SUMMARY
The objective of this study was to determine risk factors predicting seizures and damage due to seizures in a multi-ethnic systemic lupus erythematosus (SLE) cohort (PROFILE) which includes SLE patients (n=1295) from five different US institutions. Only patients with seizures after SLE diagnosis (incident) were included in the analyses of clinical seizures, 80/1295, 6.2%; but all patients (prevalent and incident) in the analyses of damage due to seizures 51/1295, 3.9%. We examined socioeconomic-demographic, clinical and genetic variables predictive of clinical seizures and damage from seizures by Cox Proportional Hazard Ratios (HR) and 95% Confidence Intervals (CI). Independent predictors of a shorter time to occurrence of clinical seizures were younger age (HR=1.0; 95% CI 0.9–1.0), having Hispanic-Texan ethnicity (HR=2.7; 95% CI 1.3–5.7) or African-American ethnicity (HR=1.8; 95% CI 1.0–3.1, and the prior occurrence of a cerebrovascular accident [CVA] (HR=3.3; 95% CI 1.6–7.1) or an episode of psychosis (HR=2.4; 95% CI 1.1–5.0), while the prior occurrence of photosensitivity (HR=0.5; 95% CI 0.3–0.9).was the only independent predictor of a longer time to the clinical occurrence of seizures Independent predictors of a shorter time to occurrence of damage due to seizures were younger age (HR=1.0 95% CI 0.9–1.0), male gender (HR=2.4; 95% CI 1.1–5.4), and the occurrence of a prior CVA (HR=2.7; 95% CI 1.0–7.0 or an episode of psychosis (HR=4.7; 95% CI 2.3–9.9). No allele from the candidate genes examined (HLA-DRB1, HLA-DQB1, FCGR2A, FCGR3A, or FCG3B) predicted clinical seizures or damage due to seizures.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a prototypic immune complex disease characterized by a variable course and outcome. Neuropsychiatric involvement is reported often, across a wide spectrum of manifestations occurring with variable frequencies across studies 1–6. The variability in reporting is also represented when focusing on seizures where the prevalence reports range from 6–51% in adult and pediatric patients 1–6.
Seizures occur in the setting of active multisystem disease or present as an isolated clinical event 7. Although antiphospholipid (APL) antibodies have been associated with seizures 4, 7, 8, there are likely to be other risk factors for the development of seizures in SLE. Familial aggregation of SLE suggests a genetic component to disease susceptibility and numerous studies have now defined genetic associations with individual candidate genes 9–12. Based on our observation that low affinity Fc receptors for IgG are associated with the development of end-stage renal disease in patients with SLE 13, we posit that a genetic component may also contribute to seizure risk.
Human familial epilepsy and other Mendelian epilepsy syndromes have defined genetic mutations14, 15. Additional evidence suggests that vaccine related encephalopathy 16 and some seizure disorders may be related to mutations in sodium channel genes.14 However, in sporadic seizures, there are no clearly defined associated genetic abnormalities including ion channel gene mutations 14.
In this study, we extend our previous reports on a multi-ethnic, multi-center cohort of SLE patients, named PROFILE17 to address potential genetic risk factors for seizures. This cohort was constituted in 1998 by combining the existing cohorts at Northwestern University (NU), Johns Hopkins University (JHU), the University of Alabama at Birmingham (UAB), The University of Texas Health Science Center at Houston (UTH) and subsequently the University of Puerto Rico Medical Sciences Campus (UPR) 13,17 with the overarching hypothesis that the patients’ genetic profile might allow for the prediction of their disease phenotype, hence the name PROFILE. Our specific hypothesis for the current study is that seizures might be related to the intensity of the immune complex mediated inflammatory disease response. Therefore, we tested several candidate genes, previously noted to be associated with renal damage in SLE, for their association with seizure occurrence.
PATIENTS AND METHODS
Institutional review board approval to constitute this cohort and to follow these patients over time was obtained at each participating institution in accordance with the declaration of Helsinki. As previously described17, PROFILE is a multi-institutional (NU, Chicago, IL; JHU, Baltimore, MD; UAB, Birmingham, AL; UTH, Houston, TX and UPR, San Juan, PR) cohort of SLE patients. PROFILE patients are those from the individual cohorts who meet the American College of Rheumatology (ACR) revised and updated classification criteria18, 19, are 16 years of age or older, and have disease duration ≤ 10 years at the time they enter this cohort. They are also of defined ethnicity [Hispanic of Mexican ancestry (residing and enrolled in Texas, hence Texan Hispanics), Hispanic of Puerto Rican ancestry (residing and enrolled in Puerto Rico, hence Puerto Rican Hispanics), African American and Caucasian], having reported all four grandparents to be of the same ethnic background. Two hundred six patients were from NU, 586 from JHU, 340 from UAB, 189 from UTH and 102 from UPR. The PROFILE database consists of those variables common to the individual cohorts which were identified after carefully mapping the different cohorts’ databases 17.
