Summary
Laminins are structural components of basement membranes. In addition, they are key extracellular-matrix regulators of cell adhesion, migration, differentiation and proliferation. This Commentary focuses on a relatively understudied aspect of laminin biology: how is laminin deposited into the extracellular matrix? This topic has fascinated researchers for some time, particularly considering the diversity of patterns of laminin that can be visualized in the matrix of cultured cells. We discuss current ideas of how laminin matrices are assembled, the role of matrix receptors in this process and how laminin-associated proteins modulate matrix deposition. We speculate on the role of signaling pathways that are involved in laminin-matrix deposition and on how laminin patterns might play an important role in specifying cell behaviors, especially directed migration. We conclude with a description of new developments in the way that laminin deposition is being studied, including the use of tagged laminin subunits that should allow the visualization of laminin-matrix deposition and assembly by living cells.
Keywords: Extracellular matrix, Integrins, Matrix receptors
Introduction
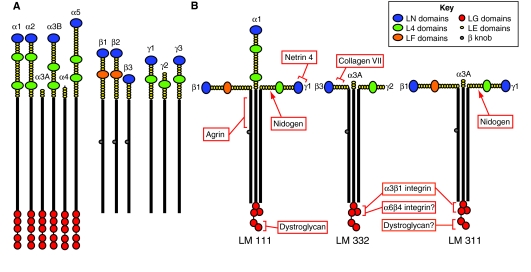
The laminins are heterotrimeric proteins of the extracellular matrix that are composed of α-, β- and γ-subunits. Currently, in mouse and human, genes encoding five α-, three β- and three γ-subunits have been identified (Fig. 1A). When known splice variants are included, these subunits assemble into at least 16 different laminin heterotrimers (Aumailley et al., 2005) (Table 1). Laminin heterotrimers are relatively large proteins (with molecular masses ranging from 400 to 900 kDa) and exist as cross-shaped molecules with two or three short arms and one long arm (Fig. 1) (Aumailley et al., 2005; Tunggal et al., 2000; Tzu and Marinkovich, 2008; Yurchenco et al., 2004). The 16 known members of the laminin family exhibit some tissue specificity and their expression is often developmentally regulated (Aumailley et al., 2005; Tunggal et al., 2000; Tzu and Marinkovich, 2008; Yurchenco et al., 2004).
Fig. 1.
Laminin subunits and examples of three heterotrimers. (A) The major laminin-subunit splice isoforms, showing some of the prominent, functionally important domains within each of the subunits (see key). (B) Organization of laminin heterotrimers. Laminins 111, 332 and 331 are depicted. Binding sites for those matrix molecules and receptors that are mentioned in the text are indicated on each diagram (red boxes).
Table 1.
The subunit composition of laminin heterotrimers
| Former designation | Subunit composition | Current designation |
|---|---|---|
| Laminin 1 | α1β1γ1 | LM 111 |
| Laminin 2 | α2β1γ1 | LM 211 |
| Laminin 3 | α1β2γ1 | LM 121 |
| Laminin 4 | α2β2γ1 | LM 221 |
| Laminin 5 or 5A | α3Aβ3γ2 | LM 332 or 3A32 |
| Laminin 5B | α3Bβ3γ2 | LM 3B32 |
| Laminin 6 or 6A | α3Aβ1γ1 | LM 311 or 3A11 |
| Laminin 7 or 7A | α3Aβ2γ1 | LM 321 or 3A21 |
| Laminin 8 | α4β1γ1 | LM 411 |
| Laminin 9 | α4β2γ1 | LM 421 |
| Laminin 10 | α5β1γ1 | LM 511 |
| Laminin 11 | α5β2γ1 | LM 521 |
| Laminin 12 | α2β1γ3 | LM 213 |
| Laminin 14 | α4β2γ3 | LM 423 |
| – | α5β2γ2 | LM 522 |
| Laminin 15 | α5β2γ3 | LM 523 |
The old and current designations for the laminin heterotrimers are indicated [adapted from (Aumailley et al., 2005)]
In keeping with the current trend of rationalizing protein nomenclature, the laminin family of proteins has recently been reclassified in a more intuitive manner (Aumailley et al., 2005). In the old system, α1β1γ1 was known as laminin-1, whereas α3β3γ2 was named laminin-5. In the new naming system, laminin trimers are known purely by their subunit composition (either including or omitting the Greek letters) such that laminin-1 is now known as laminin-111 and laminin-5 is laminin-332 (Table 1) (Aumailley et al., 2005).
Laminin trimers are assembled intracellularly. Initially, disulfide-linked dimers of the β- and γ-subunits form (Yurchenco et al., 1997); for this process, a ten-amino-acid region located toward the C-terminus of the laminin coiled-coil domain of the γ-subunit appears to be crucial (Nomizu et al., 1996; Utani et al., 1994). The βγ-dimer is retained in the cytoplasm and requires α-subunit incorporation, and therefore trimerization, to drive secretion. In addition, the α-subunit can be secreted independently as a monomer (Yurchenco et al., 1997).
In both developing and intact tissues, laminins are incorporated into basement membranes, which separate parenchymal cells from the connective tissue. Laminins play important roles in tissue morphogenesis and homeostasis by regulating tissue architecture, cell adhesion, migration and matrix-mediated signaling. For instance, mutations in the subunits of laminin-332 in humans cause a blistering disease owing to compromised adhesion of keratinocytes to the laminin-332-deficient basement membrane of the skin (McGowan and Marinkovich, 2000; Miner and Yurchenco, 2004). In mice, knockout of the α5 laminin subunit induces a variety of developmental defects, including syndactyly and aberrant lung septation, whereas mice that lack the β2 laminin subunit suffer kidney failure owing to defective glomerular filtration (Miner and Yurchenco, 2004). Examples of laminin-mediated signaling come from some of our studies, in which we have demonstrated that laminin-332 and laminin-311 activate extracellular signal-regulated kinases (ERKs) through modulation of the function of either integrin or dystroglycan receptors in epithelial cells (Gonzales et al., 1999; Jones et al., 2005).
Analyses of 2D and 3D cell cultures, mouse models and human disease emphasize the functional duality of laminins as key regulators of tissue structure and cell behavior (Aumailley et al., 2005; Tunggal et al., 2000; Tzu and Marinkovich, 2008; Yurchenco et al., 2004). By contrast, we know much less about the mechanisms by which laminins are deposited and organized into the extracellular matrix. Yet, as we will detail, laminins are deposited in diverse, exquisite arrays in the matrices of disparate cultured cells, probably reflecting differences in laminin-matrix functions. In this Commentary, our focus is the complexity of laminin-rich matrices, and we use this opportunity to speculate on the mechanisms that determine how laminin molecules are deposited by cells, particularly the role of cell-surface receptors and laminin-binding proteins in regulating such deposition. The molecular structure of laminins, which has been described in a number of recent excellent reviews, will be discussed only briefly (Aumailley et al., 2005; Tunggal et al., 2000; Tzu and Marinkovich, 2008; Yurchenco et al., 2004).
Laminin structure and domain architecture
There is a large a globular domain (termed LN) at the N-terminus of the α1, α2, α3B, α5, β1, β2, γ1 and γ3 laminin subunits, whereas this domain is absent from the N-terminus of the α3A, α4 and γ2 laminin subunits (Fig. 1A). For this reason, the former subunits have been termed `full length' whereas some authors have called the latter `truncated' (Kallunki et al., 1992); here, we will term them `headless' to avoid confusion with proteolytically processed forms.
All laminin subunits contain tandem repeats of laminin-type epidermal growth factor-like (LE) domains (Fig. 1A). Globular domains are interspersed among the LE domains: two (L4a and L4b) in the full-length α-subunits, one domain (LF) in the β1 and β2 subunits, and one (L4) in the γ-subunits (Fig. 1A). In each subunit, the final LE repeats are followed by an α-helical domain. The α-helical domains of the three subunits wind around each other to produce a trimeric, coiled-coil structure (Beck et al., 1990). In all three β-subunits, this domain is interrupted by a short stretch of amino acids termed the β-knob (Beck et al., 1990). The coiled-coil domain is probably important for orienting the large globular domain at the C-terminus of the α-subunit, facilitating its accessibility for cell binding via integrins and other cell-surface receptors, including the syndecans and dystroglycan, as well as binding to other matrix proteins (Timpl et al., 2000) (Fig. 1B). For example, Kunneken and colleagues have demonstrated that the coiled-coil domain and heterotrimerization are required for the interaction of α3β1 integrin with laminin-332 (Kunneken et al., 2004).
