Abstract
Many pathogens causing diarrhea do so by modulating ion transport in the gut. Respiratory pathogens are similarly associated with disturbances of fluid balance in the respiratory tract, although it is not known whether they too act by altering epithelial ion transport. Here we show that influenza virus A/PR/8/34 inhibits the amiloride-sensitive Na+ current across mouse tracheal epithelium with a half-time of about 60 min. We further show that the inhibitory effect of the influenza virus is caused by the binding of viral hemagglutinin to a cell-surface receptor, which then activates phospholipase C and protein kinase C. Given the importance of epithelial Na+ channels in controlling the amount of fluid in the respiratory tract, we suggest that down-regulation of Na+ channels induced by influenza virus may play a role in the fluid transport abnormalities that are associated with influenza infections.
Keywords: hemagglutinin, protein kinase C, ENaC, phospholipase C
The volume of fluid within the respiratory tract is determined by the balance between the secretion of fluid and electrolytes mediated by Cl− channels in the apical membranes of the epithelium and the absorption of fluid and electrolytes mediated by amiloride-sensitive Na+ channels also in the apical membranes of the epithelium (1, 2). Thus clearance of the respiratory tract of fluid at birth relies on activation of Na+ channels in the respiratory epithelium (3), and defective Na+ channel function, such as occurs in pseudohypoaldosteronism type I, is accompanied by defective lung fluid clearance (4–6). Conversely, the increased Na+ channel activity and decreased Cl− channel activity that accompany cystic fibrosis have been proposed to lead to depletion of lung fluid (2, 7). Sodium channel activity has also been implicated in the responses of the respiratory epithelium to hypoxia (8) and in the resolution of pulmonary edema (9).
Disordered fluid balance across the respiratory mucosa is also a major feature of respiratory infections. Influenza infections, even by the attenuated viruses used in live vaccines (10), are frequently accompanied by rhinorrhea (10, 11) and the accumulation of fluid in the eustachian tubes and middle ear (11, 12). Furthermore, fluid accumulation in the respiratory tract is an early feature of many severe respiratory infections in both humans (13, 14) and animals (15, 16). These changes in fluid transport precede the onset of morphological abnormalities in the respiratory epithelium (14).
It is well established that many bacteria causing diarrhea, including Vibrio cholerae and Escherichia coli, secrete enterotoxins that stimulate the secretion of fluid and electrolytes by the mucosa lining the gastrointestinal tract (17). It has also recently been established that rotaviruses, an important cause of diarrhea in young children, produce diarrhea as a consequence of one of their nonstructural proteins, NSP4, stimulating phospholipase C, leading to increased intracellular free Ca2+ in the gut mucosa (18–20). Thus, it occurred to us that the fluid accumulation in the respiratory tract associated with respiratory infections may be caused by altered epithelial transport.
In the present study, we used Ussing chamber methods (21) to investigate the acute effects of influenza virus on ion transport by mouse tracheal epithelium and found that the virus inhibits Na+ transport by this epithelium. To our knowledge, this is the first report of a respiratory pathogen that alters electrolyte transport by the respiratory epithelium and the first report of a pathogen that modulates epithelial Na+ channels rather than Cl− channels. Our finding that this effect of influenza virus is mediated by the hemagglutinin in its envelope suggests that other important respiratory pathogens with hemagglutins in their envelopes, such as parainfluenza viruses, may similarly inhibit Na+ transport.
Materials and Methods
Viruses.
The pneumotropic influenza virus A/PR/8/34 (PR8; H1N1) and the neurotropic influenza A virus, A/WSN/33 (WSN33; H1N1), were grown for 2 days in the allantoic cavity of 10-day embryonated hens' eggs. Aliquots of allantoic fluid containing the virus were stored at −70°C. The purified virus used in the experiments depicted in Fig. 1e was prepared by sucrose gradient centrifugation and stored in PBS. Infectivity of the influenza viruses was titered on monolayers of MDCK cells. Hemagglutinin activity was measured with chicken erythrocytes by using standard methods (22).