The variables for the current analyses include both demographic and socioeconomic elements (age, gender, education, employment, marital status, and smoking) and clinical elements [disease duration; the number of ACR criteria at entry into the cohort (seizure criterion excluded); occurrence of psychosis; cerebrovascular accident and seizure; presence of aPL syndrome (APS) which includes the following events recorded after SLE diagnosis {myocardial infarction, definite or classic angina, procedure for coronary artery disease (bypass graft), cerebrovascular event, claudication for more than six months, peripheral arterial thrombosis or peripheral venous thrombosis, but excluding pregnancy events} and aPL antibody positivity; disease damage as assessed by the Systemic Lupus International Collaborating Clinics Damage Index (SDI) (seizure criterion excluded) and medication intake (hydroxychloroquine, cyclophosphamide, azathioprine, methotrexate, mycophenolate mofetil and glucocorticoids)20]. Comorbidities [diabetes mellitus (intake of oral hypoglycemic agents and/or insulin) and hypertension (recording of three abnormal readings and/or use of antihypertensive medications)] were also noted.
Time of diagnosis was defined as the date at which a patient met four ACR classification criteria. Incident seizure involvement was defined when patients met the ACR neurological criterion for seizures and thus attributed to SLE 19, 21 either at the time of diagnosis or after diagnosis. Prevalent seizure (onset prior to diagnosis) was excluded from these analyses because disease duration was defined as the time between diagnosis and enrollment into the PROFILE cohort and the timing for Cox proportional hazards model (see below) could not be calculated if an event occurred before the time of diagnosis. Clinical seizures due to other causes such as hypertensive seizures were excluded from the analysis. Damage from seizures was defined when patients met the SDI definition of seizures {persistent seizures for more than six months requiring treatment} 22.
From the genetic domain, selected HLA-DRB1 (HLA-DRB1*08, HLA-DRB1*0301, HLA-DRB1*150), HLA-DQB1 (HLA-DQB1*0201, HLA-DQB1*0602), FCGR2A, FCGR3A and FCGR3B alleles were included. Genomic DNA was extracted using the PureGene kit (Gentra Systems, Minneapolis, MN) following the manufacturers recommendations. HLA-DRB1 and HLA-DQB1 were genotyped as we have previously described 23, 24. FCGR2A, FCGR3A and FCGR3B were genotyped as previously described and by Pyrosequencing (Biotage, Charlottesville, VA) using gene specific primers 25. The distributions of selected FCGR alleles by ethnic group have previously been reported 13.
Analyses
The common variables from the individual databases were pooled to constitute one single database 17. Descriptive statistical tests (Chi-square or Fisher’s exact test for proportions and Students’ tests for means) were used to compare variables from the different domains for incident cases of seizure involvement versus non-cases (prevalent cases with onset prior to diagnosis were excluded).
Model development
Variables with a p<0.10 in the univariable analyses and those felt to be clinically relevant, regardless of their level of significance (e.g. gender), were entered into the Cox proportional hazards regression models. Hazard ratios for ethnicity were calculated using Caucasian ethnicity as the referent group. The development of incident seizures was the dependent variable in two models (association and prediction). Similarly, the development of damage due to seizures was the dependent variable in the other two models (association and prediction). Employment was excluded from the prediction models since change in this variable may result from, rather than predict, the occurrence of seizures. Likewise, medication use was excluded from the prediction models as the precise timing for them was not available.
