Abstract
A common feature of all eukaryotic membranes is the non-random distribution of different lipid species in the lipid bilayer (lipid asymmetry). Lipid asymmetry provides the two sides of the plasma membrane with different biophysical properties and influences numerous cellular functions. Alteration of lipid asymmetry plays a prominent role during cell fusion, activation of the coagulation cascade, and recognition and removal of apoptotic cell corpses by macrophages (programmed cell clearance). Here we discuss the origin and maintenance of phospholipid asymmetry, based on recent studies in mammalian systems as well as in Caenhorhabditis elegans and other model organisms, along with emerging evidence for a conserved role of mitochondria in the loss of lipid asymmetry during apoptosis. The functional significance of lipid asymmetry and its disruption during health and disease is also discussed.
Keywords: phospholipid asymmetry, Caenhorhabditis elegans, programmed cell clearance, mitochondria, phosphatidylserine, phospholipid scramblases, aminophospholipid translocases
Introduction
A common feature of membranes in all eukaryotic cells is the non-random distribution of lipids across the bilayer. Lipid asymmetry in membranes is a consequence of multiple factors, including the biophysical properties of lipids that dictate their ability to spontaneously “flip” their polar headgroups through the hydrophobic membrane interior, and the presence of transporters (enzymes) that assist in active lipid translocation across the bilayer (see van Meer et al., 2008, for an excellent review). Moreover, this asymmetrical distribution of lipids has important functional consequences. For instance, the anionic phospholipid, phosphatidylserine (PS), is exclusively located at the cytoplasmic side of the plasma membrane in quiescent cells and is an essential co-factor for a number of membrane-bound enzymes, such as protein kinase C and Na+/K+-ATPase (Zwaal et al., 2005). However, when exposed on the cell surface, PS acts as a conserved recognition signal for phagocytes and promotes the blood coagulation cascade (Savill et al., 2002). The present review aims to discuss the origin and maintenance of phospholipid asymmetry as well as the mechanism and functional significance of its disruption in health and disease.
The origin and maintenance of phospholipid asymmetry in biological membranes
The variations and permutations of headgroups and aliphatic chains, and the occurrence of oxidatively modified lipids, allows for the existence of a multitude of individual molecular species of lipids in any eukaryotic cell (Kagan and Quinn, 2004; Wolf and Quinn, 2008). Importantly, although all lipids appear to be symmetrically distributed between the two leaflets of the endoplasmic reticulum membrane, an asymmetric distribution of lipids is seen in the Golgi, endosomal, and plasma membranes of eukaryotic cells, with sphingomyelin and glycosphingolipids residing predominantly on the non-cytosolic (luminal) side and the anionic phospholipids, phosphatidylserine (PS) and phosphatidylethanolamine (PE) enriched in the cytosolic leaflet (van Meer et al., 2008) (Figure 1). The discovery that this asymmetry results from the adenosine triphosphate (ATP)-dependent translocation of PS and PE between bilayer leaflets underscores the notion that membrane lipid asymmetry is of major physiologic importance, as it demonstrates that cells invest considerable energy in this process (Zwaal and Schroit, 1997). The identification of the specific enzyme(s) responsible for phospholipid translocation in the plasma membrane has attracted considerable attention.
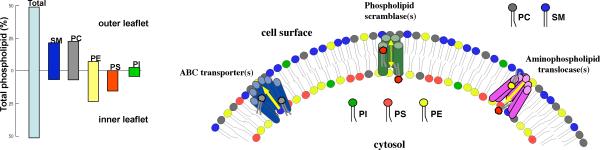
Figure 1.
Phospholipid asymmetry and related lipid-translocating enzymes. In the plasma membrane of normal eukaryotic cells, phosphatidylcholine (PC) and sphingomyelin (SM) are present predominantly in the outer leaflet. Phosphatidylethanolamine (PE) and phosphatidylinositol (PI) reside mainly in the inner leaflet, while phosphatidylserine (PS) is located almost exclusively in the inner leaflet of the plasma membrane. Phospholipid asymmetry may be maintained or altered through the action of three classes of proteins, as discussed in the present review: phospholipid scramblases, ATP-binding cassette (ABC) transporters, and aminophospholipid translocases. The graph shows the transbilayer distribution of phospholipids across the human erythrocyte membrane (adapted from Daleke 2008).
Human erythrocytes have served as a prototypical model to study the structure of the eukaryotic membrane and associated lipid asymmetry. Both energy-dependent and energy-independent transport activities have been reported to play an important role for the generation and maintenance of lipid asymmetry in these cells (Daleke, 2008). At least three types of putative lipid transporter activities have been proposed: a “flippase”, which catalyzes ATP-dependent inward transport of lipids; a “floppase”, which promotes ATP-dependent outward migration of lipids; and a “scramblase”, which stimulates bi-directional movement of lipids between the two membrane leaflets (Daleke, 2008). While the first two activities are thought to generate and maintain lipid asymmetry, bi-directional lipid scrambling promotes the collapse of this asymmetry. Numerous studies have associated a specific class of P-type ATPases (the P4 ATPases) and the ATP-binding cassette (ABC) transporter family, as well as the so-called scramblase family of proteins with each of these three classes of lipid transporters, respectively (for a review and original references, see Zwaal et al., 2005). The discovery of an aminophospholipid translocase (APLT) activity in bovine chromaffin granules led to the cloning of ATPase II (ATP8A1) (Tang et al., 1996) that is homologous to Drs2p, a trans-Golgi network-resident protein in yeast. ATP8A1 and Drs2p are founding members of a conserved subfamily of P-type ATPases that includes 5 yeast, 6 C. elegans, 4 Drosophila, and 14 human members (Lenoir et al., 2007; Darland-Ransom et al., 2008; D. Xue, unpublished observation). Approximately at the same time, Sims and colleagues purified a 37-kDa type II single-transmembrane protein from erythrocyte membranes that could mediate Ca2+- dependent movement of phospholipids between membrane leaflets and thus possesses the “phospholipid scramblase” activity (Bassé et al., 1996; Zhou et al., 1997). This phospholipid scramblase, PLSCR1, is highly conserved among different organisms (Wiedmer et al., 2000; Wang et al., 2007). A major roadblock, however, in terms of identifying the specific molecules responsible for the generation or maintenance of lipid asymmetry and for its disruption during cell activation or cell death is that there are numerous members of each family of transporters, which precludes genetic analysis of the in vivo role of each of these molecules in mammalian systems due to potential genetic redundancy. On the other hand, our recent studies in C. elegans, a model organism especially suited for powerful genetic analyses, have revealed that a worm P4 ATPase homolog, TAT-1, is required for maintenance of PS asymmetry in living cells (Darland-Ransom et al., 2008) (Figure 2). These studies also provided evidence that the disruption of phospholipid asymmetry in living cells can result in indiscriminate removal of affected cells by neighboring phagocytes. Moreover, we demonstrated that a worm phospholipid scramblase homolog, SCRM-1, is important for compromising this PS asymmetry and promoting the externalization of PS in apoptotic cells (Wang et al., 2007) (see further discussion below).
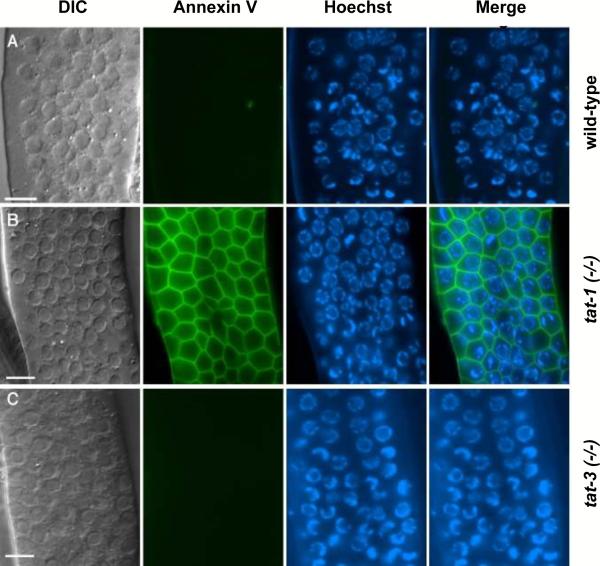
Figure 2.