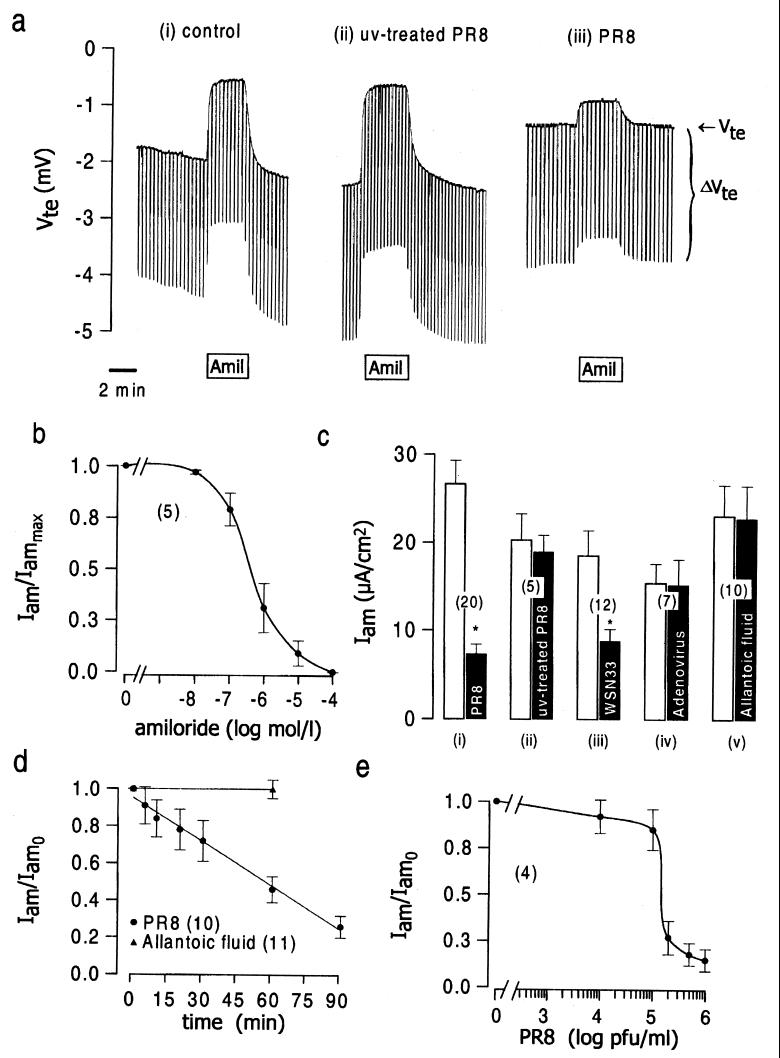
Figure 1.
The effects of influenza virus on Na+ channel activity in tracheal epithelium. (a) Original recordings showing the response of the tracheal epithelium to 10 μmol/liter amiloride under (i) control conditions, (ii) after 1 h exposure of the apical membrane to UV-inactivated PR8 (106 pfu/ml before inactivation), or (iii) after 1 h apical exposure to active PR8 virus (106 pfu/ml). (b) Concentration–response curve for amiloride. Iammax is the amiloride-sensitive current determined by using a maximal concentration of amiloride, 100 μmol/liter. (c) The effects on amiloride-sensitive current of 1 h apical treatment with: (i) active PR8 influenza virus (106 pfu/ml); (ii) UV-inactivated PRB (106 pfu/ml); (iii) active WSN33 influenza virus (106 pfu/ml); (iv) replication-deficient adenovirus, MX17 (106 pfu/ml); and (v) allantoic fluid not infected with any virus. (d) Time course of the decline produced by 106 pfu/ml PR8 in the amiloride-sensitive current. (e) Viral concentration–response curve for inhibition of the amiloride-sensitive current after apical exposure to purified PR8 for 1 h. In a–e, Iam is the amiloride-sensitive current, and Iam0 is the amiloride-sensitive current before the addition of virus.
