Abstract
The porcine small intestine submucosa, an extracellular matrix–derived bioscaffold (ECM-SIS), has been successfully used to enhance the healing of ligaments and tendons. Since the collagen fibers of ECM-SIS have an orientation of ± 30°, its application in improving the healing of the parallel-fibered ligament and tendon may not be optimal. Therefore, the objective was to improve the collagen fiber alignment of ECM-SIS in vitro with fibroblast seeding and cyclic stretch. The hypothesis was that with the synergistic effects of cell seeding and mechanical stimuli, the collagen fibers in the ECM-SIS can be remodeled and aligned, making it an improved bioscaffold with enhanced conductive properties. Three experimental groups were established: group I (n = 14), ECM-SIS was seeded with fibroblasts and cyclically stretched; group II (n = 13), ECM-SIS was seeded with fibroblasts but not cyclically stretched; and group III (n = 8), ECM-SIS was not seeded with fibroblasts but cyclically stretched. After 5 days' experiments, the scaffolds from all the three groups (n = 9 for group I; n = 8 for groups II and III) were processed for quantification of the collagen fiber orientation with a small-angle light scattering (SALS) system. For groups I and II, in which the scaffolds were seeded with fibroblasts, the cell morphology and orientation and newly produced collagen fibrils were examined with confocal fluorescent microscopy (n = 3/group) and transmission electronic microscopy (n = 2/group). The results revealed that the collagen fiber orientation in group I was more aligned closer to the stretching direction when compared to the other two groups. The mean angle decreased from 25.3° to 7.1° (p < 0.05), and the associated angular dispersion was also reduced (37.4° vs. 18.5°, p < 0.05). In contrast, groups II and III demonstrated minimal changes. The cells in group I were more aligned in the stretching direction than those in group II. Newly produced collagen fibrils could be observed along the cells in both groups I and II. This study demonstrated that a combination of fibroblast seeding and cyclic stretch could remodel and align the collagen fiber orientation in ECM-SIS bioscaffolds. The better-aligned ECM-SIS has the prospect of eliciting improved effects on enhancing the healing of ligaments and tendons.
Introduction
Ligaments and tendons are connective tissues composed of closely packed, parallel aligned collagen fiber bundles, which connect bone to bone and muscle to bone, respectively. They play an important role in maintaining the stability of joints and function of the musculoskeletal system. The alignment of the collagen fibers to the direction of tensile loading makes ligaments and tendons suitable to resist high stresses and strains.1,2 In the healing ligament and tendon, however, it has been shown that the newly synthesized extracellular matrix (ECM) is not organized and this has been correlated to their inferior stiffness and strength.1,3,4 Therefore, if the newly synthesized collagen fibers could be aligned from the initial healing process, the biomechanical properties of healing ligament and tendon could be potentially improved.
An approach to achieve this goal is to use an aligned scaffold, which can elicit such effects through a phenomenon called “contact guidance,” in which the cells align along the topographical cues of their substratum and subsequently produce new matrix in a similar orientation.5 At the same time, the biological properties of the scaffold such as biocompatibility, biodegradability, and bioactive function need to be considered for its in vivo application. Recently, an ECM bioscaffold derived from the porcine small intestine submucosa (ECM-SIS) has been used to improve the healing of ligaments and tendons.3,6–10 Its positive effects on the healing have been attributed to its unique natural ECM ultrastructure and biological properties (i.e., it is mainly composed of collagen type I and contains bioactive factors like growth factors and various cytokines). However, it has been noticed that it has only relatively aligned collagen fibers in which two distinct fiber populations are oriented at ∼ ± 30° with respect to the longitudinal axis of the intestine.11–13 Therefore, to improve the conductive effect of this ECM scaffold to guide the healing cells to be more aligned and to produce aligned matrices in the healing ligament and tendon, the objective of this study was to remodel and improve the collagen fiber alignment of the ECM-SIS in vitro by applying a combination of fibroblast seeding and cyclic stretch. We hypothesized that with the synergistic effects of seeded cells and mechanical stimuli, the collagen fibers in the bioscaffold can be remodeled to be more aligned, making it a better scaffold with enhanced conductive properties.