Exposure of PS on the surface of C. elegans germ cells in tat-1-deficient worms. Exposed gonads of the following hermaphrodite adult worms were stained with the PS-binding protein, annexin V: (A) wild-type, (B) tat-1(tm1034), and (C) tat-3(tm1275), as described in Darland-Ransom et al. (2008). Images of differential contrast interference (DIC), annexin V staining, Hoechst 33342 staining, and the merged image of annexin V plus Hoechst 33342 staining are shown. The data show that reduction of tat-1 activity, but not of related tat-3 activity is sufficient to disrupt asymmetrical PS distribution on the surface of C. elegans germ cells. Scale bars correspond to 6.5 μm.
Interestingly, the P4 ATPases belong to the superfamily of P-type ATP pumps whose members usually translocate small cations or metal ions, rather than lipids (Lenoir et al., 2007). In both cases, the net result of the action of these pumps is to maintain asymmetry or a gradient (of lipids, or ions) across a membrane, a fundamental aspect of cellular physiology that drives numerous processes in the cell. As pointed out by Lenoir and co-workers, a challenging problem is to understand how an ion pump evolved into a flippase i.e. a protein that “flips” phospholipids. In a very recent study, these investigators provided evidence that the so-called Cdc50 proteins are integral components of the P4 ATPase transport machinery and that the affinity of yeast P4 ATPase Drs2p for its Cdc50 binding partner fluctuates during the transport cycle, with the strongest interaction occurring at a point where the enzyme is loaded with phospholipid ligand (Lenoir et al., 2009). The specific interactions between P-type ATPases and Cdc50 proteins could thus suggest a basis for the transport specificity of P-type ATPases.
Because lipid asymmetry is fundamental to several cellular processes, it is important that this asymmetry is maintained throughout the life-span of the cell. Indeed, as noted above, cells invest considerable amounts of energy to generate and maintain asymmetric phospholipid distribution. However, lipid asymmetry-sensing proteins or related downstream signaling pathways have been poorly defined. Interestingly, recent studies have indicated that the pH-responsive Rim101 pathway, the protein kinase Mck1, and the transcription factor Mot3 all act in lipid asymmetry signaling in Saccharomyces cerevisiae and that the Rim101 pathway is activated in response to changes in lipid asymmetry (Ikeda et al., 2008). These studies are also suggestive of the convergence of lipid asymmetry sensing and pH adaptation through the Rim101 pathway. Whether these pathways are conserved in higher species remains to be elucidated.
Molecular mechanisms underlying the disruption of plasma membrane phospholipid asymmetry
The P4 ATPases flip lipids from the non-cytosolic (outer) leaflet to the cytosolic (inner) leaflet of the membrane, whereas the so-called ABC transporters work in the opposite direction. Moreover, ABC transporters are capable of expelling a given lipid out of the membrane (van Meer et al., 2008). Most of the mammalian ABC transporters that are involved in lipid transport have been associated with specific diseases or pathologies; however, the mechanism(s) of action and the specific substrates for many of these ABC transporters remain a matter of debate. ABCA1, for instance, is mutated in Tangier disease, a condition characterized by a severe high-density lipoprotein (HDL) deficiency, sterol deposition in tissue macrophages, and prevalent atherosclerosis (Box 1). Early studies suggested a role for ABCA1 in macrophage engulfment of apoptotic cells (Luciani and Chimini, 1996) and subsequent studies implicated ABCA1 in the disruption of phospholipid asymmetry during apoptosis (Hamon et al., 2000). In line with these studies, Wu and Horvitz (1998) reported that the C. elegans gene ced-7, encoding a protein homologous to ABCA1, functions in the engulfment of apoptotic cell corpses in the worm. They also proposed that CED-7 translocates molecules that mediate homotypic adhesion between the dying and engulfing cells. More recent studies using cells from ABCA1-deficient mice and from Tangier patients with homozygous mutations in the ABCA1 gene showed that mutations in ABCA1 do not measurably alter the rate of transbilayer movements of phospholipids (Williamson et al., 2007). These studies also discounted a role for ABCA1 in the surface externalization of annexins, phospholipid-binding proteins previously reported to be expressed both on the surface of phagocytes and on cells undergoing apoptosis (Fan et al., 2004). However, these results do not rule out a role for ABCA1 in phospholipid loading to apoA-1. Similarly, there have been conflicting reports regarding the role of C. elegans CED-7 in PS externalization in apoptotic cells. In one study (Venegas and Zhou, 2007), CED-7 was found to promote PS externalization in somatic apoptotic cells, whereas in another, CED-7 was suggested to be dispensable (Züllig et al., 2007). Obviously, the role of ABC transporters as potential transbilayer lipid transporters warrants further examination and investigation.
As shown originally for activated platelets, many cell membranes harbor a Ca2+-dependent mechanism that can rapidly move phospholipids back and forth between the two membrane leaflets (lipid scrambling), leading to a loss of membrane phospholipid asymmetry (for a review and original references, see Zwaal et al., 2004; Zwaal et al., 2005). Scott syndrome, an extremely rare congenital bleeding disorder characterized by a deficiency in platelet procoagulant activity (Weiss et al., 1979; Miletich et al., 1979), is the only known primary pathological consequence caused by an aberration in regulating membrane phospholipid asymmetry (Rosing et al., 1985) (Box 1). Despite its rare occurrence, investigations of cells from these patients have shed light on the mechanisms involved in the disruption of phospholipid asymmetry in the plasma membrane (Zwaal et al., 2004). The disorder was originally described as an isolated deficiency of platelet procoagulant activity, but the underlying defect in Ca2+-induced lipid scrambling is also evident in other cell types. Interestingly, studies in lymphocytes have shown that there are differences between Ca2+-induced lipid scrambling that occurs during cell activation and the egress of PS that occurs when cells are undergoing apoptosis. Indeed, whereas Scott syndrome cells fail to expose PS following Ca2+ influx, PS externalization is normal in apoptotic cells from these patients (Williamson et al., 2001; Martinez and Freyssinet, 2001). The identification of PLSCR1 as a protein that exhibits Ca2+-activated phospholipid scrambling activity has provided plausible candidates for studying the disruption of lipid asymmetry (Zhou et al., 1997). Indeed, PLSCR1 has been implicated in promoting PS externalization in apoptotic cells or PS exposure in neutrophils stimulated by a chemotactic peptide (Frasch et al., 2000; Frasch et al., 2004). However, in other studies, overexpression of exogenous PLSCR1 or induction of endogenous PLSCR1 expression by interferon-α failed to promote PS externalization in human lymphoma cells undergoing apoptosis (Fadeel et al., 1999). Moreover, mice deficient in PLSCR1 do not display the hemostatic abnormalities characteristic for Scott syndrome or a defect in PS externalization in activated cells (Zhou et al., 2002). In fact, recent data suggest a considerably more complex biology for PLSCR1 (see, for instance, Ben-Efraim et al., 2004; Huang et al., 2006). Nevertheless, since multiple phospholipid scramblases exist in mammals and in other organisms (Wiedmer et al., 2000; Wang et al., 2007), these results do not exclude a role for other phospholipid scramblase(s) in the process of PS externalization in the plasma membrane.