PR8 “split virus”, prepared by the Commonwealth Serum Laboratories (Melbourne, Australia), was the gift of L. E. Brown (Department of Microbiology and Immunology, University of Melbourne). The virus was produced in allantoic fluid, purified as described above, and pH adjusted to pH 8–8.5 with NaOH. It was then inactivated by incubation with 0.1% (vol/vol) β-propiolactone and disrupted by 1.6% (wt/vol) sodium taurodeoxycholate for 2 h at 37°C, after which it was dialyzed against phosphate-buffered saline (pH 7.2) with sodium azide added as a preservative (total protein 9.3 μg/ml, haemagglutinin 2.9 μg/ml). This solution was then dialyzed against phosphate-buffered saline to produce the final azide-free “split-virus” stock solution (4.8 μg/μl total protein, 1.5 μg/μl haemagglutinin).
The replication-deficient adenovirus, MX-17 (the gift of G. W. Both, Commonwealth Scientific and Industrial Research Organization, Division of Molecular Sciences, North Ryde, New South Wales), was grown and titered as described previously (23). It contains a null insert and does not express a transgene.
Cell Culture.
M-1 mouse cortical collecting duct cells were provided by C. Korbmacher (Oxford University, Oxford, U.K.) and were grown to confluence for 3 days on permeable supports (Transwell–Coll, Costar) in DMEM/F12 media containing 10% FCS; glutamine, 2 mmol/liter; penicillin, 100,000 units/liter; streptomycin, 100,000 units/liter; and dexamethasone, 0.1 μmol/liter.
Ussing Chamber Experiments.
Quackenbush (OS) mice were killed by cervical dislocation. The trachea or colon was then removed, freed of connective tissue, opened longitudinally, and divided into small pieces, which were then stored in a chilled solution containing (mmol/liter): NaCl, 145; KCl, 3.8; d-glucose, 5; MgCl2, 1; Hepes, 5; and Ca-gluconate, 1.3, pH 7.4. Each tissue piece was mounted in an Ussing chamber (21) with a circular aperture of 0.95 mm2. The apical and basolateral surfaces of the epithelium were perfused continuously at a rate of 10 to 20 ml/min (chamber volume 1 ml). The bath solution contained (mmol/liter): NaCl, 145; KH2PO4, 0.4; K2HPO4, 1.6; d-glucose, 5; MgCl2, 1; Hepes 5; and Ca-gluconate, 1.3, pH 7.4. Bath solutions were maintained at 37°C. Experiments were carried out under open-circuit conditions. Values for transepithelial potential differences (Vte) were referred to the serosal side of the epithelium. Currents were regarded as positive when conventional current flowed from apical to serosal side of the epithelium. Transepithelial resistance (Rte) was determined (21) by applying short (1-s) current pulses (▵I = 0.5 μA).
Chemicals.
3-Isobutyl-1-methylxanthine (IBMX), forskolin, 1,2-dioctanoyl-sn-glycerol, bisindolylmaleimide I (BIM), staurosporine, pertussis toxin, cytochalasin D, carbachol, chloroquine, and concanavalin A (type IV) were obtained from Sigma; U-73122 and Gö-6983 were from Calbiochem; and neuraminidase was from Boehringer Mannheim. Monoclonal antihemagglutinin antibody was a gift of L. E. Brown. It was produced as ascites fluid, partially purified by using ammonium sulfate and dialyzed extensively in PBS.
Statistical Methods.
Statistical significance was assessed by using Student's unpaired t test. In the figures, an asterisk indicates a statistically significant (P < 0.05) effect. Experiments were paired by measuring amiloride-sensitive currents before and after each maneuver. When the effects of influenza viruses were examined under different conditions, experiments were generally paired with controls from the same tissue, or, if this was impossible, with controls from the same day. Results are given as mean ± SEM.
