavior. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcoholism: Clinical and Experimental Research. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. Journal of Pharmacology and Experimental Therapeutics. 1994;271:959–968. [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behavioral and Neural Biology. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr.Opin.Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]