Summary
The maintenance of immunologic self-tolerance requires the coordination of multiple complementary systems. Studies of the Autoimmune Regulator (Aire) gene have revealed that Aire promotes self-tolerance in part by inducing the transcription of a wide array of tissue-specific antigens (TSAs), particularly in the thymus. The importance of Aire is highlighted by the fact that patients and mice defective in Aire expression develop a multi-organ autoimmune syndrome. In this review we discuss recent progress in our understanding of Aire’s control of immune tolerance at the cellular and molecular levels, and also address the potential importance of Aire expression both in the thymus and in the peripheral lymphoid organs. The detection of both Aire and TSA expression by cell populations outside of the thymus raises the possibility that such expression may play a relevant role in the maintenance of self-tolerance.
Introduction
Since its discovery as a master transcriptional regulator controlling expression of a wealth of genes within the thymus [1,2], Aire has significantly changed the face of immune tolerance theory. Named for its role as an Autoimmune Regulator, AIRE drives ectopic expression of many tissue-specific, sequestered, and otherwise “peripheral” proteins within the thymus.
Aire is primarily, but not exclusively, expressed by medullary thymic epithelial cells (mTECs), and ensures developing thymocytes are exposed to a comprehensive view of “self,” permitting early deletion of autoreactive cells before acquisition of effector functions. Although its molecular mode of action remains somewhat elusive, its influence on immune tolerance continues to expand, encompassing a multitude of cells, tissues, diseases and molecular interactions. A number of purported functions for AIRE are still hotly debated, including a role in regulatory T-cell generation; molecular mechanisms controlling its putative transcriptional regulation; its genetic targets; and its peripheral expression and impact.
AIRE is an important regulator of normal T cell development
Loss-of-function mutations in the human AIRE gene are the single causative defect in a rare, systemic autoimmune syndrome termed APECED (autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy) or APS-1 (autoimmune polyendocrinopathy syndrome type I) [3]. Patients develop a severe, complex array of symptoms, with candidiasis and insufficiency of the parathyroid and adrenal glands serving as diagnostic hallmarks. However, APECED patients commonly develop multiple other endocrinopathies including type-1 diabetes, hypogonadism, and hypothyroidism [3–5]. The collective pathologies of APECED patients are highly varied, even between siblings [6], suggesting a contributive interplay of other genetic factors or environment.
Mouse strains made deficient in Aire have been an invaluable tool enabling researchers to dissect Aire’s role in immune regulation, and more recently, to begin unraveling its somewhat elusive intracellular targets and mechanisms of action. The first described Aire-knockout mouse strain developed a relatively mild autoimmune syndrome featuring T-cell hyperactivity; production of autoantibodies to liver, testis, pancreas and adrenal glands; leukocyte infiltration of liver and ovaries; and adrenal or thymic atrophy [1]. A second knockout strain [2] revealed a more severe tolerance breakdown, featuring cellular infiltration of and autoantibody reactivity to the salivary gland, retina, stomach, pancreas and ovary.
Interestingly, the disease spectrum differs in severity and specificity of target organs depending on the genetic background of the knockout strain [1,7,8]. Non-Obese Diabetic (NOD) mice, for instance, surprisingly become resistant to type I diabetes when Aire-deficient—while insulin-producing pancreatic islets normally come under autoimmune attack in NOD mice, immune foci instead target exocrine tissue in the pancreas of Aire-knockout NODs [7,9].
Although studies of Aire knockout mice have irrefutably improved our understanding of T cell tolerance, it has been argued that differences between disease spectra of APECED patients and mice limit their relevance [4,5]. This argument centers on the generally milder murine pathology, where severe infiltration of endocrine organs does not equate to a lack of organ function, manifest as devastating polyendocrinopathy in patients [4,5]. Notable exceptions include hypogonadism and retinal autoimmunity, which occur in all Aire knockout mice generated to date, and disease severity in Aire knockout NOD mice, which waste and die within four months of birth [9]. One of the most striking similarities between murine and human pathologies paradoxically lies with the broad variability of symptoms and severity, even between first-degree relatives, or littermate inbred mice [1,4,6,10]. This suggests a conserved, probabilistic mode of action for AIRE and tells us that differences between mice and humans are a likely pairing of AIRE’s stochastic intricacies and basic physiological differences between the species.
