Summary
Muscle satellite cells are the resident stem cells of adult skeletal muscle. Here, we have examined the role of the multifunctional protein presenilin-1 (PS1) in satellite cell function. PS1 acts as a crucial component of the γ-secretase complex, which is required to cleave single-pass transmembrane proteins such as Notch and amyloid-β precursor protein. PS1, however, also functions through γ-secretase-independent pathways. Activation of satellite cells was accompanied by induction of PS1, with PS1 knockdown enhancing their myogenic differentiation, but reducing their self-renewal. Transfection with siRNA against PS1 led to accelerated myogenic differentiation during muscle regeneration in vivo. Conversely, constitutive expression of PS1 resulted in the suppression of myogenic differentiation and promotion of the self-renewal phenotype. Importantly, we found that PS1 also acts independently of its role in γ-secretase activity in controlling myogenesis, which is mediated in part by Id1 (inhibitor of DNA binding 1), a negative regulator of the myogenic regulatory factor MyoD. PS1 can control Id1, which affects satellite cell fate by regulating the transcriptional activity of MyoD. Taken together, our observations show that PS1 is a key player in the choice of satellite cell fate, acting through both γ-secretase-dependent and γ-secretase-independent mechanisms.
Keywords: Satellite cell, Myoblast, Presenilin-1, Id1, Pax7, MyoD, γ-secretase, Self-renewal, Skeletal muscle, Myogenic differentiation, Stem cell, Cell fate choice
Introduction
Muscle satellite cells are myogenic stem cells that are located between the basal lamina and the plasmalemma of myofibers (Mauro, 1961). They are necessary for postnatal muscle growth, and are responsible for maintenance, hypertrophy and repair of adult skeletal muscle. It has been shown that satellite cells are able to self-renew to maintain their population (Collins et al., 2005) and much work is currently directed at understanding how self-renewal is regulated (reviewed by Zammit, 2008).
The paired-box transcription factor Pax7 is expressed by quiescent satellite cells and is implicated in the generation of committed myogenic progenitors, but its role in the regulation of satellite cell self-renewal is in debate (Lepper et al., 2009; McKinnell et al., 2008; Olguin et al., 2007; Seale et al., 2000; Zammit et al., 2006). Following activation, satellite cells co-express Pax7 with MyoD [a member of the myogenic regulatory factor family, together with Myf5, Mrf4 and Myog (myogenin)] and proliferate. Later, satellite-cell-derived myoblasts either downregulate Pax7, maintain MyoD and induce Myog as they undergo myogenic differentiation, or they downregulate MyoD and maintain Pax7, returning to a quiescent-like state (Halevy et al., 2004; Zammit et al., 2004). Interestingly, the total number of satellite cells in adult muscles remains relatively constant after repeated muscle injury and regeneration, indicating that the self-renewal system of satellite cells is carefully coordinated (Collins et al., 2005; Yoshida et al., 1998; Zammit et al., 2004). However, the molecular mechanism of satellite cell self-renewal remains poorly understood, although recent advances have shown that Notch and canonical Wnt signalling play a role (Brack et al., 2008; Conboy and Rando, 2002; Kitzmann et al., 2006; Kuang et al., 2007; Perez-Ruiz et al., 2008).
Notch signalling controls many events, including differentiation, proliferation and apoptosis in various tissues (Hansson et al., 2004). In skeletal muscle, the Notch-signalling pathway is involved in activation, and proliferation of muscle satellite cells, and has been implicated in their self-renewal (Conboy et al., 2003; Conboy and Rando, 2002; Kitzmann et al., 2006; Kopan et al., 1994; Kuang et al., 2007; Nofziger et al., 1999; Ono et al., 2007). For example, inhibition of Notch activity enhances myogenic differentiation of murine and human myoblasts (Kitzmann et al., 2006; Kuang et al., 2007). Notch is activated by binding of members of the delta-like and Jagged families (in mammals) to its extracellular domain, which results in γ-secretase-mediated cleavage to release the Notch intracellular domain (Notch ICD) (Herreman et al., 2000; Struhl and Greenwald, 1999; Zhang et al., 2000). The Notch ICD translocates into the nucleus, where it interacts with the DNA-binding protein CSL/RBP-J (RBP-J is a member of CSL family of proteins) to regulate the transcription of target genes such as Hes1 (Jarriault et al., 1995).
Together with nicastrin, Pen-2 and Aph-1, the other crucial component of the γ-secretase complex is presenilin (reviewed by De Strooper, 2003). Presenilin-1 (PS1) and presenilin-2 (PS2) are membrane proteins that function as the catalytic subunit of the γ-secretase complex, an intramembrane protease with a number of substrates of the type I membrane protein family (De Strooper et al., 1999) (reviewed by Parks and Curtis, 2007; Vetrivel et al., 2006). For example, in addition to cleavage of activated Notch, γ-secretase targets also include, but are not limited to, amyloid precursor protein (APP), Delta, Jagged, CD44, CD43, Erb4, E-cadherin, N-cadherin and syndecan (De Strooper et al., 1999) (reviewed by Parks and Curtis, 2007; Vetrivel et al., 2006). Importantly, PS1 also functions via γ-secretase-independent pathways (Akbari et al., 2004; Esselens et al., 2004; Huppert et al., 2005; Meredith et al., 2002; Repetto et al., 2007; Tu et al., 2006; Wilson et al., 2004). For example, somitogenesis is abrogated in PS1-null mice, yet embryos lacking other essential components of the γ-secretase complex, such as nicastrin, Pen-2 and Aph-1, or null for the Notch pathway component CSL/RBP-J, still develop anterior somites in the complete absence of Notch signalling (Huppert et al., 2005). Furthermore, the roles of PS1 in Ca2+ homeostasis (Akbari et al., 2004; Tu et al., 2006); autophagy and protein degradation (Esselens et al., 2004; Repetto et al., 2007; Wilson et al., 2004); and Wnt–β-catenin signalling (Meredith et al., 2002) have all also been shown to be via γ-secretase-independent mechanisms.
Here, we sought to explore the role of PS1 in satellite cell function. We found that PS1 is strongly expressed in activated and proliferating satellite cells, where PS1 knockdown accelerates myogenic differentiation and reduces the number of cells undergoing self-renewal. By contrast, constitutive PS1 expression led to a downregulation of MyoD expression and decreased differentiation. PS1 acts via Id1 (inhibitor of DNA binding 1), a potent negative regulator of the ability of MyoD to activate its transcriptional targets. Because PS1-null mice die during embryogenesis, we used PS1–/– and/or PS2–/– murine embryonic fibroblasts (MEFs) and found that Id1 levels were low, but significantly upregulated by addition of PS1 or a mutant PS1 that lacks γ-secretase activity, but not by PS2. Taken together, these observations show that PS1 has an essential role in satellite cell function, and can act independently of γ-secretase activity by regulating Id proteins to control MyoD transcriptional activity.
Results
PS1 is induced during activation of satellite cells
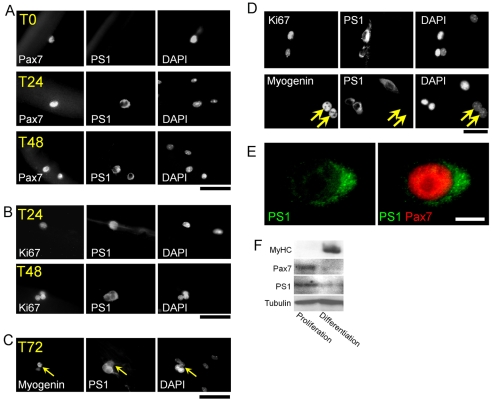
To investigate the expression dynamics of PS1 during myogenic progression, immunostaining was performed on satellite cells retained in their niche on isolated myofibres. PS1 was not detectable in Pax7+ quiescent satellite cells, analysed immediately after isolation (T0). However, culture of myofibres in plating medium for 24 hours (T24) showed that activated satellite cells induced robust PS1 expression, located in the membrane compartment (Fig. 1A,E), which remained high in proliferating Ki67+ or undifferentiated Pax7+ satellite cells at T48 (Fig. 1A,B). PS1 was then downregulated in satellite cell progeny committed to myogenic differentiation after 72 hours (T72), as shown by the presence of Myog (Fig. 1C). PS1 was clearly expressed in Ki67+ proliferating plated satellite-cell-derived myoblasts, but not in differentiating Myog+ cells, 5 days after isolation (Fig. 1D). Using western blotting, we also confirmed that PS1 is highly expressed in plated proliferating satellite-cell-derived myoblasts, and decreased in cells induced to differentiate by switching to low-serum medium (Fig. 1F).
Fig. 1.
PS1 is expressed by activated and proliferating satellite cells. Myofibres and their associated satellite cells were isolated and either immediately fixed (T0) or cultured in activation medium for either 24 hours (T24), 48 hours (T48) or 72 hours (T72) before fixation. (A) Immunostaining showed that Pax7+ satellite cells on freshly isolated myofibres (T0) did not express PS1. (B) PS1 could be detected after activation and during proliferation, as shown by the co-expression of PS1 and Ki67. (C) PS1 was downregulated as satellite-cell-derived myoblasts committed to myogenic differentiation, as demonstrated by the presence of Myog (arrows). (D) Immunocytochemistry on plated satellite-cell-derived myoblasts confirmed that expression of PS1 was associated with proliferating Ki67+ cells but not differentiating Myog+ cells (arrows). (E) High magnification immunofluorecent image to show the localisation of PS1 in plated satellite-cell-derived cells. (F) Western blot to illustrate that PS1 expression decreases after myogenic differentiation in plated satellite-cell-derived cells. Representative data of at least three independent experiments are shown. Scale bars: 60 μm (A-C), 30 μm (D) and 5 μm (E).
