Abstract
The endoplasmic reticulum (ER) Unfolded Protein Response (UPR) restores equilibrium to the ER, but prolonged expression of the UPR effector CHOP (GADD153) is cytotoxic. We found that ER stress-induced CHOP expression was suppressed by prior engagement of toll-like receptor (TLR) 3 or 4 through a TRIF-dependent pathway. TLR engagement did not suppress phosphorylation of PERK or eIF-2α, which are upstream of CHOP, but phospho-eIF-2α failed to promote translation of the CHOP activator ATF4. In mice subjected to systemic ER stress, pre-treatment with low-dose lipopolysaccharide (LPS), a TLR4 ligand, suppressed CHOP expression and apoptosis in splenic macrophages, renal tubule cells, and hepatocytes, and prevented renal dysfunction and hepatosteatosis. This protective effect of LPS did not occur in Trif−/− mice nor in wild-type mice in which CHOP expression was genetically restored. Thus, TRIF-mediated signals from TLRs selectively attenuate translational activation of ATF4 and its downstream target gene CHOP. We speculate that this mechanism evolved to promote survival of TLR-expressing cells that experience prolonged levels of physiologic ER stress in the course of the host response to invading pathogens.
The Unfolded Protein Response (UPR) is an integrated signal transduction pathway that restores equilibrium to the endoplasmic reticulum (ER) experiencing physiologic or pathophysiologic unfolded protein stress1. The UPR is initiated by the activation of three molecules, PERK, IRE1α, and ATF6. Activation of PERK leads to the phosphorylation of eIF-2α, which suppresses translation initiation of most cellular proteins but promotes translation initiation of ATF4, leading to transcription of ATF4’s downstream target CHOP (GADD153). In most circumstances, the UPR’s three known transducers are regulated by similar cues and are thus coordinately activated2. An exception to this rule is pathologically prolonged ER stress occurring in a variety of disease processes, in which sustained PERK-CHOP signaling leads to apoptosis2–8. However, there are situations in normal physiology in which ER stress is prolonged, and we wondered how the cell prevents prolonged expression of pro-apoptotic CHOP under these conditions. For example, the IRE1α-XBP-1 pathway is activated during B cell differentiation in plasma cells9, but CHOP expression is low, and evidence suggests this low level of CHOP expression promotes B cell survival10.
Another scenario in which prolonged ER stress occurs is during the response of the host to invasive organisms, as exemplified by exposure of cells to lipopolysaccharide (LPS). LPS activates TLR4 signaling, which is mediated by the MyD88-Mal and TRIF-TRAM adapters, resulting in the production of inflammatory cytokines and anti-microbial proteins11. Prolonged ER stress occurs during LPS-TLR4 signaling12,13, which likely arises from a massive increase in protein synthesis. Thus, we wondered whether CHOP expression in this setting is also suppressed.
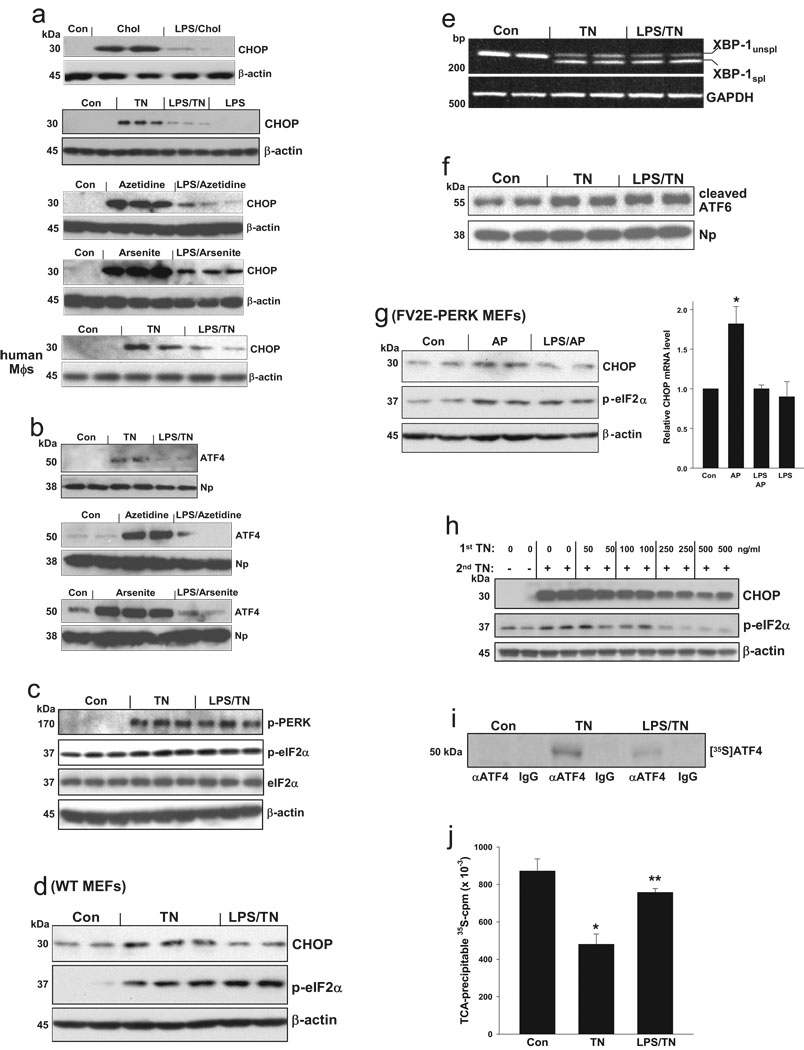
To test the hypothesis that TLR4 signaling might suppress CHOP expression during a sustained UPR response, macrophages were pre-treated in the absence or presence of a low dose of LPS that does not induce ER stress (below) and then subjected to ER stress conditions, including accumulation of lipoprotein-derived cholesterol5,14; tunicamycin, a glycosylation inhibitor15; azetidine, a proline analogue16; and arsenite, an inducer of cellular oxidant stress17. LPS pre-treatment markedly decreased CHOP protein (Fig. 1a), CHOP mRNA (Fig. S1a), and ATF4 protein (Fig. 1b). Using the time course experiment explained in the legend to Fig. S1b, we found that the ability of LPS to suppress CHOP in ER-stressed macrophages is persistent and occurs regardless of whether the LPS is added before or at the same time as the ER stressor.