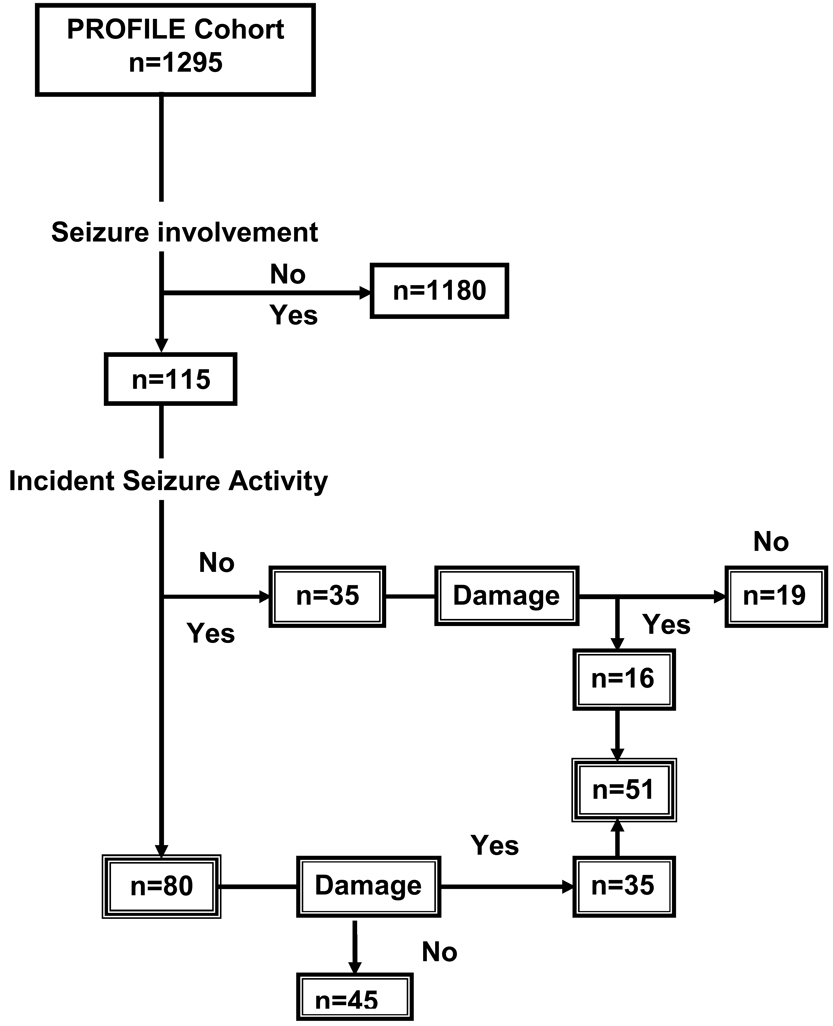
Figure 1 depicts the flow of PROFILE patients into the time-dependent analyses described.
Figure 1.
Flow Diagram of PROFILE cohort patients included in analyses.
RESULTS
Seizure Involvement
At the time of analyses, 1295 patients constituted the PROFILE cohort, 115 of whom had developed seizure involvement (Figure 1). Of these 115 patients, 35 had developed seizure involvement prior to diagnosis (prevalent cases) and were not included in the analyses presented. The 80 incident cases (those who developed seizures at the time of or after SLE diagnosis) were younger (31.4 vs. 36.4 years, p=0.0006) and less likely to be unemployed (39.5% vs. 62.0%, p < 0.0001), to be African-American (51.3% vs. 34.1%) or Hispanic-Texan (15.0% vs. 10.0%) and less likely to be Caucasian (31.3% vs. 47.6%) and Hispanic-Puerto Rican (2.5% vs. 8.3%), p = 0.0014, to have a greater number of ACR criteria (excluding the seizure criterion) (6.7 vs. 5.8, p<0.0001), to have other neurological manifestations (psychosis [10.0% vs. 2.8%, p=0.0004] and cerebrovascular event [17.5% vs. 4.0%, p < 0.0001], to have more disease damage (excluding the seizure domain) [2.4 vs. 1.2, p < 0.0001], to have sustained renal damage requiring dialysis or transplant [22.5% vs. 2.6%, p < 0.0001], and to be hypertensive (51.4% vs. 36.5%, p = 0.0083) compared to the ones who had not developed seizure involvement. We also explored the relationship of individual ACR non-CNS criteria items with the occurrence of incident seizures and only renal involvement (55% vs. 33%, p < 0.0001), and serositis (62% vs. 42%, p = 0.0010) were more common in those with seizures than those without seizures. In contrast, photosensitivity was less common in those with seizures compared to those without seizures (45% vs 59%, p = 0.0154). These data are depicted in Table 1A. There were no other differences in frequency of the remaining ACR criteria items examined in those with or without seizures (data not shown). The distribution of FCGR alleles was comparable for those patients who had developed and those who had not developed incident seizure involvement. The distribution of HLA alleles was similarly comparable for those patients with and without incident seizures (data not shown).
Table 1.