Two recent studies have provided evidence that some C. elegans phospholipid scramblases indeed are involved in PS externalization in apoptotic cells (Venegas and Zhou, 2007; Wang et al., 2007). We have found that inactivation of scrm-1, which encodes one of the eight C. elegans phospholipid scramblase homologues (named SCRM proteins), significantly reduces PS exposure on the surface of C. elegans apoptotic germ cells and compromises cell corpse engulfment (Wang et al., 2007) (Figure 3), suggesting that surface-exposed PS also serves as an “eat-me” signal for removal of apoptotic cells in C. elegans. In a similar study, Venegas and Zhou (2007) found that another C. elegans scramblase, SCRM-3 (or PLSC-1) also mediates PS externalization in apoptotic germ cells and affects their removal by phagocytes. However, loss of scrm-1 or scrm-3 only partially reduces PS exposure on the surface of apoptotic cells, indicating that additional phospholipid scramblases or lipid transporters may be involved in mediating PS externalization in C. elegans apoptotic cells. Moreover, as lipid scrambling enzymes, the activities of these scramblases need to be tightly controlled so that their activities are activated only in apoptotic cells. What could be the activation signals or mechanisms? Interestingly, we found that the C. elegans mitochondrial factor, WAH-1, a homolog of human apoptosis-inducing factor (AIF), also affects cell corpse engulfment (Wang et al., 2007). Further analysis indicated that loss of wah-1 markedly reduces PS externalization in apoptotic germ cells. However, WAH-1 by itself had no lipid scrambling activity and therefore probably needs to act through a lipid transporter. Indeed, we found that WAH-1 and SCRM-1 act in the same pathway to promote PS externalization in C. elegans apoptotic cells and that they interact with each other in vitro. Strikingly, in proteoliposome assays, WAH-1 could activate the PS scrambling activity of SCRM-1 by ten-fold and this SCRM-1-activating activity depends on specific interaction between SCRM-1 and WAH-1. Therefore, WAH-1 is an apoptotic activator and SCRM-1 is a downstream lipid-flipping enzyme that mediates PS externalization during apoptosis (Figure 4). Since the conserved BH3-only cell death initiator, EGL-1 induces the release of WAH-1 from mitochondria during apoptosis (Wang et al., 2002), this finding delineates a novel mitochondrion-to-plasma membrane signaling pathway that promotes apoptotic PS externalization. Interestingly, previous studies demonstrated that the microinjection of AIF into the cytosol of human cells induces dissipation of the mitochondrial transmembrane potential and PS externalization on the cell surface (Susin et al., 1999). Furthermore, numerous studies support the view that PS externalization occurs downstream of mitochondria in cells undergoing apoptosis (Zhuang et al., 1998; Uthaisang et al., 2003; Blom et al., 2003; Ricci et al., 2004). It will be interesting to see whether the WAH-1/SCRM-1 PS externalization pathway is conserved in mammals. Further dissection of the molecular pathways that mediate PS externalization is now possible based on the studies performed in the nematode system.
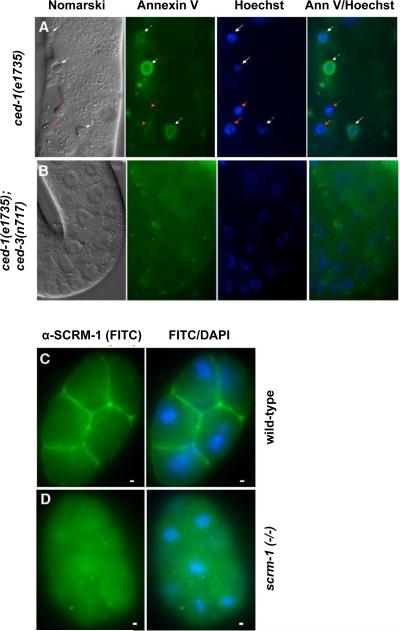
Figure 3.
wah-1 and scrm-1 promote PS exposure on the surface of apoptotic germ cells in C. elegans. (A, B) The exposed gonad of a ced-1(e1735) (A) hermaphrodite animal or a ced-1(e1735); ced-3(n717) (B) animal was stained with annexin V and Hoechst 33342. Germ cell corpses were identified by their raised-button-like morphology under Nomarski optics and the condensed Hoechst 33342 staining pattern, as detailed in Wang et al. (2007). Germ cell corpses stained with both Hoechst and annexin V are indicated by white arrows, and those that were stained with Hoechst but not with annexin V are indicated with red arrows. The scale bar represents 6.5 μm. (C, D) SCRM-1 localizes to the plasma membrane. Images of FITC (SCRM-1 antibody staining) and FITC-DAPI C. elegans 16-cell stage wild-type (C) or scrm-1(tm805) (D) embryos are shown. The scale bars correspond to 1 μm.
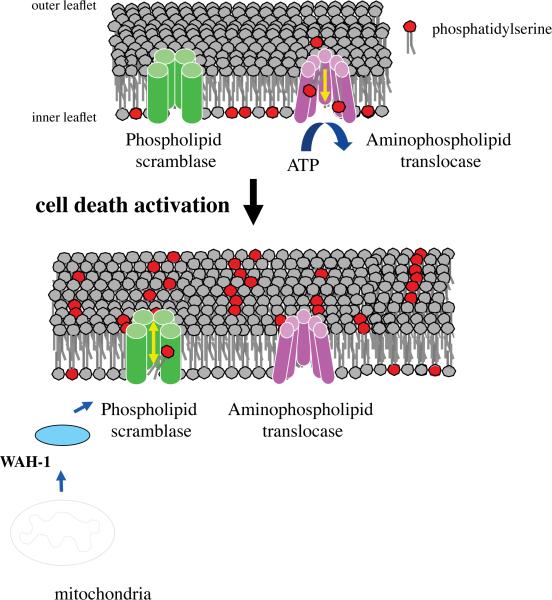
Figure 4.
Plasma membrane lipid transporters regulate the distribution of the phospholipid, PS. In quiescent cells, energy (ATP)-dependent translocation of PS from the outer to the inner leaflet of the plasma membrane serves to maintain the absolute asymmetry of PS distribution. Upon activation of the cell death program, the mitochondrial apoptogenic factor, WAH-1 (worm homolog of AIF) exits from mitochondria and activates the lipid scrambling activity of the plasma membrane phospholipid scramblase, SCRM-1. The bi-directional scrambling of phospholipids destroys the asymmetrical distribution of PS. Externalized PS serves as a signal to trigger engulfment by phagocytes through its binding to the PS receptor, PSR-1 (not shown).
The expanding role of phospholipid scrambling: implications for mitochondrial apoptosis signaling
Mice lacking PLSCR3, a member of the mammalian phospholipid scramblase family, display adiposity, dyslipidemia, and insulin resistance (Wiedmer et al., 2004), suggesting that defects in lipid scrambling may contribute to the dysregulation of lipid metabolism and the development of metabolic syndromes. PLSCR3 has also been implicated in the translocation of a phospholipid, cardiolipin (CL), from the inner to the outer mitochondrial membrane (Liu et al., 2003). CL integrates a variety of apoptosis-inducing signals at the level of mitochondria (Schug and Gottleib, 2009). For instance, apoptosis induced by tumor necrosis factor (TNF)-α and TNF-related apoptosis-inducing ligand (TRAIL) is potentiated by PLSCR3, through its effects on the distribution of CL on the mitochondrial surface (Liu et al., 2008; Ndebele et al., 2008). The latter serves as an illustrative example of the importance of the specific membrane distribution of a phospholipid for the regulation of cellular processes (apoptosis).
Further studies on the regulation of plasma membrane phospholipid asymmetry by mitochondria
Calcium has been implicated in the regulation of both cell survival and cell death (apoptosis) pathways and mitochondria participate actively in intracellular Ca2+ compartmentalization and signaling (Orrenius et al., 2003). Ca2+ ions are thought to play a role in the regulation of phospholipid asymmetry, as the activity of some scramblases is dependent on Ca2+, at least in platelets, whereas aminophospholipid translocase activity is also inhibited by Ca2+ (Zwaal and Schroit, 1997). Furthermore, in platelets, elevation of cytosolic Ca2+ with thapsigargin, an inhibitor of the sarco-endoplasmic reticulum Ca2+-ATPase, can trigger PS exposure in minutes (Dachary-Prigent et al., 1995). In thapsigargin-treated Jurkat cells, however, far fewer cells exposed PS, and the process took up to six hours (Hampton et al., 1996). In addition, removal of extracellular Ca2+, but not chelation of intracellular Ca2+, inhibited PS exposure in Fas-treated Jurkat cells by approximately 50%. Therefore, it appears that PS exposure in cells undergoing death receptor-mediated apoptosis is Ca2+-dependent, but not Ca2+-regulated, whereas the same process in platelets is Ca2+-regulated. Interestingly, antibodies to CD9, a member of the tetraspan family of membrane proteins, inhibits the prompt (within minutes) PS exposure in Jurkat cells stimulated by the calcium ionophore, A23187, but fails to block the delayed PS exposure induced by various apoptotic treatments (Li and Tait, 1998). Moreover, in lymphocytes from patients with Scott Syndrome, the scramblase activity is not activated by elevated internal Ca2+ levels but can still be induced by apoptosis (Williamson et al., 2001; Zhou et al., 1998), suggesting that at least two different pathways, one of which is Ca2+-dependent and one of which is Ca2+-independent but apoptosis-dependent, independently activate the scramblase activity in the cell. Consistent with this observation, our recent studies in C. eleggans have demonstrated that the mitochondria-derived factor, WAH-1 can activate the phospholipid scrambling activity of SCRM-1 in a Ca2+-independent manner (Wang et al., 2007). These findings further support the view that different pathways of PS externalization are initiated in cells undergoing apoptosis and following Ca2+-dependent activation.