Figure 1.
Pre-treatment of macrophages with low-dose LPS selectively suppresses the ATF4-CHOP branch of the UPR. (a–c) Murine or human macrophages were pre-treated ± 1 ng/ml LPS followed by cholesterol-loading (Chol) or 7-h treatment with 1 µg/ml tunicamycin (TN), 1 mM azetidine, or 1 µM arsenite. Extracts of cells (a) or nuclei (b) were immunoblotted for the indicated UPR effectors or loading controls; Np=nucleophosmin. See Fig. S3 for full scans of selected blots in this and other figures. The phospho-eIF-2α:total eIF-2α densitometry ratios for Con, TN, and LPS-TN were 0.66, 0.87, and 0.89, respectively (P<0.05 for TN vs. Con and LPS-TN vs. Con). (d) Murine embryonic fibroblasts were incubated for 16 h in medium alone or in medium containing 500 ng/ml LPS. The cells were then treated for 2 h with tunicamycin, and then immunoblotted for CHOP, phospho-eIF-2α, or β-actin (e) RNA from macrophages treated similar to those in (c) was analyzed by RT-PCR for unspliced (unspl) XBP-1, spliced (spl) XBP-1, and GAPDH. (f) Nuclear extracts from macrophages treated similar to those in (c) were immunoblotted for ATF6 and nucleophosmin. The ATF6:Np densitometry ratios for Con, TN, and LPS-TN were 0.76, 1.11, and 1.15, respectively. (g) FV2E-PERK MEFs were incubated for 16 h in medium ± 500 ng/ml LPS and then treated for 2 h ± 2 nM AP20187 (AP) to activate FV2E-PERK. Lysates were immunoblotted for CHOP, phospho-eIF-2α, and β-actin. The CHOP:β-actin densitometry ratios for Con, AP, and LPS-AP were 0.44, 0.74, and 0.44, respectively; the phospho-eIF-2α:β-actin ratios were 0.44, 0.88, and 0.97. RNA was assayed for CHOP mRNA by QT-PCR (*, P<0.01 vs. other values). (h) Macrophages were pre-incubated for 24 h with the indicated concentrations of tunicamycin and then treated for 16 h with 1 µg/ml tunicamycin. Cell extracts were immunoblotted for CHOP, phospho-eIF2α, and β-actin. (i) Macrophages treated similarly to those in (c) were pulse-labeled with [35S]methionine-cysteine for 20 min, followed by control (IgG) or anti-ATF4 immunoprecipitation. Autoradiograms of SD-PAGE gels are shown. (j) Proteins from cells labeled as above were precipitated with ice-cold TCA and counted for [35S]cpm. *, P<0.01 vs. Con; **, P<0.001 vs. TN.
Despite marked suppression of CHOP, LPS pre-treatment of macrophages or MEFs did not significantly suppress tunicamycin-induced phospho-PERK or phospho-eIF-2α (Fig. 1c–d and S2b), which are upstream of CHOP. The eIF-2α phosphatase GADD34, which is regulated by CHOP but also by other processes18,19, was not markedly suppressed by LPS pre-treatment (Fig. S1d), indicating that lack of suppression of phospho-eIF-2α is not simply due to less GADD34. LPS pre-treatment also did not suppress the other two branches of the UPR as indicated by similar XBP-1 splicing and cleaved nuclear ATF6 in LPS pre-treated vs. control ER-stressed cells (Fig. 1e–f). Phosphorylation of IRE1α, which is upstream of XBP-1 splicing, and expression of three chaperones downstream of XBP-1—grp78, calnexin, and calreticulin—were also not suppressed by LPS pre-treatment (data not shown). Thus, LPS suppresses ATF4 and CHOP in a highly selective manner.
To determine whether the suppression of CHOP was coupled to ER stress per se, we used a genetically altered MEF-based model described previously by Lu et al.20. In this model, PERK has been replaced by a cytoplasmic protein (FV2E-PERK), which dimerizes upon exposure to cell-permeant AP20187 (AP), leading to eIF-2α phosphorylation in the absence of ER stress. We found that AP-induced CHOP was suppressed by pre-treatment with LPS (Fig. 1g). Thus, the ability of LPS pre-treatment to suppress CHOP is uncoupled to ER stress per se, consistent with the finding that the process is not mediated by suppression of the PERK—phospho-eIF-2α pathway. Moreover, the pathway cannot be explained by “pre-conditioning,” whereupon cells pre-exposed to modest ER stress become relatively resistant to subsequent UPR activation2, because (a) ER stress is not induced by 1 ng/ml LPS in macrophages (Fig. 1a and S2); and (b) pre-conditioning, in stark contrast to the pathway described here, is associated with a suppression of all three branches of the UPR2, including phospho-eIF-2α (Fig. 1h).