Mechanisms of Tolerance in the Thymus
Although the requirement for Aire in the maintenance of normal immunologic self-tolerance was established by cloning the gene from individuals with APECED [3,11], and further supported by Aire-knockout mice, the precise mechanism of Aire-mediated tolerance remained elusive. Indeed, many elements of the immune system are grossly normal in Aire-deficient animals [1,2]. The complementary discoveries that Aire was highly expressed by mTECs [12,13], and that the thymic medulla was a site of expression of otherwise tissue-specific antigens (TSAs) such as insulin and thyroglobulin [14,15], suggested that TSA expression might be essential for self-tolerance, and that Aire might play a role in this process. Indeed, in the absence of functional AIRE, mTECs express a severely restricted array of self-antigens [2], implicating a defect in negative selection which allows a broad set of autoreactive T cells to reach the periphery. Supporting this hypothesis, transplantation of Aire-deficient thymic stroma is sufficient to induce multi-organ autoimmunity in Nude (Foxn1-knockout) mice [2], and transgenic expression of rat insulin promoter (RIP)-driven antigens like ovalbumin (OVA) and hen-egg lysozyme (HEL) induces their expression in the thymus, and tolerizes cognate TCR-transgenic thymocytes in a strictly Aire-dependent fashion [16,17].
One important and unanswered question involves the contribution of Aire to regulatory T-cell (Treg) generation. Aire-deficient mice have no gross defects in Treg number or function, and cotransplantation of Aire-replete and Aire-knockout thymi into Nude mice fails to rescue from disease [18], suggesting that dominant tolerance mediated by regulatory populations selected in an Aire-replete thymus is not sufficient to suppress autoreactive T cells exported from the cotransplant. However, transgenic expression of neo-self-antigens in thymic Aire-expressing cells also appears to skew some antigen-specific TCR-transgenic T cells toward a regulatory lineage [19]. Whether different APC populations play unique roles with regard to deletion versus regulatory T cell induction, and the role of AIRE-regulated antigens in the development of regulatory cells, remains an area of active inquiry.
Molecular Mechanisms of AIRE Function
The diversity of AIRE-regulated self-antigens incites strong interest into precisely how this single gene controls its transcriptional portfolio to represent so many tissues and organs. The predicted domain structure of the AIRE protein, particularly the putative DNA-binding SAND domain, initially suggested that AIRE might interact directly with DNA; indeed some studies have supported this through gel-shift and chromatography assays [20,21]. However AIRE’s SAND domain lacks the critical KDWK motif required for DNA-binding in other SAND domain proteins, and while some studies have reported direct association of AIRE with TSA promoter regions using ChIP of in vitro transfection systems [22], it remains unclear whether this reflects direct DNA binding or simply association as part of a larger macromolecular complex, and whether such binding is relevant in vivo. Furthermore, genomic analysis to date has failed to identify a consensus AIRE-binding site among the vast number of AIRE-regulated genes in the thymus.
AIRE’s genetic targets also provide clues to its function. In the thymus, AIRE-regulated genes show clear evidence of chromosomal clustering [23,24], suggesting that epigenetic mechanisms may play a role in their transcriptional regulation. Indeed, AIRE also appears to associate with the nuclear matrix [25], a scaffold for chromatin alteration, further supporting the idea that it may act as a broad-scale epigenetic modifier. AIRE localizes to nuclear bodies adjacent to, but distinct from, splicing factor-associated nuclear speckles. This distribution is disrupted in a dominant-negative, disease-causing G228W SAND domain mutation [26], suggesting that AIRE’s function is associated with its appropriate distribution in nuclear bodies.
Closer examination of chromosomally clustered regions of AIRE regulation, however, paints a more complex picture. For example, while the casein locus contains a family of genes uniformly expressed in mammary epithelium, its thymic expression is differently regulated. In the thymus, AIRE-induced casein genes sit directly beside AIRE-repressed casein genes, which are in turn next to genes unresponsive to AIRE. Furthermore, single-cell sorting of mammary and thymic epithelium demonstrated that while all casein locus genes are coordinately upregulated in each mammary epithelial cell, any one thymic epithelial cell expresses only a few casein genes, and the distribution appears random with regard to clustering in the locus [27]. By extrapolation from this and other studies, it seems that only ~1–2% of thymic epithelial cells express any particular TSA, with a distribution that appears stochastic in nature. AIRE-mediated regulation of these large genetic loci may therefore be more complex than simply opening the door to broad transcriptional de-repression, and may require both gene-specific interaction of specific cofactors and some stochastic element causing wide variation per cell.
