Abstract
Because technology licensed from research organizations can play a significant role in drug innovation and the generation of novel biomedical products, licensee performance under such agreements must be effectively monitored. This is necessary so that resultant benefits, including public health improvement, may be returned to the innovator(s) as well as society at large. The tasks that comprise monitoring are varied, but all come under the general heading of ‘enforcement of license provisions’.
Since 1996, the license monitoring and enforcement program established by the US National Institutes of Health (NIH) Group has collected about $US17 million in unpaid and underpaid license royalties through formal financial audits and other investigative activities. During the same period, the Office of Technology Transfer (OTT) settled more than 60 cases of suspected patent infringement, generating around 60 new licenses and collected both back and ongoing royalties. As these numbers show, an active and effective monitoring program is an essential part of any technology transfer or biomedical licensing program.
The Office of Technology Transfer (OTT) at the National Institutes of Health (NIH) has become a major licensor of biomedical technology, being responsible for the commercial licensing of biomedical inventions of NIH and US FDA scientists to companies for commercial development.[1] For the NIH, this licensing function is permitted and governed under US laws by 35 United States Code (USC) 209 and 42 USC 241 (a) and US regulations by 37 (Code of Federal Regulations) CFR part 404.[2] In 1996, the NIH established a license monitoring and enforcement group within the OTT to supplement the traditional patenting, marketing and licensing activities of the office. Since that time, the OTT’s license monitoring and enforcement group has collected about $US17 million[3] in unpaid and underpaid license royalties through formal financial audits and other investigative activities. Also during this same period, the OTT settled 60 suspected patent infringement cases, generating 60 new licenses and collected both back and ongoing license royalties. As these numbers highlight, an active and effective monitoring program has become an essential part of the technology transfer programs at the NIH.
This article explores, in detail, the realm of biomedical license monitoring at the NIH, and the results achieved to date. While our experience is limited to out-licensing of biomedical technology, we believe that our experience is typical and licensors (and licensees) of any intellectual property may find this article to be useful.
1. Rationale for Monitoring License Agreements
Many individuals who are new to biomedical technology licensing may think that a license execution itself may be the end of the process. However, the execution of a license agreement is merely the start of the process of transferring the actual technology from licensor to licensee, beginning the product development process with the goal of eventually deriving income or other benefits from the licensed patents or biological materials.
Technology license monitoring itself can be summarized as everything that needs to be done after a license is executed. The tasks that comprise monitoring are varied, but all come under the general heading of ‘enforcement of license provisions’. A list of the monitoring functions that have been adopted at the OTT and that would be applicable for similar monitoring programs is shown in table I.
Table I.
License monitoring tasks adopted at the US Office of Technology Transfer
| Regular compliance review of license agreements |
| Identifying and settling patent infringements |
| Settling of license disputes |
| Performing royalty audits |
| Collecting overdue royalty payments |
| Other royalty issues |
There are some important reasons for carefully monitoring license agreements and these are identified and discussed in sections 1.1 to 1.5.
1.1 Assuring the Technology is Being Developed
Unfortunately, exclusive licensees may not always license technology to use it in the specific development of a biomedical product. Development strategies may change over time and the licensed technology may become a blocking-strategy to prevent competitors from working in that area. Thus, the licensee may have no direct interest in using the technology, but may see an opportunity for a competitor to use the technology to develop products or services that obsolete the licensee’s business.
Other exclusive licensees may seek to license technology so that it can be resold at higher valuations to sublicensees. Sublicensing can be a legitimate business development pathway for a pharmaceutical product (e.g. from a nonprofit institution to a biotechnology company to a pharmaceutical company) and be lucrative for a licensee, but it is not in the interests of nonprofit licensors such as the NIH to provide licenses for technology blocking or solely for sublicensing.
Exclusive licensees must actually try to commercialize the licensed technology, usually according to a timetable specified in the agreement. Failure to do so may result in the termination of the license by the OTT or amendment of the license with new benchmarks, which require payment of additional fees. Licensors should also be aware that nonexclusive licensing of technology in many circumstances could also help ensure its use and development, especially for technologies such as research tools.[4]
1.2 Assuring All Users of the Technology Are Licensed
Licensors such as the OTT need to investigate cases of potential patent infringement that are reported by its inventors or by other licensees, or that are discovered through the research efforts of its own staff. Elimination of infringement is important because patent infringement deprives inventors and research organizations of royalties and it places actual licensees at a competitive disadvantage because they are paying royalties on products or services sold, while their competitors are not.