PS1 knockdown inhibits satellite cell self-renewal
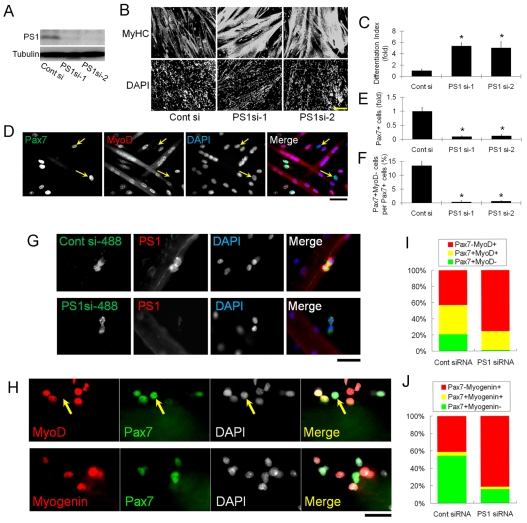
Having shown that PS1 is expressed in activated and proliferating satellite cells, we next examined its function. In order to determine the effect of knockdown of PS1 on lineage progression in satellite-cell-derived myoblasts, cells were transfected with either of two siRNA duplexes against PS1 (PS1si-1 or PS1si-2) and control siRNA. Both siRNA species targeting PS1 effectively reduced PS1 protein levels in plated satellite-cell-derived myoblasts, when assayed 48 hours after transfection using western blot (Fig. 2A). At 72 hours after transfection, reduced PS1 levels resulted in a marked promotion of differentiation, as indicated by the higher fusion index for cells transfected with PS1 siRNA than for cells transfected with control siRNA (fold change in the number of DAPI-stained nuclei of differentiated cells [as shown by the presence of myosin heavy chain (MyHC)] divided by the total number of DAPI-stained nuclei; Fig. 2B and quantified in 2C). Importantly, siRNA-mediated knockdown of PS1 drastically reduced both the number of Pax7+ cells and the percentage of cells with the Pax7+MyoD– self-renewal phenotype (Zammit et al., 2004), compared with controls (Fig. 2D and quantified in 2E,F).
Fig. 2.
PS1 is required for satellite cell self-renewal and maintenance of progenitor cells. To examine the effects of PS1 on satellite cell myogenic progression, we first knocked-down PS1 protein levels using siRNA. (A) Both PS1 siRNAs (PS1si-1 and PS1si-2) efficiently knockdown PS1 protein in plated primary satellite-cell-derived myoblasts as shown by western blotting. (B) Satellite-cell-derived myoblasts were cultured for 3 days after siRNA transfection and then immunostained for MyHC. A dramatic increase in the extent of myogenic differentiation and formation of myotubes was observed with both siRNA species directed against PS1 as compared to controls (Cont si). (C) Differentiation index quantifying the increased myogenic differentiation caused by PS1 knockdown, calculated as fold change in the number of DAPI-stained nuclei in MyHC+ cells, divided by the total number of DAPI-stained nuclei. (D) Immunocytochemistry of plated primary myoblasts was also used to investigate the presence of cells with a self-renewal phenotype (Pax7+MyoD–) 3 days after siRNA transfection (arrows indicate Pax7+MyoD– cells), and clearly demonstrate that reduced PS1 levels resulted in less Pax7+ cells (quantified in E) and self-renewal (quantified in F). (G) AlexaFluor488-conjugated control siRNA and PS1 siRNA duplexes were transfected into satellite cells retained in their niche on isolated myofibres, and knockdown of PS1 protein confirmed by immunocytochemistry 48 hours later. (H) The effects of PS1 knockdown on satellite cell fate were examined by immunostaining for Pax7, MyoD (arrows indicates Pax7+MyoD– cell) and Myog 72 hours after PS1si-1 transfection. (I,J) PS1 knockdown reduced the percentage of satellite-cell-derived myoblasts exhibiting the self-renewal phenotype (Pax7+MyoD–), while increasing the percentage of cells committed to differentiation (Pax7–MyoD+ and Pax7–Myog+). Data from at least three independent experiments is shown ± s.d. Asterisks in C, E and F indicate that data are significantly different from control values (P<0.05). Scale bars: 100 μm (B), 30 μm (D,G,H).
Transfection of PS1 siRNA was also performed to assay the effects of PS1 knockdown on the fate of satellite cells retained in their niche on the myofibre. First, we determined the transfection efficiency of siRNA into satellite cells on a myofibre by using AlexaFluor488-conjugated siRNA (Fig. 2G) and found that levels of >95% could be obtained, and that PS1 was successfully knocked down (Fig. 2G). The percentage of Pax7+MyoD– self-renewed cells was significantly decreased 72 hours after transfection with PS1 siRNA, with an increased amount of differentiation-committed Pax7–MyoD+ and Pax7–Myog+ cells present, in comparison with cells transfected with control siRNA (Fig. 2H and quantified in 2I,J). Thus, PS1 is required for satellite cell self-renewal and for inhibition of myogenic differentiation.
Constitutive PS1 expression inhibits myogenic differentiation
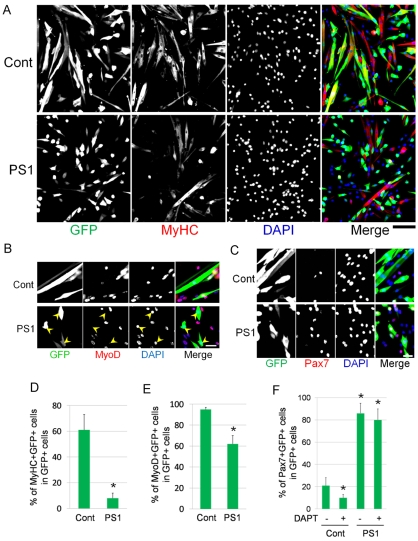
Notch is activated by the binding of its ligands, and activated Notch is then cleaved by γ-secretase to release the Notch ICD, a central step in Notch signalling. Although γ-secretase activity is dependent on PS1 function, several studies have reported that overexpression of PS1 alone does not enhance γ-secretase activity, unless in conjunction with nicastrin, Aph-1a and Pen-2 (reviewed by De Strooper, 2003; Parks and Curtis, 2007; Vetrivel et al., 2006). To investigate the effect of constitutive PS1 expression on satellite cell function, mouse PS1 cDNA was cloned to generate pMSCV-PS1-IRES-GFP or pCMV-PS1-V5. pMSCV-PS1-IRES-GFP was transfected into satellite-cell-derived myoblasts, with eGFP from the IRES-eGFP allowing transfected cells to be readily identified (transfection efficiency was >30% for both vectors). Constitutive PS1 expression led to fewer eGFP+ cells containing MyHC (8%) (Fig. 3A and quantified in 3D) or MyoD+ (62%) (Fig. 3B and quantified in 3E) compared with cells transfected with control empty pMSCV-IRES-GFP. Constitutive expression of PS1 also significantly increased the number of eGFP+ cells expressing Pax7 by approximately fourfold over parallel cultures transfected with control siRNA (Fig. 3C and quantified in 3F).
Fig. 3.
Constitutive PS1 expression leads to suppression of myogenic differentiation and augmentation of Pax7 expression. The effects of PS1 on satellite cell function were examined by constitutively expressing PS1 using expression vectors. (A,B) Primary satellite-cell-derived myoblasts were transfected with either control pMSCV-IRES-GFP (Cont) or pMSCV-PS1-IRES-GFP (PS1) vectors and, 48 hours later, immunostained for eGFP (to identify transfected cells) and either MyHC (A) or MyoD (B) (arrowheads indicate GFP+MyoD– cells in PS1-vector-transfected cells). (D,E) Constitutive PS1 expression significantly reduced the percentage of transfected cells that co-expressed either MyHC (D), or MyoD (E). (C,F) Constitutive PS1 expression also increased the percentage of satellite-cell-derived myoblasts expressing Pax7. (F) Although exposure of control pMSCV-IRES-GFP-transfected cells to 1 μM DAPT reduced the percentage of Pax7-expressing cells, DAPT inhibition of γ-secretase did not prevent the significant increase of Pax7 in transfected cells containing pMSCV-PS1-IRES-GFP vector. Data from at least three independent experiments is shown ± s.d. Asterisks in D-F indicate that data are significantly different from control values (P<0.05). Scale bars: 100 μm (A) and 30 μm (B,C).