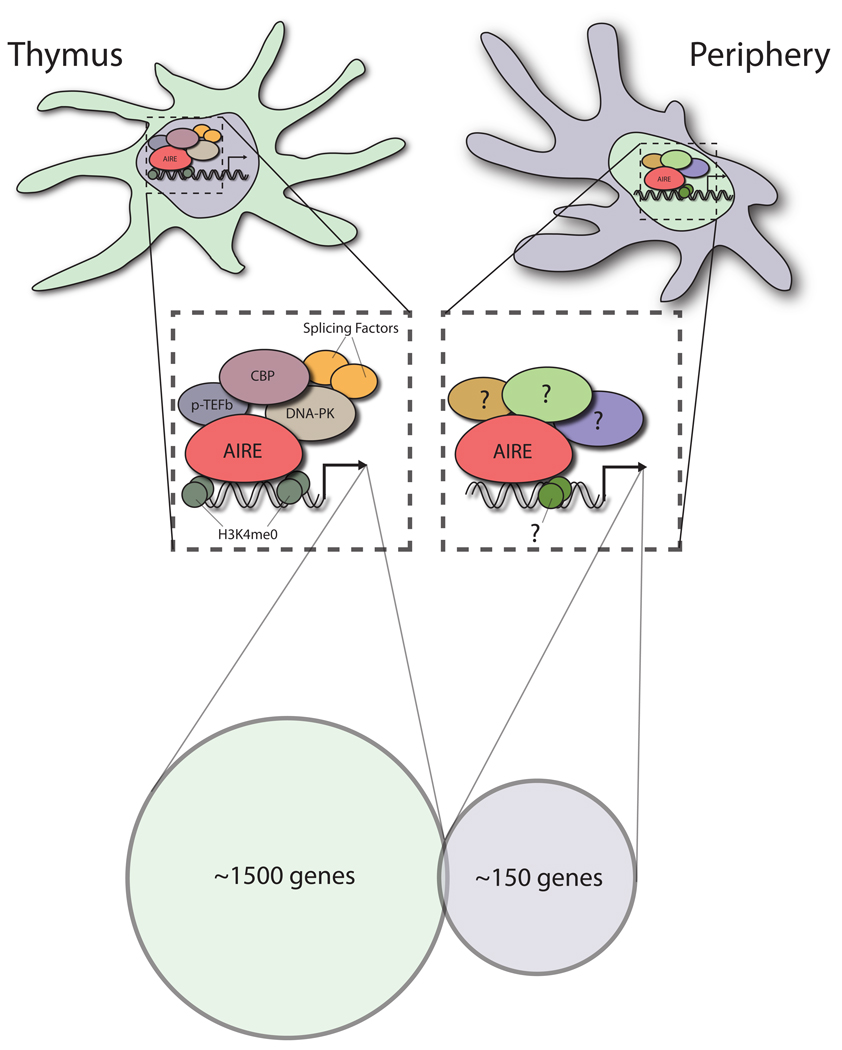
Together, these results support a model in which AIRE appears to act through epigenetic mechanisms that are necessary, but not sufficient, for expression of individual genes within a locus, and that other cofactors may be involved in determining precisely which antigens are expressed within each cell. Indeed, AIRE’s collaborative interactions with transcriptional coactivators like CBP [28], transcriptional elongation machinery such as pTEF-B [29], or DNA-specific kinases like DNA-PK [30] may play an important role in deciding which antigens are expressed in each cell (Figure 1).
Figure 1. Transcriptional activity of AIRE in mTEC’s and eTAC’s.
Shown are two of the major known Aire-expressing cell populations in the thymus (mTEC) and the periphery (eTAC). Recent work has demonstrated that AIRE promotes the transcription of an array of a complementary set of genes in the two cell populations. The number of Aire-induced genes in the periphery also appears to be smaller and may reflect a lower level of Aire expression within this cell population. As outlined in the text, AIRE has been shown to bind to and interact with a number of proteins that are involved in transcription, including CBP, P-TEFb, and DNA-PK. AIRE localizes intracellulary in mTEC’s to nuclear speckles which are enriched for a variety of splicing factors that may also participate in its regulation of transcription. The PHD1 domain of AIRE has been shown to have specificity for the H3K4me0 mark on chromatin suggesting a mechanism by which AIRE may target repressed or inactive genes. Taken together, a picture is emerging in which AIRE may promote transcription through epigenetic mechanisms and a collaboration with a variety of co-factors. The exact identity and contribution of these interactions to the regulation of AIRE-dependent transcription in eTAC’s and mTEC’s remains to be determined.