Results and Discussion
We first determined the baseline characteristics of the mouse tracheal epithelium in an Ussing chamber under open-circuit conditions. The baseline transepithelial potential difference (Vte) and transepithelial resistance were −2.65 ± 0.28 mV (n = 20) and 77.0 ± 9.2 Ωcm2 (n = 20), respectively, corresponding to an equivalent short-circuit current (Isc) of 37.4 ± 3.4 μA⋅cm−2 (n = 20). As illustrated by the representative experiment in Fig. 1a(i), exposure of the apical membrane to a maximal concentration of amiloride (10 μmol/liter, Fig. 1b) reduced the transepithelial potential difference (−0.77 ± 0.13 mV, n = 20; P < 0.05) and increased the transepithelial resistance (82.8 ± 10.2 Ωcm (2), n = 20; P < 0.05), so as to reduce the calculated short-circuit current (10.7 ± 1.9 μA⋅cm−2, n = 20; P < 0.05). We calculate that the amiloride-sensitive current, which is a measure of the activity of epithelial Na+ channels (21), was 26.7 ± 2.6 μA⋅cm−2 [n = 20; Fig. 1c(i)].
We then exposed the apical membrane of the epithelium to UV-inactivated PR8 influenza virus for 1 h. This had no significant effect on the electrical properties of the epithelium [Fig. 1a(ii) and c(ii)]. Similarly, exposure to virus-free allantoic fluid was without effect [Fig. 1c(v)]. In contrast, exposure of the apical membrane to active PR8 influenza virus for 1 h (Fig. 1a(iii) and 1c(i)] decreased the transepithelial potential difference (−1.92 ± 0.35 mV, n = 20; P < 0.05), the total short-circuit current (25.2 ± 4.1 μA⋅cm−2, n = 20; P < 0.05) and the amiloride-sensitive current (7.3 ± 1.2 μA⋅cm−2, n = 20; P < 0.05). The neurotropic influenza virus, WSN33 [106 plaque-forming units (pfu)/ml; Fig. 1c(iii)], was also inhibitory, but the replication-deficient type-5 adenovirus, MX-17, was not [106 pfu/ml; Fig. 1c(iv)]. The inhibition thus did not appear to be a nonspecific consequence of endocytosis of virus (see also Fig. 4a). Studies of the time course of the virus-induced inhibition revealed an approximately linear decline of the amiloride-sensitive short-circuit current, which had fallen to 50% of the initial value after 60 min (Fig. 1d). We further found in five experiments that 1-h incubation in virus-free media did not reverse the inhibition of the amiloride-sensitive current produced by 1-h exposure to PR8 (control amiloride-sensitive current: 37.1 ± 7.3 μA⋅cm−2; amiloride-sensitive current after 106 pfu/ml PR8 for 1 h: 11.3 ± 3.8 mA⋅cm−2; after 1 h recovery: 7.9 ± 4.0 μA⋅cm−2). The dependence of the inhibition on virus concentration is shown in Fig. 1e.
Figure 4.
Mechanism of Na+ channel inhibition by influenza virus. (a) Effect on the PR8-induced inhibition of the amiloride-sensitive current in trachea of cytochalasin D (30 μg/ml; CD); chloroquine (500 μmol/liter; CQ); amantadine (200 μmol/liter; AM) and pretreatment of the apical membrane with neuraminidase (0.1 units/ml) for 30 min at 37°C (NA). (b) Effect of “split virus” (48 μg total protein/milliliter) on the amiloride-sensitive current and its neutralization by antihemagglutinin antibody (1:100 dilution; hemagglutinin antibody). (c) Effects of staurosporine (2 μmol/liter; Stauro), bisindolylmaleimide I (0.1 μmol/liter; BIM), Gö-6983 (20 nmol/liter; Gö) and U-73122 (10 μmol/liter; U-73122) on the inhibition by 106 pfu/ml PR8 of the amiloride-sensitive current. (d) Effect of 1,2-dioctanoyl-sn-glycerol (10 μmol/liter; DOG) and BIM (0.1 μmol/liter; BIM) on the amiloride-sensitive short-circuit current.