To confirm that transfection of PS1 does not act by increasing γ-secretase activity, constitutive PS1 expression was also examined in the presence of DAPT, which is a specific pharmacological inhibitor of γ-secretase activity (Lammich et al., 2002). Importantly, the effects of PS1 on increasing the number of eGFP+ satellite-cell-derived myoblasts containing Pax7 protein, was not significantly altered when γ-secretase activity was inhibited (Fig. 3F). Because inhibition of γ-secretase normally results in a decrease in Pax7 expression, these observations indicate that PS1 is exerting its effect through a mechanism that is independent of γ-secretase activity. Taken together, these results clearly demonstrate that myogenic differentiation was suppressed by overexpression of PS1, but the number of cells expressing Pax7 significantly rose, via a mechanism independent of γ-secretase activity.
PS1 negatively regulates expression of MyoD through a γ-secretase-independent mechanism
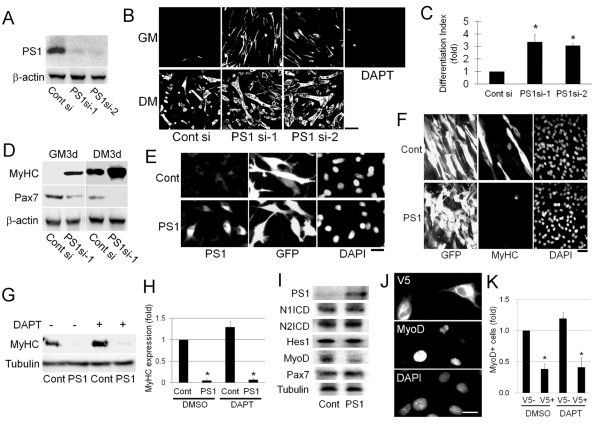
To perform detailed biochemical analysis, we also used the immortalised adult post-injury-derived C2 cell line (Yaffe and Saxel, 1977). Immunocytochemistry showed that PS1 was expressed in proliferating C2C12 myoblasts (data not shown), as observed in primary satellite-cell-derived myoblasts (Fig. 1). Both siRNAs targeting PS1 effectively reduced PS1 protein levels 48 hours after transfection in C2C12 cells (Fig. 4A) and enhanced myogenic differentiation after 72 hours in differentiation medium (DM), with larger multinucleated myotubes formed than in control cultures (Fig. 4B). Even when maintained in growth medium (GM), transfection with either of the PS1 siRNA species resulted in an induction of MyHC expression (Fig. 4B), though crucially, treatment with DAPT alone, to inhibit γ-secretase activity, did not result in MyHC expression (Fig. 4B). Immunblot analysis of C2C12 cells transfected with PS1 siRNAs also revealed a lower expression of Pax7 with both duplexes than with control siRNA (Fig. 4D).
Fig. 4.
PS1 negatively regulates MyoD expression in a γ-secretase-independent mechanism. In order to obtain sufficient material for biochemical analysis, we also used the adult mouse muscle-derived C2C12 myogenic cell line. (A) Immunoblot analysis showed that proliferating C2C12 cells expressed PS1, and that the siRNA-targeting PS1 (PS1si-1 and PS1si-2) was effective at reducing PS1 protein levels. (B) As with primary myoblasts, exposure of C2C12 myoblasts to siRNA against PS1 promoted myogenic differentiation, as shown by immunostaining for MyHC, in both growth (GM) and differentiation medium (DM) (quantified in C for DM). Importantly, treatment with DAPT in GM for 3 days to inhibit γ-secretase activity did not reproduce the effects of PS1 knockdown, being unable to induce expression of MyHC in C2C12 cells. (D) Immunblot analysis of siRNA-transfected C2C12 cells cultured in GM or DM for 3 days after transfection confirmed increased MyHC expression. Importantly, Pax7 levels were significantly reduced by PS1 knockdown under both culture conditions. (E-I) PS1 was also constitutively expressed in C2C12 myoblasts by transient transfection with PS1-expression vector (pMSCV-PS1-IRES-GFP). (F) Immunocytochemical analysis for MyHC revealed that myogenic differentiation was again inhibited. (G) Immunoblot analysis of transfected C2C12 cells constitutively expressing PS1, illustrating that inhibiting γ-secretase activity by exposure to 1 μM DAPT for 2.5 days did not alter the marked reduction in MyHC levels (quantified in H). (I) Immunoblot analysis demonstrated that constitutive PS1 expression in C2C12 cells cultured in GM for 24 hours after transfection did not affect the levels of Notch1 ICD, Notch2 ICD or Hes1, but that MyoD was significantly reduced, compared to the tubulin protein loading control. (J) Similar results were obtained using a V5-tagged PS1 expression vector transfected into C2C12 cells. Immunostaining showed that MyoD expression was reduced to a similar degree, with or without exposure to 1 μM DAPT to inhibit γ-secretase activity (quantified in K). Data from at least three independent experiments is shown ± s.d. Asterisks in C, H and K indicate that data are significantly different from control values (P<0.05). Scale bars: 100 μm (B), 20 μm (E), 30 μm (F) and 10 μm (J).
Transfection of pMSCV-PS1-IRES-GFP into C2C12 myoblasts resulted in high levels of PS1 and GFP protein in >70% of cells (Fig. 4E), allowing us to perform western blot analysis. As with primary satellite-cell-derived myoblasts, transfection with pMSCV-PS1-IRES-GFP resulted in a marked suppression of MyHC expression compared with control (Fig. 4F). Again, constitutive PS1 expression-mediated inhibition of differentiation was not influenced by the presence of DAPT to inhibit γ-secretase activity (Fig. 4G and quantified in 4H). Importantly, immunoblot analysis for Notch1 ICD, Notch2 ICD and Hes1 showed that the already-active Notch signalling in proliferating myoblasts was not further augmented by constitutive PS1 expression (Fig. 4I). Interestingly, expression of MyoD was downregulated by constitutive expression of PS1 in proliferating myoblasts, but expression of Pax7 was not affected (Fig. 4I). To check whether MyoD downregulation was influenced by γ-secretase activity, we transfected C2C12 myoblasts with pCMV-V5-tagged PS1 in the presence of DAPT to inhibit γ-secretase activity, and found that MyoD levels still decreased significantly (Fig. 4J and quantified in 4K).
Myogenic differentiation in PS1-null cells is independent of γ-secretase activity
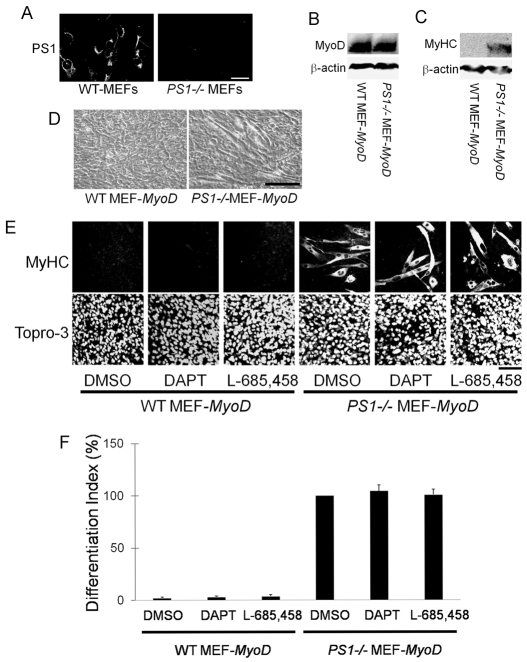
Mice homozygous for null alleles of PS1 die late in embryogenesis (Shen et al., 1997; Wong et al., 1997), precluding examination of satellite cells. To model myogenesis in PS1-null cells therefore, we used ectopic MyoD expression to initiate myogenic conversion in MEFs (Skapek et al., 1996). Immunostaining revealed robust expression of PS1 in control wild-type (WT) MEFs but not PS1–/– MEFs (Fig. 5A). γ-secretase activity was significant lower in PS1–/– MEFs than in control WT MEFs, as assessed using a γ-secretase activity detection kit (data not shown).
Fig. 5.
Induced myogenic differentiation of PS1–/– MEFs is independent of γ-secretase activity. PS1–/– mice die during embryogenesis and so, to examine myogenesis, we used MyoD-transfected MEFs. (A) Immunostaining revealed that PS1 is expressed by WT MEFs, but is absent from PS1–/– MEFs. (B,C) Immunoblot analysis demonstrated that myogenesis was effectively induced in PS1–/– MEFs by transfection of a MyoD-expression vector. β-actin was the protein loading control. (C,D) Culture in high-serum (10% FBS) medium for 5 days still resulted in MyHC expression (C) and formation of myotubes (D) in PS1–/– MEFs, but not in WT. (E,F) WT MEFs transfected with the MyoD-expression-vector and exposed to 1 μM DAPT or 1 μM L-685,458 to inhibit γ-secretase activity failed to induce MyHC expression when cultured in 10% FBS for 5 days. However, MyoD-transfected PS1–/– MEFs, maintained under identical culture conditions, differentiated and expressed MyHC, with inhibition of γ-secretase activity having no effect on the differentiation index. Data from at least three independent experiments are shown ± s.d. Scale bars: 20 μm (A), 100 μm (D) and 60 μm (E).