The coiled-coil of the α laminin subunits is followed by a globular C-terminus that can be divided into five LG domains (LG1-LG5) with LG1-3 and LG4-5 forming functionally and structurally distinct subdomains (Fig. 1). A hypothetical model constructed by Tisi and colleagues predicts that the LG1-3 domain has a cloverleaf shape, with the coiled-coil rod domain of the trimer contacting this region (Tisi et al., 2000). The relatively long linker between the LG3 and LG4 domains acts to stabilize LG4-LG5, forces the LG5 domain closer to the LG1-LG3 cloverleaf, and enables the LG4-LG5 domain to adopt a range of conformations (Timpl et al., 2000; Tisi et al., 2000). Intriguingly, the LG4-LG5 domain of both the α3 and α4 subunits can be removed by proteolytic cleavage following secretion of the laminin molecule (Amano et al., 2000; Goldfinger et al., 1998; Matsui et al., 1995; Talts et al., 2000). Loss of the LG4-LG5 domain impacts the function of the laminin heterotrimer, at least in the case of laminin-332, in which processing has been reported to modify the molecule from one that promotes migration to one that supports stable adhesion (Goldfinger et al., 1998; Tran et al., 2008). Moreover, it is possible that the cleaved LG4-LG5 fragment, under circumstances in which it is retained in tissue, stimulates migration independently of the mature laminin-332 molecule (Tran et al., 2008).
Deposition of laminin heterotrimers: the importance of patterning
Despite their relatively uniform structure, laminin heterotrimers are deposited into diverse arrays or patterns in the extracellular matrix of cultured cells. We speculate that these laminin-deposition patterns reflect differences in laminin-matrix functions and/or that they represent different stages in matrix formation. There are correlative data from a number of cell types in culture to support both speculations. For example, laminin-211 and laminin-221 are deposited as a mesh on the surface of Schwann cells and organize cell-surface receptors and cytoskeletal components (Tsiper and Yurchenco, 2002). By contrast, laminin-311 in the matrix of lung alveolar epithelial cells is organized into fibrils (DeBiase et al., 2006; Jones et al., 2005; Tsiper and Yurchenco, 2002) (Fig. 2B). A fibrillar laminin matrix is ideally suited to transmit mechanosignals in the form of stretch, and there is evidence that this occurs in lung cells (Jones et al., 2005). In the extracellular matrix of endothelial cells, the α4 laminin subunit localizes to αvβ3-integrin-rich focal adhesions (Fig. 2C,D) (Gonzales et al., 2001). In this case, laminin heterotrimers appear to be involved in the assembly of focal-contact-like structures, thereby mediating cell-matrix adhesion and potentially signaling from the matrix to the cytoplasm and vice-versa (Gonzales et al., 2001). In embryonic stem (ES) cells, laminin-111 appears as dot-like arrays, strings and plaques that might correspond to different stages in the process of laminin-matrix formation (Henry et al., 2001). Laminin-511 is deposited in punctate or linear arrays in the matrix of choriocarcinoma cells (Church and Aplin, 1998).
Fig. 2.

Laminin subunits are deposited in a variety of complex patterns in the matrix of cultured cells in vitro. (A) In the matrix of non-migrating keratinocytes, the α3 laminin subunit appears in a rosette-like pattern. (B) In alveolar epithelial cells of the lung, the α3 laminin subunit appears in fibrillar arrays. (C) In immortalized endothelial cells, the α4 laminin subunit localizes to focal-adhesion-like structures. (D) Cells shown in C are stained by an antibody against αv integrin. C and D have been adapted from Gonzales et al. (Gonzales et al., 2001), with permission. In A-D, cells were prepared for immunofluorescence and then viewed by confocal microscopy with the focal plane of the image being as close as possible to the substratum-attached surface of the cells. Scale bars: 20 μm.
Keratinocytes provide an interesting example of how laminin-deposition patterns might affect matrix function. In non-migratory human keratinocytes, we have demonstrated that laminin-332 appears in a rosette-like pattern, whereas actively migrating keratinocytes in culture assemble trails of laminin-332 over which they move in a back-and-forth pattern, as if following a railroad track (Fig. 2A and Fig. 3) (Sehgal et al., 2006). The distinct ways in which laminin-332 is deposited into the matrix of keratinocytes might simply be a secondary consequence of whether a cell is stationary or migratory. Alternatively, differences in laminin-matrix deposition by keratinocytes might be an important regulator of cell migratory behavior. There is some support for the latter possibility. For example, we showed that β4-integrin-deficient skin cells migrate in an aberrant circular pattern in vitro (Sehgal et al., 2006). However, the same cells display the motility behavior of their normal counterparts (that is, they exhibit processive migration) when plated onto laminin-332-rich matrix deposited by wild-type cells (Sehgal et al., 2006).
Fig. 3.
Imaging of laminin matrix in live motile and non-migrating keratinocytes. (A) Keratinocytes expressing YFP-tagged laminin-332 (green) were plated onto a glass coverslip and visualized by confocal microscopy 8 hours later. Phase-fluorescence overlays at the indicated time points are shown. This set of images was taken from Sehgal et al. (Sehgal et al., 2006), with permission. Scale bar: 50 μm. (B-D) A group of live, stationary keratinocytes expressing mCherry-tagged laminin-332 was viewed by confocal microscopy. The fluorescence and phase contrast images in B and C are overlaid in D. Scale bar: 20 μm.
Although the pattern of laminin-matrix deposition in diverse cultured cells is quite varied, it remains to be determined whether laminins in intact basement membranes in different tissues exhibit distinct patterns of deposition. Morphological studies of basement membranes and studies of laminin-subunit expression in tissues generally involve conventional electron microscopy and light microscopical immunolocalization of laminin antibodies in sectioned material, respectively. These techniques provide a `yes-or-no' answer as to the overall gross integrity of the basement membrane, including whether it has undergone duplication or focal loss, and provide a readout of its laminin-subunit composition. However, they do not permit high-resolution evaluation of the specific patterns in which laminin heterotrimers are incorporated into the matrix. This might explain why there are no reports of defects in laminin-332 deposition in the basement membrane of the skin of individuals whose keratinocytes lack β4 integrin, despite evidence that laminin-332 is deposited aberrantly in the matrix of keratinocytes derived from such patients in vitro (Sehgal et al., 2006; Vidal et al., 1995). Of course, as we have already mentioned, aberrant deposition might be a secondary consequence of altered migratory properties of the cells and might not be manifest in tissues in vivo. Immunoelectron microscopical analyses of laminin localization in tissue and/or the development of new high-resolution methods are required to resolve these apparent conflicts.
Self- and co-polymerization of laminins
The precise mechanisms by which cells deposit their laminin matrices are not yet fully understood. However, certain laminins self- and co-polymerize in the matrix. Self-polymerization involves the self-assembly of one specific laminin heterotrimer, by means of the short arms of its three chains, to produce a scaffold composed of that laminin isoform. By contrast, co-polymerization requires the interaction of the short arms of different laminin isoforms, generating a network that is composed of more than one type of laminin. Self- and co-polymerization of laminin heterotrimers is central to the assembly of most, if not all, complete basement membranes, and is mediated by the interaction of LN domains on the short arms of each trimer. However, such self- and/or co-polymerization is restricted to laminins containing `full-length' subunits (i.e. α1, α2, α3B or α5 and β1 or β2 and γ1 or γ3) and occurs while the laminin molecule is bound to receptors on the cell surface, such as integrins and dystroglycan (Cohen et al., 1997; Colognato and Yurchenco, 1999). Assembly can be divided into a temperature-dependent oligomerization step and a calcium-dependent polymerization step, in which calcium ions are required to induce a conformational change in the LN domains that allows them to interact (Schittny and Yurchenco, 1990).