1.3 Determining If Royalties Are Overdue
After a license agreement is executed, royalties of different types are usually due as specified in each agreement. It has been the experience of the OTT’s license monitoring and enforcement group that assisting with the collection of unpaid or late royalties is the most time-consuming task associated with technology licensing and perhaps the most frequent problem encountered with licensees.
1.4 Determining If Royalties Have Been Properly Paid
Even when a licensee pays royalties on time, it is important that the licensor determines whether or not the proper amount has been paid. Licensees may underpay (or even overpay) royalties for many reasons including confusing license language regarding royalties, misunderstandings about licensed products, poor reporting and accounting practices within companies, and many other reasons, including greed. The OTT’s monitoring group uses independent auditors who specialize in royalty audits and other tools to determine the proper royalty amounts due under each agreement.
1.5 Assuring All Licensees Are Treated Fairly
Patent infringement by unlicensed competitors unfortunately is not the only example of unfair treatment that licensees may experience. Changes in the scope of patent claims during patent prosecution, royalty overpayment, as well as changes in patent and contract law, regulatory requirements, or other factors may place a licensee in an unfair position that may need to be remedied.
2. Tracking License Compliance
Routine tracking of license compliance is the backbone of the license monitoring process. When reviewing any completed license agreement, a number of questions must be asked as shown in table II. The OTT has prepared its own License Review Summary form (see sample worksheet in figure 1) to aid in the license review process. This form prompts the reviewer to collect all of the pertinent information about the license agreement. When the form is completed, it provides a detailed picture of the license status so that all areas needing attention can be identified. The next step is to use the completed form to answer the major license compliance questions, as discussed in sections 2.1 to 2.6. A case study is presented in section 2.7.
Table II.
Tracking license compliance: questions to be asked when reviewing a completed license agreement
| Are the royalty payments up-to-date and accurate? |
| Are the progress reports up-to-date? |
| Have the performance benchmarks been met? |
| Have new patents been issued or existing patents expired? |
| Has the license expired? |
| Is the licensee using, hoarding or reselling the technology? |
| Have any sublicenses been granted? |
| Is there another potential exclusive licensee? |
Fig. 1.
The Office of Technology Transfer’s License Review Summary form. E = employee invention report number; ICD = institution, center or division; L = license number; MAR = minimum annual royalty; USPN = US patent number; USSN = US Patent Office serial number.
2.1 Are the Royalty Payments Up to Date and Accurate?
As a first step, the records of payments received are reviewed to make sure all payments including execution, minimum annual, and benchmark royalties have been paid. If there is a product on the market, licensors should confirm that earned royalties are being paid and royalty reports, detailing licensed product sales, are in the license file. Accounting figures such as these are best kept in a license database for easy access and administration. The royalty reports should include enough detail to understand how net sales were derived from gross sales numbers and to determine if any licensed products are not being reported, and should include details of any adjustments made to net sales prior to calculating the royalty due. Examples of allowed deductions include returns and allowances, packing costs, insurance costs, freight out, taxes or excise duties imposed on the transaction (if separately invoiced), and wholesaler and cash discounts in reasonable amounts.
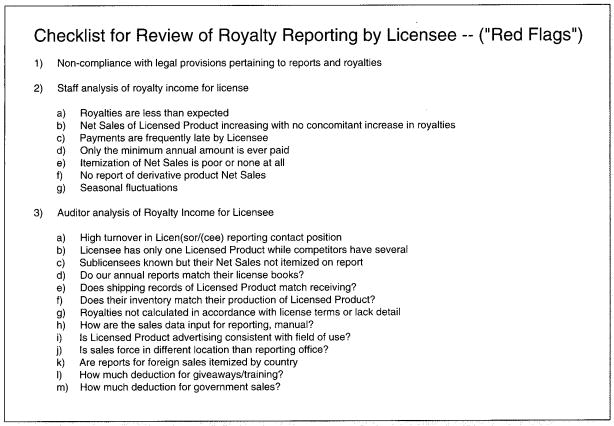
If any royalties are not paid on time, or if the royalty reports do not provide sufficient detail, a member of the monitoring group will obtain accurate sales information from the licensee and collect the appropriate royalties. A checklist for reviewing licensee royalty reporting is included (see the sample worksheet in figure 2).
Fig. 2.
The Office of Technology Transfer’s checklist for review of royalty reporting by licensee (‘red flags’).
2.2 Are the Progress Reports Up to Date?
Licensors should plan to review any progress reports received on a timely basis in order to determine if the licensee is developing the licensed product on schedule and also to determine whether sales of a licensed product are being reported. If a licensed product is being sold, the licensee is required to report the date that the product sale was initiated so the OTT can determine if earned royalties have been paid back to that date.