Interestingly, a sustained intracellular calcium level in Jurkat cells mediated through the receptor-operated calcium channel was shown to induce mitochondria-dependent PS exposure in the absence of caspase activation and without evidence of mitochondrial release of cytochrome c or AIF (Jambrina et al., 2003). However, the process required oxidative/nitrosative stress. One may thus speculate that the PS exposure evidenced in the latter model is related to oxidative/nitrosative inactivation of aminophospholipid translocase activity, as demonstrated recently in HL-60 cells (Tyurina et al., 2007). In this context, it is also interesting to note that neutrophils from patients with Barth syndrome, a rare, metabolic (mitochondrial) disorder characterized by neutropenia and cardioskeletal myopathy, expose low amounts of PS on the surface, yet do not display other features of apoptosis (Kuijpers et al., 2004). The mechanism that underlies the PS exposure in Barth syndrome neutrophils remains elusive but it has been suggested that mitochondria-derived reactive oxygen species (ROS) may act as signaling intermediates in this process (van Raam and Kuijpers, 2009). Detailed assessment of phospholipid scramblase and aminophospholipid translocase activities in neutrophils from these patients versus healthy controls could shed some light on the link between mitochondrial ROS production and the loss of phospholipid asymmetry in the plasma membrane.
Evidence has also been presented for de novo synthesis of PS during apoptosis, suggesting yet another potential role for mitochondria in regulating PS asymmetry in plasma membrane. PS synthesis takes place in the endoplasmic reticulum (ER) and results from replacement of the polar head group of preexisting phospholipids (either PC or PE) by a serine (Kuge and Nishijima, 2003). The exchange of the polar head group is catalyzed by the Ca2+-dependent serine-base exchange enzyme system. Newly synthesized PS migrates either to the innner leaflet of the plasma membrane or to mitochondria where it is decarboxylated to PE. Aussel et al. (1998) first reported a robust yet transient neosynthesis of PS in Jurkat cells triggered to undergo Fas-mediated apoptosis. PS decarboxylation, a mitochondria-specific process, was strongly inhibited in this model, and the newly synthesized PS was detectable on the cell surface. Subsequent studies from the same investigators suggested a relationship between PS synthesis through the serine-base exchange enzyme system in the ER and PS exposure at the cell surface during Fas-mediated apoptosis (Pelassy et al., 2000). Similarly, treatment of mouse thymocytes with the apoptotic stimulus, dexamethasone, enhances the activity of the serine-base exchange enzyme (Burrata et al., 2000). However, the decarboxylation of newly synthesized PS to PE was almost negligible in mouse thymocytes, indicating that while different apoptotic stimuli may trigger an increase of PS synthesis, with subsequent PS exposure, they may have specific effects on the enzymes and organelles involved in PS metabolism.
Role of plasma membrane phospholipid asymmetry for intrinsic membrane potential
The intrinsic membrane potential affects or governs a variety of biological phenomena, and it has been generally accepted that the difference in membrane potential arises from the salt ion imbalance across the plasma membrane. However, recent atomic-scale molecular dynamics simulations show that a non-zero transmembrane potential can be observed in phospholipid membranes in the absence of ions, and suggest that the asymmetric distribution of lipids across the membrane and the intrinsic membrane potential are coupled phenomena (Gurtovenko and Vattulainen, 2007). The latter findings are in agreement with classical experimental data on dipole potential measurements in asymmetric lipid membranes (Latorre and Hall, 1976). It is of interest to note that the negative charge of the surface of apoptotic cells associated with externalization of PS has been shown to trigger endothelial cell membrane hyperpolarization and cytoskeleton reorganization, events that are important for angiogenesis and vascular remodeling (Weihua et al., 2005). Moreover, the negative charge associated with the presence of PS on the cytosolic leaflet of the membrane in living cells has been shown to direct proteins with a moderately positive charge to the endocytic pathway (Yeung et al., 2008). Recent studies have shown that PS persists on phagosomes of murine macrophages as the plasma membrane invaginates and that by conferring a considerable negative charge onto the phagosomal surface, PS contributes to the recruitment of cationic proteins like the c-Src tyrosine kinase (Yeung et al., 2009).
Loss of phospholipid asymmetry and PS externalization regulate important biological processes
The loss of lipid asymmetry in the plasma membrane and the appearance of the anionic phospholipids such as PS and PE on the cell surface have been implicated in numerous biological processes, including blood coagulation, myotube formation, vesicle fusion, cell division, sperm capacitation, and phagocytic recognition and clearance of apoptotic cell corpses (for a review, see Witasp et al., 2008). Moreover, loss of PS asymmetry in the plasma membrane may also be important for some viral infections. Viruses budding off an apoptotic host cell acquire a viral envelope that also exposes PS on its external surface, and entry of vaccinia virus through macropinocytosis was recently shown to be dependent on the presence of exposed PS on the viral membrane, suggesting that certain viruses may employ apoptotic “mimicry” to enter cells (Mercer and Helenius, 2008). Apoptotic mimicry by another obligate intracellular parasite was demonstrated previously (de Freitas Balanco et al., 2001; Wanderley et al., 2006). These studies showed that exposure of PS on the surface of the disease-propagating amistigotes of Leishmania amazonensis inhibits macrophage activity and increases susceptibility to intracellular leishmanial growth. Hence, while the protozoan Trypanosoma cruzi has been reported to use apoptotic host T lymphocytes for macrophage inactivation and consequent persistence in the mammalian host (Freire de Lima et al., 2000), Leishmania amazonensis, an intra-macrophagic parasite, appears to use an apoptotic-like feature of its own cells for a similar purpose.
Binding of blood clotting enzyme complexes to phospholipid membranes is a characteristic feature of blood coagulation (Zwaal et al., 2004). PS externalization on the surface of activated platelets produces a nearly million-fold increase in the rate of thrombin formation, the key enzyme of blood coagulation. Surface exposure of PS is brought about by the activation of Ca2+-dependent plasma membrane scramblases. Moreover, and as noted above, Scott syndrome is a hematological disorder in which blood clotting does not take place due to defective phospholipid scrambling in the plasma membrane. Schoenwaelder et al. (2009) recently reported the existence of two distinct pathways regulating the procoagulant function of platelets in vitro: the classical, Ca2+-dependent pathway induced by physiological agonists, and a Bak/Bax-dependent and caspase-mediated (apoptotic-like) pathway independent of platelet activation. Whether the apoptosis-related pathway participates in externalizing PS on the surface of platelets to generate thrombin in vivo or whether it merely acts to produce a signal for the clearance of platelets by phagocytic cells remains to be investigated. Platelets are anuclear cells generated through the compartmentalized, caspase-directed death of their progenitors, megakaryocytes (de Botton et al., 2002). Interestingly, this aborted apoptosis process yields functional platelets that retain their plasma membrane phospholipid asymmetry (Clarke et al., 2003). The latter findings thus reinforce the notion that plasma membrane changes of apoptosis can be dissociated from the caspase-directed program of nuclear condensation and fragmentation (Zhuang et al., 1998).
Cell surface exposure of PS has also been shown to be part of normal physiology of skeletal muscle development and to mediate myotube formation (van den Eijnde et al., 2001). PS exposure in myoblasts is caspase-independent, occurs mainly at cell-cell contact areas, and takes place at a stage when the structural organization of the sarcomeric protein titin is initiated, prior to actual fusion of individual myoblasts into multinucleated myotubes. PS exposure is viewed as a common signal for engulfment, yet studies in mouse embryos showed that no accumulation of phagocytes occurred in areas of myoblast differentiation. As suggested by van den Eijnde et al. (2001), this may potentially be explained by the lack of secretion of chemoattractant factors for phagocytes.
Loss of phospholipid asymmetry and cell surface externalization of PS is a general feature of the phagocytosis of apoptotic cells by macrophages (Fadok et al., 1992; Martin et al., 1995; Krahling et al., 1999) and has been linked to the activation of caspases (Martin et al., 1996; Vanags et al., 1996). Apoptotic cells that fail to express PS are not efficiently engulfed by macrophages, and the clearance defect can be restored by repleting the plasma membrane of target cells with exogenous PS (Fadok et al., 2001; Kagan et al., 2002). In addition, oxidized phospholipids including oxidized PS (PS-OX) may mediate macrophage recognition (Chang et al., 1999; Matsura et al., 2002; Kagan et al., 2002; Arroyo et al., 2002; Tyurina et al., 2004). Furthermore, Kagan and associates have demonstrated that cytochrome c released from mitochondria is involved in the selective catalysis of PS oxidation during programmed cell clearance (Jiang et al., 2004), underscoring the role of mitochondria-derived factors in apoptosis-related alterations of the plasma membrane.