To test this hypothesis, we seeded fibroblasts derived from the rabbit medial collateral ligament (MCL) on ECM-SIS scaffolds and mechanically stimulated them with cyclic uniaxial stretch. The collagen fiber orientation of SIS was quantified by a small-angle light scattering (SALS) system, while the cell morphology, orientation, and the presence of newly produced collagen were visualized by confocal fluorescent microscopy and transmission electron microscopy (TEM), respectively.
Materials and Methods
Thirty-five SIS scaffolds (Cook BioTech, Indianapolis, IN) were rehydrated in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) and cut into dog bone–shaped specimens (clamp-to-clamp length was 2 cm with a width of 1 cm). The long axis of the specimen corresponded with the longitudinal axis of the small intestine, which has been shown to be the preferred direction of collagen fiber alignment.13 The scaffolds (n = 35) were randomly assigned to the following experimental groups without preselection—group I: cell seeded and cyclically stretched (n = 14); group II: cell seeded and nonstretched (n = 13); and group III: nonseeded and cyclically stretched (n = 8). For groups I and II, in which scaffolds were seeded with cells, five scaffolds from each group were used for confocal microscopy (n = 3/group) and TEM (n = 2/group) to examine cell morphology and newly produced collagen, respectively. The remaining scaffolds in these two groups (n = 9 for group I, n = 8 for group II) and the scaffolds in group III were quantitatively analyzed for fiber orientation with an SALS system. Before the test, a power analysis was done to determine the sample sizes based on a previous study on the fiber architecture of the SIS.13
For groups I and II, fibroblasts derived from skeletally mature female New Zealand White rabbit MCLs were used. Briefly, the midsubstance of MCL was dissected, rinsed with Hank's buffered salt solution (HBSS, Invitrogen), cut into small pieces in aseptic conditions, and then placed into a Petri dish with 6mL of DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1% penicillin/streptomycin (P/S; Invitrogen) for explant culture, for which standard cell culture conditions (37°C, humidified and 5% CO2) were used. The culture medium was replaced every 2 days.14 The cells were passaged after reaching 80–90% confluence. For cell seeding, the dog bone–shaped ECM-SIS scaffold was fixed on the bottom of a custom-made silicone seeding dish, which has the same dimension as the dog bone–shaped scaffold. Each bioscaffold was seeded with passage 2 fibroblasts at a density of 1.5 × 105 cells/cm2.15 After seeding on the scaffolds, the fibroblasts were allowed 24 h to attach.
The cell-seeded scaffolds from groups I and II as well as the scaffold from group III were clamped into custom-made clamps and then transferred to the individual stations of a custom-designed cyclic stretching tissue culture (CSTC) system. The scaffolds in group I were subjected to cyclic stretch for 5 days, while those in group II were not stretched but kept in the same condition as group I. For group III in which no fibroblasts were seeded, the scaffolds were cyclically stretched for 5 days as those in group I.
The CSTC system was developed for the purpose of studying the remodeling of ECM scaffolds. This system is capable of independently applying precise cyclic displacement waveforms to each specimen.15 The CSTC system (Fig. 1) consists of three independent stretching chambers, which can apply precise displacement profiles while measuring the load in each scaffold. The samples were mounted on custom-made clamps and immersed in culture medium supplemented with 50 μg/mL ascorbic acid. Immediately before cyclic stretch, all samples were preloaded with 0.05 N to establish a standardized starting point, and then subjected to a cyclic stretching regimen of 2 h, allowed to rest 2 h, stretched another 2h, and finally rested for 18 h. This stretching pattern was repeated for 5 consecutive days. The elongation and frequency were set at 10% and 0.5Hz, respectively. The culture medium was changed every 2 days.
FIG. 1.