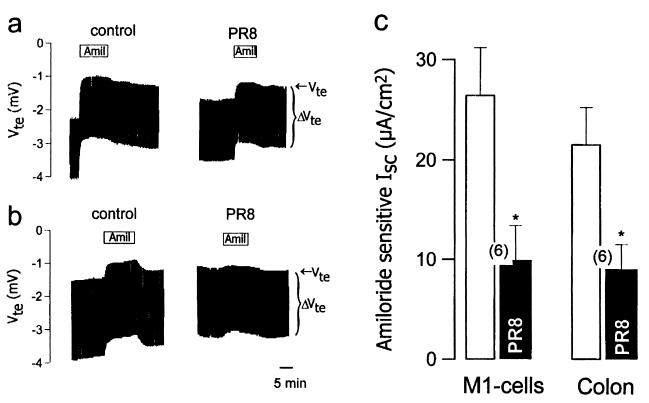
Exposure of the tracheal epithelium to a mixture of forskolin (10 μmol/liter) and IBMX (100 μmol/liter; Fig. 2 a and c), which increases intracellular cyclic AMP, or to carbachol (100 μmol/liter; Fig. 2 b and d), which increases intracellular Ca2+, has been shown to increase transepithelial potential difference and short-circuit current by activation of Cl− secretion (21, 24). PR8 virus had no significant effect on the time course (Fig. 2 a and b) or the size of these responses (Fig. 2 c and d). A component of the short-circuit current in tracheal epithelia is also known to be carried by the Na+-glucose cotransporter (25). We thus examined whether PR8 virus affected the component of the short-circuit current that was sensitive to isomolar replacement of glucose in the apical bathing solution by mannitol. We found that it did not (control glucose-sensitive current: 7.3 ± 0.6 μA⋅cm−2, n = 5; glucose-sensitive current after 1 h 106 pfu/ml PR8: 7.5 ± 1.3 μA⋅cm−2, n = 5). We next examined whether other amiloride-sensitive Na+-transporting epithelia were also sensitive to influenza virus. We found that PR8 virus inhibited amiloride-sensitive currents in both the M1 mouse collecting duct cell line (Fig. 3 a and c) and in mouse colonic epithelium (Fig. 3 b and c).
Figure 2.
Effects of influenza virus on responses of tracheal epithelium to stimulants. (a) Original recordings showing the effects of 10 μmol/liter forskolin plus 100 μmol/liter IBMX, before and after 1 h apical exposure to 106 pfu/ml PR8. (b) Original recordings showing the effects of 100 μmol/liter carbachol before and after 1 h apical exposure to 106 pfu/ml PR8. (c and d) The effects of 1 h apical exposure to 106 pfu/ml PR8 virus on the current activated by 10 μmol/liter forskolin plus 100 μmol/liter IBMX (c) and by 100 μmol/liter carbachol (d).
Figure 3.
Effects of influenza virus on nonrespiratory epithelia. (a) Original recordings showing the effect of 10 μmol/liter amiloride on M1 mouse collecting duct cells before and after 1 h apical exposure to 106 pfu/ml PR8. (b) Original recordings showing the effect of 10 μmol/liter amiloride on mouse colonic epithelium before and after 1 h apical exposure to 106 pfu/ml PR8. (c) Effect of 106 pfu/ml PR8 on the amiloride-sensitive current in M1 cells and mouse colonic epithelium.