Transient transfection with a MyoD expression vector resulted in robust MyoD expression in both WT and PS1–/– MEFs (Fig. 5B). Myogenic differentiation in vitro is suppressed by culture in medium containing high levels of serum (Kodaira et al., 2006), and we observed no signs of myogenic differentiation in WT MEFs maintained in 10% FBS for 5 days after transfection with MyoD (Fig. 5C,D). By contrast, robust MyHC expression and formation of multinucleated myotubes occurred in MyoD-transfected PS1–/– MEFs maintained under identical high-serum conditions (Fig. 5C,D). Under low-serum (2% FBS) conditions, MyoD-transfected PS1–/– MEFs also had higher MyHC levels than control-transfected WT MEFs (data not shown). We next investigated whether this MyoD-induced myogenesis in PS1–/– MEFs could be `rescued' by PS1. Transfection with PS1 expression vectors completely inhibited the MyoD-induced myogenic differentiation of PS1–/– MEFs (Fig. 7K).
Fig. 7.
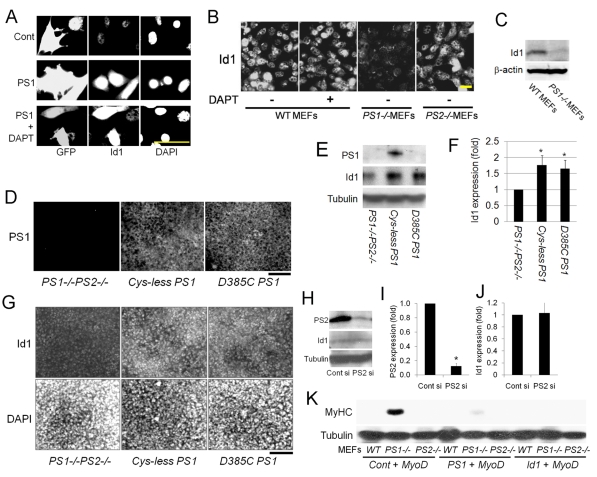
PS1 controls Id1 expression in non-muscle cells via a γ-secretase-activity-independent mechanism. (A) PS1-regulated Id1 expression was not restricted to myogenic cells: in the mesenchymal stem cell line 10T1/2, immunostaining showed that constitutive PS1 expression increased Id1 protein levels, which was also independent of γ-secretase activity, as shown by exposure to 1 μM DAPT for 6 hours. (B) Immunostaining showed that reduction in Id1 levels was specific to PS1–/– MEFs because PS2–/– MEFs had robust Id1 levels, comparable to WT MEFs. Exposure of WT MEFs to 1 μM DAPT for 12 hours did not result in a reduction in Id1 levels. (C) Although Id1 was clearly present in WT MEFs, immunoblot analysis demonstrated that Id1 was expressed at very low levels from PS1–/– MEFs. β-actin was used as a control for protein loading. (D) Immunostaining showed that anti-PS1 (N-terminal) antibody recognised both stably inserted D385C PS1 (PS1 mutant with no γ-secretase activity) and control Cys-less PS1 in PS1–/–PS2–/– MEFs. (E) Immunoblot analysis revealed that PS1 protein in D385C-PS1-inserted PS1–/–PS2–/– MEFs was not detected by anti-PS1 (cleaved loop domain) antibody, which recognised the Cys-less PS1-inserted PS1–/–PS2–/– MEFs. (E,F). Id1 protein was increased in both Cys-less PS1 and D385C-PS1 expressing PS1–/–PS2–/– MEFs (quantified in F). (G) Immunostaining confirmed that D385C PS1 inserted into PS1–/–PS2–/– MEFs can upregulate Id1 protein, as does the control Cys-less PS1-inserted PS1–/–PS2–/– MEFs. (H) Immunoblot analysis illustrates that specific siRNA-mediated knockdown of PS2 does not affect the level of Id1 protein in C2C12 myoblasts (quantified in I,J). (K) Immunoblotting 5 days after transfection illustrated that the presence of a MyoD-expression vector resulted in myogenic differentiation in PS1–/– MEFs maintained in high-serum culture conditions, as shown by the presence of MyHC. By contrast, WT or PS2–/– MEFs did not undergo myogenic conversion. Importantly, co-transfection of the MyoD expression vector with either a PS1-expression vector, or an Id1-expression vector, largely blocked this induction of MyHC in PS1–/– MEFs. Tubulin was used as an internal control for protein loading. Data from at least three independent experiments are shown ± s.d. Asterisks in F, I and J indicate whether data are significantly different from control values (P<0.05). Scale bars: 30 μm (A), 10 μm (B) and 100 μm (D,G).
To determine whether this process is independent of γ-secretase activity, we used the γ-secretase inhibitors DAPT and L-685,458 on MyoD-transfected PS1–/– MEFs and WT MEFs. γ-secretase activity was significantly decreased in WT MEFs (∼93%) after 1 μM DAPT treatment, as determined using a γ-secretase activity detection kit (data not shown). We found that neither treatment with DAPT (even up to 50 μM; data not shown) nor L-685,458 could induce myogenic differentiation in MyoD-expressing WT MEFs in high-serum medium, or changed high MyHC expression levels in MyoD-containing PS1–/– MEFs (Fig. 5E and quantified in 5F). Together, these observations suggest that the MyoD-induced myogenic program is inhibited by PS1 in a manner that is independent of γ-secretase activity.
PS1 regulates Id1 expression in myogenic cells
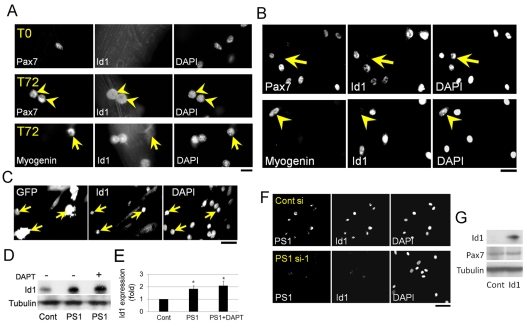
Despite the high-serum culture conditions, our results show that myogenic differentiation is clearly induced by ectopic MyoD expression in PS1–/– MEFs (Fig. 5). BMP (bone morphogenetic protein) signalling contributes to serum-induced inhibition of myogenic differentiation in vitro (Kodaira et al., 2006) and Id1 is a major BMP signalling downstream target gene, known to be a potent negative regulator of MyoD in myoblasts (Benezra et al., 1990; Jen et al., 1992; Katagiri et al., 2002). We hypothesised therefore, that Id1 might also be regulated by PS1. To test this, immunostaining was performed to investigate whether satellite cells express Id1 during myogenic progression. Id1 was not detectable in Pax7+ quiescent satellite cells (T0), but was upregulated in activated and proliferating satellite cells (T72; Fig. 6A). Id1 was then downregulated in myoblasts committing to myogenic differentiation, as shown by the presence of Myog or absence of Pax7 (arrow; Fig. 6A,B), showing that the Id1 expression profile mirrors that of PS1 (Fig. 1).
Fig. 6.
PS1 regulates the expression of Id1, to function independently of Notch signalling. (A) Immunocytochemical analysis for Pax7 and Id1 on satellite cells associated with a myofibre demonstrated that Id1 was not present in quiescent satellite cells (T0). By 72 hours (T72) after isolation, Id1 was robustly expressed in Pax7-expressing cells (arrowheads), but downregulated in satellite-cell-derived myoblasts as they commit to myogenic differentiation (arrows), as shown by co-immunostaining for Id1 and Myog. (B) In plated satellite-cell-derived myoblasts, again Id1 expression was associated with undifferentiated cells (arrows, Pax7– cells; arrowheads, Myog+ cells). (C) Constitutive expression of PS1 by transfection of satellite-cell-derived myoblasts with pMSCV-PS1-IRES-eGFP resulted in eGFP (PS1)-containing cells also having robust Id1 expression, revealed by immunostaining (arrows, GFP+Id1+ cells). (D) Immunoblotting of C2C12 myoblasts transfected with a PS1-expression vector (PSI) showed that Id1 levels were significantly increased. This PS1-induced increase in Id1 levels was not affected by exposure to 1 μM DAPT for 6 hours to inhibit γ-secretase activity (quantified in E). (F) The effect of PS1 siRNA on expression of Id1 in satellite-cell-derived myoblasts was analysed by immunostaining, and demonstrated that PS1 and Id1 were colocalised in cells transfected with control siRNA (Cont), but revealed that siRNA-mediated knockdown of PS1 resulted in less Id1 expression. (G) Immunoblotting analysis showed that constitutive Id1 expression did not change Pax7 expression levels in C2C12 myoblasts transfected with the pCMV-Id1 expression vector. Data from at least three independent experiments are shown ± s.d. Asterisks in E indicate that data are significantly different from control values (P<0.05). Scale bars: 10 μm (A), 20 μm (B), 30 μm (C) and 50 μm (F).
To determine whether the levels of PS1 and Id1 were causally linked in myoblasts, we transfected satellite-cell-derived myoblasts with pMSCV-PS1-IRES-GFP and found high Id1 levels in eGFP+ (PS1-expressing) cells (Fig. 6C). Constitutive PS1 expression in C2C12 cells also resulted in upregulation of the level of Id1 protein on immunoblots (Fig. 6D and quantified in 6E). Consistent with the observations that PS1 promotes Id1, knockdown of PS1 levels using siRNA in C2C12 myoblasts, led to a dramatic decrease in Id1 expression levels (Fig. 6F). Constitutive PS1 expression did not influence Pax7 protein levels (Fig. 4I), and in accord with this, increased Id1, did not change Pax7 expression in C2C12 myoblasts transfected with pCMV-Id1 (Fig. 6G).