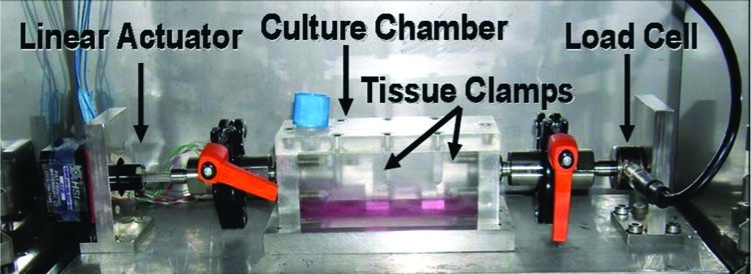
Cyclic stretching tissue culture (CSTC) system. Color images available online at www.liebertonline.com/ten.
An SALS system was used to quantify the collagen fiber orientation of the ECM-SIS before and after experimentation (group I: n = 9; group II: n = 8; group III: n = 8). The SALS system is an accepted method to quantify collagen fiber orientation and operates on the principle of single-slit light diffraction of a laser beam.16 The SALS device passes a 4-mW unpolarized helium-neon laser beam through the tissue, which was chosen because its wavelength (632.8 nm) is within an order of magnitude of the collagen fiber diameter. The frequency distribution obtained represents the scattered light intensity distribution I(ф). From the I(ф), the collagen fiber orientation is described by фc, where фc is the median angle of the I(ф) at a single scanned point, and describes the main vector of alignment at each scanned point.
For each SIS sample, a total of 1000 scan points at the midsubstance (about 0.8×1.25 cm) were scanned before and after experimentation using the SALS system. The collagen fiber orientation at each point was expressed as the angle with respect to the longitudinal axis of the SIS. The average of these 1000 angles represented the mean collagen fiber orientation of each SIS sample. The mean collagen fiber orientation and the associated angular dispersion (represented by the circular standard deviation of the angles) of each experimental group before and after experimentation were compared statistically to evaluate the effect of seeding and/or stretching. The differences in mean collagen fiber orientation and the associated angular dispersion were used to quantify the ECM remodeling.
It should be noted that the collagen fiber orientations obtained fall into the category of circular data, for which conventional methods of calculating arithmetic mean and performing inferential analysis do not apply. Thus, statistical methods for circular data were employed in the present study. Specifically, a second-order analysis for circular data was conducted for the mean angle of collagen fiber orientation, as the mean angle of each group was the mean of the mean angle of each specimen.17 In addition, a Watson–Williams test was performed on the angular dispersion. The significance level was set at 0.05.
Another parameter called the orientation index (OI) reflecting the fiber organization was also calculated. The OI represents the angle containing 50% of the fibers at one scan point. Thus, a highly organized collagen fiber network at the point of laser beam intersection will have a lower OI, while randomly oriented fibers will have larger values. The OI distribution was portrayed on a schematic graph with a color scale ranging from purple (low OI, high fiber organization) to blue (high OI, low fiber organization).
To evaluate cell morphology and orientation and to determine the presence of newly produced collagen, scaffolds from groups I and II were processed for confocal fluorescent microscopy (n = 3/group) and TEM (n = 2/group) after 5 day's experimentation, respectively. For confocal fluorescent microscopy, the ECM-SIS was fixed in 4% paraformaldehyde and cell membranes were permeabilized with 0.1% Triton X-100 for 10 min. The specimens were then incubated with rhodamine phalloidin ( Molecular Probes, Eugene, OR) for 1h and then with Sytox Green (Molecular Probes) for 15 min at room temperature to stain actin filaments and the nuclei, respectively. The specimens were then viewed under a Bioptechs Confocal System featuring an Olympus IX70 inverted microscope. Images from at least four randomly chosen regions in each specimen were captured and recorded in LaserSharp software via a Radiance liquid light acquisition system.