Phosphatidylserine receptors: lessons emerging from studies in mice and other model organisms
PS externalization serves as an important and conserved recognition signal during apoptosis (Fadeel and Xue, 2006), and recent studies have identified several different PS receptors on macrophages, including T cell immunoglobulin- and mucin-domain-containing molecule-4 (Tim-4) and Tim-1 (Miyanishi et al., 2007; Kobayashi et al., 2007; Ichimura et al., 2008), the G protein-coupled receptor, brain-specific angiogenesis inhibitor 1 (BAI1) (Park et al., 2007), and stabilin 2 (Park et al., 2008), in addition to the original PS receptor (PSR) that was cloned almost 10 years ago by Fadok et al. (2000). Importantly, studies in mice that are deficient for PSR (Li et al., 2003; Kunisaki et al., 2004) or the PS-binding protein, milk fat globule-epidermal growth factor 8 (MFG-E8) (Hanayama et al., 2004) have demonstrated the occurrence of numerous unengulfed apoptotic cells and autoimmune disease, thus providing compelling evidence that PS-dependent programmed cell clearance is required for maintenance of tissue homeostasis. Injection of an MFG-E8 mutant protein that binds to PS but fails to bridge the apoptotic cells to macrophage receptors also results in impairment of apoptotic cell engulfment and production of autoantibodies in mice (Asano et al., 2004). Furthermore, inactivation of the C. elegans PSR homolog, PSR-1, also results in an engulfment defect and PSR-1 is found to act upstream of the CED-5/CED-12 engulfment pathway and may transduce the PS “eat-me” signal through its interacting proteins, CED-5 and CED-12 (Wang et al., 2003). Similarly, knockdown of the zebrafish PSR homolog (zfpsr) by a PSR morpholino oligonucleotide led to accumulation of a large number of dead apoptotic cells during development (Hong et al., 2004). Moreover, the Drosophila PSR homolog (dPSR) plays an important role in mediating the removal of growth-disadvantaged cells that have reduced ribosomal protein gene dose by neighboring wild-type cells through a phagocytic mechanism (Li and Baker, 2007), although in a different study no obvious defect in engulfment of apoptotic cells was found in dPSR-deficient flies and instead dPSR appeared to function to inhibit apoptosis (Krieser et al., 2007). In a third study involving PSR-deficient mice, Böse et al. (2004) reported that PSR-deficient macrophages were impaired in pro- and anti-inflammatory cytokine signaling after stimulation with apoptotic cells, but they could not document any impairment of apoptotic cell engulfment. Therefore, one may conclude that PSR plays a conserved role in phagocytosis of apoptotic or non-apoptotic cells in multiple organisms but may not be the dominant receptor for phagocytosis. It also should be noted that PSR is so far the only conserved candidate PS receptor, as the other proposed PS receptors discussed above are not found in worms or fruit flies. Further analysis of PSR, and in particular the cellular localization pattern of the endogenous PSR protein will be critical for understanding how PSR is involved in phagocytosis and animal development.
Despite the prevailing view that PS exposure on the plasma membrane induces phagocytosis, recent studies of TAT-1-deficient worms have shown that the loss of living cells with ectopic exposure of PS appears to be random and non-exhaustive but is completely dependent on the activity of PSR-1 and another worm phagocyte receptor CED-1, which acts in a different engulfment pathway from PSR-1 (Darland-Ransom et al., 2008). This raises the possibility that engagement of a single cell surface receptor for PS may not suffice to trigger phagocytosis, and that other “eat-me” signals or multiple receptors or pathways need to be engaged for efficient removal of apoptotic cells. This observation may also explain the weak engulfment defect observed in PSR-deficient animals, as discussed above. Our previous studies in mammalian model systems have suggested that oxidation of PS may be required for efficient macrophage clearance of apoptotic cell corpses (Kagan et al., 2002). Interestingly, the murine scavenger receptor, CD36 was shown to specifically recognize oxidized forms of PS (Greenberg et al., 2006) and the CD36-related receptor, croquemort, is also implicated in apoptotic corpse clearance in Drosophila (Franc et al., 1999). It is thus possible that different macrophage receptors may have very specific preferences for certain species of lipid generated during apoptosis (Fadeel et al., 2007). Alternatively, or additionally, other engulfment signals or pathways may also be required for efficient engulfment of apoptotic cell corpses. We recently reported that PS externalization alone is not sufficient for macrophage disposal of neutrophils (Jitkaew et al., 2009). However, addition of recombinant MFG-E8, a PS-binding protein, restored engulfment of target cells, thus enforcing the view that bridging molecules such as MFG-E8 may activate a different signaling pathway that is required for opsonization and efficient engulfment of target cells. Besides being a binding partner for the integrin receptor, MFG-E8 may also bind transglutaminase 2 (TG2) on macrophages (Toth et al., 2009). Indeed, the presence of TG2 was shown to be important for the formation of an efficient phagocytic “portal” for internalization of apoptotic cells. Therefore, a single recognition signal may trigger the activation of multiple pathways through different receptors, which coordinately may lead to efficient engulfment of cells with surface exposed PS.
Novel pathways of phosphatidylserine externalization: role in the resolution of inflammation
While the externalization of PS has been linked to the apoptotic program and is frequently employed as a marker of apoptotic cell death (van Genderen et al., 2006), several studies have demonstrated that caspase-independent and/or non-apoptotic pathways of PS exposure also exist. For instance, PS exposure is seen during B cell activation (Dillon et al., 2000) and mast cell degranulation (Martin et al., 2000) and transient PS exposure is also observed during stimulation of neutrophils with the chemotactic peptide formylated Met-Leu-Phe (fMLP) (Frasch et al., 2004). Our previous work demonstrated that neutrophils possess two different pathways of PS exposure: a caspase-dependent pathway associated with induction of constitutive apoptosis, and a ROS-dependent, caspase-independent pathway following neutrophil activation (Fadeel et al., 1998). More recently, we have identified an additional, novel pathway of PS externalization in primary human neutrophils that is neither caspase-dependent nor ROS-dependent but is induced by neighboring phagocytes in a manner that is dependent on cell-cell contact (Jitkaew et al., 2009). In another recent study, activated macrophages were shown to cause inhibition of aminophospholipid translocation in the plasma membrane of neighboring, “innocent” target cells via a nitrosative stress-dependent mechanism, resulting in PS exposure on the surface of the latter cells and subsequent engulfment by macrophages (Tyurina et al., 2007). PS-dependent clearance of apoptotic cells by macrophages plays an active role in the resolution of inflammation, through production of anti-inflammatory cytokines such as transforming growth factor-β and down-regulation of pro-inflammatory mediators such as tumor necrosis factor-α (Voll et al., 1997; Fadok et al., 1998). The novel observation of macrophage-induced PS exposure in bystander neutrophils (Jitkaew et al., 2009) and other target cells (Tyurina et al., 2007) suggests that activated macrophages may engage in a PS-dependent feedback mechanism that potentially could serve to limit excessive inflammatory responses.
Concluding remarks
Several putative lipid transporter activities have been implicated in the generation, maintenance, and alteration of phospholipid asymmetry in the plasma membrane, which are critical for maintaining cell integrity and physiology and for regulating multiple important cellular events. However, it has been difficult to assign these activities to specific molecular entities. Recent studies in the model organism C. elegans have revealed a role for the P4 ATPase homolog, TAT-1, in the maintenance of phospholipid asymmetry in living cells, and have unearthed a novel mitochondria-to-plasma membrane signaling pathway that promotes the disruption of lipid asymmetry in apoptotic cells. The latter pathway involves the WAH-1 (worm homolog of AIF)-dependent activation of SCRM-1, a homolog of the mammalian phospholipid scramblases, in the plasma membrane (see Figure 4, for a schematic diagram). The mechanisms of lipid asymmetry and its disruption are thus beginning to unravel. Collectively, studies in this exciting field of research have served to elucidate how crucial biological functions are encoded into the vectorial distribution of plasma membrane phospholipids.
Box 1 Examples of human diseases associated with defects in lipid transporters.