| Table 1A. Baseline Socioeconomic,, Demographic and Clinical Features of PROFILE Patients. Clinical Definition of Seizures | ||||
|---|---|---|---|---|
| Variable | Yes n=80 |
No n=1215 |
p value | |
| Age, years, mean (SD) | 31.4 (11.8) | 36.4 (12.3) | 0.0006 | |
| Gender, % women | 87.5 | 91.0 | 0.2924 | |
| Ethnicity, % | ||||
| Hispanic-Texan | 15.0 | 10.0 | 0.0014 | |
| Hispanic-Puerto Rican | 2.5 | 8.3 | ||
| African American | 51.3 | 34.1 | ||
| Caucasian | 31.3 | 47.6 | ||
| Employment, % working | 39.5 | 62.0 | <0.0001 | |
| Education, years, mean (SD) | 13.2 (3.5) | 13.7 (2.9) | 0.2062 | |
| Marital status, % married | 33.3 | 42.1 | 0.1278 | |
| Smoking, % | 11.5 | 16.6 | 0.2414 | |
| Disease duration, TD-T0,years, mean | 2.1 (3.1) | 1.9 (3.2) | 0.6030 | |
| ACR* criteria #, mean (SD) | 6.7 (1.6) | 5.8 (1.5) | <0.0001 | |
| ACR serositis criteria, % | 61.30 | 42.5 | 0.0010 | |
| ACR renal criteria, % | 55.0 | 33.1 | < 0.0001 | |
| ACR photosensitivity criteria, % | 45.0 | 58.8 | 0.0154 | |
| SLICC† Damage Index score, mean (SD) | 2.4 (2.5) | 1.2 (1.9) | <0.0001 | |
| Renal Damage, % (dialysis or transplant) | 22.5 | 2.6 | <0.0001 | |
| Antiphospholipid syndrome‡, % | 23.1 | 9.4 | 0.0001 | |
| Comorbidities, % | ||||
| Hypertension | 51.4 | 36.5 | 0.0083 | |
| Diabetes | 7.5 | 6.1 | 0.6155 | |
| CNS¶-related manifestations, % | ||||
| Psychosis | 10.0 | 2.8 | 0.0004 | |
| Cerebrovascular accident | 17.5 | 4.0 | <0.0001 | |
| Medications, % | ||||
| Low dose aspirin | 35.0 | 28.6 | 0.2256 | |
| Hydroxychloroquine | 81.3 | 84.9 | 0.3784 | |
| Glucocorticoid (oral)‡ | 97.5 | 80.6 | 0.0046 | |
| Glucocorticoid (IV pulse) | 58.8 | 24.2 | <0.0001 | |
| Azathioprine | 40.0 | 25.9 | 0.0056 | |
| Mycophenolate mofetil | 27.9 | 17.3 | 0.0195 | |
| Cyclophosphamide | 61.3 | 20.1 | <0.0001 | |
| Methotrexate | 17.5 | 15.8 | 0.6807 | |
| FCGR polymorphisms | ||||
| FCGR2A*HH | 8.3 | 18.2 | ||
| FCGR2A*HR/RR | 91.7 | 81.8 | 0.1301 | |
| FCGR3A*TT | 51.4 | 46.2 | ||
| FCGR3A*TG/GG | 48.6 | 53.8 | 0.5200 | |
| FCGR3B*11 | 28.1 | 19.3 | ||
| FCGR3B*12/22 | 71.9 | 80.1 | 0.2691 | |
| Table 1B. Baseline Socioeconomic Demographic and Clinical Features of PROFILE Patients. Damage Definition of Seizures | ||||
|---|---|---|---|---|
| Variable | Yes n=51 |
No n=1244 |
p value | |
| Age, years, mean (SD) | 31.4 (10.5) | 36.4 (12.4) | 0.0053 | |
| Gender, % women | 82.4 | 91.0 | 0.0376 | |
| Ethnicity, % | ||||
| Hispanic-Texan | 11.8 | 10.4 | ||
| Hispanic-Puerto Rican | 2.0 | 8.1 | ||
| African American | 54.9 | 34.5 | 0.0127 | |
| Caucasian | 31.4 | 47.0 | ||
| Employment, % working | 40.8 | 61.0 | 0.0046 | |
| Education, years, mean (SD) | 13.1 (3.0) | 13.6 (3.0) | 0.1709 | |
| Marital status, % married | 29.4 | 41.9 | 0.0752 | |
| Smoking, % | 21.6 | 16.3 | 0.3154 | |
| Disease duration, TD-T0 years, mean (SD) | 1.6 (1.9) | 1.9 (3.4) | 0.4760 | |
| ACR* criteria #, mean (SD) | 6.7 (1.9) | 5.8 (1.5) | <0.0001 | |
| SLICC† Damage Index score, mean (SD) | 3.1 (2.2) | 1.2 (1.9) | <0.0001 | |
| Renal Damage, % (dialysis or transplant) | 14.9 | 3.4 | <0.0001 | |
| Antiphospholipid syndrome‡, % | 24.0 | 9.8 | 0.0012 | |
| Comorbidities, % | ||||
| Hypertension | 54.9 | 36.9 | 0.0095 | |
| Diabetes* | 7.8 | 6.0 | 0.5957 | |
| CNS¶-related manifestations, % | ||||
| Psychosis | 17.7 | 2.9 | <0.0001 | |
| Cerebrovascular accident | 15.7 | 4.5 | 0.0003 | |
| Medications, % | ||||
| Low dose aspirin | 39.2 | 28.7 | 0.1051 | |
| Hydroxychloroquine | 82.4 | 84.8 | 0.6331 | |
| Glucocorticoid (oral)‡ | 100.0 | 86.9 | 0.0059 | |
| Glucocorticoid (IV pulse) | 66.7 | 24.9 | <0.0001 | |
| Azathioprine | 35.3 | 26.3 | 0.1538 | |
| Mycophenolate mofetil | 22.4 | 18.0 | 0.4313 | |
| Cyclophosphamide | 54.9 | 21.4 | <0.0001 | |
| Methotrexate | 15.7 | 15.8 | 0.9771 | |
| FCGR polymorphisms | ||||
| FCGR2A*HH | 11.4 | 17.8 | 0.6087 | |
| FCGR2A*HR/RR | 88.6 | 82.2 | ||
| FCGR3A*TT | 50.0 | 46.6 | 0.8898 | |
| FCGR3A*TG/GG | 50.0 | 53.3 | ||
| FCGR3B*11 | 21.2 | 19.9 | 0.4236 | |
| FCGR3B*12/22 | 78.8 | 80.1 | ||
American College of Rheumatology;
Systemic Lupus International Collaborating Clinics (seizures excluded);
modified definition without pregnancy data; Fisher’s exact text;
central nervous system.