In support of such epigenetic mechanisms, a number of recent studies have shown that the PHD1 domain of AIRE, a highly conserved Zn-finger domain [31] and a common site of causative mutation in APECED patients [32], binds to histone H3 in a methylation-sensitive manner [33,34]. Methylation of lysine 4 in the amino-terminus of histone H3 generally reflects the accessibility of a particular chromatin region, with unmethylated histones marking regions that are transcriptionally inactive, and trimethylated histones marking regions of high transcriptional activity. AIRE’s PHD1 domain appears to have a selective affinity for binding unmethylated H3K4, as recent crystal structures have confirmed [35,36]. This suggests an attractive hypothesis: that AIRE may be built to selectively recognize and bind to transcriptionally inactive heterochromatic regions via their unmethylated H3K4 subunits, recruiting additional machinery to help facilitate site-specific transcription in these regions.
AIRE: Beyond Autoantigens
The kaleidoscope of genes regulated by AIRE was first revealed with microarrays of mTECs from wildtype and Aire-knockout mice [2,18]. Although Aire deficiency has a profound effect on expression of many genes in mTECs, its function may also extend beyond TSA expression. For example, using a sensitive negative selection system where the exogenous antigen OVA is transgenically expressed in the thymus, Anderson et al. [18] reported impaired deletion of OVA-specific thymocytes in Aire knockout mice despite the fact that OVA expression was not reduced. Indeed, Aire-deficient mTECs showed altered intrinsic interaction with thymocytes in this system despite the presence of OVA [18]. Similarly, thymic expression of the autoantigen α-fodrin was not impaired in Aire-knockout mice, despite demonstrable autorecognition in the periphery [37]. Furthermore, expression of various genes involved in antigen presentation and chemokine expression appears to be controlled by AIRE [18,24].
AIRE expression is also used to define end-stage matured mTECs [38,39] and an active role for AIRE in this maturation has been proposed [40,41]. Loss of Aire appears to alter thymic architecture [40] and skew mTEC development toward the proliferative MHC class IIhigh CD80high subset [39,42]. These thymic abnormalities may occur as a result of perturbed thymocyte migration and selection; a theory supported by several recent studies, which together showed that the number of mature mTECs is governed by direct interactions with positively selected, autoreactive CD4+ thymocytes [43–45]. The presence of autoreactive cells may therefore create a driving force to develop the very cell type capable of inducing their deletion. Expression of Aire speeds apoptosis in transfected cell lines and marks post-mitotoic mTECs that die quickly [39], suggesting that it may precipitate the death of mTECs and perhaps aid in the dispersal of tissue-specific antigens. This raises the important and unresolved question of whether Aire-expressing mTECs directly delete autoreactive T cells or simply serve as antigen reservoirs for other APC populations.
Extrathymic AIRE and Promiscuous Gene Expression
The precise distribution of Aire transcript and protein expression outside the thymus has remained controversial. All accounts concur on the high expression levels of Aire within mTECs, and nearly all on the presence of detectable transcript in secondary lymphoid organs and/or spermatogonia [2,46–48]. But whether such extrathymic transcripts are translated into functional protein, the identity of peripheral Aire+ cells, and the role and physiologic relevance of extrathymic Aire remain contentious. Aire transcript and protein are reportedly expressed by spermatogonia and spermatocytes, and loss of Aire in these cells appears to correlate with T-cell independent deficiencies in scheduled and sporadic apoptosis [46]. These defects are particularly interesting given recent reports of AIRE acting as a pro-apoptotic factor in mTECs [39].
In secondary lymphoid organs of mice and humans, Aire transcript has been detected in monocyte/dendritic cell lineages [47,48], and a cell-intrinsic effect on BAFF production from dendritic cells, independent of thymic Aire expression, has been reported [49]. Recent evidence also suggests that Aire is expressed in the lymphoid stroma. Lee et al. [50] and Gardner et al. [51] both reported detection of Aire transcript among CD45-negative lymphoid stroma, and AIRE protein has also been shown in nuclear bodies in secondary lymphoid organs of mice [51] and humans [52]; however, there are some conflicting data on this [42].