In polymerization assays in vitro using full-length laminin isoforms or fragments, Cheng and coworkers demonstrated that laminin-111 is able to drive its polymerization with any other laminin, provided the latter is composed of full-length subunits (Cheng et al., 1997). Indeed, they have proposed the `three-arm-assembly hypothesis' in which only those heterotrimers composed of three full-length subunits are capable of self- and co-polymerization (Cheng et al., 1997). Thus, according to this hypothesis, the headless α3A- and α4-subunit-containing laminins would be incapable of self-polymerization or co-polymerization with other laminins. For example, in congenital muscular dystrophy caused by loss of α2 laminin expression, overexpression of the α4 laminin subunit is unable to compensate for this loss, which might in part be due to the inability of α4-containing heterotrimers to self-polymerize (Patton et al., 1997). Recent studies by McKee and coworkers have shown that the addition of a polypeptide containing the short arm of α1 laminin allows a polymerization-incompetent α1 laminin subunit that lacks the LN domain to polymerize with its β1 and γ1 partners (McKee et al., 2009; McKee et al., 2007). By contrast, Odenthal and coworkers used recombinant laminin fragments composed of the LN domain and one or more LE modules to assay the ability of these fragments to homo- or heterodimerize (Odenthal et al., 2004). Their results indicate that the β3 LN domain is able to bind the γ1 and γ3 LN domains, suggesting that even those laminin heteotrimers that contain a minimum of one LN domain might possess the ability to self- or co-polymerize (Odenthal et al., 2004). If this is the case, then this raises the possibility that complexes of laminin-332 with laminin-311 or of laminin-411 with laminin-511 exist. There is some support for this, as a complex of laminins 332, 311 and 321 has been isolated from the basement membrane of human amnion (Champliaud et al., 1996). However, whether the laminins in this complex exist as a co-polymer or whether they simply piggyback on other non-laminin molecules in the basement membrane remains to be determined.
Regulation of laminin deposition in the matrix by cell-surface receptors
Following their secretion as intact heterotrimers, laminins are deposited into the extracellular matrix. As described above, some laminin molecules are subsequently proteolytically processed (see Goldfinger et al., 1998; Marinkovich et al., 1992; Matsui et al., 1995; Talts et al., 2000) and, in processed laminins such as laminin-332, this processing event might modulate binding of the laminin to cell-surface receptors (Goldfinger et al., 1998). Regardless of processing, however, emerging data indicate that deposition and/or formation of laminin matrices is regulated by cell-surface receptors, including integrins, dystroglycan and syndecans, and that other matrix components stabilize laminin incorporation into the extracellular matrix.
Regulation of laminin deposition by integrins
We and other authors have speculated that integrins regulate the deposition of laminins by signaling to Rho GTPases, which are known to be key regulators of cell adhesion, spreading and migration, primarily through their effects on the actin cytoskeleton (Ridley, 2001a; Ridley, 2001b; deHart and Jones, 2004; Hamelers et al., 2005; O'Brien et al., 2001; Sehgal et al., 2006). Data from our own laboratory indicate that α3β1 integrin modulates deposition of laminin-332 matrix in vitro (deHart et al., 2003; deHart and Jones, 2004; Sehgal et al., 2006). In particular, we have presented evidence that laminin-332 is deposited in spikes and arrowheads by α3-integrin-null keratinocytes (most probably reflective of their migratory behavior), whereas their normal counterparts deposit laminin-332 in diffuse `arcs' (deHart et al., 2003). Moreover, some workers have reported that deposition of laminin-332 requires signaling from α3β1 integrin to the guanine-nucleotide exchange factor Tiam1, an activator of Rho-like GTPases (Hamelers et al., 2005). However, this remains controversial because Margadant and coworkers have recently reported that deposition of laminin-332 is normal in the healing wounds of the skin of mice in which there is an epidermis-targeted deletion of α3 integrin (Margadant et al., 2009). The ability of such cells to deposit an apparently normal laminin matrix might be the result of an upregulation of α6β1 integrin expression (Margadant et al., 2009).
There is evidence that a second integrin heterodimer, α6β4, also has a role in laminin-332 deposition and/or matrix formation in keratinocytes (Sehgal et al., 2006). As mentioned above, migrating keratinocytes assemble trails of laminin-332 (Fig. 3A) (Frank and Carter, 2004; Sehgal et al., 2006). By contrast, we have demonstrated that β4-integrin-deficient keratinocytes deposit circular arrays of laminin-332 and move in a circular fashion over this matrix (Sehgal et al., 2006). The loss of β4 integrin in these cells correlates with low Rac1 activity (Russell et al., 2003). This is consistent with published data indicating that α6β4 integrin not only co-precipitates with Rac1 from extracts of keratinocytes, but might also regulate Rac1 activation (Mainiero et al., 1997; Sehgal et al., 2006; Zahir et al., 2003). One consequence of the decrease in Rac1 activity is a loss of front-to-back cell polarity (Raftopoulou and Hall, 2004). Hence, the difference in the pattern of laminin-332 deposition between wild-type and β4-integrin-deficient cells might be the consequence of the non-polarized cell phenotype exhibited by β4-integrin-deficient cells. However, as detailed above, work in our laboratory has demonstrated that β4-integrin-deficient cells migrate in a similar manner to wild-type cells if plated onto the laminin-332-rich matrix of normal keratinocytes (Sehgal et al., 2006). This raises the intriguing possibility that β4 integrin, through regulating Rac1 activity, might specify the precise way in which laminin-332 is assembled and/or organized following its deposition by keratinocytes (Sehgal et al., 2006).
Clearly this is a classic `chicken-and-egg' conundrum – does β4 integrin determine cell polarity and thereby indirectly regulate matrix deposition, or does β4 integrin directly regulate matrix deposition, which in turn specifies polarity? There is not yet a definitive answer to this question. Moreover, if β4 integrin regulates laminin deposition through its interaction with and activation of Rac1, how does it do so? It has already been established that decreased Rac1 activity has effects on the actin cytoskeleton (Raftopoulou and Hall, 2004; Ridley, 2001a; Ridley, 2001b). In keratinocytes, loss of Rac1 activity leads to reduced activation of the Slingshot phosphatases and their substrate, the actin-severing protein cofilin (Kligys et al., 2007). These observations suggest to us the possibility that the dynamic activity of integrins in keratinocytes, in response to actin reorganization regulated by Rac1 and cofilin signaling, modulates the conformation and/or organization of freshly secreted laminin-332 heterotrimers such that deposits of laminin-332 are `converted' into trails (Sehgal et al., 2006).
This is clearly a highly speculative model. However, this mechanism has a precedent. In the final stage of formation of fibronectin fibrils, integrins are moved by actin stress fibers and induce conformational changes in their fibronectin ligand (Leiss et al., 2008; Wierzbicka-Patynowski and Schwarzbauer, 2003). Receptor-mediated `stretching' of the fibronectin molecule exposes sites that are involved in fibronectin-fibronectin self-association, leading to the formation of fibrils (Leiss et al., 2008). Of course, whether integrins actually mediate conformational changes in laminin molecules as we are hypothesizing requires experimental verification.
Dystroglycan and laminin matrix formation
The role of the transmembrane protein dystroglycan in laminin-matrix formation is somewhat controversial. One group has presented evidence that laminin-111 subunits are localized normally in the subendodermal basement membranes of dystroglycan-null mouse embryoid bodies (Li et al., 2002). By contrast, others have reported that binding of the extracellular α-dystroglycan subunit to laminin is a crucial step in the formation of laminin-111-rich matrices of ES cells, in deposition of laminin-211 on the surface of myotubes and Schwann cells, and in polymerization of laminin-111 and matrix formation in breast epithelial cells (Cohen et al., 1997; Colognato and Yurchenco, 1999; Henry and Campbell, 1998; Henry et al., 2001; Montanaro et al., 1999; Tsiper and Yurchenco, 2002; Weir et al., 2006). In mouse, there is evidence for the importance of dystroglycan in laminin-matrix deposition, because embryos null for dystroglycan die in the early stages of development, possibly as a result of abnormal formation of a primitive basement membrane termed Reichert's membrane (Williamson et al., 1997).