2.3 Have the Performance Benchmarks Been Met?
If any benchmark requirements are overdue, the OTT will call the licensee’s contact person and discuss the situation. It is important to get as much material in writing as possible to document any progress toward benchmarks. If any benchmarks are more than one year overdue, it is critical to alert the licensee and find out if the licensee wants to keep and amend the license (requiring payment of additional royalties) or terminate it.
2.4 Have Any Sublicenses Been Granted?
Although under many license agreements, including those from the US government, licensees are required to request approval before sublicensing any patent rights, this requirement may be overlooked by licensees in their haste to complete a transaction.[5] When unsure, it is recommended to call the licensee’s contact person or their general counsel and request a list of sublicensees, along with executed copies of the sublicenses.
2.5 Have Any of the Patent Rights Lapsed or Expired?
Using database records, the OTT monitoring specialists check to confirm whether maintenance fees or annuities have been paid on the licensed patent rights. If in doubt, it may be necessary to query the relevant patent office. Using database records, it is also possible to calculate the expiration dates for each patent in a license agreement to determine if any have naturally expired.
2.6 Has the License Expired or Been Terminated?
For this, the OTT would determine the expiration date of the agreement, based on the language in the agreement and/or the patent expiration dates. Database or license records should also be checked to see if the license agreement has been prematurely terminated with or without cause. For example, biomedical companies may typically terminate NIH license agreements for any reason with only 60 days’ written notice.
When the above information is complete, a determination about the licensee’s compliance can be made. If it is found, for example, that the licensee is not in compliance with the license agreement, the following questions would typically be asked by the NIH before taking further action:
How important is the licensee’s product from a public health point of view?
What are the licensee’s true intentions for the technology (if no product is available yet)?
Are there other licensees or potential licensees for this technology?
Other licensors of biomedical technology may have different questions based upon their institutional goals for their own licensing program. Using the answers to the above questions, the OTT would determine what alternatives are to be offered before notifying the licensee in writing about the noncompliance under the license. In the most severe cases, the notification letter will provide the official notice that the licensee is in default of a material provision of the agreement, effectively beginning the termination process. Such a letter should describe the default in detail and cite the pertinent paragraph(s) in the agreement covering the situation. This letter can also suggest how the licensee can attain compliance, which may involve negotiating new deadlines for completion of data, reports or payments, and will vary with each situation.
2.7 Case Study 1
An exclusive biomedical licensee issued a press release announcing that their licensed product had failed to receive FDA approval following clinical trials. Anticipating that the licensee would now terminate the license, the OTT reviewed the license file. The licensee was delinquent in paying its current minimum annual royalty and had not submitted a recent progress report. When contacted about these problems, the licensee stated that the ‘licensed product’ really did not fall under the licensed claims, even though previous progress reports inferred that it did. Thus, no licensed product was ever developed and no performance benchmarks were met. Since the licensee had never developed a licensed product, they reasoned that they never needed the license and so now did not owe reports or royalties.[6]
The OTT carefully investigated the product that was developed and the licensee’s history. The product that had entered clinical trials was indeed slightly different from the claimed compositions and, as a result, the licensee should have reported this fact to the OTT and terminated the agreement many years before. They apparently chose to keep it in force to make the technology unavailable to competitors. The investigation concluded with the licensee’s termination of the license and payment of the overdue minimum annual royalty. Lesson learned from the licensor’s perspective: trust, but verify.
3. Performing Royalty Audits
Royalty audits of license agreements are designed to keep the licensee honest and assure a licensor such as the NIH that the payments reported and made are fair and correct. Although this would seem to be a fairly simple process, in practice it can be complex, expensive, and difficult. Persistence and a suspicious nature are traits that help lead to successful royalty audits. Table III lists the major issues that initiate a royalty audit and the key information an auditor needs to discover; this is further discussed in section 3.1 and section 3.2.
Table III.
Performing royalty audits: major issues that initiate a royalty audit and the key information an auditor needs to discover
| Choosing licenses for audit |
| Annual earned royalties greater than $US100 000 |
| History of late, variable or rapidly changing payments |
| Reported sales differ from that reported to others |
| Key information from an audit |
| Gross sales broken out by product number |
| Testing of gross sales by invoice comparison |
| Listing and review of entire licensed product line |
| Listing and review of all countries where licensed products are sold |
| Review of adjustments made to gross sales to obtain net sales |
3.1 Choosing Licensees for Audit
Since the average royalty audit, performed by a contractor, may well cost $US20 000 or more, it is prudent to audit licensees where the potential recovery could possibly cover the cost of the audit. As a result, those licenses for which the average annual royalty paid is at least $US100 000 are the best candidates for auditing, which would likely include virtually any biomedical-related product license agreement. License agreements with incomes <$US100 000 per year may be audited through ‘desk audits’ by internal staff that can request and analyze standard information from the licensee without a site visit.