Recent evidence now suggests that the secondary lymphoid organs may also be a site of promiscuous expression for tissue-specific antigens, though the nature and relevance of this phenomenon remains contentious. By analogy, however, the discovery of TSA expression in the thymus provides a useful historical precedent. When tissue-specific antigen promoters such as insulin and elastase I were first exploited to drive expression of exogenous T antigens in target tissues, a number of these antigens were, surprisingly, found to be “promiscuously” expressed in the thymus. [14,53]. These observations led a number of groups to suggest that such promiscuous gene expression might be an important property of thymic stroma, and might promote negative selection of antigen-specific T cells.
Suggestively, a number of recent reports have observed similar phenomena mediated by radioresistant cells of the secondary lymphoid organs. Lee et al. [50], using a transgenic system to express a truncated form of cytosolic OVA under the control of the intestinal fatty-acid binding protein promoter, found that OVA was promiscuously expressed by lymph node stroma, and that adoptive transfer of OVA-specific CD8 T cells induced rapid proliferation and subsequent deletion mediated by a radioresistant population. Similarly, Nichols et al. [54] showed that endogenous tyrosinase, a melanocyte-specific antigen, was expressed by radioresistant cells in secondary lymphoid organs, inducing rapid proliferation and subsequent deletion of adoptively transferred tyrosinase-specific CD8 T cells. Finally Gardner et al. [51], using a transgenic Aire reporter, identified a population of radioresistant cells in secondary lymphoid organs expressing nuclear AIRE protein and a host of AIRE-regulated TSAs. Transgenic self-antigen expression in these cells induced a similarly rapid proliferation and subsequent deletion of cognate CD8 T cells. Together, these results suggest the lymphoid stroma as a site of immunologically relevant expression of both Aire and TSAs.
Aire Expression in Secondary Lymphoid Organs: The Debate Continues
While our groups and others have generated evidence suggesting that extrathymic AIRE and lymphoid stroma may play a role in ectopic TSA expression and immune tolerance, our respective approaches have thus far identified distinct cell populations. Lee et al. [50] characterized a population of UEA1+ gp38+ MHC class II+ LNSCs which ectopically expressed transgenic antigen, resulting in deletion of cognate T cells. In the same study, LNSCs were shown to ectopically express a number of TSAs relevant to autoimmunity, including those from the eye, thyroid, pancreas and central nervous system [50,55]. These cells, which upregulated PD-L1 to prevent CD8 T cell activation during chronic viral infection [56], are presumably part of the lymph node fibroblastic reticular cell (FRC) network. Far from a static scaffold, LN FRC form a matchmaking network for immune responses, expressing chemokines to attract and guide T cells and DC along processes rich in extracellular matrix, to meet in the paracortex [57,58]. They also deliver cytokines, chemokines and soluble antigen from lymph along specialized conduits to the rest of the organ [59]. Importantly, presentation of antigen via MHC on stromal cells in these studies was tolerogenic without contribution from dendritic cells. The mechanisms involved are under investigation, but certainly a blockade of PD-L1 rescued autoreactive CD8+ T cells, which became activated and caused disease [60].
By comparison, a recent study by Gardner et al. [51] using an Aire reporter construct showed expression of both Aire transcript and nuclear AIRE protein in a unique population of tolerogenic stromal cells, termed eTACs (extra-thymic Aire-expressing cells). These cells, which express the epithelial marker EpCAM, are UEA1- and gp38-negative and phenotypically distinct from FRC populations. While expressing some markers reminiscent of mTECs (MHC class II, EpCAM), these cells appear to lack expression of canonical costimulatory molecules CD80 and CD86. This study also described weak expression of the Aire reporter in a subset of CD11c+ dendritic cells, though AIRE protein was not specifically detected in this population, and deletional tolerance of cognate T cells did not depend on it. As in the thymus, comparison of eTACs from WT and Aire-knockout mice demonstrated that Aire regulates a set of TSAs that includes several important autoantigens, as well as genes important in antigen processing and presentation, suggesting that Aire expression has broad transcriptional consequences for TSA expression in the periphery. Surprisingly, the genes regulated by AIRE in eTACs had virtually no overlap with AIRE-regulated genes in the thymus, suggesting a complementary role in the maintenance of self-tolerance (Figure 1). In addition, this lack of overlap reinforces the idea that AIRE-regulation of transcription is complex and may vary between cell types because of changes in the array of transcriptional or epigenetic factors that differ between the two cell populations.