The time course of the inhibition (Fig. 1d) suggested that it was a consequence of one of the early steps in the infection process (26). These early steps include: (i) the binding of hemagglutinin in the viral coat to sialic acid residues on a receptor protein in the apical membrane, which can be inhibited by pretreatment with neuraminidase (27); (ii) the endocytosis of the virus-receptor complex, which can be blocked by cytochalasin D (28); and (iii) the uncoating of the virus because of the movement of H+ through the M2 protein in the viral coat, which can be blocked by amantadine (29, 30) or by chloroquine, which dissipates the endosomal pH gradient (31). Of the four agents that block one or the other of these early steps in the infection process, only neuraminidase prevented the inhibition of the amiloride-sensitive current by PR8 virus (Fig. 4a). In addition, a “split-virus” preparation, in which the virus had been disrupted with detergent (see Materials and Methods) to render it inactive but still capable of binding to the epithelium, was also inhibitory (Fig. 4b). This inhibition could be prevented by a monoclonal antibody directed against hemagglutinin (Fig. 4b). Thus the Na+ channel inhibition produced by influenza virus seemed likely to be caused by hemagglutinin binding to a receptor in the apical membrane of the respiratory epithelium. To substantiate further this possibility, we examined whether the hemagglutinating lectin, concanavalin A (50 μg/ml), could inhibit the amiloride-sensitive Na+ current. We found that it did, and that the time course of this inhibition was similar to that observed with influenza virus (control amiloride-sensitive current: 35.5 ± 9.1 μA⋅cm−2, n = 5; after concanavalin A for 10 min: 31.5 ± 5.3 μA⋅cm−2, n = 5; after concanavalin A for 1 h: 16.5 ± 6.7 μA⋅cm−2, n = 5).
Finally, we examined the intracellular mechanisms of the inhibition produced by the influenza virus (Fig. 4c). The inhibitor of phospholipase Cβ, U-73122, blocked the response, as did a broad-spectrum inhibitor of protein kinase C, BIM, and the selective inhibitor of the α and β isoforms of protein kinase C, Gö-6983. Staurosporine, a nonselective inhibitor of serine and threonine kinases, partially prevented the inhibitory effect of the virus, but also itself significantly reduced the amiloride-sensitive current (Fig. 4c). Because Na+ channels in respiratory epithelium have not previously been shown to be regulated by protein kinase C, we checked and confirmed that the activator of protein kinase C, 1,2-dioctanoyl-sn-glycerol, inhibits the amiloride-sensitive current (Fig. 4d), whereas inhibition of protein kinase C with BIM stimulates it (Fig. 4d). Pertussis toxin (300 ng/ml for 3 h) did not prevent the inhibitory effect of the virus, although, like staurosporine, it reduced the baseline amiloride-sensitive current (data not shown).
In this paper, we show that influenza virus inhibits the activity of epithelial Na+ channels in respiratory and other epithelia. This inhibition is caused by the hemagglutinin in the viral coat and does not require endocytosis of the virus or virus entry into the cytoplasm. The effect is mediated by phospholipase C and protein kinase C, recalling the mechanism by which influenza virus stimulates B lymphocyte proliferation (32) and depresses neutrophil function (33, 34). Our observation that concanavalin A inhibits amiloride-sensitive Na+ channels with a similar time course to the virus gives further support to the primary role of the viral hemagglutinin in evoking the inhibition. The inhibition of amiloride-sensitive Na+ channels produced by influenza is not attributable, however, to a redistribution of membrane proteins (“capping”) such as occurs in lymphocytes after exposure to concanavalin A (35). Capping in lymphocytes after exposure to concanavalin A and other agents that crosslink receptors is inhibited by microfilament inhibitors such as cytochalasin D (36, 37), whereas the inhibitory effect of influenza virus on Na+ channels is not.
This is, to our knowledge, the first report of any viral or microbial pathogen that regulates amiloride-sensitive Na+ channels. Given that the concentration range over which influenza virus inhibits epithelial Na+ channels (Fig. 1e) is 10- to 1,000-fold lower than the viral concentrations measured in the nasal turbinates and lungs of mice infected for 3–4 days with influenza (38–41) and the role of these channels in controlling the amount of fluid in the respiratory tract (4, 42), the present findings provide an explanation for the accumulation of fluid in the respiratory tract that is a feature of influenza infections (11, 13–16). The role of epithelial Na+ channels in maintaining the middle ear cavities free of fluid (43, 44) suggests that down-regulation of Na+ channel activity may also underlie the known association between influenza infections and otitis media (11, 45). Finally, our observation that influenza hemagglutinin inhibits Na+ channel activity may point the way to the development of a novel alternative to the use of Na+ channel blockers such as amiloride in the treatment of the Na+ channel overactivity seen in cystic fibrosis (46).