Other important factors to consider in choosing licensees for audit are a history of late payments, payments that vary significantly or payment amounts that are rapidly decreasing. Another royalty compliance ‘red flag’ are sales reports that differ from those reports to other sources, such as the US Security and Exchange Commission (SEC)[7] or the press or other public sources.[8] A checklist for review of royalty reporting by the licensee is shown in figure 2.
3.2 What a Royalty Audit Must Include
A royalty audit must begin with a list of the known licensed products and their variations. The auditor should begin with an accounting of gross sales of licensed products. Without gross sales figures, there is no way to verify that all of the adjustments made to arrive at net sales are accurate. The gross sales must be broken down by product number so that a reviewer can account for the sales of all licensed products. Otherwise, it is impossible to determine whether or not a licensee is omitting products from the royalty report. Gross sales numbers should also be tested by random comparison to actual invoices to verify their accuracy.
Throughout this process, the auditor should compare the product descriptions to the starting list and seek immediate clarification of any discrepancies. The auditor must also list all of the countries where licensed products are sold and review it carefully against the gross sales figures. The auditor should follow up by reviewing all adjustments made to gross sales, by the licensee, to arrive at the net sales subject to royalty. Any discrepancies found by the auditor should be documented in the final report. Royalty auditors should also be instructed to request detailed product literature from the licensee such as marketing brochures and product inserts, because they can be useful in determining whether sales of any licensed products have been omitted from past royalty reports.
4. Typical Findings in a Royalty Audit
Some examples of the audit findings for license agreements that have been reported to the OTT and could occur for any licensor are discussed in sections 4.1 to 4.3.
4.1 Misinterpretation by the Licensee of What Constitutes a Licensed Product
Because of a poor understanding of patent claims or contract language, a licensee may omit certain products that they feel are not covered by the license agreement, resulting in underpayment of earned royalties. Examples of this type of error by the licensee include the following assumptions: that some forms of the product are exempt from royalty; that sales in certain countries are exempt from royalty; that certain types of sales are exempt; and that no royalty is due on sales of products covered by pending patent claims.
4.2 Poor Understanding of Product Specifications
Sometimes royalties are incorrectly calculated based on the company internal transfer price (often arbitrary) of the licensed product, rather than the higher price obtained when the product actually leaves the company via sale to an unrelated party. Also, simple errors can be the cause of certain licensed product sales being unreported. An example of when this can occur is where foreign sales are reported to a US home office only in summary form. In such cases, omitted product sales and mathematical errors are difficult to catch because the raw numbers are not always reviewed before they are reported to the licensor.
4.3 Royalty Audit Collection
In the OTT’s experience, the cost of license royalty audits depends on the amount of travel required, the amount of on-site time required, and the complexity of the data analysis. For the NIH, the typical audit recovery to date has been in the $US 100 000 to $US200 000 range and with the highest single recovery so far being $US10 000 000. While recoveries can be substantial, collecting the underpaid royalties can require as much time and effort, or more, than the audit required. Convincing the licensee to pay the amounts reported by the auditor may require license termination, addition of interest and penalties, or litigation. Licensees may also choose to first pay the requested amount, to avoid interest or termination, and then sue to recover the payment.
5. Collecting Overdue Royalty Payments
Unpaid or underpaid royalties can be a significant source of royalty income, but establishing how much is overdue and invoicing the licensee is just the beginning. The job really is incomplete until the licensee’s payment has been cleared by the bank. The general process that the OTT employs is described in table IV. A case study is presented in section 5.1.
Table IV.
Collecting overdue royalties: general process for licensors
| Find responsible person at licensee (hardest part) |
| Establish reason(s) for non-payment |
| Settle any problems or disputes preventing payment |
| Follow up frequently until payment is sent |
| Use an effective collection letter |
Finding the responsible person at the licensee is the hardest and most critical part of the collection process. If you deal with the wrong person, weeks or months of effort may be wasted when you are referred to another contact at the company. Usually, a senior employee in business development, licensing or finance is the person who can translate your invoice, phone calls and follow-up letters into a payment. Once found, the responsible person should be used to establish the reason(s) for nonpayment. Reasons such as ignorance of the license or inadvertent missing of the payment date do not require any specific follow-up, but sometimes licensees refuse payment because they have a problem with the license or the licensor. Their nonpayment may be one way to express their dissatisfaction without confronting the licensor. The problem may be license terms, once deemed fair, but now considered to be onerous, or lack of responsiveness to inquiries made to the licensor’s staff. If any such issues preventing payment are discovered, they should be considered carefully and settled promptly, if possible. Compromise solutions should be considered if the licensee’s wishes cannot be granted exactly as requested.