Molecular genetic analyses in breast epithelial cells have demonstrated that the extracellular domain of dystroglycan can mediate laminin deposition, even when most of the cytoplasmic region has been deleted, implying that the role of dystroglycan is primarily structural in this cell type (Weir et al., 2006). Dystroglycan might also function as a regulator of laminin deposition in myoblasts because decreased expression of α-dystroglycan correlates with decreased laminin-211 deposition on the surface of myotubes (Montanaro et al., 1999). In addition, there is evidence that dystroglycan cooperates with integrin receptors in mediating laminin deposition (Colognato and Yurchenco, 1999; Henry and Campbell, 1998; Henry et al., 2001; Lohikangas et al., 2001). For example, Weir and coworkers have suggested that, initially, dystroglycan interacts with the LG domains of monomeric laminin-111 and that the resulting receptor-ligand complex recruits β1-subunit-containing integrins, which then induce laminin polymerization (Weir et al., 2006).
Other cell-surface receptors
In addition to integrins and dystroglycan, members of the syndecan family of cell-surface receptors also modulate laminin-matrix deposition. Chinese hamster ovary (CHO) cells that lack syndecan-2 expression lose the ability to rearrange laminin into fibers (Klass et al., 2000). Moreover, syndecan-1 appears to contribute to the regulation of laminin-332 deposition, because laminin-332 appears in arrowhead-like arrays in the matrix of syndecan-1-null keratinocytes, whereas laminin-332 is deposited as cloud-like trails in the matrix of wild-type cells (Stepp et al., 2007).
In summary, it is clear that cell-surface receptors play an important role in regulating laminin-matrix deposition. However, non-receptor molecules also modulate laminin-matrix assembly and functions, as we will discuss next.
Laminin-interacting proteins stabilize the basement membrane and might modulate laminin deposition or matrix assembly
Following their secretion and deposition, laminins interact with several matrix proteins, and this might affect laminin deposition. Here, we focus our discussion on those molecules that have been shown to influence laminin-matrix deposition and/or stabilization. These include nidogen (also known as entactin), the heparan sulfate proteoglycans perlecan and agrin, the netrins and collagen VII.
Nidogens
Members of the nidogen family of sulfated glycoproteins, which consists of nidogen 1 and nidogen 2, are capable of interacting with a variety of extracellular-matrix proteins including laminin, perlecan, collagen IV and fibulin (Kohfeldt et al., 1998; Mann et al., 1989). For this reason, one of their primary functions is to stabilize the extracellular matrix. Nidogen 1 has been shown to interact with an LE module in the laminin γ1 subunit (Fig. 1B), as well as with type IV collagen. Blocking laminin-nidogen interaction leads to disruption of early lung, kidney and salivary gland development in organ culture (Ekbloom et al., 1994; Kadoya et al., 1997; Mayer et al., 1993; Poschl et al., 1994; Takagi et al., 2003) and defects in matrix assembly in F9-teratocarcinoma-derived embryoid bodies (Tunggal et al., 2003). By contrast, the presence of a recombinant laminin γ1 fragment containing LE and L4 domains in 3D co-cultures of keratinocytes and fibroblasts results in the disruption of basement-membrane formation, alterations in expression patterns of matrix components, and loss of recognizable hemidesmosomes (Breitkreutz et al., 2004).
Mice that lack expression of either nidogen 1 or nidogen 2 have normal life spans and are relatively healthy and fertile (Murshed et al., 2000; Schymeinsky et al., 2002). Functional basement membranes are also deposited during early embryogenesis in nidogen 1 and 2 double-knockout mice, suggesting that other matrix components compensate for the lack of both nidogens, or that there is direct binding of laminin to type IV collagen that can bypass nidogens (Bader et al., 2005). However, these nidogen double-knockout mice, which either die before birth or within 24 hours of birth, display abnormalities in the basement membranes of several tissues (Bader et al., 2005). For example, capillary basement membranes are disorganized, with a loss of expression of laminin-411 (Mokkapati et al., 2008). These defects probably reflect both a loss of extracellular-matrix structure or stability and alterations in matrix-mediated signaling (Bose et al., 2006).
Heparan sulfate proteoglycans
One of the major heparan sulfate proteoglycans of the extracellular matrixis is perlecan. Perlecan contains five distinct domains, including one region that is comprised of Ig-like repeats that are sites of interaction with several matrix molecules including the nidogens, fibronectin, heparin, dystroglycan and collagen IV (Costell et al., 1999; Kallunki and Tryggvason, 1992; Noonan et al., 1991). Laminin-111, through its interactions with nidogen 1, can also form ternary complexes with perlecan (Hopf et al., 1999). Despite these many interactions, perlecan has been shown not to be essential for matrix assembly in either perlecan-null mouse embryos or in a perlecan-deficient keratinocyte skin-equivalent model. However, perlecan is crucial for maintaining the integrity of the basement membrane, especially in tissues such as the heart that are subject to mechanical stress (Costell et al., 1999; Sher et al., 2006). Moreover, on the basis of the temporal appearance of perlecan during deposition of laminin-311 matrix, we have proposed that perlecan might nucleate the formation of fibrillar forms of laminin (Jones et al., 2005).
Agrin, a second heparan sulfate proteoglycan, binds to laminins 111, 211 and 221 and is believed to play an important role in the formation of the neuromuscular junction (Denzer et al., 1995; Denzer et al., 1998; Moll et al., 2001; Ruegg and Bixby, 1998). Agrin is expressed as one of two isoforms. The long-N-terminal isoform contains a domain that interacts with the coiled-coil central region of laminins, and is distributed across most tissues (Mascarenhas et al., 1993; Stetefeld et al., 2001) (Fig. 1B). By contrast, the short-N-terminal isoform is found primarily in the nervous system (Burgess et al., 2000; Denzer et al., 1995). The C-terminal laminin globular domain region of agrin contains sites of interaction with dystroglycan and is required for heparan binding (Bezakova and Ruegg, 2003; Gesemann et al., 1998). On the basis of its intermolecular interactions, one might assume that agrin has a role in laminin-matrix formation and/or basement-membrane assembly. However, further evaluation will be required to determine whether this is the case.
Netrins
The netrins share homology with the laminins, as they also contain LN and LE domains (Yurchenco and Wadsworth, 2004). They were originally identified as molecules involved in neuronal guidance (Yurchenco and Wadsworth, 2004). Recent data suggest that the netrins might also regulate the functions of the laminins with which they interact (Schneiders et al., 2007; Yurchenco and Wadsworth, 2004). One netrin family member (netrin-4) interacts with the short arms of both the laminin γ1 and γ3 subunits through its LN domain. (Schneiders et al., 2007) (Fig. 1B). This is thought to promote laminin-111 polymerization, such that expression of a truncated form of netrin-4 inhibits laminin-111 self-assembly in vitro (Schneiders et al., 2007). Therefore, netrin-4 probably plays a role in the formation of basement membrane. In addition, a complex of netrin-4, laminin γ1 subunit and α6β1 integrin has also been identified in mouse neural stem cells (Staquicini, 2009). This complex has been shown to promote stem-cell proliferation and migration, both processes that are influenced by the laminin matrix.
Laminin N-terminus proteins
Recently, we have identified a novel family of alternative splice isoforms derived from the 5′ end of the LAMA3 and LAMA5 genes (Hamill et al., 2009). These transcripts encode short proteins consisting of a LN domain followed by a short stretch of LE repeats. They are distinct from other laminin proteins in that they lack the LCC domain and cannot assemble as a heterotrimer. For this reason, we termed these laminin N-terminus (LaNt) proteins. Functional studies of one of these novel proteins (LaNt α31) suggest that it plays a role in keratinocyte adhesion, spreading and migration, although the precise mechanisms by which it acts have yet to be defined (Hamill et al., 2009). However, because of their domain composition and structural similarity to the netrins, we speculate that the LaNts are involved in regulating laminin-network formation through competition or enhancement of LN-LN domain interactions.