For TEM, the ECM-SIS was fixed for at least 1 h in Karnovsky's fixative. The specimens were washed with three changes of 0.1 M sodium cacodylate buffer pH 7.4 and postfixed for 1 h in a solution containing 1% OsO4 buffered with 0.1 M sodium cacodylate (pH 7.4). Thereafter washed in three changes of dH2O and dehydrated in EtOH (50%, 70%, 80%, 90%, and three changes of 100%), the specimens were infiltrated overnight in a 1:2 mixture of Epon-Araldite embedding resin (Electron Microscopy Sciences, Fort Washington, PA) and propylene oxide (PO) for 24 h, followed by a 1:1 mixture of resin and PO for 24 h, and finally a 2:1 mixture of resin and PO overnight. The following day, the 2:1 mixture was removed and replaced with 100% Epon-Araldite. The specimens were infiltrated for an additional 8h, placed in flat embedding molds, and polymerized for 48 h at 60°C. The embedded specimens were thin sectioned (0.1 mm) using a Reichert-Jung Ultracut E (Leica Microsystems AG, Wetzlar, Germany) and a DDK diamond knife (Delaware Diamond Knives, Wilmington, DE). Thin sections were stained with 1% uranyl acetate and Reynolds lead citrate and viewed on a Hitachi H-7100 TEM (Hitachi High Technologies America, Pleasanton, CA) operating at 50 kV. Digital images were obtained from at least four randomly chosen regions by using an AMT Advantage 10 CCD Camera System (Advanced Microscopy Techniques, Danvers, MA) and NIH Image software at both 20,000× and 70,000× magnification.
Results
There were no tears or failures of the scaffolds during or following the cyclic stretching. The quantitative data from SALS showed that the initial fiber orientation of all ECM-SIS scaffolds in the three groups was not significantly different (p > 0.05; Table 1), in which group I had a mean angle of 25.3° with an angular dispersion of 37.4°, group II had a mean angle of 26.1° with an angular dispersion of 30.1°, and group III had a mean angle of 8.9° with an angular dispersion of 12.8°. The mean angle of all the scaffolds in the three groups before experiments, which represented the inherent collagen fiber orientation of the ECM-SIS, was 2.8 ± 27.0°. After 5 days of stretch, the fiber orientation of the scaffolds in group I was significantly improved. The mean angle of the fibers was decreased from initial 25.3° to 7.1° after cell seeding and stretch (p < 0.05), which indicated that in group I the orientation of fibers of the scaffolds had become closer to the stretching direction. In addition, the angular dispersion decreased from the initial 37.4° to 18.5° (p < 0.05), indicating that the variance of the angles had become smaller (Table 1).In contrast, for the scaffolds in group II, the mean angle of the fiber orientation and the angular dispersion did not show significant improvement after only cell seeding by the current sample size. The mean angle was 26.1° versus 20.5° (p > 0.05), and the angular dispersion was 30.1° versus 24.8° (p > 0.05). Similarly, the two parameters of fiber orientation for the scaffolds in group III did not show significant changes either after only stretch by the current sample size. The mean angle was 8.9° versus 10.2° (p > 0.05), and the angular dispersion was 12.8° versus 10.2° (p > 0.05). A power analysis using the current data of groups II and III showed that to detect a statistical significance (as in group I), 81 and 257 samples would be needed, respectively.
Table 1.
The Effects of Cell Seeding and Cyclic Stretching on the Orientation of Collagen Fibers in the ECM Bioscaffold as Quantified by SALS
| |
Mean angle |
Angular dispersion |
||
|---|---|---|---|---|
| Groups | Before | After | Before | After |
| I (seeded and cyclically stretched) | 25.3° | 7.1°a | 37.4° | 18.5°a |
| II (seeded and nonstretched) | 26.1° | 20.5° | 30.1° | 24.8° |
| III (nonseeded and cyclically stretched) | 8.9° | 10.2° | 12.8° | 10.2° |
Indicate significant difference before and after stretching. ECM, extracellular matrix; SALS, small-angle light scattering.