Mutations in the Atp8B1 gene encoding a putative member of the flippase family are the cause of progressive familial intrahepatic cholestasis type I (Klomp et al., 2000), originally described as “Byler disease” in an Amish kindred. The disease primarily manifests itself as a chronic intrahepatic cholestasis, which progresses to severe, end-stage liver disease before adolescence. The patients display impaired hepatobiliary bile salt secretion and normal serum cholesterol. Overexpression of Atp8B1 was shown to result in energy-dependent PS translocation in a model cell line (Ujhazy et al., 2001). However, it is not known whether the absence of the putative aminophospolipid translocase activity is related to the pathogenesis of this severe disease.
Mutations in the ATP-binding cassette (ABC) transporter, ABCA1, a candidate floppase, are responsible for Tangier disease (Bodzioch et al., 1999; Brooks-Wilson et al., 1999; Rust et al., 1999), an autosomal recessive disorder characterized by the absence of high-density lipoprotein (HDL), reduced levels of low-density lipoprotein (LDL), and mildly elevated triglycerides. Tangier patients are at high risk for developing premature coronary artery disease (atherosclerosis). Mice with a targeted inactivation of ABCA1 are unable to load cholesterol and phospholipids to apoA-1, implicating ABCA1 in the efflux of these lipids from cells (Orso et al., 2000; McNeish et al., 2000).
Scott syndrome is a rare congenital bleeding disorder, characterized by an impaired ability of the patients' platelets to promote blood coagulation (Weiss et al., 1979). Platelets from these individuals are deficient for Ca2+-induced phospholipid scramblase activity in the plasma membrane, resulting in a reduced exposure of procoagulant PS on the outer surface of the cell (reviewed in Zwaal et al., 2004). The molecular lesions responsible for the underlying genetic defect have yet to be determined.
Acknowledgements
This work was supported by grants from the Swedish Research Council, National Institutes of Health (GM059083 and GM079097), and Human Frontier Science Program (RGP0016/2005-C). B.F. is the recipient of a Senior Investigator Award from the Swedish Cancer Foundation. D.X is a Foreign Adjunct Professor of Karolinska Institutet. We thank our long-term collaborators, Prof. Valerian Kagan, University of Pittsburgh, and Prof. Peter Quinn, King's College London, for valuable discussions.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Arroyo A, Modriansky M, Serinkan FB, Bello RI, Matsura T, Jiang J, Tyurin VA, Tyurina YY, Fadeel B, Kagan VE. NADPH oxidase-dependent oxidation and externalization of phosphatidylserine during apoptosis in Me2SO-differentiated HL-60 cells. Role in phagocytic clearance. J Biol Chem. 2002;277:49965–49975. doi: 10.1074/jbc.M204513200. [DOI] [PubMed] [Google Scholar]
- 2.Arur S, Uche UE, Rezaul K, Fong M, Scranton V, Cowan AE, Mohler W, Han DK. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 3.Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 2004;200:459–467. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aussel C, Pelassy C, Breittmayer JP. CD95 (Fas/APO-1) induces an increased phosphatidylserine synthesis that precedes its externalization during programmed cell death. FEBS Lett. 1998;431:195–199. doi: 10.1016/s0014-5793(98)00748-0. [DOI] [PubMed] [Google Scholar]
- 5.Bassé F, Stout JG, Sims PJ, Wiedmer T. Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem. 1996;271:17205–17210. doi: 10.1074/jbc.271.29.17205. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Efraim I, Zhou Q, Wiedmer T, Gerace L, Sims PJ. Phospholipid scramblase 1 is imported into the nucleus by a receptor-mediated pathway and interacts with DNA. Biochemistry. 2004;43:3518–3526. doi: 10.1021/bi0356911. [DOI] [PubMed] [Google Scholar]
- 7.Blom WM, de Bont HJ, Nagelkerke JF. Regional loss of the mitochondrial membrane potential in the hepatocyte is rapidly followed by externalization of phosphatidylserines at that specific site during apoptosis. J Biol Chem. 2003;278:12467–12474. doi: 10.1074/jbc.M201264200. [DOI] [PubMed] [Google Scholar]
- 8.Bodzioch M, Orso E, Klucken T, Langmann T, Böttcher L, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 9.Böse J, Gruber AD, Helming L, Schiebe S, Wegener I, Hafner M, Beales M, Köntgen F, Lengeling A. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J Biol. 2004;3:15. doi: 10.1186/jbiol10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 11.Buratta S, Migliorati G, Marchetti C, Mambrini R, Riccardi C, Mozzi R. Dexamethasone increases the incorporation of [3H]serine into phosphatidylserine and the activity of serine base exchange enzyme in mouse thymocytes: a possible relation between serine base exchange enzyme and apoptosis. Mol Cell Biochem. 2000;211:61–67. doi: 10.1023/a:1007102531404. [DOI] [PubMed] [Google Scholar]
- 12.Chang MK, Bergmark C, Laurila A, Hörkkö S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke MC, Savill J, Jones DB, Noble BS, Brown SB. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol. 2003;160:577–587. doi: 10.1083/jcb.200210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dachary-Prigent J, Pasquet JM, Freyssinet JM, Nurden AT. Calcium involvement in aminophospholipid exposure and microparticle formation during platelet activation: a study using Ca2+-ATPase inhibitors. Biochemistry. 1995;34:11625–11634. doi: 10.1021/bi00036a039. [DOI] [PubMed] [Google Scholar]
- 15.Daleke DL. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol. 2008;15:191–195. doi: 10.1097/MOH.0b013e3282f97af7. [DOI] [PubMed] [Google Scholar]
- 16.Darland-Ransom M, Wang X, Sun CL, Mapes J, Gengyo-Ando K, Mitani S, Xue D. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320:528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- 17.de Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, Kroemer G, Vainchenker W, Debili N. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–1317. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 18.de Freitas Balanco JM, Moreira ME, Bonomo A, Bozza PT, Amarante-Mendes G, Pirmez C, Barcinski MA. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001;11:1870–1873. doi: 10.1016/s0960-9822(01)00563-2. [DOI] [PubMed] [Google Scholar]
- 19.Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164:1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 20.Fadeel B, Åhlin A, Henter J-I, Orrenius S, Hampton MB. Involvement of caspases in neutrophil apoptosis: regulation by reactive oxygen species. Blood. 1998;92:4808–4818. [PubMed] [Google Scholar]
- 21.Fadeel B, Gleiss B, Högstrand K, Chandra J, Wiedmer T, Sims PJ, Henter J-I, Orrenius S, Samali A. Phosphatidylserine exposure during apoptosis is a cell type-specific event and does not correlate with plasma membrane phospholipid scramblase expression. Biochem Biophys Res Commun. 1999;266:504–511. doi: 10.1006/bbrc.1999.1820. [DOI] [PubMed] [Google Scholar]
- 22.Fadeel B, Karpova MB, Enoksson M, Orrenius S. Phosphatidylserine externalization in cardiolipin-deficient cells. Blood. 2004;104:1582–1583. doi: 10.1182/blood-2004-03-0840. [DOI] [PubMed] [Google Scholar]
- 23.Fadeel B, Quinn P, Xue D, Kagan V. Fat(al) attraction: oxidized lipids act as “eat-me” signals. HFSP J. 2007;1:225–229. doi: 10.2976/1.2800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadeel B, Xue D. PS externalization: from corpse clearance to drug delivery. Cell Death Diff. 2006;13:360–362. doi: 10.1038/sj.cdd.4401836. [DOI] [PubMed] [Google Scholar]
- 25.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 27.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 28.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 29.Fan X, Krahling S, Smith D, Williamson P, Schlegel RA. Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell. 2004;15:2863–2872. doi: 10.1091/mbc.E03-09-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 1999;284:1991–1994. doi: 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- 31.Frasch SC, Henson PM, Kailey JM, Richter DA, Janes MS, Fadok VA, Bratton DL. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cδ. J Biol Chem. 2000;275:23065–23073. doi: 10.1074/jbc.M003116200. [DOI] [PubMed] [Google Scholar]