Collagen VII
Collagen VII, a component of the anchoring fibrils of the skin, interacts with the β3 subunit of laminin-332 (Chen et al., 1999; Rousselle et al., 1997). Keratinocytes that lack collagen VII do deposit laminin-332 into the basement membrane of skin grafts, indicating that collagen VII is not necessary for deposition of laminin-332 matrix (Chen et al., 2002). However, evidence from corneal tissue reconstitution studies indicates that collagen-VII-containing anchoring fibrils might nucleate assembly of hemidesmosomes at specific discrete sites along the basement membrane (Gipson et al., 1983; Jones et al., 1998). If collagen VII does nucleate hemidesmosomes, this suggests the intriguing possibility that collagen VII functions to target deposition of, or locally organize, laminin-332. This is highly speculative. However, there is some indirect support for this possibility. Immunoelectron microscopy reveals that there is a discontinuous distribution of laminin-332 along the basement membrane of stratified squamous epithelia, with laminin-332 being localized immediately beneath the plaques of hemidesmosomes (Jones et al., 1994).
In summary, laminin-associated matrix proteins in concert with cell-surface receptors are key to proper assembly and/or deposition of laminin matrices. They function to fine-tune both matrix formation and its function.
Conclusions and future directions
Our Commentary has focused primarily on the molecules involved in the deposition and organization of laminin proteins in the extracellular matrix surrounding cells. Although at least some laminins self-polymerize, the assembly of complex laminin-rich matrices requires the coordinate activity of cell-surface receptors and of laminin-associated and laminin-related proteins. Future studies will presumably dissect the precise molecular mechanisms underlying the manner in which laminins are patterned and assess whether such patterning has important functional consequences. To conclude, we will discuss some directions in which future studies might go.
As mentioned above, laminin-matrix formation might involve changes in the conformation of laminin heterotrimers. The development of conformation-dependent monoclonal antibodies against laminins might allow testing of such a possibility, as conformation-dependent antibodies have proved invaluable in dissecting structural and functional modifications in a variety of proteins, including the tau protein and αIIbβ3 integrin (Dabadie et al., 2001; Wolozin et al., 1986).
Several studies have demonstrated that processing of some laminin subunits not only influences the behavior of cells, but might also play a role in matrix deposition and/or stabilization. For example, the α3, α4, β3 and γ2 laminin subunits have all been shown to undergo proteolytic cleavage, in vitro and in vivo (Giannelli et al., 1997; Talts et al., 2000; Tsubota et al., 2000; Udayakumar et al., 2003). With regard to the α3 and γ2 laminin subunits, there is experimental evidence that only their unprocessed versions are properly incorporated into laminin-332 matrix (Gagnoux-Palacios et al., 2001; Sigle et al., 2004). One assumes that the domains that are released following cleavage `encode' important matrix-assembly information. Uncovering the particular way in which these domains function might provide valuable new insight into the assembly of higher-order laminin structures.
A relatively newly discovered role for laminin and laminin-rich matrices is their capacity to act as inducers of either the growth or differentiation of both adult and ES cells. For example, laminin and laminin-rich Matrigel support human embryonic-cell differentiation into neural progenitors and neurons (Ma et al., 2008). In addition, the growth and chondrogenic differentiation of mesenchymal stem cells is enhanced by laminin-332 (Hashimoto et al., 2006). Defining precisely how laminin matrices are able to induce signaling that regulates proliferation, differentiation, adhesion and migration of both adult and embryonic cells will continue to be an exciting avenue of investigation.
We have recently described a new approach for analyzing formation of the laminin matrix. This involves visualizing the deposition of fluorescently tagged laminin subunits onto the substrate of live cells (Fig. 3A,B) (Hopkinson et al., 2008; Sehgal et al., 2006). Using these tagged molecules, one can observe the deposition of nascent laminin subunits together with their receptor molecules in real time. This should allow characterization of the precise steps that lead to laminin-matrix deposition and formation by cells in vitro and might also indicate how particular receptors function in this complex process. Indeed, this approach to the study of laminin-matrix formation is already providing new insights. For example, we have used live-cell imaging of tagged laminin subunits to demonstrate that truncated versions of the α3 laminin subunit are incorporated into the matrix of epithelial cells, at least in cells that also express wild-type laminin-332 subunits (Hopkinson et al., 2008). In addition, we have shown that matrix deposition is regulated by a feedback loop when cells are plated on, or move onto, an already assembled lawn of matrix proteins (Hopkinson et al., 2008). Imaging laminin-matrix formation in live cells is a perfect complement to the many biochemical and molecular approaches to the study of matrix formation and protein interactions that have been applied over the last 20 years.
Work in the Jones laboratory is supported by grants from the NIH (RO1AR054184 and RO1HL092963). Deposited in PMC for release after 12 months.
References
- Amano, S., Scott, I. A., Takahara, K., Koch, M., Gerecke, D. R., Keene, D. R., Hudson, D. L., Nishiyama, T., Lee, S., Greenspan, D. S. et al. (2000). Bone morphogenetic protein-1 (BMP-1) is an extracellular processing enzyme of the laminin 5 γ2 chain. J. Biol. Chem. 275, 22728-22735. [DOI] [PubMed] [Google Scholar]
- Aumailley, M., Bruckner-Tuderman, L., Carter, W. G., Deutzmann, R., Edgar, D., Ekblom, P., Engel, J., Engvall, E., Hohenester, E., Jones, J. C. R. et al. (2005). A simplified laminin nomenclature. Matrix Biol. 24, 326-332. [DOI] [PubMed] [Google Scholar]
- Bader, B. L., Smyth, N., Nedbal, S., Miosge, N., Baranowsky, A., Mokkapati, S., Murshed, M. and Nischt, R. (2005). Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell. Biol. 25, 6846-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, K., Hunter, I. and Engel, J. (1990). Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 4, 148-160. [DOI] [PubMed] [Google Scholar]
- Bezakova, G. and Ruegg, M. A. (2003). New insights into the roles of agrin. Nat. Rev. Mol. Cell. Biol. 4, 295-308. [DOI] [PubMed] [Google Scholar]
- Bose, K., Nischt, R., Page, A., Bader, B. L., Paulsson, M. and Smyth, N. (2006). Loss of nidogen-1 and -2 results in syndactyly changes in limb development. J. Biol. Chem. 281, 39620-39629. [DOI] [PubMed] [Google Scholar]
- Breitkreutz, D., Mirancea, N., Schmidt, C., Beck, R. and Werner, U. (2004). Inhibition of basement membrane formation by a nidogen-binding laminin γ1-chain fragment in human skin-organotypic cocultures. J. Cell Sci. 117, 2611-2622. [DOI] [PubMed] [Google Scholar]
- Burgess, R. W., Skarnes, W. C. and Sanes, J. R. (2000). Agrin isoforms with distinct amino termini: differential expression, localization, and function. J. Cell Biol. 151, 41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champliaud, M. F., Lunstrum, G. P., Rousselle, P., Nishiyama, T., Keene, D. R. and Burgeson, R. E. (1996). Human amnion contains a novel laminin variant, laminin-7, which like laminin-6, covalently associates with laminin-5 to promote stable epithelial-stromal attachment. J. Cell Biol. 132, 1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M., Marinkovich, P. M., Jones, J. C. R., O'Toole, E. A., Li, Y.-Y. and Woodley, D. T. (1999). NC1 domain of type VII collagen binds to the β3 chain of laminin 5 via a unique subdomain within the fibrogenic-like repeats. J. Invest. Dermatol. 112, 177-183. [DOI] [PubMed] [Google Scholar]