Acknowledgments
This project was supported by the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. D.I.C. and G.K. are Fellows of The Medical Foundation of the University of Sydney (Sydney, Australia).
Abbreviations
- IBMX
3-isobutyl-1-methylxanthine
- BIM
bisindolylmaleimide I
- pfu
plaque-forming unit
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 9827.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160041997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160041997
References
- 1.Matalon S, O'Brodovich H. Annu Rev Physiol. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 2.Boucher R C. J Physiol (London) 1999;516:631–638. doi: 10.1111/j.1469-7793.1999.0631u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olver R E, Ramsden C A, Strang L B, Walters D V. J Physiol (London) 1986;376:321–340. doi: 10.1113/jphysiol.1986.sp016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, et al. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- 5.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier B C. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 6.Barker P M, Nguyen M S, Gatzy J T, Grubb B, Norman H, Hummler E, Rossier B, Boucher R C, Koller B. J Clin Invest. 1998;102:1634–1640. doi: 10.1172/JCI3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsui H, Grubb B R, Tarran R, Randell S H, Gatzy J T, Davis C W, Boucher R C. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 8.Matalon S, Yue G. Am J Physiol. 1997;272:L407–L412. doi: 10.1152/ajplung.1997.272.3.L407. [DOI] [PubMed] [Google Scholar]
- 9.Modelska K, Matthay M A, McElroy M C, Pittet J F. Am J Physiol. 1997;273:305–314. doi: 10.1152/ajplung.1997.273.2.L305. [DOI] [PubMed] [Google Scholar]
- 10.Belshe R B, Mendelman P M, Treanor J, King J, Gruber W C, Piedra P, Bernstein D I, Hayden F G, Kotloff K, Zangwill K, et al. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 11.Doyle W J, Skoner D P, Hayden F, Buchman C A, Seroky J T, Fireman P. Ann Otol Rhinol Laryngol. 1994;103:59–69. doi: 10.1177/000348949410300111. [DOI] [PubMed] [Google Scholar]
- 12.Giebink G S, Ripley M L, Wright P F. Ann Otol Rhinol Laryngol. 1987;96:199–206. doi: 10.1177/000348948709600212. [DOI] [PubMed] [Google Scholar]
- 13.Suarez D L, Perdue M L, Cox N, Rowe T, Bender C, Huang J, Swayne D E. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winternitz M G, Wason I M, McNamara F P. The Pathology of Influenza. New Haven, CT: Yale Univ. Press; 1920. pp. 14–15. [Google Scholar]
- 15.Kudlacz E M, Baugh L E, Porter W P, Kenny M T, Farrell A M. Lab Anim Sci. 1993;43:445–453. [PubMed] [Google Scholar]
- 16.Parusov V N, Zhunko V V. Biulletin Eksperimentalnoi Biologii i Meditsiny. 1977;83:243–244. [PubMed] [Google Scholar]
- 17.Popoff M R. Toxicon. 1998;36:665–685. doi: 10.1016/s0041-0101(97)00100-1. [DOI] [PubMed] [Google Scholar]
- 18.Ball J M, Tian P, Zeng C Q, Morris A P, Estes M K. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Zeng C Q, Ball J M, Estes M K, Morris A P. Proc Natl Acad Sci USA. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Zeng C Q, Dong Y, Ball J M, Saif L J, Morris A P, Estes M K. J Virol. 1998;72:3666–3672. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazekas de St Groth S, Webster R G. J Exp Med. 1966;124:347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poronnik P, O'Mullane L M, Harding E A, Greger R, Cook D I. Cell Calcium. 1998;24:97–103. doi: 10.1016/s0143-4160(98)90077-x. [DOI] [PubMed] [Google Scholar]