American College of Rheumatology;
Systemic Lupus International Collaborating Clinics (seizures excluded);
modified definition without pregnancy data; Fisher’s exact text;
central nervous system.
Damage Definition of Seizure Involvement
As shown in Figure 1, 51 patients, from a total of 115 patients with seizure involvement (incidence and prevalent cases), met the definition of damage due to seizures20 after diagnosis. As shown in Tables 1B, patients meeting this definition were younger (31.4 vs. 36.4 years, p=0.0053), less likely to be unemployed (40.8% vs. 61.0%, p=0.0046), to have a greater number of ACR criteria (excluding the seizure criterion) (6.7 vs. 5.8, p<0.0001), to have renal damage requiring dialysis or transplant [ 14.9 % vs. 3.4 %, p < 0.0001], and to have accrued more damage (excluding the seizure domain item) (3.1 vs. 1.2, p<0.0001) than those who did not have seizure-related damage. Within the different ethnic groups, damage due to seizures was more likely to occur among the African-Americans than among patients in the other ethnic groups (28 of 51 patients, 55%, p=0.0127). We also explored the relationship of individual ACR non-central nervous systemc (CNS) criteria items with the occurrence of damage due to seizures and only renal involvement (51% vs. 34%, p < 0.0001) was more common in those with damage due to seizures compared with those without damage due to seizures. There were no other differences in the frequency of the remaining ACR criteria items between those with or without seizures (data not shown).
Factors associated with the time to occurrence of clinical seizures
Risk factors associated with the time to occurrence of seizure involvement were identified in univariable analyses and were combined with other clinically relevant factors for testing in a multivariable regression model. Events considered risk factors, such as cerebrovascular accident, were only included in the models if they occurred before the seizure date. The risk factor variables identified from the univariate analyses for inclusion in the multivariable analysis were younger age (HR=1.0; 95% CI 0.9–1.0), Hispanic-Texan ethnicity (HR=2.6; 95%CI 1.3–5.1), African-American ethnicity (HR=2.3; 95% CI 1.4–3.7), shorter disease duration (HR=0.97; 95% CI 0.9–1.0), higher number of ACR criteria (HR=1.3; 95% CI 1.1–1.5), the occurrence of serositis as defined by ACR criteria (HR=1.8; 95% CI 1.2–2.9), the occurrence of renal disease by ACR critieria (HR=2.1; 95% CI 1.4–3.3), the occurrence of a cerebrovascular accident (HR=3.9; 95% CI 2.2–6.9), the occurrence of psychosis (HR=3.3 ; 95% CI 1.5–6.9), the occurrence of renal damage requiring dialysis or transplant (HR=3.8; 95% CI 2.3–6.5), the lower occurrence of photosensivity by ACR criteria (HR=0.5; 95% CI 0.3–0.8), higher damage accrual (HR=1.2; 95% CI 1.1–1.3), APS (HR=2.2; 95% CI 1.3–3.7), and the use of intravenous pulse glucocorticoids (HR=3.7; 95% CI 2.3–5.7) and cyclophosphamide (HR= 5.3; 95% CI 3.4–8.3), while the following variables were not significant: Hispanic Puerto Rican ethnicity, employment, marital status, education, smoking, diabetes, any of the genetic polymorphisms tested, and the use of low dose aspirin, hydroxychloroquine, or methotrexate. Oral glucocorticoids, azathioprine, mycophenolate mofetil and renal involvement were excluded from the final model because these variables were correlated with other variables. In the final multivariable analysis, only the following variables were associated with a shorter time to clinical occurrence of seizures: shorter disease duration (HR-0.91; 95% CI 0.83–0.98), occurrence of renal damage (HR=2.3; 95% CI 1.32–4.0), occurrence of a cerebrovascular accident (HR=4.0; 95% CI 2.0–8.2), and the use of intravenous glucocorticoids (HR=2.0; 95% CI 1.2–3.4), and cyclophosphamide (HR=2.7; 95% CI 1.6–4.8), while the remaining variables were not significant.