Follow-up is the second most critical part of the collection process. The licensee should be followed up frequently until all overdue payments are received. ‘Frequent’ means at least once per month and more often when discussions reach a critical stage or deadlines are approaching. A deadline should be assigned to all requests and followed-up just before and just after the deadline. Consequences for missing a deadline should be established, such as loss of the privilege of licensing additional technologies or referring the matter to a collection agent.[9] Once referred to collection by the NIH, such debts incur additional interest and penalties and the overdue amounts are reported to major credit bureaus. This is an effective means of collecting royalties due for license agreements but only after trying other solutions and allowing at least three requests for payment (usually one month apart) to be sent.
5.1 Case Study 2
A company licensee terminated their license agreement still owing $US60 000 and refused written and oral requests to pay it. The OTT met with the licensee and established that the Chief Executive Officer (CEO) was disappointed in the scientific results obtained with the licensed technology. He saw the license royalties as a guarantee of specific results, even though the licensed technology itself was only early-stage. The OTT informed the CEO that his reason for nonpayment was not based on patent law, contract law or fairness and so was irrelevant to the nonpayment issue. Such behavior is sometimes referred to as ‘corporate pouting’. The licensee still refused to pay, so the OTT requested the payment be partially offset by a $US40 000 payment owed to the company from another part of the NIH. The remaining $US20 000 was sent to US federal debt collection and was paid a few months later.[10]
6. Identifying and Settling Suspected Patent Infringement
Licensors can learn of potential patent infringement from a number of sources, some of which are summarized in table V. One source is complaints from existing licensees who do not want to be at a financial disadvantage versus their competitors, because they have to add the cost of license royalties to their products. Another source is warnings from inventors who are interested in seeing that all users of their technology are licensed. Routine web searches by technology area or company and review of pharma/biotech press releases will identify a partial list of specific technology users. Review of US FDA approvals, bulletins, advisory committee meetings and guidelines also helps to identify infringing and potentially infringing companies.[11] Whatever the source of information, the goal of a biomedical licensing program is to negotiate a license agreement for what appears to be an infringing product or activity.
Table V.
Sources for identifying potential patent infringement
| Complaints from licensees |
| Heads up (alerting someone) from inventors |
| Routine web or DIALOG® searchesa |
| Review of pharma and biotech press releases |
| Review of US FDA information |
Searching for legal, corporate and financial information about a company.
Once a suspected patent infringement is identified, the company must be given notice, in writing, of that infringement. Back royalties can typically only be collected for a maximum of 6 years prior to the date of an initial infringement-warning letter.[12] A model letter can be crafted with the assistance of legal counsel. If the technology already has licensees who have the right to enforce and sublicense the patent, this letter should be sent from them. For the NIH, the letter would typically include a description of the patent(s), a description of the suspected infringing products or services, a copy of the patent(s) and a license application form. A courier or registered mail should be used to confirm receipt, but in our experience, registered mail is less reliable.
It is important to follow-up on the warning letter within 1 month to prevent the suspected infringing company from ignoring the issue and thus to clearly send the message that the issue is important and that the patent owner is diligent and persistent. Without good follow-up, about half of the warning letters will be ignored.
With the infringing company’s direct attention, various incentives may be offered to quickly settle the suspected infringement and thus avoid litigation. One suggestion is to forgive any potential interest or penalties on back royalties in return for a prompt settlement. For small, private companies, it is also useful to point out the adverse effect that an unsettled infringement dispute will have on the resale value of that company. With a public company that is using the unlicensed technology in a major product, the senior management of the firm should be reminded that patent infringement has been the subject of fraud litigation by shareholders or other investors. For unlicensed products, it is also possible to notify distributors or corporate customers that the use or sale of such products results in a potential royalty liability until someone in the distribution chain executes a license and pays past and future royalties. If all else fails litigation can be considered, which for the NIH means a request from the Office of the General Counsel to the US Department of Justice to sue the company for patent infringement. Similarly, other licensors would seek to hire their own litigation counsel to initiate such action.
