Abstract
In the present voltammetric study, we have characterized cocaine-induced changes in evoked dopamine release and uptake in the striatum of freely moving mice in real time. Cocaine induced marked dopamine uptake inhibition measured as apparent Km changes, producing a maximal effect 20 minutes following a single injection (15 mg/kg i.p.). Changes in uptake were paralleled by increases in evoked dopamine release per stimulus pulse, revealing a high correlation between these two parameters following cocaine administration. This initial characterization of cocaine effects on striatal dopamine transmission in the commonly used C57BL/6 mouse strain provides a basis for future voltammetric studies using genetic mouse models.
Keywords: Cocaine, freely-moving mouse, voltammetry, dopamine
Cocaine increases extracellular dopamine levels by inhibiting the uptake of dopamine through dopamine transporters located on presynaptic terminals [1]. Many of the characteristic behavioral effects associated with cocaine, such as psychomotor activation [2], stereotypic movements [3] and reinforcement [4] are tightly linked to levels of dopamine uptake inhibition. Although several studies have documented the time-course of cocaine-induced changes in dopamine uptake in vivo using rats [5;6], a time-course of this effect has never been reported in mice.
Advances in molecular biology have allowed the production of many different strains of genetically modified mice, which provide researchers with animal models to study different human diseases, including psychiatric disorders such as drug addiction. These mouse models can help to further clarify the neurochemical mechanisms of addictive drugs. Here, we applied fast-scan cyclic voltammetry [1;2] to study the time-course of cocaine-induced changes in dopamine uptake and stimulated dopamine release in the striatum of freely moving C57BL/6 mice, a commonly used mouse strain. Our approach clearly demonstrates that real time dopamine measurements can be conducted in freely moving mice, and opens the door to future analyses in transgenic and knockout mice.
All voltammetric recordings were preformed in freely moving male mice (C57BL/6, 8-12 weeks old, n=5). The experimental protocol adhered to National Institutes of Health Animal Care guidelines and was approved by the Wake Forest University Institutional Animal Care and Use Committee.
Surgery for implantation of a stimulating electrode, a reference electrode and a guide cannula for the micromanipulator was carried out as previously described in rats [7]. Mice were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and placed in a stereotaxic frame. A hole for the guide cannula (Bioanalytical Systems, West Lafayette, IN) was drilled according to coordinates from a mouse brain atlas (AP +1.0, L +1.3 mm from bregma). A Ag/AgCl reference electrode was implanted in the contralateral superficial cortex. A bipolar stimulating electrode was lowered to the VTA/SN area ipsilateral to the guide cannula at 3 mm posterior and 1.0 mm lateral to bregma. A newly designed lightweight micromanipulator (0.95 g) capable of inserting large diameter (1.2-mm) glass capillary carbon-fiber electrodes in the mouse brain through the guide cannula was constructed for this study. The head-mounted voltammetric amplifier (UNC Electronics Design Facility, Chapel Hill, NC) was miniaturized for use with mice. Dopamine was evoked by electrical stimulation of the VTA/SN and monitored in the dorsal striatum using fast-scan cyclic voltammetry. Voltammetric recordings were made at the carbon-fiber microelectrode every 100 ms by applying a triangle waveform (-0.4 to +1.3 V, 300 V/s). Upon establishment of stable baseline signals, stimulation (24 pulses, 60 Hz, 120 μA, 2 ms/phase, biphasic) was applied every 10 minutes for 20 minutes before and 2 hours after cocaine (15 mg/kg, i.p.) administration. Cocaine HCl, obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, N.C., USA), was dissolved in a solution of sterilized 0.9% saline, passed through a microfilter (0.45 μm pore size) and diluted to a solution of 2.5 mg/ml for these experiments. All statistics were performed using SigmaPlot (version 11). Evoked dopamine levels and uptake parameters were statistically analyzed using an ANOVA with repeated measures. Correlations are reported as Pearson's r values.