Factors associated with the time to occurrence of damage due to seizures
Risk factors associated with the time to occurrence of damage due to seizure involvement were identified in univariable analyses and were combined with other clinically relevant variables for testing in a multiple regression model.. Events considered risk factors, such as cerebrovascular accident, were only included in the models if they occurred before the seizure date. The risk factor variables identified from the univariate analyses for inclusion in the multivariate model were younger age (HR=1.0; 95% CI 0.9–1.0), male gender (HR=2.3; 95% CI 1.2–4.7), African-American ethnicity (HR=2.4; 95% CI 1.3–4.5), shorter disease duration (HR=0.9; 95% CI 0.8–1.0), higher number of ACR criteria (HR=1.3; 95% CI 1.1–1.5), higher damage accrual (HR=1.2; 95% CI 1.2–1.3), APS (HR=2.3; 95% CI 1.1–4.4), the occurrence of hypertension (HR=1.8; 05% CI 1.0–3.2), the occurrence of renal involvement by ACR criteria (HR=1.82; 95% CI 1.05–3.17), the occurrence of psychosis (HR=6.2; 95% CI 3.1–12.9), the occurrence of a cerebrovascular accident (HR=3.3; 95% CI 1.5–6.9), the occurrence of renal damage requiring dialysis or transplant (HR=4.1; 95% CI 1.8–9.1), the use of intravenous glucocorticoids (HR=5.1; 95% CI 2.9–9.2) and cyclophosphamide (HR=4.0; 95% CI 2.3–6.9), while the following variables were not significant: Hispanic-Texan or Hispanic Puerto Rican ethnicities, employment, education, marital status, smoking, diabetes, any of the genetic polymorphisms tested, and the use of low dose aspirin, hydroxychloroquine, azathioprine, mycophenolate mofetil, and methotrexate. In the final multivariable analysis, only the following variables were independently associated with a shorter time to occurrence of damage due to seizures: younger age (HR=1.0; 95% CI 0.95–1.0), shorter disease duration (HR=0.9; 95% CI 0.8–1.0), occurrence of psychosis (HR=3.9; 95% CI 1.8–8.2), and use of intravenous glucocorticoids (HR=3.5; 95% CI 1.8–6.9), while the remaining ones were not significant.
Factors predicting time to occurrence of clinical seizures
Risk factors predicting time to occurrence of incident seizure involvement were identified in univariable analyses and were combined with other clinically relevant variables such as gender, but not employment and medication variables as previously noted. Events considered risk factors, such as cerebrovascular accident, were only included in the models if they occurred before the seizure date. In the final multivariable analysis, only the following variables were independent predictors of shorter time to occurrence of clinical seizure involvement: younger age (HR=1.0; 95% CI 0.9–1.0), Hispanic-Texan ethnicity (HR=2.7; 95% CI 1.3–5.7), African-American ethnicity (HR=1.8; 95% CI 1.0–3.1, occurrence of a prior cerebrovascular accident (HR=3.3; 95% CI 1.6–7.1) or prior episode of psychosis (HR=2.4; 95% CI 1.1–5.0), while the prior occurrence of photosensitivity (HR=0.5; 95% CI 0.3–0.9) predicted a longer time to occurrence of clinical seizures. The remaining variables tested were not significant: gender, Hispanic-Puerto Rican ethnicity, disease duration, ACR criteria or damage accrual indices modified to exclude parameters entered separately into the model such as renal involvement or renal damage, serositis, APS or hypertension..
Factors predicting time to occurrence of damage due to seizures
Risk factors predicting the time to occurrence of damage due to seizure involvement were identified in univariable analyses and were combined with other clinically relevant variables such as gender, but not employment and medication variables as previously noted. Events considered risk factors, such as cerebrovascular accident, were only included in the models if they occurred before the seizure date. In the final multivariable analysie, only the following variables were independent predictors of a shorter time to occurrence of damage due to seizures: younger age (HR=1.0 95% CI 0.9–1.0), male gender (HR=2.4; 95% CI 1.1–5.4), and occurrence of a prior cerebrovascular event (HR=2.7; 95% CI 1.0–7.0) or prior episode of psychosis (HR=4.7; 95% CI 2.3–9.9. The remaining variables tested were not significant: ethnicity, disease duration, ACR criteria number, damage accrual, renal damage, APS or hypertension..