- Chen, M., Kasahara, N., Keene, D. R., Chan, L., Hoeffler, W. K., Finlay, D., Barcova, M., Cannon, P. M., Mazurek, C. and Woodley, D. T. (2002). Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa. Nat. Genet. 32, 670-675. [DOI] [PubMed] [Google Scholar]
- Cheng, Y. S., Champliaud, M. F., Burgeson, R. E., Marinkovich, M. P. and Yurchenco, P. D. (1997). Self-assembly of laminin isoforms. J. Biol. Chem. 272, 31525-31532. [DOI] [PubMed] [Google Scholar]
- Church, H. J. and Aplin, J. D. (1998). BeWo choriocarcinoma cells produce laminin 10. Biochem. J. 332, 491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. W., Jacobson, C., Yurchenco, P. D., Morris, G. E. and Carbonetto, S. (1997). Laminin-induced clustering of dystroglycan on embryonic muscle cells: comparison with agrin-induced clustering. J. Cell Biol. 136, 1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H. and Yurchenco, P. D. (1999). The laminin alpha2 expressed by dystrophic dy(2J) mice is defective in its ability to form polymers. Curr. Biol. 18, 1327-1330. [DOI] [PubMed] [Google Scholar]
- Costell, M., Gustafsson, E., Aszódi, A., Mörgelin, M., Bloch, W., Hunziker, E., Addicks, K., Timpl, R. and Fässler, R. (1999). Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 147, 1109-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabadie, M., Valli, N., Jacobin, M. J., Laroche-Traineau, J., Barat, J. L., Ducassou, D., Nurden, A. T. and Clofent-Sanchez, G. (2001). Characterisation, cloning and sequencing of a conformation-dependent monoclonal antibody to the αIIbβ3 integrin: interest for use in thrombus detection. Platelets 12, 395-405. [DOI] [PubMed] [Google Scholar]
- DeBiase, P. J., Lane, K., Budinger, S., Ridge, K., Wilson, M. and Jones, J. C. R. (2006). Laminin-311 (laminin-6) fiber assembly by type I-like alveolar cells. J. Histo. Cytochem. 54, 665-672. [DOI] [PubMed] [Google Scholar]
- deHart, G. W. and Jones, J. C. R. (2004). Myosin-mediated cytoskeleton contraction and Rho GTPases regulate laminin-5 matrix assembly. Cell Motil. Cyto. 57, 107-117. [DOI] [PubMed] [Google Scholar]
- deHart, G. W., Healy, K. E. and Jones, J. C. R. (2003). The role of α3β1 integrin in determining the supramolecular organization of laminin-5 in the extracellular matrix of keratinocytes. Exp. Cell Res. 283, 67-79. [DOI] [PubMed] [Google Scholar]
- Denzer, A. J., Gesemann, M., Schumacher, B. and Ruegg, M. A. (1995). An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J. Cell Biol. 131, 1547-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer, A. J., Schulthess, T., Fauser, C., Schumacher, B., Kammerer, R. A., Engel, J. and Ruegg, M. A. (1998). Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO J. 17, 335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbloom, P., Ekbloom, M., Fecker, L., Klein, G., Zhang, H. Y., Kadoya, Y., Chu, M. L., Mayer, U. and Timpl, R. (1994). Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development 120, 2003-2014. [DOI] [PubMed] [Google Scholar]
- Frank, D. E. and Carter, W. G. (2004). Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J. Cell Sci. 117, 1351-1363. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios, L., Allegra, M., Spirito, F., Pommeret, O., Romero, C., Ortonne, J. P. and Meneguzzi, G. (2001). The short arm of the laminin gamma2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J. Cell Biol. 153, 835-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesemann, M., Brancaccio, A., Schumacher, B. and Ruegg, M. A. (1998). Agrin Is a high-affinity binding protein of dystroglycan in non-muscle tissue. J. Biol. Chem. 273, 600-605. [DOI] [PubMed] [Google Scholar]
- Giannelli, G., Falk-Marzillier, J., Schiraldi, O., Stetler-Stevenson, W. G. and Quaranta, V. (1997). Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277, 225-228. [DOI] [PubMed] [Google Scholar]
- Gipson, I. K., Grill, S. M., Spurr, S. J. and Brennan, S. J. (1983). Hemidesmosome formation in vitro. J. Cell Biol. , 97, 849-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger, L. E., Stack, M. S. and Jones, J. C. R. (1998). Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J. Cell Biol. 141, 255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, M., Haan, K., Baker, S. E., Fitchmun, M. I., Todorov, I., Weitzman, S. and Jones, J. C. R. (1999). A cell signal pathway involving laminin-5, α3β1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol. Biol. Cell 10, 259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, M., Weksler, B., Tsuruta, D., Goldman, R. D., Yoon, K. J., Hopkinson, S. B., Flitney, F. W. and Jones, J. C. R. (2001). Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell 12, 85-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelers, I. H. L., Olivo, C., Mertens, A. E. E., Pegtel, D. M., van der Kammen, R. A., Sonnenberg, A. and Collard, J. G. (2005). The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading, and cell migration. J. Cell Biol. 171, 871-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill, K. J., Langbein, L., Jones, J. C. R. and McLean, W. H. I. (2009). Identification of a novel family of laminin N-terminal alternate splice isoforms: structural and functional characterization. J. Biol. Chem. [Epub ahead of print] doi; 10.1074/jbc.M109.052811 [DOI] [PMC free article] [PubMed]
- Hashimoto, J., Kariya, Y. and Miyazaki, K. (2006). Regulation of proliferation and chondrogenic differentiation of human mesenchymal stem cells by laminin-5 (laminin-332). Stem Cells 24, 2346-2354. [DOI] [PubMed] [Google Scholar]
- Henry, M. D. and Campbell, K. P. (1998). A role for dystroglycan in basement membrane assembly. Cell 95, 859-870. [DOI] [PubMed] [Google Scholar]
- Henry, M. D., Satz, J. S., Brakebusch, C., Costell, M., Gustafsson, E., Fassler, R. and Campbell, K. P. (2001). Distinct roles for dystroglycan, β1 integrin and perlecan in cell surface laminin organization. J. Cell Sci. 114, 1137-1144. [DOI] [PubMed] [Google Scholar]
- Hopf, M., Goring, W., Kohfeldt, E., Yamada, Y. and Timpl, R. (1999). Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2, and heparin. Eur. J. Biochem. 259, 917-926. [DOI] [PubMed] [Google Scholar]
- Hopkinson, S. B., DeBiase, P. J., Kligys, K. H. K. and Jones, J. C. R. (2008). Fluorescently tagged laminin subunits facilitate analyses of the properties, assembly and processing of laminins in live and fixed lung epithelial cells and keratinocytes. Matrix Biol. 27, 640-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. C. R., Asmuth, J., Baker, S. E., Langhofer, M., Roth, S. I. and Hopkinson, S. B. (1994). Hemidesmosomes: Extracellular Matrix/Intermediate Filament Connectors. Exp. Cell Res. 213, 1-11. [DOI] [PubMed] [Google Scholar]
- Jones, J. C. R., Hopkinson, S. B. and Goldfinger, L. E. (1998). Structure and assembly of hemidesmosomes. BioEssays 20, 488-494. [DOI] [PubMed] [Google Scholar]
- Jones, J. C. R., Lane, K., Hopkinson, S. B., Lecuona, E., Geiger, R. C., Dean, D. A., Correa-Meyer, E., Gonzales, M., Campbell, K., Sznajder, J. I. et al. (2005). Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechano-signal transduction via a dystroglycan-dependent, integrin-independent, mechanism. J. Cell Sci. 118, 2557-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya, Y., Salmwirta, K., Talts, J. F., Kadoya, K., Mayer, U., Timpl, R. and Ekbloom, P. (1997). Importance of nidogen binding to laminin gamma 1 for branching morphogenesis in the submadibular gland. Development 124, 683-691. [DOI] [PubMed] [Google Scholar]