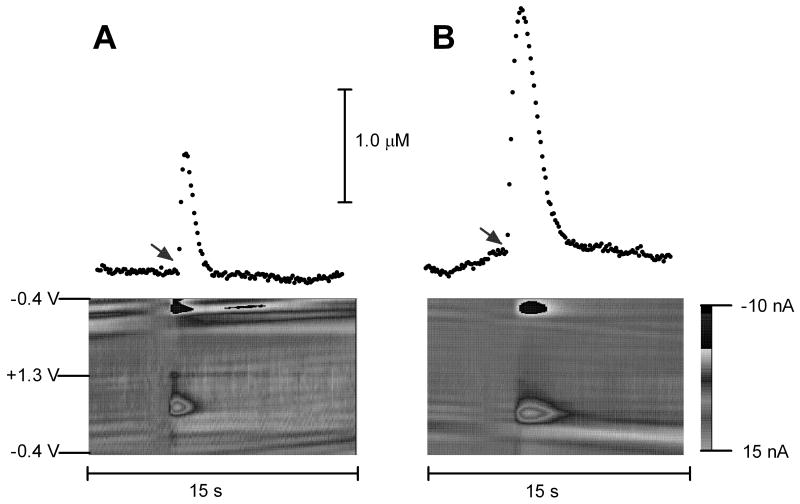
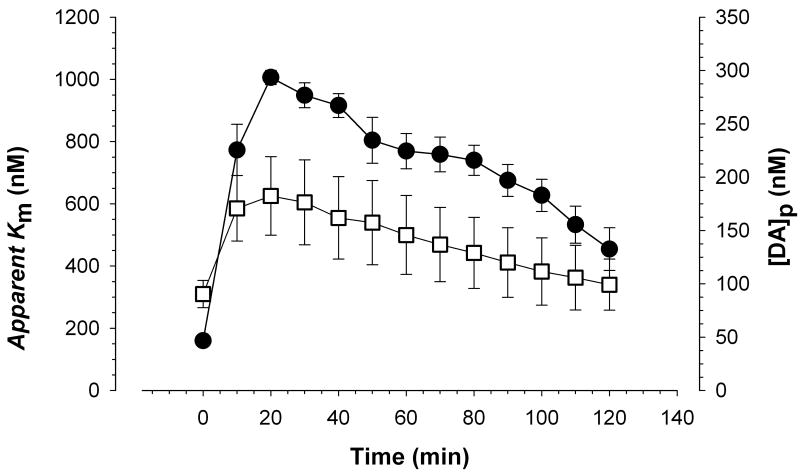
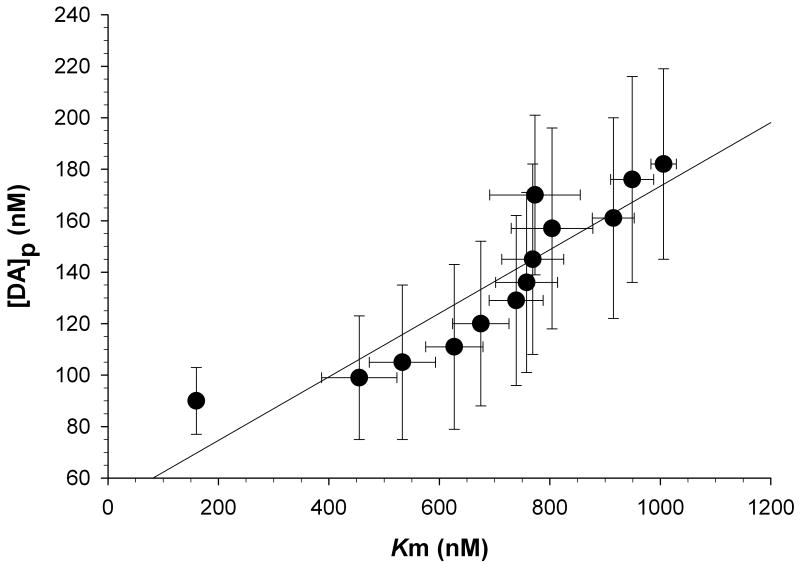
Stimulated extracellular dopamine efflux was detected in the dorsal striatum of freely-moving mice as first reported by Yavich and Tiihonen [8]. As illustrated in Figure 1, electrical stimulation of the VTA/SN area resulted in a rapid increase in striatal extracellular dopamine (∼1 μM) prior to cocaine administration. The observed maximal amplitude of the evoked dopamine signal approximately doubled (∼2 μM) 10 minutes after a single cocaine injection (15 mg/kg i.p.). Figure 2 shows changes in the parameters of apparent Km (filled circles) and DAp (open squares) over a 2 hour time-course after a single cocaine injection (15 mg/kg i.p.). Cocaine resulted in a significant increase in DAp (F(12,64) = 7.895; p<0.01), which reflects the concentration of dopamine released per stimulus pulse, and apparent Km (F(12,64) = 25.685; p<0.01), which represents the affinity of dopamine for the dopamine transporter. Increased apparent Km values represent greater uptake inhibition. Maximal apparent Km (1006.2 nM ± SEM 23.0) and DAp (182.4 nM ± SEM 36.9) values were recorded 20 minutes following cocaine administration and then gradually returned to baseline values. The apparent Km and DAp data are replotted in figure 3 to emphasize the relationship between these two parameters. As illustrated in Figure 3, apparent Km and DAp are highly correlated (r = 0.91) in the presence of cocaine. Consistent with competitive uptake inhibition, cocaine did not significantly change Vmax (data not shown), which reflects the maximal velocity of dopamine uptake. The average Vmax in the dorsal striatum was 3427 nM/s ± SEM 353.5.
Figure 1.
(top) Representative concentration-time plots of electrically evoked dopamine measured in the dorsal striatum before (left) and 10 minutes after (right) a single injection of cocaine (15 mg/kg i.p.). Arrows indicate the onset of electrical stimulation.(bottom) Representative color plots – which topographically depict the voltammetric data with time on the x-axis, applied scan potential on the y-axis and background-subtracted faradaic current shown on the z-axis in pseudo-color – are illustrated before (left) and after (right) cocaine administration.