The fiber organization as reflected by the OI was also improved for the scaffolds in group I when compared to those in the other two groups (Fig. 2). From the scanning images, it could be observed that the collagen fibers of the scaffolds in group I were more organized as indicated by the large area of the red to purple coloration (low OI), as compared to the images before experiments, which showed yellow to blue coloration (high OI) (Fig. 2A). Comparatively, the scaffolds in groups II and III did not show the predominant change after only stretching or seeding, respectively (Fig. 2B, C). For these two groups, colors on the schematic scanning images from before and after the experimentation did not show much change, indicating that the organization of the fibers at individual points were not improved (Fig. 2B, C).
FIG. 2.
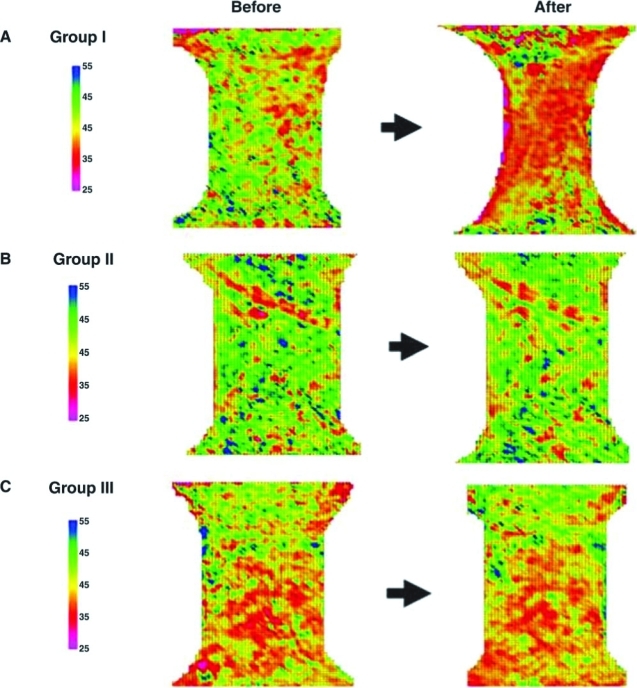
Schematic scanning images of SALS for ECM-SIS before and after cell seeding and/or cyclic stretch. Representative samples are shown in (A) group I, (B) group II, and (C) group III. Color images available online at www.liebertonline.com/ten.
With the confocal fluorescent microscopy, the cell morphology and orientation could be observed in the two cell-seeded groups. The fibroblasts in group I became aligned along the stretching direction after cyclic stretch, while those in group II without stretch remained unoriented. As shown in Figure 3, both the green-colored nuclei and the red-colored intracellular actin filaments were more aligned along the stretch direction in group I. Comparatively, the majority of the nuclei and actin filament in group II did not have any noticeable preferred orientation.
FIG. 3.
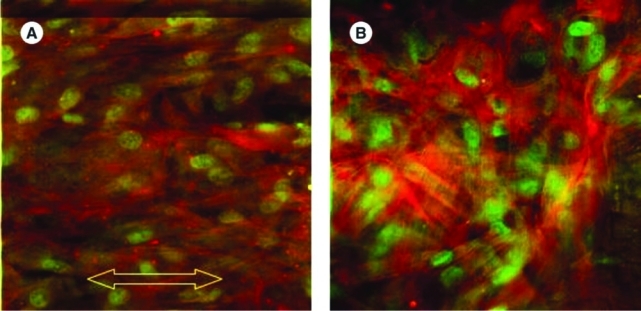
Fluorescent staining of the cells seeded on the ECM-SIS observed under confocal fluorescent microscope. (A) Group I, in which the arrow indicates the stretching direction; (B) group II. Actin fibers were stained in red, and nuclei were stained in green. Color images available online at www.liebertonline.com/ten.
In addition, TEM revealed that in both cell-seeded groups (groups I and II), some fibroblasts had migrated into the scaffolds and those that remained on the surface of the SIS appeared flat and stacked, forming cell layers. Newly produced collagen fibrils that appeared as collagen fibrils with a much smaller diameter than those of the ECM-SIS scaffold could also be observed between the fibroblasts (Fig. 4A, B).
FIG. 4.