- Kallunki, P. and Tryggvason, K. (1992). Human basement membrane heparan sulfate proteoglycan core protein: a 467-kD protein containing multiple domains resembling elements of the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J. Cell Biol. 116, 559-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallunki, P., Sainio, K., Eddy, R., Byers, M., Kallunki, T., Sariola, H., Beck, K., Hirvonen, H., Shows, T. B. and Tryggvason, K. (1992). A truncated laminin chain homologous to the B2 chain: structure, spatial expression, and chromosomal assignment. J. Cell Biol. 119, 679-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass, C. M., Couchman, J. R. and Woods, A. (2000). Control of extracellular matrix assembly by syndecan-2 proteoglycan. J. Cell Sci. 113, 493-506. [DOI] [PubMed] [Google Scholar]
- Kligys, K., Claiborne, J. N., DeBiase, P., Hopkinson, S. B., Mizuno, K. and Jones, J. C. R. (2007). The Slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization and motility behavior of keratinocytes. J. Biol. Chem. 282, 32520-32528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohfeldt, E., Sasaki, T., Göhring, W. and Timpl, R. (1998). Nidogen-2: a new basement membrane protein with diverse binding properties. J. Mol. Biol. 282, 99-109. [DOI] [PubMed] [Google Scholar]
- Kunneken, K., Pohlentz, G., Schmidt-Hederich, A., Odenthal, U., Smyth, N., Peter-Katalinic, J., Bruckner, P. and Eble, J. A. (2004). Recombinant human laminin-5 domains - effects of heterotrimerization, proteolytic processing, and N-glycosylation on α3β1 integrin binding. J. Biol. Chem. 279, 5184-5193. [DOI] [PubMed] [Google Scholar]
- Leiss, M., Beckmann, K., Giros, A., Costell, M. and Fassler, R. (2008). The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr. Opin. Cell Biol. 20, 502-507. [DOI] [PubMed] [Google Scholar]
- Li, S., Harrison, D., Carbonetto, S., Fassler, R., Smyth, N. and Edgar, D. (2002). Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157, 1279-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohikangas, L., Gullberg, D. and Johansson, S. (2001). Assembly of laminin polymers is dependent on β1-integrins. Exp. Cell Res. 265, 135-144. [DOI] [PubMed] [Google Scholar]
- Ma, W., Tavakoli, T., Derby, E., Serebryakova, Y., Rao, M. and Mattson, M. (2008). Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev. Biol. 8, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainiero, F., Murgia, C., Wary, K. K., Curatola, A. M., Pepe, A., Blumenberg, M., Westwick, J. K., Der, C. J. and Giancotti, F. G. (1997). The coupling of α6β4 integrin to the Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 16, 2365-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, K., Deutzmann, R., Aumailley, M., Timpl, R., Raimondi, L., Yamada, Y., Pan, T.-C., Conway, D. and Chu, M.-L. (1989). Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J. 8, 65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant, C., Raymond, K., Kreft, M., Sachs, N., Janssen, H. and Sonnenberg, A. (2009). Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin. J. Cell Sci. 122, 278-288. [DOI] [PubMed] [Google Scholar]
- Marinkovich, M. P., Lunstrum, G. P. and Burgeson, R. E. (1992). The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J. Biol. Chem. 267, 17900-17906. [PubMed] [Google Scholar]
- Mascarenhas, J. B., Rüegg, M. A., Winzen, U., Halfter, W., Engel, J. and Stetefeld, J. (1993). Mapping of the laminin-binding site of the N-terminal agrin domain (NtA). EMBO J. 22, 529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, C., Wang, C. K., Nelson, C. F., Bauer, E. A. and Hoeffler, W. K. (1995). The assembly of laminin-5 subunits. J. Biol. Chem. 270, 23496-23503. [DOI] [PubMed] [Google Scholar]
- Mayer, U., Nischt, R., Poschl, E., Mann, K., Fukuda, K., Gerl, M., Yamada, Y. and Timpl, R. (1993). A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J. 12, 1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan, K. and Marinkovich, M. P. (2000). Laminins and human disease. Micro. Res. Tech. 51, 262-279. [DOI] [PubMed] [Google Scholar]
- McKee, K. K., Harrison, D., Capizzi, S. and Yurchenco, P. D. (2007). Role of laminin terminal globular domains in basement membrane assembly. J. Biol. Chem. 282, 21437-21447. [DOI] [PubMed] [Google Scholar]
- McKee, K. K., Capizzi, S. and Yurchenco, P. D. (2009). Scaffold-forming and adhesive contributions of synthetic laminin-binding proteins to basement membrane assembly. J. Biol. Chem. 284, 8984-8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J. H. and Yurchenco, P. D. (2004). Laminin function in tissue morphogenesis. Ann. Rev. Cell Dev. Biol. 20, 255-284. [DOI] [PubMed] [Google Scholar]
- Mokkapati, S., Baranowsky, A., Mirancea, N. and Smyth, N. (2008). Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J. Invest. Dermatol. 128, 2259-2267. [DOI] [PubMed] [Google Scholar]
- Moll, J., Barzaghi, P., Lin, S., Bezakova, G., Lochmüller, H., Engvall, E., Müller, U. and Ruegg, M. A. (2001). An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature 413, 302-307. [DOI] [PubMed] [Google Scholar]
- Montanaro, F., Lindenbaum, M. and Carbonetto, S. (1999). α-Dystroglycan is a laminin receptor involved in extracellular matrix assembly on myotubes and muscle cell viability. J. Cell Biol. 145, 1325-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed, M., Smyth, N., Miosge, N., Karolat, T., Krieg, M., Paulsson, M. and Nischt, R. (2000). The absence of nidogen 1 does not affect murine basement membrane formation. Mol. Cell. Biol. 20, 7007-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomizu, M., Utani, A., Beck, K., Otaka, A., Roller, P. P. and Yamada, Y. (1996). Mechanism of laminin chain assembly into a triple-stranded coiled-coil structure. Biochem. 35, 2885-2893. [DOI] [PubMed] [Google Scholar]
- Noonan, D. M., Fulle, A., Valente, P., Cai, S., Horigan, E., Sasaki, M., Yamada, Y. and Hassell, J. R. (1991). The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J. Biol. Chem. 266, 22939-22947. [PubMed] [Google Scholar]
- O'Brien, L. E., Jou, T. S., Pollack, A. L., Zhang, Q., Hansen, S. H., Yurchenco, P. and Mostov, K. E. (2001). Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3, 831-838. [DOI] [PubMed] [Google Scholar]
- Odenthal, U., Haehn, S., Tunggal., P, Merkl, B., Schomburg, D., Frie, C., Paulsson, M. and Smyth, N. (2004). Molecular analysis of laminin N-terminal domains mediating self-interactions. J. Biol. Chem. 279, 44504-44512. [DOI] [PubMed] [Google Scholar]
- Patton, B. L., Miner, J. H., Chiu, A. Y. and Sanes, J. (1997). Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 139, 1507-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl, E., Fox, J. W., Block, D., Mayer, U. and Timpl, R. (1994). Two non-contiguous regions contribute to nidogen binding to a single EGF-like motif on the laminin gamma 1 chain. EMBO J. 13, 3741-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftopoulou, M. and Hall, A. (2004). Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23-32. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J. (2001a). Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471-477. [DOI] [PubMed] [Google Scholar]
- Ridley, A. J. (2001b). Rho GTPases and cell migration. J. Cell Sci. 114, 2713-2722. [DOI] [PubMed] [Google Scholar]
- Rousselle, P., Keene, D. R., Ruggiero, F., Champliaud, M. F., Rest, M. and Burgeson, R. E. (1997). Laminin 5 binds the NC-1 domain of type VII collagen J. Cell Biol. 138, 719-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg, M. A. and Bixby, J. L. (1998). Agrin orchestrates synaptic differentiation at the vertebrate neuromuscular junction. Trends Neurosci. 21, 22-27. [DOI] [PubMed] [Google Scholar]