Figure 2.
time-course of dopamine uptake inhibition and evoked dopamine release after cocaine administration Changes in apparent Km (filled circles) and DAp (open squares) following administration of cocaine (15 mg/kg, i.p.) are shown over 2 hours. Data are expressed as means ± SEM. Maximal apparent Km (1006.2 nM ± SEM 23.0) and DAp (182.4 nM ± SEM 36.9) occurred 20 minutes following cocaine administration.
Figure 3.
Dopamine uptake inhibition and evoked release are correlative parameters in the presence of cocaine. A high correlation coefficient was found (r = 0.91) between apparent Km and DAp when compared across all time-points following a single injection of cocaine (15mg/kg i.p.). Data were grouped into 10-minute bins of time following cocaine administration and are expressed as means ± SEM.
While measuring dopamine dynamics in the striatum of freely moving mice we observed that cocaine markedly decreased the uptake of dopamine by increasing apparent Km without significantly changing the maximal uptake rate (Vmax). When maximal dopamine uptake inhibition (apparent Km) and maximal dopamine per pulse (DAp) were observed, the uptake rate (Vmax) remained unchanged (3427 nM/s ± SEM 353.5). These data are in agreement with previous in vitro studies performed in striatal slices from mice [9] and in vivo studies using anesthetized and freely moving rats [10;11]. Dopamine uptake inhibition (apparent Km) reached a maximum at 20 minutes, which coincides with previous reports showing maximal behavioral activation and extracellular dopamine concentrations occur between 20-30 minutes after a single intraperitoneal injection of 15 mg/kg cocaine in both mice and rats [12;13]. The apparent Km gradually returned to baseline values within approximately 2 h. These changes were accompanied by a parallel decrease in behavioral activity. These data further confirmed the critical role of the dopamine transporter in cocaine-induced psychomotor activation.