DISCUSSION
We have previously documented that renal involvement is more frequent among SLE patients of African American and Texan Hispanic (primarily Mexican) ancestry 13. In the current study, we show that incident seizures also occur more often in these two ethnic groups and that African-American patients were more likely to have disease damage due to seizures. In contrast to our findings that demonstrated low affinity Fc receptors for IgG (FCGR) involvement when assessing disease damage focusing on the development of end-stage renal disease in patients with SLE 13, we were unable to show a similar relationship between the development of incident seizure or damage due to seizures in patients with SLE and candidate gene allelic polymorphisms of HLA-DRB1, HLA-DQB1, FCGR2A, FCGR3A and FCGR3B. The absence of a genetic association with seizure involvement in our cohort may represent a power issue; given the exploratory nature of our analyses, however, a formal power calculation was not done a priori. Alternatively, it is possible that we did not test for the appropriate candidate gene(s). However, the known genetic associations with epilepsy are rare and usually familial or associated with ion channel gene mutations as described earlier 14, 15. It is unlikely that we would find these latter abnormalities in SLE as our approach was to target previously identified candidate genes and their alleles associated with the immune response in SLE.
We also noted that younger age was a significant factor in these analyses as has been noted in previous studies 5, 8 We and others have observed that shorter disease duration contributes to both seizure occurrence and damage due to seizures suggesting that seizures are a relatively early occurring event in the disease course of lupus 5, 8.
We did not note a relationship between education and seizure onset. Nevertheless, given that ethnicity is not merely a biological construct but one that also encompasses ancestry, geography, history, values, culture and language, the role of socioeconomic factors in the development of seizures or damage due to seizures should not be easily dismissed 26.
We have confirmed previous findings related to associations between damage accrual, clinical seizures or damage due to seizures 7, between a previous cerebrovascular accident and clinical seizures 8, as well as the observation that male gender may have a role in increasing the risk of damage due to seizures 7. In contrast to our study where a previous episode of psychosis predicted damage due to seizures, another study only showed a relationship between clinical seizures and psychosis 7. We were unable to show a relationship between APS and clinical seizure or damage due to seizures.
Our study has several limitations. First, it includes SLE patients recruited at academic health centers and thus, they may not be representative of the overall lupus population; this may make our data less generalizable than if our patients have been recruited from the community. Second, because different disease activity indices such as the SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) 27, 28 and the SLAM-R (Systemic Lupus Activity Measure-Revised) 27, 28 had been obtained at the different US centers and could not be pooled, we were unable to assess the relationship of ongoing disease activity with the occurrence of seizure involvement. Third, we were unable to include imaging studies, such as Magnetic Resonance Imaging [MRI] or autoantibody data, such as anti-dsDNA, anti-ribosomal or aPL antibodies at the time of the event because they were not obtained at the time of seizure occurrence in all patients nor read in a centralized laboratory utilized by all sites contributing patients to the cohort. Therefore, we could not compare our study with others that have demonstrated a relationship between aPL antibody or the presence of a lupus anticoagulant with seizures 4, 7, 8. Fourth, we do not have information on the types of seizures that occurred. Finally, seizure involvement occurred prior to SLE diagnosis in 35 (3.9%) patients in the combined cohort. Since the time of diagnosis was the onset date that was used as the anchor for the Cox proportional hazards analyses, we were unable to include the prevalent seizures in the model. The occurrence of seizures prior to diagnosis has been recognized and the frequency of these early onset seizures is consistent with previous studies 6, 8.
Despite these limitations, our data are quite relevant to clinicians caring for patients from ethnic minorities, particularly young Hispanics (primarily of Mexican and Central American ancestry) and African Americans who tend to have a lower socioeconomic status than the Caucasian majority and who have now been shown to have more severe disease with respect to end-stage kidney involvement and seizures. A genetic risk factor has been identified for end-stage kidney disease in SLE 13, but the current study does not support a similar association between the tested genetic polymorphisms and the occurrence of seizures or of damage due to seizures.
ACKNOWLEDGEMENTS
We thank Martha L. Sanchez, M.D., M.P.H., Ellen D. Sowell, AA and Maria A. Tyson, AA at UAB; Bhavna Gowda, M.D., M.P.H. at JHU, Charmayne Dunlop-Thomas, M.S., Katie Arrigo, B.A., Ahn Chang, B.S. and Sue Cunanan, B.S. at NU; Carmine Pinilla, M.T. at UPR and Robert Sandoval, B.S. at UTH for their assistance with all aspects of the study.
Supported by Grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) P01AR49084 (UAB, JHU, NU, and UTH); AR43727 and General Clinical Research Centers (GCRC) M01-RR00052 (JHU); GCRC M01- RR00032 (UAB); NIAMS K24-AR002138, P60AR048098 and GCRC M01-RR00048 (NU); National Center for Research Resources (NCRR/NIH) RCMI Clinical Research Infrastructure Initiative (RCRII) award 1P20-RR11126 (UPR) and GCRC M01-RR02558 (UTH).