As expected21,22, ATF4 mRNA did not change with tunicamycin treatment, and we found that ATF4 mRNA levels were not significantly altered by pre-treatment with LPS (Fig. S1c). Despite stable levels of ATF4 mRNA, the incorporation of [35S]methionine-cysteine into ATF4 protein after a 20-min labeling period was markedly reduced when exposure to tunicamycin was preceded by LPS (Fig. 1i). Given the brevity of the labeling period, this observation suggests that LPS affects rates of ATF4 translation and not protein stability. An effect of LPS on ER stress-mediated translational control is further supported by the observation that LPS pre-treatment interfered with the suppression of global protein synthesis that is normally observed following induction of ER stress (Fig. 1j). The global protein data also indicate that the decrease in [35S]-labeled ATF4 in the LPS-TN cells cannot be explained by dilution of the radiolabeled charged tRNA(Met) pool. These data suggest the LPS pre-treated cells become “resistant” to the translational effects of phospho-eIF-2α, which both prevents ATF4-CHOP expression and maintains global protein synthesis.
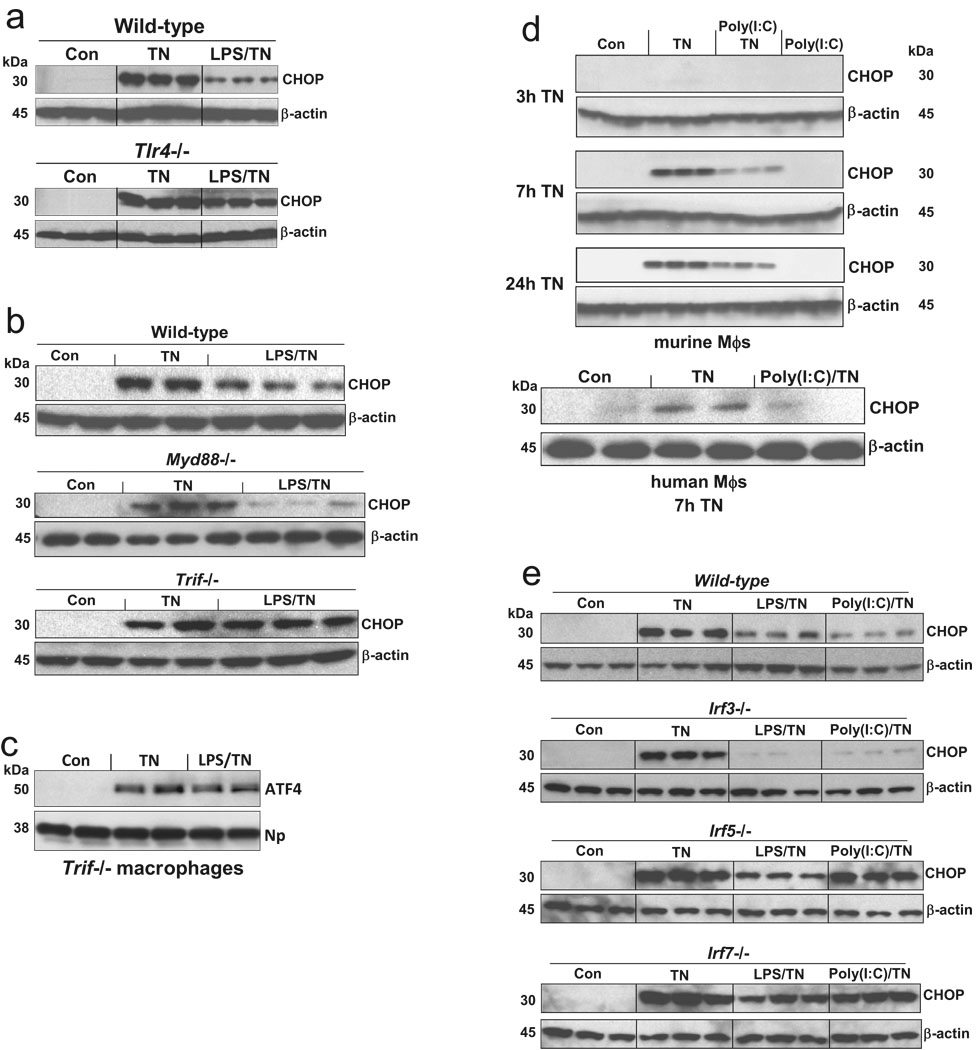
We used peritoneal macrophages from a series of gene-targeted mice to map the TLR pathway involved in suppression of CHOP by LPS pre-treatment. The marked suppression of CHOP by LPS in wild-type macrophage was almost completely absent in Tlr4−/− and Trif−/− macrophages, but not in Myd88−/− macrophages (Fig. 2a–c). Similar data were observed with macrophages from Tram−/− mice (data not shown). Furthermore, pre-treatment of macrophages with the TLR3 ligand poly(I:C), which only uses TRIF signaling11, markedly suppressed tunicamycin-induced CHOP expression (Fig. 2d).
Figure 2.