6.1 Case Study 3
A medium-sized company appeared to be infringing certain patents for diagnostic tests, but when contacted, they refused to discuss a license citing ‘exhaustion of patent rights’. Their rationale was that since they were buying their antigen, a component of the kit, from an existing OTT licensee, they had effectively paid a royalty on their finished product and could not be required to pay that royalty again. The OTT pointed out to the company that the patents at issue contained claims to complete kits (not licensed by the antigen supplier), as well as to the antigens themselves. The OTT agreed that the company did not owe additional royalties on the antigen and incorporated into their license agreement a provision to allow the deduction of antigen costs from the net sales numbers, prior to calculation of the royalty due. The company settled with the OTT, paid $US420 000 in back royalties and continues to pay substantial annual royalties.[13]
7. Settling License Disputes
License disputes are not always easy to identify and may at first appear to be a different problem. For example, nonpayment of royalties by a licensee may be precipitated by a license dispute known only to the licensee. Our general approach is outlined in table VI. Once a dispute is discovered, the licensee’s complaint should be analyzed in detail. In considering the licensee’s complaint, the contract law issues should be clearly separated from the patent law issues. A fair solution to the issue should be offered, in the interest of a quick settlement. If the dispute persists, an outside legal opinion should be sought and a solution in line with that opinion offered. The offer should be discussed in person, if necessary. If an agreement is reached, the license should be amended to incorporate the solution. If no agreement can be reached, the license should be terminated and any unpaid royalties collected.
Table VI.
Settling license disputes: general approach for licensors
| Analyze licensee’s complaint in detail |
| Separate contract law from patent law issues |
| Offer a compromise solution if dispute is valid |
| If dispute persists, seek outside opinion |
| Offer a solution in line with opinion |
| Discuss in person if necessary |
| Amend license to incorporate solution or terminate license |
7.1 Case Study 4
A large pharmaceutical firm stopped paying significant annual royalties over a license dispute. On the surface, the dispute seemed to involve the validity of the licensed patents, but a better understanding revealed that the licensee was seeking a type of ‘revenge’ for the NIH not assisting in their own separate litigation concerning a closely related technology. The OTT offered various settlement terms and all were rejected. Various solutions were considered, including offsetting the owed royalties against payments owed to the licensee. Finally, the OTT sent a termination letter giving the licensee thirty days to cure the problem. On day twenty-nine, a settlement meeting was called and the parties were able to agree on a solution, in part because the OTT understood and discussed the underlying reasons. About $US170000 in back royalties were collected along with the potential for substantial future royalties.[14]
8. Other Biomedical License Compliance Problems
8.1 Reporting
The previous sections of this article discussed some of the major license compliance problems that the OTT (and similarly other licensors) can encounter, but there are also some minor problems related to royalty reporting that can be significant because of their frequency.
8.1.1 No Report
Licensees sometimes fail to provide a report by the due date. The reason for this may be a lack of funds to pay the royalties, a lack of awareness of the deadline (i.e., after a sale or merger of a business or a change of employees) or simply forgetting. The licensee should simply be sent a reminder and warned of possible license termination.
8.1.2 No Sales Figures
A royalty report may include just a payment and a letter specifying the amount of royalties the company feels is due. However, if the gross sales figures (broken down by product number) and all of the calculations leading from gross sales to royalty owed are not included in the report, there is no way to judge the accuracy of the royalty payment. The licensee should be sent a sample royalty worksheet and reminded of possible license termination.
8.1.3 Incorrect Mathematics
It is important to check the mathematics in each royalty report. This is usually done by the Royalties Coordinator’s staff. The licensee should be notified of any discrepancy and billed for any shortfall or refunded any overage.
8.1.4 Indecipherable Reports
Some reports cannot be deciphered because they contain no license reference number or licensed product name or description. The categories on the royalty worksheet may be cryptic or the entire report may bear no resemblance to anything previously submitted by the licensee. The licensee should be sent a sample royalty worksheet (see figure 3) and reminded of possible license termination.
Fig. 3.