PS1 regulates Id1 expression with a γ-secretase independent mechanism
Constitutive PS1 expression still caused an increase in Id1 in C2C12, even when γ-secretase activity was inhibited using DAPT (Fig. 6D,E). This response was not restricted to myogenic cells, however: in the mesenchymal stem cell line 10T1/2, constitutive PS1 expression again increased Id1 protein levels, independently of γ-secretase activity (Fig. 7A). To further examine PS1 regulation of Id1, we performed immunocytochemical analysis to assay Id1 protein in PS1–/– MEFs, and found that Id1 expression was lower in PS1–/– MEFs than in WT MEFs. This was again independent of γ-secretase activity because inhibition with DAPT did not influence Id1 expression in WT MEFs (Fig. 7B). This decreased level of Id1 protein in PS1–/– MEFs was also confirmed by immunoblotting (Fig. 7C). Because MyoD-induced myogenesis in PS1–/– MEFs could be `rescued' by PS1 (Fig. 7K), we also transfeced PS1–/– MEFs with the Id1 expression vector, pCMV-Id1, and again found a complete inhibition of MyoD-induced myogenic differentiation in high-serum medium (Fig. 7K).
To separately confirm that PS1 regulates Id1 in a γ-secretase-activity-independent manner, we used a mutant PS1 (D385C PS1) that encodes a PS1 protein with an Asp385 mutation that completely abolishes γ-secretase activity but still allows complex formation (Tolia et al., 2006). PS1–/–PS2–/– MEFs stably expressing D385C PS1 and another line carrying a control PS1 (Cys-less PS1) with full γ-secretase activity, both produced protein, as shown by immunostaining with PS1 (N-terminal) antibody (Fig. 7D). As expected, D385C PS1 was not recognised by an anti-PS1 (cleaved loop domain) antibody that detected the Cys-less PS1 control (Fig. 7E) (Tolia et al., 2006). Crucially, Id1 protein was increased in both PS1–/–PS2–/– MEFs expressing D385C-PS1 and control PS1–/–PS2–/– MEFs expressing Cys-less PS1 (Fig. 7E-G), demonstrating again that γ-secretase activity is not required for PS1-mediated Id1 upregulation.
PS1, but not PS2, regulates Id1 expression
There is evidence that PS1 and PS2 might have partially overlapping functions (reviewed by Vetrivel et al., 2006). However, the reduced Id1 levels found in PS1–/– MEFs was not observed in PS2–/– MEFs, whose Id1 levels were comparable with WT MEFs (Fig. 7B). To specifically test the effects of PS2 on Id1 expression, we performed immunoblot analysis in C2C12 myoblasts transfected with PS2 siRNA and found that the significantly reduced PS2 protein levels did not affect Id1 (Fig. 7H quantified in 7I,J), in contrast to the effects of PS1 siRNA (Fig. 6F). Furthermore, PS2–/– MEFs are refractory to MyoD-induced myogenic differentiation in high-serum culture conditions, in contrast to PS1–/– MEFs, confirming that there are non-redundant functions between the PS1 and PS2 (Fig. 7K).
PS1 knockdown accelerates myogenic differentiation in regenerating skeletal muscle in vivo
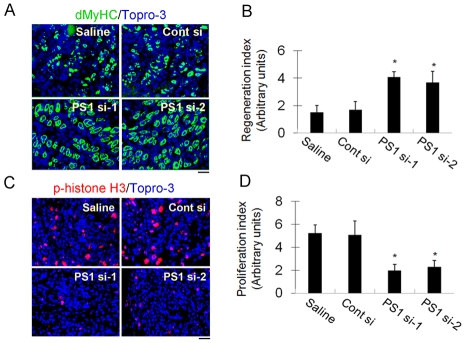
Finally, we evaluated the significance of PS1 to regenerating muscle in vivo. Cardiotoxin was used to induce muscle damage in the gastrocnemius of adult C57BL/6N mice and 12 hours later, the muscle was directly injected with 50 μl of 10 μM siRNA in Atelocollagen, a potent siRNA delivery mediator in vivo (Kinouchi et al., 2008). Four different conditions were used: saline control, control siRNA, PS1 si-1 and PS1 si-2. Regenerating muscles were isolated 3.5 days after cardiotoxin injection and frozen in liquid nitrogen before being cryosectioned. Muscle regeneration was assayed by immunostaining muscle sections for developmental MyHC (dMyHC), which is transiently expressed in regenerating myofibres, and phosphorylated histone H3 (p-histone H3) was used as a proliferation marker (Fig. 8). PS1 knockdown resulted in a significant induction of dMyHC expression (Fig. 8A and quantified in 8B) and a significant reduction in the number of cells containing p-histone H3 (Fig. 8C and quantified in 8D) compared with control siRNA. These observations indicate that PS1 is required for maintenance of proliferating cells and inhibition of myogenic differentiation during muscle regeneration in vivo as well as in vitro.
Fig. 8.
PS1 maintains proliferation and inhibits precocious differentiation during muscle regeneration in vivo. Regeneration was induced in mouse gastrocnemius muscles by injection of cardiotoxin and, 12 hours later, injection of 50 μl of 10 μM siRNA in Atelocollagen. We employed four different conditions: saline control, control siRNA, PS1si-1 and PS1si-2. Then, 3.5 days after cardiotoxin injection, the muscles were removed, frozen and cryosectioned. (A,B) Muscle regeneration was assayed by immunostaining with Topro-3 for developmental MyHC (dMyHC), which is transiently expressed in regenerating muscle. PS1 knockdown resulted in a significant induction of dMyHC expression compared to controls (quantified in B). (C,D) Immunostaining for phosphorylated histone H3 (p-histone H3) was used as a proliferation marker. A significant reduction of p-histone H3+ cells was observed in muscles exposed to siRNAs against PS1, compared with controls (quantified in D). The bar charts of regeneration index (B) or proliferation index (D) were calculated from the staining intensity of dMyHC-stained regenerating fibres (from A) or the number of p-histone H3-stained proliferating cells (from C), divided by the total number of nuclei in the field of randomly selected sections (n=5 for each condition). Values are means ± s.d. Asterisks in B and D indicate that data are significantly different from values obtained using control siRNA (P<0.05). Scale bars: 70 μm.
Discussion
Understanding how the decision between self-renewal and differentiation is regulated in satellite cells is central to understanding how skeletal muscle maintains a viable stem cell compartment. Here, we show that the multifunctional protein PS1 is able to direct muscle satellite cells away from myogenic differentiation and towards the self-renewal phenotype. Although PS1 is a key component of the γ-secretase complex, we found that PS1 also acts through γ-secretase-independent mechanisms to affect satellite cell fate, by controlling Id1 protein.
Satellite cells are normally mitotically quiescent in mature muscle, so must first be activated to enter the cell cycle and generate myoblasts (Zammit, 2008). Important pathways associated with activation include sphingolipid (Nagata et al., 2006b; Nagata et al., 2006a), p38MAPK (Jones et al., 2005) and Notch signalling (Conboy and Rando, 2002). Notch signalling in proliferating satellite cells plays a role in expanding the satellite cell pool after activation, while preventing precocious differentiation (Conboy et al., 2003; Conboy and Rando, 2002; Kitzmann et al., 2006; Kopan et al., 1994; Kuang et al., 2007; Nofziger et al., 1999). When PS1 is knocked down using siRNA, we observe an increase in cells undergoing myogenic differentiation, with fewer expressing Pax7; a similar phenotype to that observed when Notch signalling is prevented by inhibiting γ-secretase activity using pharmacogical reagents such as DAPT and L-685,458 (Kitzmann et al., 2006; Kuang et al., 2007). Considering the crucial role of PS1 in the γ-secretase complex, PS1 knockdown will directly affect Notch signalling and so some effects on satellite cells are due to perturbation of this pathway. Importantly, not all effects of siRNA knockdown of PS1 in myoblasts could be reproduced by simply inhibiting γ-secretase activity. For example, PS1 knockdown in myoblasts maintained even under high serum conditions, resulted in a high degree of myogenic differentiation, an observation not repeated when only γ-secretase activity was inhibited (Fig. 4B). Therefore, PS1 is also operating independently of its role in the γ-secretase complex. We found that PS1 knockdown also reduces the level of Id1 protein in C2C12 myoblasts. Id1 has been shown to dimerise with basic helix-loop-helix transcription factors such as MyoD, and to inhibit their transcriptional activity (Benezra et al., 1990; Jen et al., 1992). Therefore, reduced Id1 levels would alleviate a block on MyoD transcriptional activity and so lead to induction of Myog and myogenic differentiation. Myog has been shown to indirectly inhibit Pax7 expression (Olguin et al., 2007), thus providing a mechanism by which differentiation and self-renewal are linked through MyoD transcriptional activity. Furthermore, it has been reported that inhibition of myogenic differentiation by forced expression of Notch ICD is not accompanied by Id1 induction in C2C12 myoblasts (Nofziger et al., 1999), again indicating that this role of PS1 is not operating through Notch signalling.