The ability of low-dose LPS to suppress tunicamycin-induced CHOP is dependent on the TRIF branch of TLR signaling. (a–b, e), Bone marrow-derived macrophages from wild-type or the indicated gene-targeted mice were pre-incubated for 24 h in control medium or medium containing 1 ng/ml LPS and then incubated for 10 h with medium alone or medium containing 1 ng/ml tunicamycin. The cells were then immunoblotted for CHOP and β-actin. (c) Macrophages from Trif−/− mice were pre-incubated for 24 h in the absence or presence of 1 ng/ml LPS and then treated in the absence or presence of 1 µg/ml tunicamycin (TN). Nuclear extracts were immunoblotted for ATF4 and nucleophosmin (Np) as a loading control. This experiment was conducted in parallel with, and should be compared with, the experiment with wild-type macrophages in Fig. 1b, top blot. (d) Murine peritoneal macrophages or human THP-1 cell-derived macrophages were pre-treated for 24 h with 2.5 µg/ml poly(I:C) instead of LPS and then incubated with tunicamycin for the indicated times and immunoblotted for CHOP and β-actin as above. (e) As in panels a–b for the indicated gene-targeted mice, using either LPS or poly(I:C) pre-incubation.
A major signal transducer downstream of the TRIF branch is IRF3, which subsequently induces the expression of type 1 interferons11. However, macrophages from Irf3−/− mice showed normal suppression of tunicamycin-induced CHOP by LPS or poly(I:C) pre-treatment (Fig. 2e, upper blots). Moreover, immunoneutralization of interferon-α and interferon-β had no effect on CHOP suppression (data not shown). Consistent with this finding, siRNA-mediated silencing of TANK-binding kinase 1 (TBK1), a kinase that mediates TRIF signaling11, did not block the suppression of CHOP by LPS (data not shown). Two other signal transducers that can be downstream of TRIF during TLR3 or TLR4 signaling are IRF5 and IRF723–25. LPS, and especially poly(I:C), were unable to fully suppress tunicamycin-induced CHOP in macrophages deficient in either of these molecules (Fig. 2e, lower blots). In summary, LPS and poly(I:C) suppress CHOP in ER-stressed macrophages through a TLR—TRIF pathway that likely involves IRF5 and IRF7 but not IRF3.
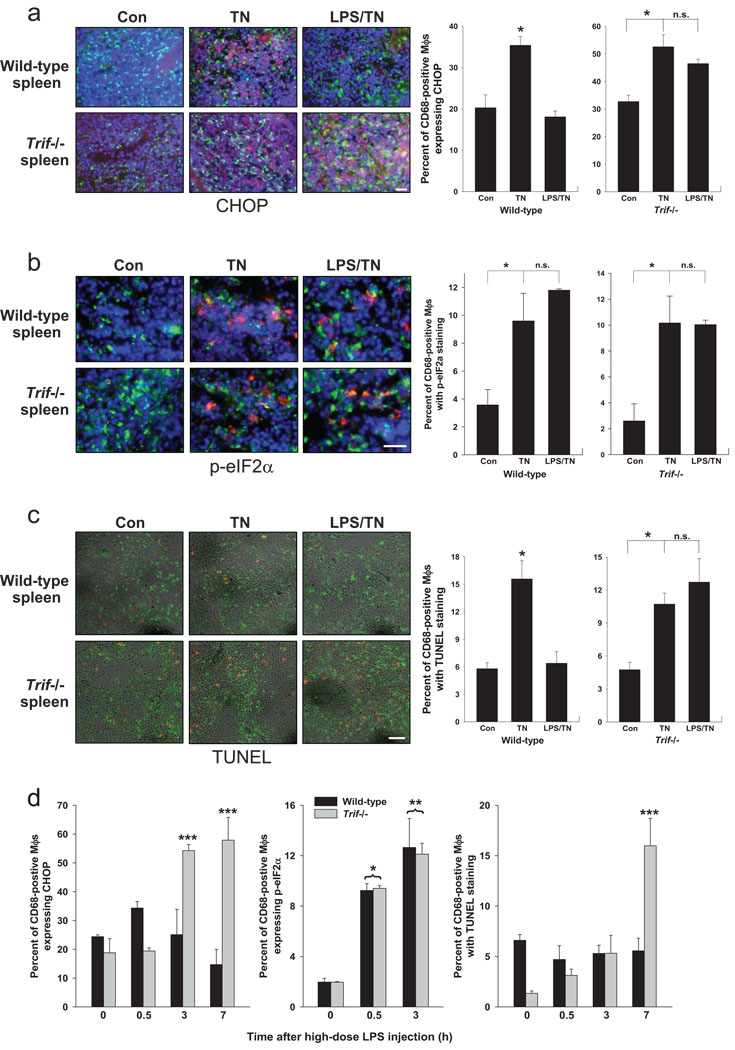
To test this pathway in vivo, wild-type and Trif−/− mice were pre-treated with 80 µg/kg/day of LPS, which did not cause any observable adverse effects in the mice, or vehicle control for two consecutive days and then exposed to an intraperitoneal injection of tunicamycin for 12 h to induce systemic ER stress3. CHOP was induced in macrophages by tunicamycin treatment, and LPS suppressed this induction in wild-type, but not Trif−/−, mice (Fig. 3a). Importantly, LPS pre-treatment did not suppress phospho-eIF-2α (Fig. 3b), consistent with the mechanism elucidated in cultured cells (above). Apoptosis, as assessed by TUNEL staining, followed the same pattern as CHOP, namely, induction by ER stress and suppression by LPS in wild-type but not Trif−/− mice (Fig. 3c).
Figure 3.