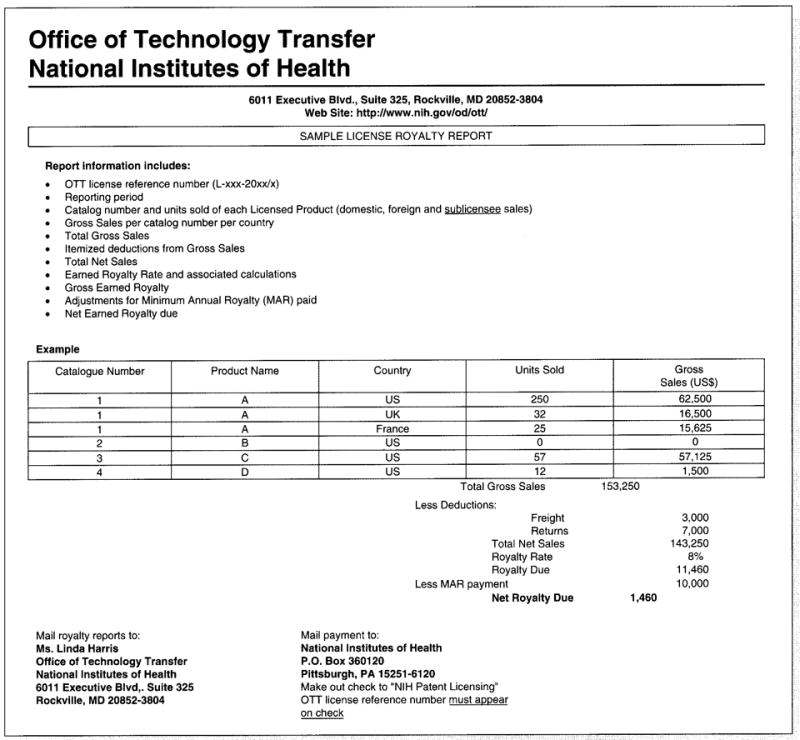
The Office of Technology Transfer’s License Sample Royalty Report form.
8.1.5 Improper Sales Exemption
Sales of products may be exempt from earned royalty obligations to certain purchasers in accordance with the law or the specific terms of the license agreement. In such circumstances, it is important that the licensee be able to document that any exempt sales reported were indeed made to appropriate customers and in the proper manner in order to be relieved of the royalty obligation to the licensor.
8.1.6 Improperly Withheld Income Tax
Some foreign countries require licensees to withhold income tax from royalty payments and the withholding rate is usually around 10%. NIH licenses generally do not allow any taxes to be deducted from royalty payments and the US government is not liable for income taxes on royalty income in most countries by treaty provisions. Licensees should be invoiced for any improperly withheld amounts and may be referred to a collection agency if the debt is not paid. It is also helpful to assist the licensee with any documents needed to obtain a refund should any overpayments be made to local tax authorities.
8.1.7 Inappropriate Deductions
Royalty report deductions must be scrutinized for anything that is not allowed under the license agreement. Typical allowed deductions are returns and allowances, packing costs, insurance costs, freight out (the cost of shipping goods to a distributor or customer), taxes or excise duties imposed on the transaction (if separately invoiced), and wholesaler and cash discounts in reasonable and customary amounts. Some licensees take a ‘standard’ deduction of about 5% in lieu of itemized deductions, which should be reviewed against actual deductions allowed in the license agreement for products with significant sales. Disallowed deductions are invoiced and collected.
8.1.8 Unidentified or Misdirected Payments
Sometimes checks or wire transfer payments are received without identification as to the specific license agreement or even the licensee. Others are sent to the wrong banking institution despite explicit invoice instructions. Without proper identification, the payment cannot be charged to the appropriate account. The problem is usually solvable but the licensee is responsible for retrieving the errant payments and assuring that the proper amount is received on time.
8.1.9 Overpayment/Underpayment
The monitoring group should assist accounting or bookkeeping staff as needed in the collection of underpaid royalties and in identifying the correct payment amounts in some cases. These problems can be minimized by providing licensees with specific payment instructions. While individual licensors will want to develop a template to fit their own situation, an example of the OTT’s payment instruction form is shown in figure 4. Key elements that all licensors should consider with their own payment form are as follows: (i) wire transfer instructions; (ii) overnight mail address; and (iii) having separate contact points for receipt of payments from the licensing office.
Fig. 4.
The Office of Technology Transfer’s payment instruction form.
8.2 Bankruptcy
License agreements typically require licensees to notify licensors of bankruptcy filings, but in practice this rarely occurs. Usually licensors (as unsecured creditors) hear of bankruptcy filings after the fact and then have to get in line with other licensee’s creditors to request unpaid royalties. In the US, companies using Chapter 11 bankruptcy filings[15] are protected from their creditors, including the US government, but are allowed to continue operating their business in the hope of reorganizing and someday emerging from bankruptcy.[15] If a licensee wishes to sell off that portion of the business that includes the license agreement, licensors should try to negotiate a settlement with the licensee at that time. The reasons are: there is money available to pay a settlement (proceeds of the sale); the licensee may need to restructure the license or clear part of the debt to make the business desirable to the buyer; and such a settlement preserves the license agreement for the buyer. The OTT also routinely files papers with the appropriate bankruptcy court, to protect the US government’s collection rights, in case a settlement cannot be reached.