The relationship between these populations, and the physiologic relevance of extrathymic AIRE in a non-transgenic context, remain to be defined. The limited evidence available offers only a few clues. First, the immunologically relevant role of AIRE appears to be restricted to radioresistant populations [2]. Second, the fact that transplantation of Aire-deficient thymic stroma into Nude (Foxn1 knockout) hosts is sufficient to induce Aire-like autoimmunity [2] suggests that peripheral Aire expression may not be sufficient to compensate for loss of thymic Aire. This is neither surprising nor inconsistent with the idea that central and peripheral AIRE may play unique and complementary roles, and that multiple overlapping systems are required to maintain normal self-tolerance. Furthermore, because we know nothing about the Foxn1-dependence of eTAC development, it is not clear whether the Nude mouse presents the best system for investigating these issues. Careful comparison of the relative spectra of autoimmune disease observed upon loss of central or peripheral Aire, particularly in autoimmune-prone strains, remains to be done. Such experiments are eagerly anticipated, though the proper experimental approach to test this has been challenging, moreso given the recent report by Guerau-de-Arellano and colleagues that the critical time for Aire expression may be early in perinatal development [61].
The study by Guerau-de-Arellano et al. also comments on Aire mRNA expression in secondary lymphoid stroma and its relevance [61]. Using a Tetracycline-responsive transgenic system designed to allow temporal control of Aire expression in an otherwise Aire-deficient mouse, the authors reported detecting transgene expression in lymph nodes and spleen, but argued that the absence of Tetracycline-responsiveness of this transgenic Aire in lymph nodes, despite the observed autoimmunity in these mice, suggests that peripheral Aire expression is not relevant to disease progression. However, factors such as overexpression of the transgene, its imprecise tissue-specificity (which, for example, is also expressed in cortical epithelium), and the fact that splenic Aire levels do respond to Tetracycline administration, all cast doubt on the relevance of these conclusions to endogenous peripheral Aire. Ultimately, peripheral Aire must be assessed using experiments appropriate to the task.
Conclusions
The study of Aire continues to yield groundbreaking insights into the mechanisms of immunologic tolerance, though each step forward seems to raise as many questions as it answers. In the thymus, the relationship between deletion and generation of regulatory populations remains an active area of inquiry, and recent evidence that AIRE may also promote tolerance by means independent of TSA expression has raised important new questions. At the molecular level, recent work identified a role for AIRE in binding histones in a methylation-sensitive manner, suggesting an attractive mechanism by which AIRE may recognize and promote expression in otherwise transcriptionally inactive regions of the genome. Analysis of AIRE-regulated loci in single cells has shown that notions of broad epigenetic deregulation by AIRE are oversimplified, and that additional stochastic and regulatory mechanisms appear to be at play. Clearly, much exciting work remains to determine the mechanisms behind AIRE’s role as a transcriptional regulator and mediator of self-tolerance.
In the periphery, the role of Aire remains incompletely understood, though increasing evidence suggests that secondary lymphoid stroma is the site of both ectopic TSA expression and Aire expression at transcript and protein levels. Growing evidence also suggests that a network of TSA-expressing stroma may play a role in the maintenance of immunologic tolerance, and that self-antigen expression in the periphery may complement self-antigen expression in the thymus, providing a safety net to eliminate autoreactive T cells that evade thymic negative selection. Further research is, of course, necessary to understand the relationship between peripheral AIRE and TSA expression, and between these identified populations, and the ultimate relevance of these phenomena. We look forward to the coming research into the respective roles and relevance of these populations in preventing autoimmunity, and anticipate rapid progress in this emerging field.