REFERENCES
- 1.Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001 Oct;45(5):419–423. doi: 10.1002/1529-0131(200110)45:5<419::aid-art360>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002 Apr 23;58(8):1214–1220. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, McCurdy G, Fougere L, Douglas JA, Thompson K. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol. 2004 Nov;31(11):2156–2162. [PubMed] [Google Scholar]
- 4.Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol. 2003 May;30(5):985–992. [PubMed] [Google Scholar]
- 5.Sibbitt WL, Jr, Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002 Jul;29(7):1536–1542. [PubMed] [Google Scholar]
- 6.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007 Jan;56(1):265–273. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- 7.Mikdashi J, Krumholz A, Handwerger B. Factors at diagnosis predict subsequent occurrence of seizures in systemic lupus erythematosus. Neurology. 2005 Jun 28;64(12):2102–2107. doi: 10.1212/01.WNL.0000165959.98370.D5. [DOI] [PubMed] [Google Scholar]
- 8.Appenzeller S, Cendes F, Costallat LT. Epileptic seizures in systemic lupus erythematosus. Neurology. 2004 Nov 23;63(10):1808–1812. doi: 10.1212/01.wnl.0000144178.32208.4f. [DOI] [PubMed] [Google Scholar]
- 9.Nath SK, Kilpatrick J, Harley JB. Genetics of human systemic lupus erythematosus: the emerging picture. Curr Opin Immunol. 2004 Dec;16(6):794–800. doi: 10.1016/j.coi.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Croker JA, Kimberly RP. SLE: challenges and candidates in human disease. Trends Immunol. 2005 Nov;26(11):580–586. doi: 10.1016/j.it.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Croker JA, Kimberly RP. Genetics of susceptibility and severity in systemic lupus erythematosus. Curr Opin Rheumatol. 2005 Sep;17(5):529–537. doi: 10.1097/01.bor.0000169360.15701.27. [DOI] [PubMed] [Google Scholar]
- 12.Graham RR, Kozyrev SV, Baechler EC, et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006 May;38(5):550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 13.Alarcon GS, McGwin G, Jr, Petri M, et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med. 2006 Oct;3(10):e396. doi: 10.1371/journal.pmed.0030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbull J, Lohi H, Kearney JA, et al. Sacred disease secrets revealed: the genetics of human epilepsy. Hum Mol Genet. 2005 Oct 15;14(Spec No 2):2491–2500. [PubMed] [Google Scholar]
- 15.Ferraro TN, Dlugos DJ, Buono RJ. Role of genetics in the diagnosis and treatment of epilepsy. Expert Rev Neurother. 2006 Dec;6(12):1789–1800. doi: 10.1586/14737175.6.12.1789. [DOI] [PubMed] [Google Scholar]
- 16.Berkovic SF, Harkin L, McMahon JM, et al. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006 Jun;5(6):488–492. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon GS, McGwin G, Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11(2):95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classifcation of systemic lupus erythematosus. Arthritis Rheum. 1998;41(4):751. doi: 10.1002/1529-0131(199804)41:4<751::aid-art30>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of SLE. Arthritis and Rheumatism. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Gladman DD, Goldsmith CH, Urowitz MB, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000 Feb;27(2):373–376. [PubMed] [Google Scholar]
- 21.Hochberg M. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis and Rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 22.Gladman DD, Ginzler E, Goldsmith C, et al. The development and initial validation of the SLICC/ACR damage index for SLE. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 23.Reveille JD, Durban E, MacLeod-St Clair MJ, et al. Association of amino acid sequences in the HLA-DQB1 first domain with antitopoisomerase I autoantibody response in scleroderma (progressive systemic sclerosis) J Clin Invest. 1992 Sep;90(3):973–980. doi: 10.1172/JCI115974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh SG, Bodmer JG. HLA class II region nucleotide sequences, 1995. Tissue Antigens. 1995 Sep;46(3 Pt 2):258–280. doi: 10.1111/j.1399-0039.1995.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 25.Edberg JC, Langefeld CD, Wu J, et al. Genetic linkage and association of Fcgamma receptor IIIA (CD16A) on chromosome 1q23 with human systemic lupus erythematosus. Arthritis Rheum. 2002 Aug;46(8):2132–2140. doi: 10.1002/art.10438. [DOI] [PubMed] [Google Scholar]
- 26.Brooks K, Fessler B, Bastian H, Alarcon G. Sociocultural issues in clinical research. Arthritis Care Res. 2001;45:203–207. doi: 10.1002/1529-0131(200104)45:2<203::AID-ANR174>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Gladman DD, Goldsmith CH, Urowitz MB, et al. Sensitivity to change of 3 Systemic Lupus Erythematosus Disease Activity Indices: international validation. J Rheumatol. 1994 Aug;21(8):1468–1471. [PubMed] [Google Scholar]
- 28.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis and Rheumatism. 1989;32:1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]