LPS treatment of tunicamycin-treated mice suppresses CHOP expression in splenic macrophages. Wild-type or Trif−/− mice were injected i.v. with 80 µg/kg LPS or vehicle control intravenously for 2 consecutive days. Mice were then injected with 1 mg/kg tunicamycin (TN) intraperitoneally and sacrificed 12 h later. Spleen cryosections were stained for (a) CHOP (red), CD68 as a macrophage marker (green), and DAPI as a stain for nuclei (blue); (b) phospho-eIF-2α (red in cytoplasm), CD68 (green), and DAPI (blue); or (c) TUNEL (red) and CD68 (green). Quantification of percent of CD68-positive macrophages that stained positively for CHOP, phospho-eIF-2α, or TUNEL are shown in the bar graphs in (a) and (b), respectively. For all three sets of data, the asterisk indicates P<0.05; n.s. = non-significant. (d) CHOP expression and apoptosis in splenic macrophages are suppressed in a TRIF-dependent manner in mice treated with high-dose LPS. Wild-type or Trif−/− mice were injected i.p. with 5 mg/kg LPS or vehicle control. At the indicated timepoints, CD68-positive splenic cells were assayed for phospho-eIF-2α, CHOP expression, and TUNEL-positive cells. *, P<0.02 vs. zero timepoint for both wild-type and Trif−/−; **, P<0.05 vs. zero timepoint for both wild-type and Trif−/−; ***, P<0.05 for Trif−/− vs. both wild-type and Trif−/− at zero timepoint and for wild-type at the same timepoint.
Sepsis, as modeled by treatment with high-dose LPS, leads to prolonged ER stress12,13. Successful host defense would likely call for prolonged UPR activation to handle increased protein load but suppression of prolonged CHOP expression. The pathway described here might enable high-dose LPS to act as both an activator of the UPR and a selective suppressor of CHOP. To test this idea, wild-type and Trif−/− mice were injected with 5 mg/kg LPS, followed by examination of CHOP and phospho-eIF-2α expression and apoptosis in splenic macrophages. Treatment with this higher dose of LPS resulted in only a slight increase in CHOP expression in wild-type mice at the 30-min timepoint, and it was suppressed thereafter (Fig. 3d, left graph). In stark contrast, CHOP expression rose dramatically after 30 min in the splenic macrophages of Trif−/− mice. Despite the lack of increase in CHOP expression in wild-type splenic macrophages, phospho-eIF-2α was markedly increased at both 30 min and 3 h (Fig. 3d, middle graph), consistent with the ability of high-dose LPS to trigger the UPR and indicative of selective suppression of CHOP. The later rise in CHOP in Trif−/− but not wild-type splenic macrophages correlated with a sharp rise in apoptosis (Fig. 3d, right graph). Thus, during UPR activation by high-dose LPS in vivo, splenic macrophage CHOP is selectively suppressed in a TRIF-dependent manner, and this response is associated with a protection against splenic macrophage apoptosis.
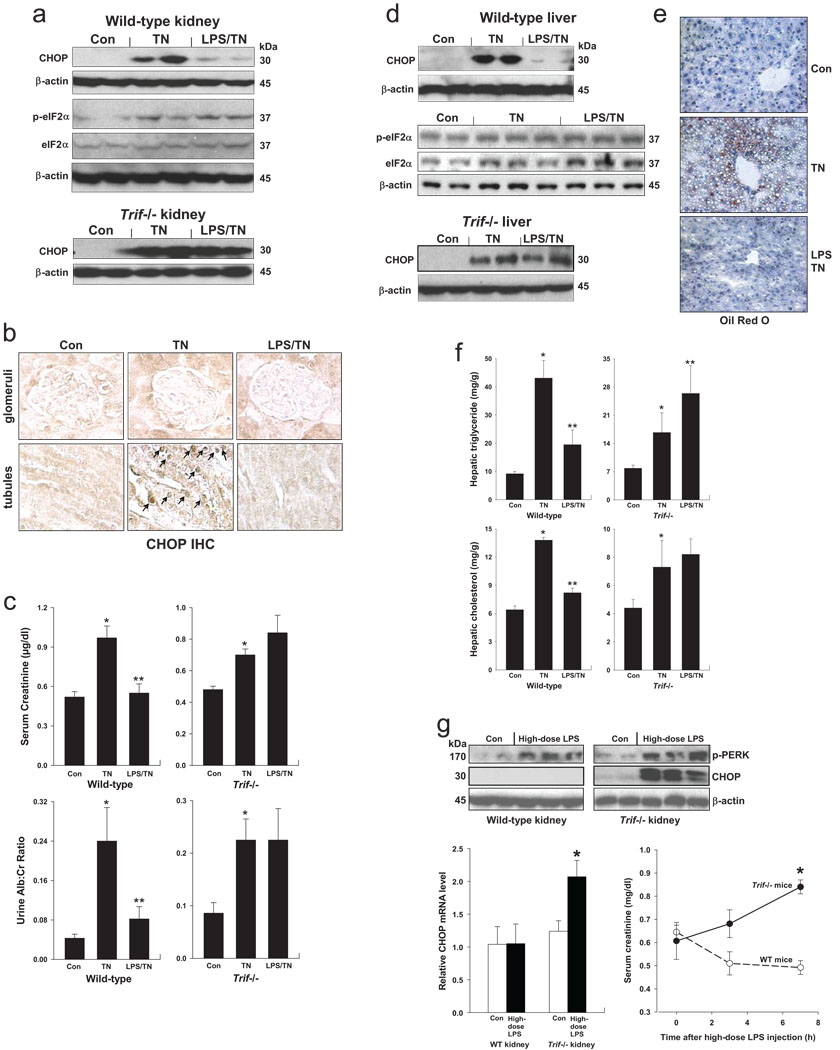
The renal proximal tubular epithelium responds to infectious insults through a TLR4-induced inflammatory response26. Moreover, the renal tubular epithelium is highly responsive to ER stress inducers, which is associated with renal dysfunction in a number of renal diseases3,27. We verified that both CHOP and phospho-eIF-2α were increased in the kidneys of tunicamycin-treated, with CHOP expression primarily in tubular cells (Fig. 4a–b). Pre-treatment with low-dose LPS markedly suppressed CHOP but not phospho-eIF-2α in the kidneys of WT mice, and CHOP suppression did not occur in Trif−/− mice (Fig. 4a). Two measures of renal function, serum creatinine and urine albumin:creatinine ratio, were abnormal in tunicamycin-treated wild-type mice but not in mice pre-treated with LPS, and this protective effect of LPS was not seen in Trif−/− mice (Fig. 4c).