Several studies have reported that overexpression of PS1 alone is insufficient for enhancing γ-secretase activity, without concomitant overexpression of other members of the complex, including nicastrin, Aph-1 and Pen-2 (reviewed by De Strooper, 2003; Parks and Curtis, 2007; Vetrivel et al., 2006). Therefore, when we used expression vectors to constitutively express only PS1, γ-secretase activity should not have been enhanced. Indeed, myoblasts with constitutive expression of PS1 did not show altered levels of the ICD of either Notch1 or Notch2, or of Hes1 (Fig. 4I). However, to ensure that the effects of PS1 were uncoupled from any caused by increasing γ-secretase activity, we also employed, in parallel, potent inhibitors of γ-secretase activity (DAPT and L-685,458).
Constitutive expression of PS1 resulted in approximately fourfold more transfected satellite-cell-derived myoblasts with Pax7 protein than found in controls, and a significant inhibition of myogenic differentiation. Importantly, inhibiting γ-secretase activity with DAPT did not prevent these effects of constitutive PS1 expression, showing that PS1 acts independently of Notch signalling. Constitutive PS1 expression causes Id1 levels to be increased, presumably allowing more Id1 dimerisation with MyoD and inhibition of myogenic differentiation. Therefore, the failure of MyoD to induce Myog probably prevents the inhibition of Pax7 (Olguin et al., 2007).
We further explored the effects of PS1 on myogenesis using mouse cells with two null alleles of PS1 (De Strooper et al., 1999; Herreman et al., 2000). PS1-null mice die in utero and so we examined MyoD-induced myogenesis in PS1–/– MEFs. Ectopic MyoD was able to efficiently induce myogenesis in PS1–/– MEFs maintained under high-serum conditions, and inhibition of γ-secretase activity with either DAPT or L-685,458 did not prevent this. By contrast, MyoD could not induce myogenesis under similar conditions in WT or PS2–/– MEFs (Fig. 7K), with or without concomitant γ-secretase inhibition. Because WT and PS2–/– MEFs had robust Id1 expression but PS1–/– MEFs did not, and co-transfection of MyoD with either PS1 or Id1 prevented myogenic differentiation of PS1–/– MEFs, these observations indicate that PS1, but not PS2, is able to prevent myogenesis by promoting Id1 function. We also independently confirmed that γ-secretase activity is not required for Id1 upregulation by PS1, by using a PS1 mutant that completely lacks γ-secretase activity (Tolia et al., 2006) yet still increases Id1 levels (Fig. 7).
We have recently shown that β-catenin, probably via canonical Wnt signalling, can influence satellite cell fate, with increased β-catenin levels promoting the self-renewal phenotype (Perez-Ruiz et al., 2008). Interestingly, it has been shown that PS1 can interact with various armadillo family members, including β-catenin (reviewed by Parks and Curtis, 2007; Vetrivel et al., 2006). PS1 binds β-catenin and can regulate β-catenin stability, in both positive and negative ways (Meredith et al., 2002; Vetrivel et al., 2006; Zhang et al., 1998). Specific inhibition of BMP signalling results in myogenic differentiation in high-serum culture conditions (Kodaira et al., 2006), and Id1 is a major effector of BMP signalling (Katagiri et al., 2002). We did not find any differences between WT and PS1–/– MEFs in the expression of proteins known to be upstream of Id1, such as pSmad1/5/8 and Egr-1 (our unpublished observations). Pharmacological blockade has shown that interactions between PS1 and β-catenin are independent of γ-secretase activity (Meredith et al., 2002) and, as mentioned above, Notch ICD is not accompanied by Id1 induction in C2C12 myoblasts (Nofziger et al., 1999). Thus, speculatively, PS1 might operate through its effects on β-catenin stability to affect canonical Wnt signalling to control Id1, independently of its γ-secretase activity.
PS1 is also known to regulate Ca2+ homeostasis through γ-secretase-independent mechanisms (Akbari et al., 2004; Tu et al., 2006). In regenerating muscles, Id1 can be negatively regulated by Calcineurin, a Ca2+-calmodulin-dependent serine/threonine protein phosphatase (Sakuma et al., 2005), so PS1 might also control Id1 protein through the regulation of Ca2+ homeostasis. Id1 protein is also known to be controlled by protein stabilisation (Bounpheng et al., 1999), so PS1 might also more directly control Id1 expression through such a mechanism.
Taken together, our data provide evidence of a novel mechanism operating in stem cells, whereby PS1 controls Id1 to regulate the transcriptional activity of bHLH transcription factors, operating independently of γ-secretase activity. In the neural system for example, PS1 is essential for maintenance of the neural progenitor cell pool and for preventing neural differentiation in the developing brain (Hitoshi et al., 2002). In adults, proliferating neural progenitor cells strongly express PS1, but mature neurones do not (Wen et al., 2002). Importantly, Id1 negatively regulates neurogenic transcription factors such as Mash1 in brain (Nakashima et al., 2001; Vinals et al., 2004), paralleling its actions on MyoD in muscle. Speculatively therefore, PS1 might have a common role in maintaining the progenitor cell pool via regulation of Id1 in both muscle and brain. The demonstration of a PS1-Id1 signalling network might also provide new insight into the pathogenesis of mutated PS1-related early-onset familial Alzheimer's disease (reviewed by Parks and Curtis, 2007; Vetrivel et al., 2006).
In conclusion, our study shows that PS1 acts as a potent regulator of fate choice in muscle satellite cells. Undoubtedly, some of the effects of PS1 on satellite cell function are due to its role as a crucial component of the γ-secretase complex, central to Notch signalling. However, PS1 also operates in a γ-secretase-independent manner to control MyoD, and our results show that this is probably achieved through regulation of Id1. The mechanisms that promote satellite cell self-renewal for the maintenance of the stem cell pool and those that prevent precocious myogenic differentiation must be linked and feedback on each other to carefully regulate the extent of differentiation for repair, versus maintenance, of a viable stem cell pool, able to respond to future needs. PS1 control of MyoD transcriptional activity would appear to be one of these links between differentiation and self-renewal mechanisms.
Materials and Methods
Isolation and culture of primary satellite cells and myoblasts
Adult (8-12 weeks old) C57BL10 mice were killed by cervical dislocation, and the extensor digitorum longus (EDL) muscles isolated and digested in collagenase as previously described (Beauchamp et al., 2000). Myofibres and associated satellite cells were isolated and cultured in plating medium (DMEM supplemented with 10% horse serum, 0.5% chicken embryonic extract, 4 mM L-glutamine and 1% penicillin-streptomycin) at 37°C in 5% CO2, as described previously (Perez-Ruiz et al., 2008). Satellite cells were removed from myofibres by enzymatic treatment with 0.125% trypsin-EDTA solution for 10 minutes at 37°C and maintained in high-serum-containing medium (DMEM supplemented with 20% FBS, 1% chicken embryo extract, 10 ng/ml FGF, 4 mM L-glutamine and 1% penicillin-streptomycin). This medium supported both proliferation and differentiation of satellite cell progeny when bFGF was removed. The C2 (Yaffe and Saxel, 1977) subclone C2C12 and 10T1/2 cell lines were obtained from Riken Cell Bank (Tsukuba, Japan). C2C12 cells were maintained in growth medium (GM; F-10 medium supplemented with 20% FBS and antibiotics). For myogenic differentiation (both C2 and satellite cells), the culture medium was replaced with differentiation medium (DM; DMEM containing 2% horse serum and antibiotics) for 72 hours at 37°C. 10T1/2 cells, WT, PS1–/–, PS2–/–, PS1–/–PS2–/– MEFs and PS1–/–PS2–/– MEFs expressing Cys-less PS1 or D385C PS1 (Herreman et al., 1999; Herreman et al., 2003) were maintained in DMEM containing 10% FBS and antibiotics.
Antibodies and Reagents
Antibodies were obtained from the following sources: mouse and rabbit anti-PS1 antibodies from Millipore (Bedford, MA); mouse anti-GFP from Roche (Basel, Switzerland); rabbit anti-PS2 antibody from Abcam; mouse anti-Notch1 antibody from BD Biosciences; rat anti-Ki67 from DAKO; goat anti-PS1 antibody, rabbit anti-Id1 antibody, rabbit anti-Hes1 antibody goat anti-Notch2 antibody and rabbit anti-MyoD antibody were obtained from Santa Cruz (Santa Cruz, CA); mouse anti-developmental myosin heavy chain (dMyHC) antibody from Novocastra (Newcastle, UK); mouse anti-MyHC (MF20), anti-Pax7 antibody, anti-Myog antibody (F5D) and anti-tubulin antibody (E7) were obtained from the DSHB (Iowa City, IA); rabbit anti-p-histone H3 antibody from Cell Signaling Technology (Beverly, MA) and Topro-3, rabbit anti-GFP antibody and mouse anti-V5 antibody from Invitrogen (Carlsbad, CA). Mounting medium containing DAPI was purchased from Vector Laboratories (Burlingame, CA). Nuclei were counterstained with either DAPI or Topro-3. DAPT and L-685,468 were purchased from Peptide Institute (Osaka, Japan) and dissolved and applied in DMSO. γ-secretase activity was measured using a γ-secretase activity detection kit according to the manufacturer's instructions (R&D Systems).