In this study both apparent Km and DAp were determined using a model developed by Wightman and colleagues, in which electrically stimulated dopamine concentrations are described as a delicate balance between release and uptake [14]. One aspect that merits additional discussion is the possibility that the changes in electrically-evoked dopamine concentrations observed after cocaine can be influenced by alterations in both dopamine uptake and release. In addition to delaying uptake, cocaine can increase the amount of dopamine detected during the stimulus train by promoting dopamine release from reserve pools of dopamine-containing vesicles [15]. Additionally, electrically-stimulated dopamine release is also subject to D2 dopamine receptor-mediated autoinhibition [16-18] which would have the opposite effect of reducing evoked dopamine concentrations during dopamine transporter inhibition. In light of this complicated action of cocaine on electrically-evoked dopamine release, the observation of a high correlation between changes in an apparent Km and DAp during the drug time course is obviously important. The strong temporal association between these parameters suggests that the effect of cocaine on the evoked dopamine release can be preferentially attributed to changes in dopamine uptake. The fact that the increase in electrically-evoked dopamine levels following cocaine was not observed in mice with a genetic deletion of the dopamine transporter [19;20] supports this notion. However, the transporter knockout mice have many other alterations in dopamine storage and release [21], which make direct comparisons to wild-type mice difficult.
In conclusion, the present data provide the first characterization of cocaine-induced changes in dopamine uptake and evoked dopamine concentrations in freely moving mice. A time-course of cocaine induced changes in apparent Km and DAp was documented, revealing a tight correlation between the two parameters. This work provides both a methodology and a baseline standard for future pharmacological studies using genetic mouse models of drug addiction.
Acknowledgments
This work was supported by Wake Forest University Cross-Campus Collaborative Fund Award (EAB, KDB) and National Institute of Health grants F31DA024525 (EBO) DA021634 (EAB) DA018815, AA014091 and AA013900 (SRJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 2.Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- 3.Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oleson EB, Talluri S, Childers SR, Smith JE, Roberts DC, Bonin KD, Budygin EA. Dopamine uptake changes associated with cocaine self-administration. Neuropsychopharmacology. 2009;34:1174–1184. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espana RA, Roberts DC, Jones SR. Short-acting cocaine and long-acting GBR-12909 both elicit rapid dopamine uptake inhibition following intravenous delivery. Neuroscience. 2008;155:250–257. doi: 10.1016/j.neuroscience.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yavich L, Tiihonen J. In vivo voltammetry with removable carbon fibre electrodes in freely-moving mice: dopamine release during intracranial self-stimulation. J Neurosci Methods. 2000;104:55–63. doi: 10.1016/s0165-0270(00)00321-6. [DOI] [PubMed] [Google Scholar]
- 9.John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 12.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank ST, Krumm B, Spanagel R. Cocaine-induced dopamine overflow within the nucleus accumbens measured by in vivo microdialysis: a meta-analysis. Synapse. 2008;62:243–252. doi: 10.1002/syn.20489. [DOI] [PubMed] [Google Scholar]
- 14.Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- 15.Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22:8002–8009. doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Reith ME, Walker QD, Kuhn CM, Carroll FI, Garris PA. Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study. J Neurosci. 2002;22:6272–6281. doi: 10.1523/JNEUROSCI.22-14-06272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budygin EA, John CE, Mateo Y, Jones SR. Lack of cocaine effect on dopamine clearance in the core and shell of the nucleus accumbens of dopamine transporter knock-out mice. J Neurosci. 2002;22:RC222. doi: 10.1523/JNEUROSCI.22-10-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]