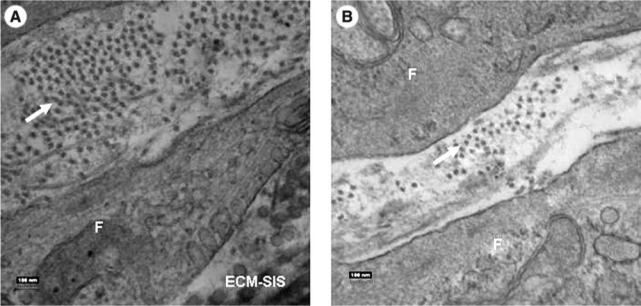
Transmission electron microscopy of ECM-SIS after cell seeding. (A) Newly produced collagen in group I as indicated by the arrow; (B) Newly produced collagen in group II as indicated by the arrow. Magnification at 70,000. “F” indicates fibroblast.
Discussion
This study showed that a combination of fibroblast seeding and cyclic uniaxial stretch could effectively improve the collagen fiber alignment in the ECM-SIS bioscaffold toward the axis of the applied stretch, while mechanical stretch or cell seeding alone was not sufficient to affect the ultrastructure of the scaffold. These findings support our hypothesis and suggest that the dynamic cell–matrix interaction under mechanical stimuli was necessary to stimulate the cells to effectively remodel the matrix fibers to align along the stretching direction.18
Our data emphasized the importance of the synergistic effects of cells and mechanical stimuli in the process of matrix remodeling. Studies have shown that cells have the tendency to avoid axial surface strain.5,19–21 When cells were embedded in a fibrous substrate, the cells tended to align along the fibers in the stretching direction (stress shielding) and remodel the matrix rapidly by upregulating matrix metalloproteinases (MMPs).18,22–24 Our results are in congruency with those in these studies as we observed that the fibroblasts seeded on the ECM-SIS bioscaffolds became aligned in the stretching direction. Moreover, it was documented that cell orientation determined the alignment of the newly produced collagen fibers.25,26 Therefore, a possible mechanism for our findings is that under mechanical stimuli, the seeded fibroblasts tended to align in the stretching direction, broke down the fibers of the scaffold that were not in the same orientation, and simultaneously produced new fibers that aligned to the stretching direction, by which the fibers in the scaffold finally became more aligned and more organized. The possible alterations of the levels of MMPs, which are considered as an indication for the matrix remodeling, will be investigated in our subsequent studies.
In the study, the collagen fiber orientation in the ECM-SIS scaffolds prior to experimentation was found to be 2.8 ± 27.0° as measured by SALS, which is similar to the published results from previous studies (±30°).13 With cell seeding and cyclic stretch, the orientation and organization of the collagen fibers in the scaffold were significantly improved, as confirmed by both quantitative and morphological fiber orientation results. This improvement in the fiber architecture in the ECM-SIS bioscaffold has an important implication in its application in repairing injured ligaments and tendons in that the remodeled bioscaffold gains further topographic advantages in addition to its unique biological properties, which would increase its existing repairing capability through the effects of contact guidance.
The phenomenon of contact guidance has been documented in previous studies using various substrates such as microgrooved substrates, orientated collagen fibrils, and fibronectin fibers.24,27,28 It was found that cellular behavior such as movement and arrangement can be affected by the topographical morphology of their substratum. Further, it was also shown that fibroblasts that were aligned through contact guidance were also producing aligned collagen.25,26 These findings support our study to create a bioactive scaffold with aligned fibers to meet the special needs in repairing injured ligaments and tendons, which normally have a unique structure of parallel-aligned collagen fibers to sustain high stresses and strains. The remodeled ECM-SIS bioscaffold could provide a better contact guidance for the healing cells in the injured ligaments and tendons, and thus improve the alignment of the healing cells. Consequently, the newly deposited ECM will align in the fiber direction. By this way, the biomechanical properties of healing ligaments and tendons could be better improved.