Acknowledgements
The authors thank members of the Turley and Anderson labs for helpful discussions. This work was supported by grants from the American Diabetes Association, the UCSF Medical Scientist Training Program, the NIAID, and the Burroughs Wellcome Fund to JMG and MSA; from the NIDDK and NIAID to SJT; and ALF was supported by an Australian NHMRC CJ Martin Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune responses. Human Molecular Genetics. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Venanzi ES, Klein L, Chen SY, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Projection of an immunological self-shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 3.Aaltonen J, Bjorses P, Perheentupa J, Horelli-Kuitunen N, Palotie A, Peltonen L, Lee YS, Francis F, HenningSteffen, Thiel C, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 4.Pontynen N, Miettinen A, Arstila TP, Kampe O, Alimohammadi M, Vaarala O, Peltonen L, Ulmanen I. Aire deficient mice do not develop the same profile of tissue-specific autoantibodies as APECED patients. J Autoimmunity. 2006;27:96–104. doi: 10.1016/j.jaut.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Kekalainen E, Miettinen A, Arstila TP. Does the deficiency of Aire in mice really resemble human APECED? Nat Rev Immunol. 2007;7 doi: 10.1038/nri2136-c1. [DOI] [PubMed] [Google Scholar]
- 6.Myhre AG, Bjorses P, Dalen A, Husebye ES. Three sisters with Addison's disease. J Clin Endocrinol Metab. 1998;83:4204–4206. doi: 10.1210/jcem.83.12.5332. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubert F-X, Kinkel SA, Crewther PE, Cannon P, Webster KE, Link M, Uibo R, O'Bryan MK, Meager A, Forehan SP, et al. Aire-deficient C57Bl/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009;182:3902–3918. doi: 10.4049/jimmunol.0802124. [DOI] [PubMed] [Google Scholar]
- 9.Niki S, Oshikawa K, Mouri Y, Hirota F, Matsushima A, Yano M, Han M, Bando Y, Izumi K, Matsumoto M, et al. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J Clin Invest. 2006;116:1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venanzi ES, Melamed R, Mathis D, Benoist C. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc Natl Acad Sci U S A. 2008;105:15860–15865. doi: 10.1073/pnas.0808070105. • This work shows that the transcription of Aire-regulated TSAs is somewhat
- 11.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 12.Blechschmidt K, Schweiger M, Wertz K, Poulson R, Christensen HM, Rosenthal A, Lehrach H, Yaspo ML. The mouse Aire gene: comparative genomic sequencing, gene organization, and expression. Genome Res. 1999;9:158–166. [PMC free article] [PubMed] [Google Scholar]
- 13.Heino M, Peterson P, Kudoh J, Nagamine K, Lagerstedt A, Ovod V, Ranki A, Rantala I, Nieminen M, Tuukkanen J, et al. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257:821–825. doi: 10.1006/bbrc.1999.0308. [DOI] [PubMed] [Google Scholar]
- 14.Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci U S A. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 16.Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. Gene dosage--limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 18.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 19. Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. •• This study shows that mTECs can induce the positive selection of FoxP3-expressing T regulatory cells in a T-cell-receptor transgenic model.
- 20.Kumar PG, Laloraya M, Wang CY, Ruan QG, Davoodi-Semiromi A, Kao KJ, She JX. The autoimmune regulator (AIRE) is a DNA-binding protein. J Biol Chem. 2001;276:41357–41364. doi: 10.1074/jbc.M104898200. [DOI] [PubMed] [Google Scholar]
- 21.Purohit S, Kumar PG, Laloraya M, She JX. Mapping DNA-binding domains of the autoimmune regulator protein. Biochem Biophys Res Commun. 2005;327:939–944. doi: 10.1016/j.bbrc.2004.12.093. [DOI] [PubMed] [Google Scholar]
- 22.Ruan QG, Tung K, Eisenman D, Setiady Y, Eckenrode S, Yi B, Purohit S, Zheng WP, Zhang Y, Peltonen L, et al. The autoimmune regulator directly controls the expression of genes critical for thymic epithelial function. J Immunol. 2007;178:7173–7180. doi: 10.4049/jimmunol.178.11.7173. [DOI] [PubMed] [Google Scholar]
- 23.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnnidis JB, Venanzi ES, Taxman DJ, Ting JP-Y, Benoist C, Mathis D. Chromosomal clustering of genes controlled by the aire transcription factor. Proc Natl Acad Sci U S A. 2005;102:7233–7238. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao Y, Kupfer R, Stewart BJ, Williams-Skipp C, Crowell CK, Patel DD, Sain S, Scheinman RI. AIRE recruits multiple transcriptional components to specific genomic regions through tethering to nuclear matrix. Mol Immunol. 2006;43:335–345. doi: 10.1016/j.molimm.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 26. Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL, Devoss JJ, Johannes KP, Lu W, Gardner J, et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J Clin Invest. 2008;118:1712–1726. doi: 10.1172/JCI34523. •• This study describes a dominant-negative allele of Aire harboring a point mutation in the SAND domain, and highlights the importance of quantitative changes in thymic TSA expression in autoimmunity.