- Russell, A. J., Fincher, E. F., Millman, L., Smith, R., Vela, V., Waterman, E. A., Dey, C. N., Guide, S., Weaver, V. M. and Marinkovich, M. P. (2003). α6β4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of α3β1 integrin. J. Cell Sci. 116, 3543-3556. [DOI] [PubMed] [Google Scholar]
- Schittny, J. C. and Yurchenco, P. D. (1990). Terminal short arm domains of basement membrane laminin are critical for its self-assembly. J. Cell Biol. 110, 825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiders, F. I., Maertens, B., Böse, K., Li, Y., Brunken, W. J., Paulsson, M., Smyth, N. and Koch, M. (2007). Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J. Biol. Chem. 282, 23750-23758. [DOI] [PubMed] [Google Scholar]
- Schymeinsky, J., Nedbal, S., Miosge, N., Poschl, E., Rao, C., Beier, D. R., Skarnes, W. C., Timpl, R. and Bader, B. (2002). Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol. Cell. Biol. 22, 6820-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal, B. U., Debiase, P., Matzno, S., Chew, T.-L., Claiborne, J. N., Hopkinson, S. B., Russell, A., Marinkovich, P. M. and Jones, J. C. R. (2006). Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 (laminin-5) matrix organization. J. Biol. Chem. 281, 35487-35498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher, I., Zisman-Rozen, S., Eliahu, L., Whitelock, J. M., Maas-Szabowski, N., Yamada, Y., Breitkreutz, D., Fusenig, N. E., Arikawa-Hirasawa, E., Iozzo, R. V. et al. (2006). Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J. Biol. Chem. 281, 5178-5187. [DOI] [PubMed] [Google Scholar]
- Sigle, R. O., Gil, S. G., Bhattacharya, M., Ryan, M. C., Yang, T.-M., Brown, T. A., Boutaud, A., Miyashita, Y., Olerud, J. and Carter, W. G. (2004). Globular domains 4/5 of the laminin α3 chain mediate deposition of precursor laminin 5. J. Cell Sci. 11, 4481-4494. [DOI] [PubMed] [Google Scholar]
- Staquicini, F. I., Dias-Neto, E., Li, J., Snyder, E. Y., Sidman, R. L., Pasqualini, R. and Arap, W. (2009). Discovery of a functional protein complex of netrin-4, laminin γ1 chain, and integrin α6β1 in mouse neural stem cells. Proc. Natl. Acad. Sci. USA 106, 2903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp, M. A., Liu, Y., Pal-Ghosh, S., Jurjus, R. A., Tadvalkar, G., Sekaran, A., LoSicco, K., Jiang, L., Larsen, M., Li, L. et al. (2007). Reduced migration, altered matrix and enhanced TGF1 signaling are signatures of mouse keratinocytes lacking Sdc1. J. Cell Sci. 120, 2851-2863. [DOI] [PubMed] [Google Scholar]
- Stetefeld, J., Jenny, M., Schulthess, T., Landwehr, R., Schumacher, B., Frank, S., Rüegg, M. A., Engel, J. and Kammerer, R. A. (2001). The laminin-binding domain of agrin is structurally related to N-TIMP-1. Nat. Struct. Biol. 8, 705-709. [DOI] [PubMed] [Google Scholar]
- Takagi, J., Yang, T., Liu, J., Wang, J. and Springer, T. A. (2003). Complex between nidogen and laminin fragments reveals a paradigmatic β-propellor interface. Nature 424, 969-974. [DOI] [PubMed] [Google Scholar]
- Talts, J. F., Sasaki, T., Miosge, N., Gohring, W., Mann, K., Mayne, R. and Timpl, R. (2000). Structural and functional analysis of the recombinant G domain of the laminin α4 chain and its proteolytic processing in tissues. J. Biol. Chem. 275, 35192-35199. [DOI] [PubMed] [Google Scholar]
- Timpl, R., Tisi, D., Talts, J. F., Andac, Z., Sasaki, T. and Hohenester, E. (2000). Structure and function of laminin LG modules. Matrix Biol. 19, 309-317. [DOI] [PubMed] [Google Scholar]
- Tisi, D., Talts, J. F., Timpl, R. and Hohenester, E. (2000). Structure of the C-terminal laminin G-like domain pair of the laminin alpha2 chain harbouring binding sites for alpha-dystroglycan and heparin. EMBO J. 19, 1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, M., Rousselle, P., Nokelainen, P., Tallapragada, S., Nguyen, N. T., Fincher, E. F. and Marinkovich, M. P. (2008). Targeting a tumor-specific laminin domain critical for human carcinogenesis. Cancer Res. 68, 2885-2894. [DOI] [PubMed] [Google Scholar]
- Tsiper, M. V. and Yurchenco, P. D. (2002). Laminin assembles into separate basement membrane and fibrillar matrices in Schwann cells. J. Cell Sci. 115, 1005-1015. [DOI] [PubMed] [Google Scholar]
- Tsubota, Y., Mizushima, H., Hirosaki, T., Higashi, S., Yasumitsu, H. and Miyazaki, K. (2000). Isolation and activity of proteolytic fragment of laminin-5 α3 chain. Biochem. Biophys. Res. Commun. 278, 614-620. [DOI] [PubMed] [Google Scholar]
- Tunggal, P., Smyth, N., Paulsson, M. and Ott, M. C. (2000). Laminins: structural and genetic regulation. Micro. Res. Tech. 51, 214-227. [DOI] [PubMed] [Google Scholar]
- Tunggal, P., Wartenberg, M., Paulsson, M. and Smyth, N. (2003). Expression of the nidogen-binding site on laminin γ1 chain disturbs basement membrane formation and maintenance in F9 embryoid bodies. J. Cell Sci. 116, 803-812. [DOI] [PubMed] [Google Scholar]
- Tzu, J. and Marinkovich, M. P. (2008). Bridging structure with function: Structural, regulatory, and developmental role of laminins. Int. J. Biochem. Cell Biol. 40, 199-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udayakumar, T. S., Chen, M. L., Bair, E. L., Von Bredow, D. C., Cress, A. E., Nagle, R. B. and Bowden, G. T. (2003). Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 63, 2292-2299. [PubMed] [Google Scholar]
- Utani, A., Nomizu, M., Timpl, R., Roller, P. P. and Yamada, Y. (1994). Laminin chain assembly. Specific sequences at the C terminus of the long arm are required for the formation of specific double- and triple-stranded coiled-coil structures. J. Biol. Chem. 269, 19167-19175. [PubMed] [Google Scholar]
- Vidal, F., Aberdam, D., Miquel, C., Christiano, A. M., Pulkkinen, L., Uitto, J., Ortonne, J.-P. and Meneguzzi, G. (1995). Integrin β4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat. Genet. 10, 229-234. [DOI] [PubMed] [Google Scholar]
- Weir, L., Oppizzi, M. L., Henry, M. D., Onishi, A., Campbell, K. P., Bissell, M. J. and Muschler, J. (2006). Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. J. Cell Sci. 119, 4047-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski, I. and Schwarzbauer, J. E. (2003). The ins and outs of fibronectin matrix assembly. J. Cell Sci. 116, 3269-3276. [DOI] [PubMed] [Google Scholar]
- Williamson, R. A., Henry, M. D., Daniels, K. J., Hrstka, R. F., Sunada, Y., Ibraghimov-Beskrovnaya, O. and Campbell, K. P. (1997). Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum. Mol. Genet. 6, 831-841. [DOI] [PubMed] [Google Scholar]
- Wolozin, B. L., Pruchnicki, A., Dickson, D. W. and Davies, P. (1986). A neuronal antigen in the brains of Alzheimer patients. Science 232, 648-650. [DOI] [PubMed] [Google Scholar]
- Yurchenco, P. D., Quan, Y., Colognato, P., Mathus, T., Harrison, D., Yamada, Y. and O'Rear, J. (1997). The α chain of laminin 1 is independently secreted and drives secretion of its β and γ chain partners. Proc. Natl. Acac. Sci. USA 94, 10189-10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco, P. D., Amenta, P. S. and Patton, B. L. (2004). Basement membrane assembly, stability and activities through the developmental lens.. Matrix Biol. 22, 521-538. [DOI] [PubMed] [Google Scholar]
- Yurchenco, P. D. and Wadsworth, W. G. (2004). Assembly and tissue functions of early embryonic laminins and netrins. Curr. Op. Cell Biol. 6, 572-579. [DOI] [PubMed] [Google Scholar]
- Zahir, N., Lakins, J. N., Russell, A., Ming, W., Chatterjee, C., Rozenberg, G. I., Marinkovich, M. P. and Weaver, V. M. (2003). Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J. Cell Biol. 163, 1397-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]