Figure 4.
LPS treatment of tunicamycin-treated mice suppresses renal tubular and hepatic CHOP induction, renal dysfunction, and hepatosteatosis. Wild-type or Trif−/− mice were injected i.v. with 80 µg/kg LPS or vehicle control intravenously for 2 consecutive days. (NB: In this experiment, the Trif−/− mice also had a deficiency of its co-adaptor TRAM; cells from Trif−/−, Tram−/−, and Trif-Tram−/− mice behave similarly in terms of CHOP suppression by LPS pre-treatment.) Mice were then injected with 1 mg/kg tunicamycin (TN) intraperitoneally and sacrificed 48 h later. (a–b) Kidney extracts were assayed for CHOP, phospho-eIF-2α, and total eIF-2α expression, and kidney sections were immunostained for CHOP (CHOP IHC). In (a), the average phospho-eIF-2α:total eIF-2α densitometry ratios for the Con, TN, and LPS-TN groups were 0.26, 0.59, and 0.56, respectively. In (b), the arrows depict CHOP-positive nuclei in renal tubular cells. (c) Serum creatinine levels and urine albumin levels (normalized to urine creatinine) were determined for all groups of mice. *, P<0.01 vs. Con; **, P<0.001 vs. TN. (d–f) The livers were assayed for CHOP, phospho-eIF-2α, and total eIF-2α expression; Oil Red O staining; and triglyceride and cholesterol mass. In (e), the average phospho-eIF-2α:total eIF-2α densitometry ratios for the Con, TN, and LPS-TN groups were 0.59, 0.64, and 0.86, respectively. In (f), *, P<0.01 vs. Con; **, P<0.001 vs. TN. (g) Treatment of mice with high-dose LPS activates renal PERK, but CHOP is suppressed and renal function is preserved in a TRIF-dependent manner. Wild-type or Trif−/− mice were injected i.p. with 5 mg/kg LPS or vehicle control. Seven hours later, the kidneys were assayed for phospho-PERK and CHOP by immunoblot and for CHOP mRNA by RT-QPCR. *, P<0.01 vs. Con. Serum creatinine levels were measured at the indicated times after LPS treatment. *, P<0.01 vs. WT mice.
Hepatocytes also express TLR4 and respond to LPS28,29, and they are susceptible to ER stress-induced pathology, including CHOP-induced hepatic steatosis30,31. In tunicamycin-treated mice, CHOP was suppressed by low-dose LPS pre-treatment in wild-type but not Trif−/− liver (Fig. 4d). There was considerable basal expression of phospho-eIF-2α, and it increased only slightly in the tunicamycin-treated mice, but it was not suppressed—and was actually slightly increased—by LPS (Fig. 4d). Livers from the tunicamycin-treated mice contained vacuolated hepatocytes that stained with Oil Red O, indicative of hepatosteatosis, which was suppressed by low-dose LPS pre-treatment (Fig. 4e). Consistent with these data, the livers of tunicamycin-treated mice had an increase in triglyceride and cholesterol content compared with control mice, and LPS pre-treatment partially restored lipid levels to the control values in wild-type but not Trif−/− mice (Fig. 4f).
We re-examined the kidney findings in the model of high-dose LPS-induced ER stress described above. Wild-type and Trif−/− mice were injected with 5 mg/kg LPS, followed by examination of renal CHOP expression and renal function. As shown in Fig. 4g, LPS treatment resulted in phosphorylation of PERK in both wild-type and Trif−/− kidneys. CHOP was not induced by LPS treatment in wild-type kidney, but was robust in the Trif−/− kidneys. Most importantly, serum creatinine did not rise in wild-type mice for as long as 7 h after treatment with high-dose LPS, but serum creatinine did increase in the TRIF-deficient mice.