- 32.Frasch SC, Henson PM, Nagaosa K, Fessler MB, Borregaard N, Bratton DL. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J Biol Chem. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 33.Freire de Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurtovenko AA, Vattulainen I. Lipid transmembrane asymmetry and intrinsic membrane potential: two sides of the same coin. J Am Chem Soc. 2007;129:5358–5359. doi: 10.1021/ja070949m. [DOI] [PubMed] [Google Scholar]
- 36.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 37.Hampton MB, Vanags DM, Pörn-Ares MI, Orrenius S. Involvement of extracellular calcium in phosphatidylserine exposure during apoptosis. FEBS Lett. 1996;399:277–282. doi: 10.1016/s0014-5793(96)01341-5. [DOI] [PubMed] [Google Scholar]
- 38.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 39.Hong JR, Lin GH, Lin CJ, Wang WP, Lee CC, Lin TL, Wu JL. Phosphatidylserine receptor is required for the engulfment of dead apoptotic cells and for normal embryonic development in zebrafish. Development. 2004;131:5417–5427. doi: 10.1242/dev.01409. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Zhao Q, Zhou CX, Gu ZM, Li D, Xu HZ, Wiedmer T, Sims PJ, Zhao KW, Chen GQ. Antileukemic roles of human phospholipid scramblase 1 gene, evidence from inducible PLSCR1-expressing leukemic cells. Oncogene. 2006;25:6618–6627. doi: 10.1038/sj.onc.1209677. [DOI] [PubMed] [Google Scholar]
- 41.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeda M, Kihara A, Denpoh A, Igarashi Y. The rim101 pathway is involved in rsb1 expression induced by altered lipid asymmetry. Mol Biol Cell. 2008;19:1922–1931. doi: 10.1091/mbc.E07-08-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jambrina E, Alonso R, Alcalde M, del Carmen Rodríguez M, Serrano A, Martínez-A C, García-Sancho J, Izquierdo M. Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J Biol Chem. 2003;278:14134–14145. doi: 10.1074/jbc.M211388200. [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, Kini V, Belikova N, Serinkan BF, Borisenko GG, Tyurina YY, Tyurin VA, Kagan VE. Cytochrome c release is required for phosphatidylserine peroxidation during Fas-triggered apoptosis in lung epithelial A549 cells. Lipids. 2004;39:1133–1142. doi: 10.1007/s11745-004-1340-1. [DOI] [PubMed] [Google Scholar]
- 45.Jitkaew S, Witasp E, Zhang S, Kagan VE, Fadeel B. Induction of caspase- and reactive oxygen species-independent phosphatidylserine externalization in primary human neutrophils: role in macrophage recognition and engulfment. J Leukoc Biol. 2009;85:427–437. doi: 10.1189/jlb.0408232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kagan VE, Gleiss B, Tyurina YY, Tyurin VA, Elenström-Magnusson C, Liu SX, Serinkan FB, Arroyo A, Chandra J, Orrenius S, Fadeel B. A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002;169:487–499. doi: 10.4049/jimmunol.169.1.487. [DOI] [PubMed] [Google Scholar]
- 47.Kagan VE, Quinn PJ. Toward oxidative lipidomics of cell signaling. Antioxid Redox Signal. 2004;6:199–202. doi: 10.1089/152308604322899260. [DOI] [PubMed] [Google Scholar]
- 48.Klomp LW, Bull LN, Knisely AS, van Der Doelen MA, Juijn JA, Berger R, Forget S, Nielsen IM, Eiberg H, Houwen RH. A missense mutation in FIC1 is associated with greenland familial cholestasis. Hepatology. 2000;32:1337–1341. doi: 10.1053/jhep.2000.20520. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi N, Karisola P, Peña-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krahling S, Callahan MK, Williamson P, Schlegel RA. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6:183–189. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- 51.Krieser RJ, Moore FE, Dresnek D, Pellock BJ, Patel R, Huang A, Brachmann C, White K. The Drosophila homolog of the putative phosphatidylserine receptor functions to inhibit apoptosis. Development. 2007;134:2407–2414. doi: 10.1242/dev.02860. [DOI] [PubMed] [Google Scholar]
- 52.Kuge O, Nishijima M. Biosynthetic regulation and intracellular transport of phosphatidylserine in mammalian cells. J Biochem. 2003;133:397–403. doi: 10.1093/jb/mvg052. [DOI] [PubMed] [Google Scholar]
- 53.Kuijpers TW, Maianski NA, Tool AT, Becker K, Plecko B, Valianpour F, Wanders RJ, Pereira R, Van Hove J, Verhoeven AJ, Roos D, Baas F, Barth PG. Neutrophils in Barth syndrome (BTHS) avidly bind annexin-V in the absence of apoptosis. Blood. 2004;103:3915–3923. doi: 10.1182/blood-2003-11-3940. [DOI] [PubMed] [Google Scholar]
- 54.Kunisaki Y, Masuko S, Noda M, Inayoshi A, Sanui T, Harada M, Sasazuki T, Fukui Y. Defective fetal liver erythropoiesis and T lymphopoiesis in mice lacking the phosphatidylserine receptor. Blood. 2004;103:3362–3364. doi: 10.1182/blood-2003-09-3245. [DOI] [PubMed] [Google Scholar]
- 55.Latorre R, Hall JE. Dipole potential measurements in asymmetric membranes. Nature. 1976;264:361–363. doi: 10.1038/264361a0. [DOI] [PubMed] [Google Scholar]
- 56.Lenoir G, Williamson P, Holthuis JC. On the origin of lipid asymmetry: the flip side of ion transport. Curr Opin Chem Biol. 2007;11:654–661. doi: 10.1016/j.cbpa.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Lenoir G, Williamson P, Puts CF, Holthuis JC. Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter DRS2P. J Biol Chem. 2009;284:17956–17967. doi: 10.1074/jbc.M109.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li W, Baker NE. Engulfment is required for cell competition. Cell. 2007;129:1215–1225. doi: 10.1016/j.cell.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 59.Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302:1560–1563. doi: 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Tait JF. Regulatory effect of CD9 on calcium-stimulated phosphatidylserine exposure in Jurkat T lymphocytes. Arch Biochem Biophys. 1998;351:89–95. doi: 10.1006/abbi.1997.0535. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Dai Q, Chen J, Durrant D, Freeman A, Liu T, Grossman D, Lee RM. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol Cancer Res. 2003;1:892–902. [PubMed] [Google Scholar]
- 62.Liu J, Epand RF, Durrant D, Grossman D, Chi NW, Epand RM, Lee RM. Role of phospholipid scramblase 3 in the regulation of tumor necrosis factor-alpha-induced apoptosis. Biochemistry. 2008;47:4518–4529. doi: 10.1021/bi701962c. [DOI] [PubMed] [Google Scholar]
- 63.Luciani MF, Chimini G. The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 1996;15:226–235. [PMC free article] [PubMed] [Google Scholar]
- 64.Martin SJ, Finucane DM, Amarante-Mendes GP, O'Brien GA, Green DR. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. J Biol Chem. 1996;271:28753–28756. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- 65.Martin S, Pombo I, Poncet P, David B, Arock M, Blank U. Immunologic stimulation of mast cells leads to the reversible exposure of phosphatidylserine in the absence of apoptosis. Int Arch Allergy Immunol. 2000;123:249–258. doi: 10.1159/000024451. [DOI] [PubMed] [Google Scholar]
- 66.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martínez MC, Freyssinet JM. Deciphering the plasma membrane hallmarks of apoptotic cells: phosphatidylserine transverse redistribution and calcium entry. BMC Cell Biol. 2001;2:20. doi: 10.1186/1471-2121-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsura T, Serinkan BF, Jiang J, Kagan VE. Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett. 2002;524:25–30. doi: 10.1016/s0014-5793(02)02990-3. [DOI] [PubMed] [Google Scholar]
- 69.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Nat Acad Sci USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miletich JP, Kane WH, Hofmann SL, Stanford N, Majerus PW. Deficiency of factor Xa-factor Va binding sites on the platelets of a patient with a bleeding disorder. Blood. 1979;54:1015–1022. [PubMed] [Google Scholar]