A limitation of this study is that only one set of frequency and magnitude of the cyclic stretching and stretching duration was chosen to remodel the scaffold. Thus, it is necessary to expand this treatment regimen to get more optimal values. Nevertheless, the chosen regimen was able to successfully remodel an ECM-scaffold following cell seeding and cyclic stretching.
This study provides the basis for in vitro modification of SIS scaffold for the enhancement of its application in vivo to improve the healing of ligaments and tendons. Further, the use of SALS provides a novel method to detect changes or improvements in the ultrastructure of ECM-bioscaffolds. In the future, the stretching regimen will be optimized and the in vivo effects of the remodeled SIS scaffold will be tested.
Acknowledgments
The authors wish to express their gratitude to the technical assistance of Kira Lathrop and to Dr. David Merryman, Dr. Tom Gilbert, and Cook Biotech for supplying the ECM-SIS; the financial support of NIH #AR39683, ASIAM, and the Dutch Anna Fund is gratefully acknowledged.
References
- 1.Frank C. Woo S.L. Amiel D. Harwood F. Gomez M. Akeson W. Medial collateral ligament healing. A multidisciplinary assessment in rabbits. Am J Sports Med. 1983;11:379. doi: 10.1177/036354658301100602. [DOI] [PubMed] [Google Scholar]
- 2.Woo S.L. Gomez M.A. Sites T.J. Newton P.O. Orlando C.A. Akeson W.H. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am. 1987;69:1200. [PubMed] [Google Scholar]
- 3.Woo S.L.-Y. Takakura Y. Liang R. Jia F. Moon D.K. Treatment with bioscaffold enhances the fibril morphology and the collagen composition of healing medial collateral ligament in rabbits. Tissue Eng. 2006;12:159. doi: 10.1089/ten.2006.12.159. [DOI] [PubMed] [Google Scholar]
- 4.Niyibizi C. Kavalkovich K. Yamaji T. Woo S.L. Type V collagen is increased during rabbit medial collateral ligament healing. Knee Surg Sports Traumatol Arthrosc. 2000;8:281. doi: 10.1007/s001670000134. [DOI] [PubMed] [Google Scholar]
- 5.Wang J.H. Grood E.S. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect Tissue Res. 2000;41:29. doi: 10.3109/03008200009005639. [DOI] [PubMed] [Google Scholar]
- 6.Musahl V. Abramowitch S.D. Gilbert T.W. Tsuda E. Wang J.H. Badylak S.F. Woo S.L. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament—a functional tissue engineering study in rabbits. J Orthop Res. 2004;22:214. doi: 10.1016/S0736-0266(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 7.Perry S.M. Gupta R.R. van Kleunen J. Ramsey M.L. Soslowsky L.J. Glaser D.L. Use of small intestine submucosa in a rat model of acute and chronic rotator cuff tear. J Shoulder Elbow Surg. 2007;16:S179. doi: 10.1016/j.jse.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Badylak S.F. Tullius R. Kokini K. Shelbourne K.D. Klootwyk T. Voytik S.L. Kraine M.R. Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29:977. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 9.Karaoglu S.. B.F M. Woo S.L. Fu Y.C. Liang R. Abramowitch S.D. Use of a bioscaffold to improve healing of a patellar tendon defect after graft harvest for ACL reconstruction: a study in rabbits. J Orthop Res. 2008;26:255. doi: 10.1002/jor.20471. [DOI] [PubMed] [Google Scholar]
- 10.Ledet E.H. Carl A.L. DiRisio D.J. Tymeson M.P. Andersen L.B. Sheehan C.E. Kallakury B. Slivka M. Serhan H. A pilot study to evaluate the effectiveness of small intestinal submucosa used to repair spinal ligaments in the goat. Spine J. 2002;2:188. doi: 10.1016/s1529-9430(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 11.Hodde J.P. Record R.D. Liang H.A. Badylak S.F. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 12.Voytik-Harbin S.L. Brightman A.O. Kraine M.R. Waisner B. Badylak S.F. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478. [PubMed] [Google Scholar]