Immunoblot analysis
Immunoblot analysis was performed as previously described (Ono et al., 2006). Rabbit or mouse anti-PS1 (recognise loop domain), anti-PS2, anti-MyHC, anti-tubulin, anti-Pax7, anti-Notch1, anti-Notch2, anti-Hes1, anti-MyoD, anti-Id1 or anti-β-actin antibody were applied at 4°C overnight. Horseradish-peroxidase-conjugated secondary antibodies were used for visualisation by chemiluminescence with a digital luminescent image analyser LAS-1000 (Fujifilm, Tokyo, Japan).
Immunostaining
Immunocytochemistry was performed as previously described (Ono et al., 2007). Primary antibodies were used in PBS as follows: goat anti-PS1 (recognises N-terminal), anti-Id1, anti-Ki67, anti-MyHC, anti-Pax7, anti-Myog, mouse anti-GFP, rabbit anti-GFP, anti-V5 or anti-MyoD antibody at 4°C overnight. For immunohistochemistry, frozen muscle cross-sections were fixed with cold acetone, blocked with M.O.M kit (Vector Laboratories) and incubated with either anti-dMyHC or p-histone H3 antibody. Immunostained myofibres and plated cells were viewed on a Zeiss Axiophot 200M using Plan-Neofluar lenses, or on a Nikon C1si confocal using Plan-Fluor lenses. Digital images were acquired with a Zeiss AxioCam HRm Charge-Coupled Device using AxioVision software version 4.4. Images were optimised globally and assembled into figures using Adobe Photoshop.
RNA interference in vitro
The transfection of siRNA (Stealth siRNA; Invitrogen) into C2C12 myoblasts and primary muscle progenitors cells was performed using Lipofectamine 2000 reagent (Invitrogen) as previously described (Ono et al., 2007). Transfection of siRNA into single myofibres was carried out 20-24 hours after isolation. All samples were examined 72 hours after the transfection. The following siRNA sequences were used: PS1 siRNA-1, 5′-ACTCTCTTTCCAGCTCTTATCTATT-3′; PS1 siRNA-2, 5′-GCACCTTTGTCCTACTTCCAGAATG-3′; and PS2 siRNA, 5′-CCACUAUCAAGUCUGUGCGUUUCUA-3′. The control siRNA sequence and AlexaFlour488-conjugated siRNA were purchased from Invitrogen.
Plasmid construction and transfection
PS1 cDNA was cloned into pMSCV-IRES-GFP (Zammit et al., 2006) or pCMV-V5-expression vectors to generate pMSCV-PS1-IRES-GFP or pCMV-PS1-V5 respectively. Transfection was performed once or twice (10 hours after the first transfection) using Lipofectamine 2000 (Invitrogen) or Lipofectamine LTX (Invitrogen) with Plus Reagents (Invitrogen) in accordance with the manufacturer's instructions.
Muscle injury and in vivo siRNA transfection
Male 8-week old C57BL/6N mice were used according to the Guidelines and Regulations for Laboratory Animal Care of Tohoku University Graduate School of Medicine. Muscle damage was induced by direct intramuscular injection of 50 μl of 10 μM cardiotoxin (Sigma) into the belly of gastrocnemius muscle using a 29G 1/2 insulin syringe. For in vivo siRNA transfection, siRNA duplexes were incubated with Atelocollagen (Koken, Japan) according to the manufacturer's instructions.
Statistical analysis
Data are presented as mean ± standard deviation. Comparisons among groups were determined by the Student's t-test. P values of <0.05 were considered to be statistically significant.
We would like to thank: Bart De Strooper (Center for Human Genetics, Katholieke Universiteit Leuven) for kindly providing the Cys-less PS1 and D385C PS1 constructs, and WT, PS1–/–, PS2–/–, PS1–/–PS2–/– MEFs and PS1–/–PS2–/– MEFs expressing Cys-less PS1 or D385C PS1; Douglas Melton and Robert Benezra for generously sharing constructs pCMV-MyoD and pcDNA3-mId1, respectively through Addgene; the Pax7, Myog, tubulin and MF20 antibodies, developed by A. Kawakami, W. E. Wright, M. Klymkowsky and D. A. Fischman, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa; and Frederico Calhabeu and Paul Knopp for much help. Y.O. received support from Tohoku University research fellowships and is now funded by the Muscular Dystrophy Campaign (grant number RA3/737). V.F.G. is supported by the Medical Research Council (grant number G0700307). This work was supported in part by a research grant from the Uehara Memorial Foundation. The laboratory of P.S.Z. is also supported by the Association of International Cancer Research and the Wellcome Trust. Deposited in PMC for release after 6 months.
References
- Akbari, Y., Hitt, B. D., Murphy, M. P., Dagher, N. N., Tseng, B. P., Green, K. N., Golde, T. E. and LaFerla, F. M. (2004). Presenilin regulates capacitative calcium entry dependently and independently of gamma-secretase activity. Biochem. Biophys. Res. Commun. 322, 1145-1152. [DOI] [PubMed] [Google Scholar]
- Beauchamp, J. R., Heslop, L., Yu, D. S., Tajbakhsh, S., Kelly, R. G., Wernig, A., Buckingham, M. E., Partridge, T. A. and Zammit, P. S. (2000). Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 151, 1221-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra, R., Davis, R. L., Lockshon, D., Turner, D. L. and Weintraub, H. (1990). The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61, 49-59. [DOI] [PubMed] [Google Scholar]
- Bounpheng, M. A., Dimas, J. J., Dodds, S. G. and Christy, B. A. (1999). Degradation of Id proteins by the ubiquitin-proteasome pathway. FASEB J. 13, 2257-2264. [PubMed] [Google Scholar]
- Brack, A. S., Conboy, I. M., Conboy, M. J., Shen, J. and Rando, T. A. (2008). A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50-59. [DOI] [PubMed] [Google Scholar]
- Collins, C. A., Olsen, I., Zammit, P. S., Heslop, L., Petrie, A., Partridge, T. A. and Morgan, J. E. (2005). Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289-301. [DOI] [PubMed] [Google Scholar]
- Conboy, I. M. and Rando, T. A. (2002). The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397-409. [DOI] [PubMed] [Google Scholar]
- Conboy, I. M., Conboy, M. J., Smythe, G. M. and Rando, T. A. (2003). Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575-1577. [DOI] [PubMed] [Google Scholar]
- De Strooper, B. (2003). Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron 38, 9-12. [DOI] [PubMed] [Google Scholar]
- De Strooper, B., Annaert, W., Cupers, P., Saftig, P., Craessaerts, K., Mumm, J. S., Schroeter, E. H., Schrijvers, V., Wolfe, M. S., Ray, W. J. et al. (1999). A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518-522. [DOI] [PubMed] [Google Scholar]
- Esselens, C., Oorschot, V., Baert, V., Raemaekers, T., Spittaels, K., Serneels, L., Zheng, H., Saftig, P., De Strooper, B., Klumperman, J. et al. (2004). Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J. Cell Biol. 166, 1041-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy, O., Piestun, Y., Allouh, M. Z., Rosser, B. W., Rinkevich, Y., Reshef, R., Rozenboim, I., Wleklinski-Lee, M. and Yablonka-Reuveni, Z. (2004). Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev. Dyn. 231, 489-502. [DOI] [PubMed] [Google Scholar]
- Hansson, E. M., Lendahl, U. and Chapman, G. (2004). Notch signaling in development and disease. Semin. Cancer Biol. 14, 320-328. [DOI] [PubMed] [Google Scholar]
- Herreman, A., Hartmann, D., Annaert, W., Saftig, P., Craessaerts, K., Serneels, L., Umans, L., Schrijvers, V., Checler, F., Vanderstichele, H. et al. (1999). Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc. Natl. Acad. Sci. USA 21, 11872-11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman, A., Serneels, L., Annaert, W., Collen, D., Schoonjans, L. and De Strooper, B. (2000). Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat. Cell Biol. 2, 461-462. [DOI] [PubMed] [Google Scholar]
- Herreman, A., Van Gassen, G., Bentahir, M., Nyabi, O., Craessaerts, K., Mueller, U., Annaert, W. and De Strooper, B. (2003). gamma-Secretase activity requires the presenilin-dependent trafficking of nicastrin through the Golgi apparatus but not its complex glycosylation. J. Cell Sci. 116, 1127-1136. [DOI] [PubMed] [Google Scholar]
- Hitoshi, S., Alexson, T., Tropepe, V., Donoviel, D., Elia, A. J., Nye, J. S., Conlon, R. A., Mak, T. W., Bernstein, A. and van der Kooy, D. (2002). Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 16, 846-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert, S. S., Ilagan, M. X., De Strooper, B. and Kopan, R. (2005). Analysis of Notch function in presomitic mesoderm suggests a gamma-secretase-independent role for presenilins in somite differentiation. Dev. Cell 8, 677-688. [DOI] [PubMed] [Google Scholar]
- Jarriault, S., Brou, C., Logeat, F., Schroeter, E. H., Kopan, R. and Israel, A. (1995). Signalling downstream of activated mammalian Notch. Nature 377, 355-358. [DOI] [PubMed] [Google Scholar]
- Jen, Y., Weintraub, H. and Benezra, R. (1992). Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 6, 1466-1479. [DOI] [PubMed] [Google Scholar]