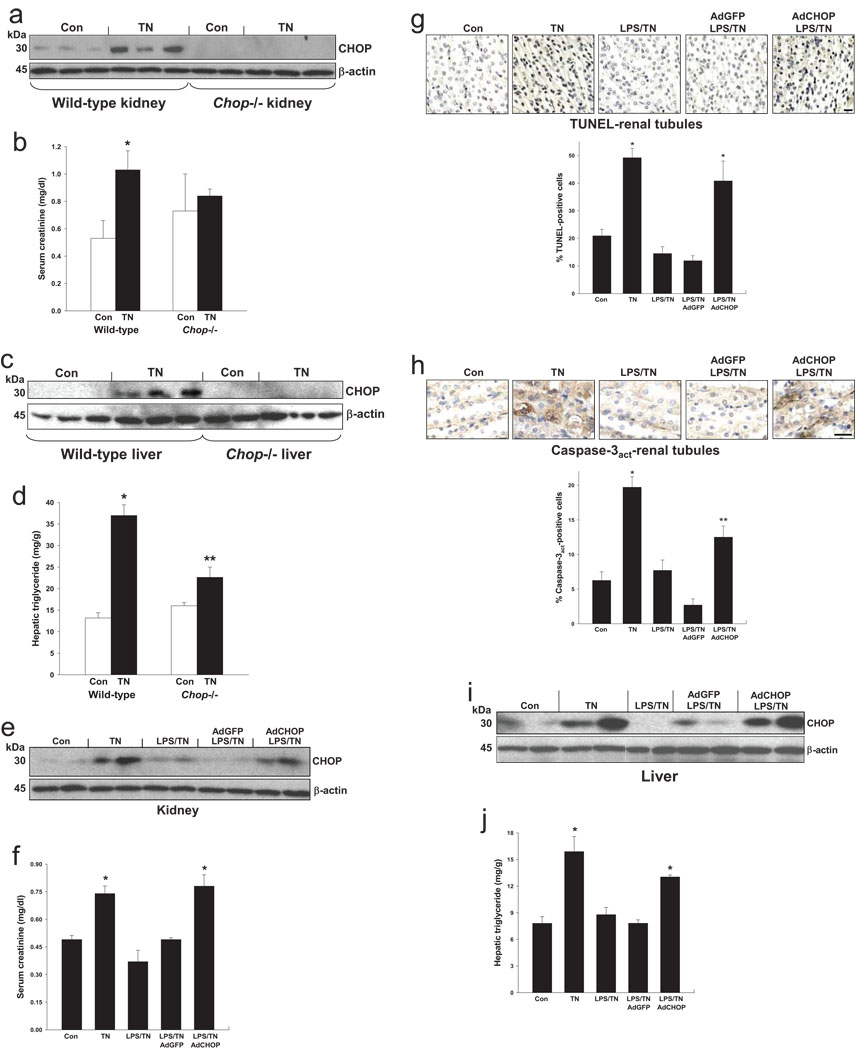
To test the concept that the protective effect of LPS in tunicamycin-treated mice is due CHOP suppression, we compared tunicamycin-induced renal dysfunction and hepatosteatosis in wild-type vs. Chop−/− mice. As above, tunicamycin treatment increased renal CHOP expression and serum creatinine, which was not seen in Chop−/− mice (Fig. 5a–b). Similarly, hepatic CHOP induction in tunicamycin-treated mice was associated with an increase in hepatic triglyceride content, which was abrogated in Chop−/− mice (Fig. 5c–d). We next used CHOP cDNA-containing adenovirus (adeno-CHOP) to restore CHOP expression in LPS-pre-treated, ER-stressed mice to a level similar to that in mice not pre-treated with LPS (Fig. 5e). Restoration of CHOP prevented LPS-mediated protection from renal dysfunction and tubular apoptosis (Fig. 5f–h). Treatment of non-ER-stressed mice with adeno-CHOP led to a 2-3-fold increase renal tubular apoptosis (data not shown). Adeno-CHOP also blocked LPS-mediated protection from hepatosteatosis in ER-stressed mice (Fig. 5i–j). Thus, suppression of CHOP expression is the mechanism whereby low-dose LPS prevents ER stress-induced renal tubular cell apoptosis, renal dysfunction, and hepatosteatosis.
Figure 5.
Protection from tunicamycin-induced renal dysfunction and hepatosteatosis by pre-treatment with low-dose LPS is due to suppression of CHOP. (a–d) Wild-type or Chop−/− mice were injected with 1 mg/kg tunicamycin (TN) intraperitoneally and sacrificed 48 h later. In (a–b), kidneys were collected and subjected to immunoblot for CHOP and β-actin, and serum was assayed for creatinine concentration. *, P<0.01 vs. Con; the two values for the Chop−/− mice were not statistically different. In (c–d), livers were collected and subjected to immunoblot for CHOP and β-actin, and extracts were assayed for triglyceride mass. *, P<0.001 vs. Con; **, P<0.01 vs. wild-type TN value. (e-j) Mice were injected i.v. with 80 µg/kg LPS or vehicle control intravenously for 2 consecutive days. On the second day, some mice were injected i.v. with either GFP-expressing adenovirus (AdGFP) or with CHOP-expressing adenovirus (AdCHOP). Mice were then injected with 1 mg/kg tunicamycin (TN) intraperitoneally and sacrificed 48 h later. In (e–f), expression of renal CHOP and serum creatinine levels were determined. *, P<0.01 vs. Con and AdGFP. In (g–h), renal tubule sections were stained for TUNEL or activated caspase-3 and quantified for percent-positive cells. *, P<0.001 vs. Con; **, P=0.01 vs. control and <0.001 vs. AdGFP. In (i–j), liver extracts were assayed for CHOP expression and triglyceride mass. *, P<0.01 vs. Con and AdGFP.
The coordinate expression of all three branches of the UPR contributes to the adaptation to physiologic stressors that would otherwise perturb the equilibrium of various ER functions1. The CHOP segment of the PERK branch presents a special situation in this process, because prolonged expression of CHOP triggers cell death2–7. Therefore, in cases of prolonged physiologic ER stress, such as occurs during processes that entail a high level of protein synthesis, prolonged CHOP expression would not be desirable and, indeed, CHOP has been found to be suppressed under these conditions10 (data herein). Several mechanisms have been proposed, including dephosphorylation of phospho-eIF-2α by CHOP-induced GADD341; inhibition of the kinase domains of PKR and PERK by ATF6-induced P58IPK 32,33; and suppression of all UPR branches by prior low levels of ER stress2. However, the lack of suppression of P-PERK and phospho-eIF-2α by LPS indicate that none of these mechanisms are responsible for ATF4-CHOP suppression by TLR-TRIF signaling. Indeed, the pathway described herein may uniquely allow cells to benefit from intact PERK activity34 while avoiding the detrimental effects of CHOP.