- 13.Sacks M.S. Gloeckner D.C. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J Biomed Mater Res. 1999;46:1. doi: 10.1002/(sici)1097-4636(199907)46:1<1::aid-jbm1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Ross S.M. Joshi R. Frank C.B. Establishment and comparison of fibroblast cell lines from the medial collateral and anterior cruciate ligaments of the rabbit. In Vitro Cell Dev Biol. 1990;26:579. doi: 10.1007/BF02624206. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert T.W. Stewart-Akers A.M. Sydeski J. Nguyen T.D. Badylak S.F. Woo S.L. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Eng. 2007;13:1313. doi: 10.1089/ten.2006.0318. [DOI] [PubMed] [Google Scholar]
- 16.Sacks M.S. Smith D.B. Hiester E.D. A small angle light scattering device for planar connective tissue micro-structural analysis. Ann Biomed Eng. 1997;25:678. doi: 10.1007/BF02684845. [DOI] [PubMed] [Google Scholar]
- 17.Zar J.H. Upper Saddler River. fourth. N.J.: Prentice Hal; 1999. Biostatistical Analysis. [Google Scholar]
- 18.Prajapati R.T. Chavally-Mis B. Herbage D. Eastwood M. Brown R.A. Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen. 2000;8:226. doi: 10.1046/j.1524-475x.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 19.Neidlinger-Wilke C. Grood E. Claes L. Brand R. Fibroblast orientation to stretch begins within three hours. J Orthop Res. 2002;20:953. doi: 10.1016/S0736-0266(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 20.Buckley M.J. Banes A.J. Levin L.G. Sumpio B.E. Sato M. Jordan R. Gilbert J. Link G.W. Tran Son Tay R. Osteoblasts increase their rate of division and align in response to cyclic, mechanical tension in vitro. Bone Miner. 1988;4:225. [PubMed] [Google Scholar]
- 21.Dartsch P.C. Hammerle H. Orientation response of arterial smooth muscle cells to mechanical stimulation. Eur J Cell Biol. 1986;41:339. [PubMed] [Google Scholar]
- 22.Huang D. Chang T.R. Aggarwal A. Lee R.C. Ehrlich H.P. Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast-populated collagen matrices. Ann Biomed Eng. 1993;21:289. doi: 10.1007/BF02368184. [DOI] [PubMed] [Google Scholar]
- 23.Eastwood M. Mudera V.C. McGrouther D.A. Brown R.A. Effect of precise mechanical loading on fibroblast populated collagen lattices: morphological changes. Cell Motil Cytoskeleton. 1998;40:13. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Mudera V.C. Pleass R. Eastwood M. Tarnuzzer R. Schultz G. Khaw P. McGrouther D.A. Brown R.A. Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell Motil Cytoskeleton. 2000;45:1. doi: 10.1002/(SICI)1097-0169(200001)45:1<1::AID-CM1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Wang J.H. Jia F. Gilbert T.W. Woo S.L. Cell orientation determines the alignment of cell-produced collagenous matrix. J Biomech. 2003;36:97. doi: 10.1016/s0021-9290(02)00233-6. [DOI] [PubMed] [Google Scholar]
- 26.den Braber E.T. de Ruijter J.E. Ginsel L.A. von Recum A.F. Jansen J.A. Orientation of ECM protein deposition, fibroblast cytoskeleton, and attachment complex components on silicone microgrooved surfaces. J Biomed Mater Res. 1998;40:291. doi: 10.1002/(sici)1097-4636(199805)40:2<291::aid-jbm14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Ejim O.S. Blunn G.W. Brown R.A. Production of artificial-orientated mats and strands from plasma fibronectin: a morphological study. Biomaterials. 1993;14:743. doi: 10.1016/0142-9612(93)90038-4. [DOI] [PubMed] [Google Scholar]
- 28.Guido S. Tranquillo R.T. A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J Cell Sci. 1993;105(Pt 2):317. doi: 10.1242/jcs.105.2.317. [DOI] [PubMed] [Google Scholar]