- 71.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 72.Ndebele K, Gona P, Jin TG, Benhaga N, Chalah A, Degli-Esposti M, Khosravi-Far R. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced mitochondrial pathway to apoptosis and caspase activation is potentiated by phospholipid scramblase-3. Apoptosis. 2008;13:845–856. doi: 10.1007/s10495-008-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 74.Orso E, Broccardo C, Kaminski WE, Böttcher A, Liebisch G, Drobnik W, Götz A, Chambenoit O, Diederich W, Langmann T, Spruss T, Luciani MF, Rothe G, Lackner KJ, Chimini G, Schmitz G. Transport of lipids from Golgi to plasma membrane is defective in Tangier disease patients and Abc1-deficient mice. Nat Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 75.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, Kim IS. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 76.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 77.Pelassy C, Breittmayer JP, Aussel C. Regulation of phosphatidylserine exposure at the cell surface by the serine-base exchange enzyme system during CD95-induced apoptosis. Biochem Pharmacol. 2000;59:855–863. doi: 10.1016/s0006-2952(99)00383-4. [DOI] [PubMed] [Google Scholar]
- 78.Ricci JE, Muñoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Rosing J, Bevers EM, Comfurius P, Hemker HC, van Dieijen G, Weiss HJ, Zwaal RF. Impaired factor X and prothrombin activation associated with decreased phospholipid exposure in platelets from a patient with a bleeding disorder. Blood. 1985;65:1557–1561. [PubMed] [Google Scholar]
- 80.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 81.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 82.Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, O'Reilly LA, Henley KJ, Ono A, Hsiao S, Willcox A, Roberts AW, Huang DC, Salem HH, Kile BT, Jackson SP. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114:663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 83.Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009 May 18; doi: 10.1016/j.bbamem.2009.05.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 84.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 85.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 86.Toth B, Garabuczi E, Sarang Z, Vereb G, Vámosi G, Aeschlimann D, Blaskó B, Bécsi B, Erdõdi F, Lacy-Hulbert A, Zhang A, Falasca L, Birge RB, Balajthy Z, Melino G, Fésüs L, Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- 87.Tyurina YY, Basova LV, Konduru NV, Tyurin VA, Potapovich AI, Cai P, Bayir H, Stoyanovsky D, Pitt BR, Shvedova AA, Fadeel B, Kagan VE. Nitrosative stress inhibits the aminophospholipid translocase resulting in phosphatidylserine externalization and macrophage engulfment: implications for the resolution of inflammation. J Biol Chem. 2007;282:8498–8509. doi: 10.1074/jbc.M606950200. [DOI] [PubMed] [Google Scholar]
- 88.Tyurina YY, Serinkan FB, Tyurin VA, Kini V, Yalowich JC, Schroit AJ, Fadeel B, Kagan VE. Lipid antioxidant, etoposide, inhibits phosphatidylserine externalization and macrophage clearance of apoptotic cells by preventing phosphatidylserine oxidation. J Biol Chem. 2004;279:6056–6064. doi: 10.1074/jbc.M309929200. [DOI] [PubMed] [Google Scholar]
- 89.Ujhazy P, Ortiz D, Misra S, Li S, Moseley J, Jones H, Arias IM. Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology. 2001;34:768–775. doi: 10.1053/jhep.2001.27663. [DOI] [PubMed] [Google Scholar]
- 90.van Genderen H, Kenis H, Lux P, Ungeth L, Maassen C, Deckers N, Narula J, Hofstra L, Reutelingsperger C. In vitro measurement of cell death with the annexin A5 affinity assay. Nat Protoc. 2006;1:363–367. doi: 10.1038/nprot.2006.55. [DOI] [PubMed] [Google Scholar]
- 91.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Raam BJ, Kuijpers TW. Mitochondrial defects lie at the basis of neutropenia in Barth syndrome. Curr Opin Hematol. 2009;16:14–19. doi: 10.1097/MOH.0b013e32831c83f3. [DOI] [PubMed] [Google Scholar]
- 93.Vanags DM, Pörn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271:31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 94.Venegas V, Zhou Z. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol Biol Cell. 2007;18:3180–3192. doi: 10.1091/mbc.E07-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, van Heerde WL, Henfling ME, Vermeij-Keers C, Schutte B, Borgers M, Ramaekers FC. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–3642. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 96.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 97.Wanderley JL, Moreira ME, Benjamin A, Bonomo AC, Barcinski MA. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol. 2006;176:1834–1839. doi: 10.4049/jimmunol.176.3.1834. [DOI] [PubMed] [Google Scholar]
- 98.Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C, Shi Y, Kobayashi T, Shi Y, Mitani S, Xie XS, Xue D. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol. 2007;9:541–549. doi: 10.1038/ncb1574. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Wu YC, Fadok VA, Lee MC, Gengyo-Ando K, Cheng LC, Ledwich D, Hsu PK, Chen JY, Chou BK, Henson P, Mitani S, Xue D. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science. 2003;302:1563–1566. doi: 10.1126/science.1087641. [DOI] [PubMed] [Google Scholar]
- 100.Wang X, Yang C, Chai J, Shi Y, Xue D. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science. 2002;298:1587–1592. doi: 10.1126/science.1076194. [DOI] [PubMed] [Google Scholar]
- 101.Weihua Z, Tsan R, Schroit AJ, Fidler IJ. Apoptotic cells initiate endothelial cell sprouting via electrostatic signaling. Cancer Res. 2005;65:11529–11535. doi: 10.1158/0008-5472.CAN-05-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiss HJ, Vicic WJ, Lages BA, Rogers J. Isolated deficiency of platelet procoagulant activity. Am J Med. 1979;67:206–213. doi: 10.1016/0002-9343(79)90392-9. [DOI] [PubMed] [Google Scholar]
- 103.Wiedmer T, Zhou Q, Kwoh DY, Sims PJ. Identification of three new members of the phospholipid scramblase gene family. Biochim Biophys Acta. 2000;1467:244–253. doi: 10.1016/s0005-2736(00)00236-4. [DOI] [PubMed] [Google Scholar]
- 104.Wiedmer T, Zhao J, Li L, Zhou Q, Hevener A, Olefsky JM, Curtiss LK, Sims PJ. Adiposity, dyslipidemia, and insulin resistance in mice with targeted deletion of phospholipid scramblase 3 (PLSCR3) Proc Natl Acad Sci USA. 2004;101:13296–13301. doi: 10.1073/pnas.0405354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williamson P, Christie A, Kohlin T, Schlegel RA, Comfurius P, Harmsma M, Zwaal RF, Bevers EM. Phospholipid scramblase activation pathways in lymphocytes. Biochemistry. 2001;40:8065–8072. doi: 10.1021/bi001929z. [DOI] [PubMed] [Google Scholar]
- 106.Williamson P, Halleck MS, Malowitz J, Ng S, Fan X, Krahling S, Remaley AT, Schlegel RA. Transbilayer phospholipid movements in ABCA1-deficient cells. PLoS ONE. 2007;2:e729. doi: 10.1371/journal.pone.0000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Witasp E, Kagan V, Fadeel B. Programmed cell clearance: molecular mechanisms and role in autoimmue disease, chronic inflammation, and anti-cancer immune responses. Curr Immunol Rev. 2008;4:53–69. [Google Scholar]
- 108.Wolf C, Quinn PJ. Lipidomics: practical aspects and applications. Prog Lipid Res. 2008;47:15–36. doi: 10.1016/j.plipres.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 109.Wu YC, Horvitz HR. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell. 1998;93:951–960. doi: 10.1016/s0092-8674(00)81201-5. [DOI] [PubMed] [Google Scholar]
- 110.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 111.Yeung T, Heit B, Dubuisson JF, Fairn GD, Chiu B, Inman R, Kapus A, Swanson M, Grinstein S. Contribution of phosphatidylserine to membrane surface charge and protein targeting during phagosome maturation. J Cell Biol. 2009;185:917–928. doi: 10.1083/jcb.200903020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Q, Zhao J, Wiedmer T, Sims PJ. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood. 2002;99:4030–4038. doi: 10.1182/blood-2001-12-0271. [DOI] [PubMed] [Google Scholar]
- 114.Zhuang J, Ren Y, Snowden RT, Zhu H, Gogvadze V, Savill JS, Cohen GM. Dissociation of phagocyte recognition of cells undergoing apoptosis from other features of the apoptotic program. J Biol Chem. 1998;273:15628–15632. doi: 10.1074/jbc.273.25.15628. [DOI] [PubMed] [Google Scholar]
- 115.Züllig S, Neukomm LJ, Jovanovic M, Charette SJ, Lyssenko NN, Halleck MS, Reutelingsperger CP, Schlegel RA, Hengartner MO. Aminophospholipid translocase TAT-1 promotes phosphatidylserine exposure during C. elegans apoptosis. Curr Biol. 2007;17:994–999. doi: 10.1016/j.cub.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 116.Zwaal RF, Comfurius P, Bevers EM. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim Biophys Acta. 2004;1636:119–128. doi: 10.1016/j.bbalip.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]