- 24.Boucher R C, Gatzy J T. J Appl Physiol. 1982;52:893–901. doi: 10.1152/jappl.1982.52.4.893. [DOI] [PubMed] [Google Scholar]
- 25.Joris L, Quinton P M. Pflügers Arch. 1989;415:118–120. doi: 10.1007/BF00373149. [DOI] [PubMed] [Google Scholar]
- 26.Lamb R A, Krug R M. In: Fields Virology. 3rd Ed. Fields B N, Knipe D M, Howley P M, Charnock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Philadelphia: Lippincott; 1996. pp. 1353–1395. [Google Scholar]
- 27.Matlin K S, Reggio H, Helenius A, Simons K. J Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb T A, Ivanov D D, Adesnik M, Sabatini D D. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharton S A, Belshe R B, Skehel J J, Hay A J. J Gen Physiol. 1994;75:945–948. doi: 10.1099/0022-1317-75-4-945. [DOI] [PubMed] [Google Scholar]
- 30.Ogden D, Chizhmakov I V, Geraghty F M, Hay A J. Methods Enzymol. 1999;294:490–506. doi: 10.1016/s0076-6879(99)94029-6. [DOI] [PubMed] [Google Scholar]
- 31.Shibata M, Aoki H, Tsurumi T, Sugiura Y, Nishiyama Y, Suzuki S, Maeno K. J Gen Virol. 1983;64:1149–1156. doi: 10.1099/0022-1317-64-5-1149. [DOI] [PubMed] [Google Scholar]
- 32.Rott O, Charreire J, Semichon M, Bismuth G, Cash E. J Immunol. 1995;154:2092–2103. [PubMed] [Google Scholar]
- 33.Cassidy L F, Lyles D S, Abramson J S. J Immunol. 1989;142:4401–4406. [PubMed] [Google Scholar]
- 34.Hartshorn K L, Collamer M, White M R, Schwartz J H, Tauber A I. Blood. 1990;75:218–226. [PubMed] [Google Scholar]
- 35.Taylor R B, Duffus P H, Raff M C, de Petris S. Nat New Biol. 1971;233:225–229. doi: 10.1038/newbio233225a0. [DOI] [PubMed] [Google Scholar]
- 36.Bourguignon L Y, Walker G, Huang H S. J Immunol. 1990;144:2242–2252. [PubMed] [Google Scholar]
- 37.de Petris S. Nature (London) 1974;250:54–56. doi: 10.1038/250054a0. [DOI] [PubMed] [Google Scholar]
- 38.Matsuki N, Ogasawara K, Takami K, Namba K, Takahashi A, Fukui Y, Sasazuki T, Iwabuchi K, Good R A, Onoe K. Vaccine. 1999;17:1161–1168. doi: 10.1016/s0264-410x(98)00336-3. [DOI] [PubMed] [Google Scholar]
- 39.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGreger M, Cooley A J, Kawaoka Y. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castrucci M R, Kawaoka Y. J Virol. 1993;67:759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castrucci M R, Bilsel P, Kawaoka Y. J Virol. 1992;66:4647–4653. doi: 10.1128/jvi.66.8.4647-4653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthay M A, Wiener-Kronish J P. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 43.Herman P, Tan C T, Portier F, Clerici C, Escoubet B, Friedlander G, Tran Ba Huy P. Kidney Int. 1998;65:S94–S97. [PubMed] [Google Scholar]
- 44.Portier F, van den Abbeele T, Lecain E, Sauvaget E, Escoubet B, Huy P T, Herman P. Am J Physiol. 1999;276:C312–C317. doi: 10.1152/ajpcell.1999.276.2.C312. [DOI] [PubMed] [Google Scholar]
- 45.Doyle W J, Skoner D P, Alper C M, Allen G, Moody S A, Seroky J T, Hayden F G. J Infect Dis. 1998;177:1260–1265. doi: 10.1086/515294. [DOI] [PubMed] [Google Scholar]
- 46.Tomkiewicz R P, App E M, Zayas J G, Ramirez O, Church N, Boucher R C, Knowles M R, King M. Am Rev Respir Dis. 1993;148:1002–1007. doi: 10.1164/ajrccm/148.4_Pt_1.1002. [DOI] [PubMed] [Google Scholar]