Under Chapter 7 bankruptcy filings,[15] a company is usually liquidated, that is, its physical assets are sold, usually at auction, to recover their residual value. Under this plan, most creditors do not recover anything. However, it is important to look carefully at Chapter 7 filings to see what is actually occurring and follow-up as necessary. For instance, a company can be sold after filing for Chapter 7 protection, and the proceeds of the sale become an additional asset available to the creditors. For example, about $US48 000 was recently collected by the OTT, on a debt of $US110 000, from a licensee that was sold while in Chapter 7.
9. Conclusion
This article has reviewed the scope and some of the experiences of a biomedical license agreement monitoring program established at the NIH. The experience of this program has been that license agreement monitoring, done properly, can consume at least as much time and manpower as the initial patenting, marketing, and licensing activities combined. All aspects of license compliance must be constantly reviewed to ensure that licensees are meeting their obligations under the agreements.
In addition, licensors have an obligation to treat licensees fairly by eliminating infringement of licensed technologies and conducting good-faith negotiations to cure troubled agreements. Royalty audits and effective collection of overdue royalties, even from bankrupt companies, are essential in order to receive the proper financial return from licensees. In the biomedical area, technology licensing can play a significant role in drug or other research innovations and thus the generation of novel biomedical products for human health. An effective monitoring program thus is an essential part of any technology licensing operation and helps to ensure a proper return on the technology investment in terms human health, product development, financial return or other organizational goals.
Acknowledgments
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
References
- 1.Ferguson SM. Products, partners and public health: transfer of biomedical technologies from the US government. J Biolaw Bus. 2002:39. [PMC free article] [PubMed] [Google Scholar]
- 2.Keller GH. Biomedical technology transfer in the government sector. ASM News. 1998;64 (8):454–7. [Google Scholar]
- 3.Office of Technology Transfer PHS/NIH. Monitoring and enforcement figures as tabulated by the authors: official royalty figures for the entire NIH licensing program. [Accessed 2003 Sep 31]; [online]. Available from URL: http://www.ott.nih.gov/about_nih/statistics.aspx.
- 4.Ferguson SM, Kim JP. Distribution and licensing of drug discovery tools: NIH perspective. Drug Discov Today. 2002;7 (2):1102–6. doi: 10.1016/s1359-6446(02)02499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Archives and Records Administration. Code of federal regulations: 37 CFR (Code of Federal Regulation) Part 404.5(b)(4) [Accessed 2003 Sep 3]; [online]. Available from URL: http://www.access.gpo.gov/nara/cfr.
- 6.National Institutes of Health. The Office of Technology Transfer: case study I. Rockville (MD): National Institutes of Health; 2003. (Data on file) [Google Scholar]
- 7.US Securities and Exchange Commission. The EDGAR database: the archive of historical EDGAR documents. [Accessed 2003 Sep 3]; [online]. Available from URL: http://www.sec.gov/cgi-bin/srch-edgar.
- 8.RECAP. Recombinant capital. [Accessed 2003 Sep 3]; [online]. Available from URL: http://www.recap.com.
- 9.US Department of Treasury. News from the Office of Legislative and Public Affairs: the Debt Collection Improvement Act of 1996 (DCIA) [Accessed 2003 Sep 3]; [online]. Available from URL: http://www.fms.treas.gov/news/factsheets/dcia.html.
- 10.National Institutes of Health. The Office of Technology Transfer: case study 2. Rockville (MD): National Institutes of Health; 2003. (Data on file) [Google Scholar]
- 11.US Food and Drug Administration. [Accessed 2003 Sep 3];Department of Health and Human Services. [online]. Available from URL: http://www.fda.gov/search.html.
- 12.Converium. Doctrine of laches and patent infringement litigation. [Accessed 2003 Sep 3]; [online]. Available from URL: http://www.converium.com/web/converium/converium.nsf/ar-ticles/5731FF9F4372B6ED85256B43006EA07D.
- 13.National Institutes of Health; (Data on file) The Office of Technology Transfer: case study 3. Rockville (MD): National Institutes of Health; 2003. [Google Scholar]
- 14.National Institutes of Health. The Office of Technology Transfer: case study 4. Rockville (MD): National Institutes of Health; 2003. (Data on file) [Google Scholar]
- 15.Free Advice. Chapter 7 and chapter 11 business bankruptcy. [Accessed 2003 Sep 3]; [online]. Available from URL: http://bankruptcy-law.freeadvice.com/business_bankruptcy/