- Jones, N. C., Tyner, K. J., Nibarger, L., Stanley, H. M., Cornelison, D. D., Fedorov, Y. V. and Olwin, B. B. (2005). The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 169, 105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri, T., Imada, M., Yanai, T., Suda, T., Takahashi, N. and Kamijo, R. (2002). Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 7, 949-960. [DOI] [PubMed] [Google Scholar]
- Kinouchi, N., Ohsawa, Y., Ishimaru, N., Ohuchi, H., Sunada, Y., Hayashi, Y., Tanimoto, Y., Moriyama, K. and Noji, S. (2008). Atelocollagen-mediated local and systemic applications of myostatin-targeting siRNA increase skeletal muscle mass. Gene Ther. 15, 1126-1130. [DOI] [PubMed] [Google Scholar]
- Kitzmann, M., Bonnieu, A., Duret, C., Vernus, B., Barro, M., Laoudj-Chenivesse, D., Verdi, J. M. and Carnac, G. (2006). Inhibition of Notch signaling induces myotube hypertrophy by recruiting a subpopulation of reserve cells. J. Cell Physiol. 208, 538-548. [DOI] [PubMed] [Google Scholar]
- Kodaira, K., Imada, M., Goto, M., Tomoyasu, A., Fukuda, T., Kamijo, R., Suda, T., Higashio, K. and Katagiri, T. (2006). Purification and identification of a BMP-like factor from bovine serum. Biochem. Biophys. Res. Commun. 345, 1224-1231. [DOI] [PubMed] [Google Scholar]
- Kopan, R., Nye, J. S. and Weintraub, H. (1994). The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development 120, 2385-2396. [DOI] [PubMed] [Google Scholar]
- Kuang, S., Kuroda, K., Le Grand, F. and Rudnicki, M. A. (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammich, S., Okochi, M., Takeda, M., Kaether, C., Capell, A., Zimmer, A. K., Edbauer, D., Walter, J., Steiner, H. and Haass, C. (2002). Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J. Biol. Chem. 277, 44754-44759. [DOI] [PubMed] [Google Scholar]
- Lepper, C., Conway, S. J. and Fan, C. M. (2009). Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro, A. (1961). Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell, I. W., Ishibashi, J., Le Grand, F., Punch, V. G., Addicks, G. C., Greenblatt, J. F., Dilworth, F. J. and Rudnicki, M. A. (2008). Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat. Cell Biol. 10, 77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith, J. E., Jr., Wang, Q., Mitchell, T. J., Olson, R. E., Zaczek, R., Stern, A. M. and Seiffert, D. (2002). Gamma-secretase activity is not involved in presenilin-mediated regulation of beta-catenin. Biochem. Biophys. Res. Commun. 299, 744-750. [DOI] [PubMed] [Google Scholar]
- Nagata, Y., Kobayashi, H., Umeda, M., Ohta, N., Kawashima, S., Zammit, P. S. and Matsuda, R. (2006a). Sphingomyelin levels in the plasma membrane correlate with the activation state of muscle satellite cells. J. Histochem. Cytochem. 54, 375-384. [DOI] [PubMed] [Google Scholar]
- Nagata, Y., Partridge, T. A., Matsuda, R. and Zammit, P. S. (2006b). Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J. Cell Biol. 174, 245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, K., Takizawa, T., Ochiai, W., Yanagisawa, M., Hisatsune, T., Nakafuku, M., Miyazono, K., Kishimoto, T., Kageyama, R. and Taga, T. (2001). BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc. Natl. Acad. Sci. USA 98, 5868-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofziger, D., Miyamoto, A., Lyons, K. M. and Weinmaster, G. (1999). Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development 126, 1689-1702. [DOI] [PubMed] [Google Scholar]
- Olguin, H. C., Yang, Z., Tapscott, S. J. and Olwin, B. B. (2007). Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J. Cell Biol. 177, 769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, Y., Sensui, H., Sakamoto, Y. and Nagatomi, R. (2006). Knockdown of hypoxia-inducible factor-1alpha by siRNA inhibits C2C12 myoblast differentiation. J. Cell Biochem. 98, 642-649. [DOI] [PubMed] [Google Scholar]
- Ono, Y., Sensui, H., Okutsu, S. and Nagatomi, R. (2007). Notch2 negatively regulates myofibroblastic differentiation of myoblasts. J. Cell Physiol. 210, 358-369. [DOI] [PubMed] [Google Scholar]
- Parks, A. L. and Curtis, D. (2007). Presenilin diversifies its portfolio. Trends Genet. 23, 140-150. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz, A., Ono, Y., Gnocchi, V. F. and Zammit, P. S. (2008). beta-Catenin promotes self-renewal of skeletal-muscle satellite cells. J. Cell Sci. 121, 1373-1382. [DOI] [PubMed] [Google Scholar]
- Repetto, E., Yoon, I. S., Zheng, H. and Kang, D. E. (2007). Presenilin 1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J. Biol. Chem. 282, 31504-31516. [DOI] [PubMed] [Google Scholar]
- Sakuma, K., Nakao, R., Aoi, W., Inashima, S., Fujikawa, T., Hirata, M., Sano, M. and Yasuhara, M. (2005). Cyclosporin A treatment upregulates Id1 and Smad3 expression and delays skeletal muscle regeneration. Acta Neuropathol. 110, 269-280. [DOI] [PubMed] [Google Scholar]
- Seale, P., Sabourin, L. A., Girgis-Gabardo, A., Mansouri, A., Gruss, P. and Rudnicki, M. A. (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777-786. [DOI] [PubMed] [Google Scholar]
- Shen, J., Bronson, R. T., Chen, D. F., Xia, W., Selkoe, D. J. and Tonegawa, S. (1997). Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89, 629-639. [DOI] [PubMed] [Google Scholar]
- Skapek, S. X., Rhee, J., Kim, P. S., Novitch, B. G. and Lassar, A. B. (1996). Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRB hyperphosphorylation. Mol. Cell. Biol. 16, 7043-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl, G. and Greenwald, I. (1999). Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398, 522-525. [DOI] [PubMed] [Google Scholar]
- Tolia, A., Chavez-Gutierrez, L. and De Strooper, B. (2006). Contribution of presenilin transmembrane domains 6 and 7 to a water-containing cavity in the gamma-secretase complex. J. Biol. Chem. 281, 27633-27642. [DOI] [PubMed] [Google Scholar]
- Tu, H., Nelson, O., Bezprozvanny, A., Wang, Z., Lee, S. F., Hao, Y. H., Serneels, L., De Strooper, B., Yu, G. and Bezprozvanny, I. (2006). Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell 126, 981-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel, K. S., Zhang, Y. W., Xu, H. and Thinakaran, G. (2006). Pathological and physiological functions of presenilins. Mol Neurodegener. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinals, F., Reiriz, J., Ambrosio, S., Bartrons, R., Rosa, J. L. and Ventura, F. (2004). BMP-2 decreases Mash1 stability by increasing Id1 expression. EMBO J. 23, 3527-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, P. H., Friedrich, V. L., Jr., Shioi, J., Robakis, N. K. and Elder, G. A. (2002). Presenilin-1 is expressed in neural progenitor cells in the hippocampus of adult mice. Neurosci. Lett. 318, 53-56. [DOI] [PubMed] [Google Scholar]
- Wilson, C. A., Murphy, D. D., Giasson, B. I., Zhang, B., Trojanowski, J. Q. and Lee, V. M. (2004). Degradative organelles containing mislocalized alpha-and beta-synuclein proliferate in presenilin-1 null neurons. J. Cell Biol. 165, 335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, P. C., Zheng, H., Chen, H., Becher, M. W., Sirinathsinghji, D. J., Trumbauer, M. E., Chen, H. Y., Price, D. L., Van der Ploeg, L. H. and Sisodia, S. S. (1997). Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387, 288-292. [DOI] [PubMed] [Google Scholar]
- Yaffe, D. and Saxel, O. (1977). Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725-727. [DOI] [PubMed] [Google Scholar]
- Yoshida, N., Yoshida, S., Koishi, K., Masuda, K. and Nabeshima, Y. (1998). Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates `reserve cells'. J. Cell Sci. 111, 769-779. [DOI] [PubMed] [Google Scholar]
- Zammit, P. S. (2008). All muscle satellite cells are equal, but are some more equal than others? J. Cell Sci. 121, 2975-2982. [DOI] [PubMed] [Google Scholar]
- Zammit, P. S., Golding, J. P., Nagata, Y., Hudon, V., Partridge, T. A. and Beauchamp, J. R. (2004). Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 166, 347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit, P. S., Relaix, F., Nagata, Y., Ruiz, A. P., Collins, C. A., Partridge, T. A. and Beauchamp, J. R. (2006). Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 119, 1824-1832. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Hartmann, H., Do, V. M., Abramowski, D., Sturchler-Pierrat, C., Staufenbiel, M., Sommer, B., van de Wetering, M., Clevers, H., Saftig, P. et al. (1998). Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395, 698-702. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Nadeau, P., Song, W., Donoviel, D., Yuan, M., Bernstein, A. and Yankner, B. A. (2000). Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat. Cell Biol. 2, 463-465. [DOI] [PubMed] [Google Scholar]