The critical role of TLRs in innate immunity has led us to speculate that the pathway revealed herein may protect cells from physiologic prolonged ER stress associated with host defense. Using high-dose LPS as a model of sepsis, we showed that disabling the TLR-CHOP suppression pathway by TRIF deficiency resulted in detrimental effects in splenic macrophages and the kidney. However, further in-vivo models will be needed to further expand the possible scenarios in which TLR-TRIF signaling is critical to prevent the detrimental effects of prolonged ER stress. Moreover, once the downstream signaling pathways are further elucidated, other non-TLR pathways leading to suppression of ATF4 translation may be revealed, which in turn might suggest additional scenarios in which the fundamental principles of this pathway come into play in vivo.
A key mechanistic question that arises from our findings is how TLR-TRIF signaling suppresses ATF4 translation but not global translation in the face of phosphorylated eIF-2α. In order for phospho-eIF-2α to influence rates of translation initiation, the phosphorylation event must be sensed by eIF-2B, the GTP-exchange factor for eIF-2. Thus, it is possible that TLR-TRIF signaling may modulate such sensing by affecting known (i.e., eIF-2B or eIF-2 components) or yet to be discovered components. Interference with this mechanism could account for the effects of LPS on both the normally-observed up-regulation of ATF4 and the global down-regulation of protein synthesis.
In summary, the data in this report reveal a TLR-UPR crosstalk pathway in which pre-exposure of cells to activators of TLR-TRIF signaling selectively suppresses ATF4-CHOP expression in the setting of prolonged ER stress. This mechanism could uniquely enable the beneficial aspects of prolonged physiologic ER stress without the detrimental effects of prolonged CHOP expression. Failure of this adaptive pathway may help explain diseases driven by excess CHOP expression4,6,8, and selective targeting of this pathway may suggest new strategies to kill cancer cells that have adapted to prolonged ER stress35.
METHODS
Methods and associated references are available in the online version of the paper at http://www.nature.com/naturecellbiology/
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a German Research Foundation Grant to B.D.; NIH grants HL75662 and HL57560 to I.T.; and DK47119 and ES08681 to D.R. We thank Drs. Alice Prince and Vincent Racaniello (Columbia University) for helpful discussions related to the high-dose LPS mouse experiments and biological effects of TRIF signaling, respectively.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski DT, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS. Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oyadomari S, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng B, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 6.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin. Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorp E, et al. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metabolism. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 10.Skalet AH, et al. Rapid B Cell Receptor-induced Unfolded Protein Response in Nonsecretory B Cells Correlates with Pro- Versus Antiapoptotic Cell Fate. J. Biol. Chem. 2005;280:39762–39771. doi: 10.1074/jbc.M502640200. [DOI] [PubMed] [Google Scholar]
- 11.Kenny EF, O’Neill LA. Signalling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Ma T, et al. The endoplasmic reticulum stress-mediated apoptosis signal pathway is involved in sepsis-induced abnormal lymphocyte apoptosis. Eur. Surg. Res. 2008;41:219–225. doi: 10.1159/000135631. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu N, Kasai A, Hayakawa K, Yao J, Kitamura M. Real-time detection and continuous monitoring of ER stress in vitro and in vivo by ES-TRAP: evidence for systemic, transient ER stress during endotoxemia. Nucleic Acids Res. 2006;34:e93. doi: 10.1093/nar/gkl515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, et al. Enrichment of endoplasmic reticulum with cholesterol inhibits SERCA2b activity in parallel with increased order of membrane lipids. Implications for depletion of ER calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 15.Duksin D, Seiberg M, Mahoney WC. Inhibition of protein glycosylation and selective cytotoxicity toward virally transformed fibroblasts caused by B3-tunicamycin. Eur. J. Biochem. 1982;129:77–80. doi: 10.1111/j.1432-1033.1982.tb07022.x. [DOI] [PubMed] [Google Scholar]
- 16.Watowich SS, Morimoto RI. Complex regulation of heat shock- and glucose-responsive genes in human cells. Mol. Cell Biol. 1988;8:393–405. doi: 10.1128/mcb.8.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol. Chem. 2009;284:6661–6673. doi: 10.1074/jbc.M806735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu PD, et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaoka A, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang X, et al. Cooperation between MyD88 and TRIF pathways in TLR synergy via IRF5 activation. Biochem. Biophys. Res. Commun. 2007;354:1045–1051. doi: 10.1016/j.bbrc.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald KA, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp. Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfs TG, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J. Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura M. Endoplasmic reticulum stress in the kidney. Clin. Exp. Nephrol. 2008;12:317–325. doi: 10.1007/s10157-008-0060-7. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol. Pharm. Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 29.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 30.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutkowski DT, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan W, et al. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van HR, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J. Biol. Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi Y, et al. Endoplasmic reticulum (ER) chaperone regulation and survival of cells compensating for deficiency in the ER stress response kinase, PERK. J. Biol. Chem. 2008;283:17020–17029. doi: 10.1074/jbc.M802466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boelens J, Lust S, Offner F, Bracke ME, Vanhoecke BW. Review. The endoplasmic reticulum: a target for new anticancer drugs. In Vivo. 2007;21:215–226. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.