- 27. Derbinski J, Pinto S, Rosch S, Hexel K, Kyewski B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci U S A. 2008;105:657–662. doi: 10.1073/pnas.0707486105. •• By studying promiscuous gene expression at a single-cell level, these authors show that TSA transcription is more likely to be stochastic rather than developmentally driven.
- 28.Pitkanen J, Doucas V, Sternsdorf T, Nakajima T, Aratani S, Jensen K, Will H, Vahamurto P, Ollila J, Vihinen M, et al. The autoimmune regulator protein has transcriptional transactivating properties and interacts with the common coactivator CREB-binding protein. J Biol Chem. 2000;275:16802–16809. doi: 10.1074/jbc.M908944199. [DOI] [PubMed] [Google Scholar]
- 29. Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–8823. doi: 10.1128/MCB.01085-07. #x02022; These authors show that AIRE can participate in transcriptional elongation and suggest that this may be part of the mechanism by which AIRE drives transcription of such a broad array of target genes.
- 30.Liiv I, Rebane A, Org T, Saare M, Maslovskaja J, Kisand K, Juronen E, Valmu L, Bottomley MJ, Kalkkinen N, et al. DNA-PK contributes to the phosphorylation of AIRE: importance in transcriptional activity. Biochim Biophys Acta. 2008;1783:74–83. doi: 10.1016/j.bbamcr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saltis M, Criscitiello MF, Ohta Y, Keefe M, Trede NS, Goitsuka R, Flajnik MF. Evolutionarily conserved and divergent regions of the autoimmune regulator (Aire) gene: a comparative analysis. Immunogenetics. 2008;60:105–114. doi: 10.1007/s00251-007-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halonen M, Kangas H, Ruppell T, Ilmarinen T, Ollila J, Kolmer M, Vihinen M, Palvimo J, Saarela J, Ulmanen I, et al. APECED-causing mutations in AIRE reveal the functional domains of the protein. Hum Mutat. 2004;23:245–257. doi: 10.1002/humu.20003. [DOI] [PubMed] [Google Scholar]
- 33. Org T, Chignola F, Hetenyi C, Gaetani M, Rebane A, Liiv I, Maran U, Mollica L, Bottomley MJ, Musco G, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9:370–376. doi: 10.1038/embor.2008.11. •• (See below, reference 34)
- 34. Koh AS, Kuo AJ, Park SY, Cheung P, Abramson J, Bua D, Carney D, Shoelson SE, Gozani O, Kingston RE, et al. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc Natl Acad Sci U S A. 2008;105:15878–15883. doi: 10.1073/pnas.0808470105. •• These two studies show that a PHD finger domain of AIRE specifically interacts with histone H3 unmethylated at lysine 4, suggesting an epigenetic link to the mechanism of AIRE's transcriptional activity.
- 35.Chignola F, Gaetani M, Rebane A, Org T, Mollica L, Zucchelli C, Spitaleri A, Mannella V, Peterson P, Musco G. The solution structure of the first PHD finger of autoimmune regulator in complex with non-modified histone H3 tail reveals the antagonistic role of H3R2 methylation. Nucleic Acids Res. 2009;37:2951–2961. doi: 10.1093/nar/gkp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravarty S, Zeng L, Zhou MM. Structure and site-specific recognition of histone H3 by the PHD finger of human autoimmune regulator. Structure. 2009;17:670–679. doi: 10.1016/j.str.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher AL, Lowen TE, Sakkal S, Reiseger JJ, Hammett MV, Seach N, Scott HS, Boyd RL, Chidgey AP. Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells following cyclosporine, cyclophosphamide and dexamethasone treatment. J Immunol. 2009 doi: 10.4049/jimmunol.0900225. In Press. [DOI] [PubMed] [Google Scholar]
- 39. Gray D, Abramson J, Benoist C, Mathis Dc. Proliferative arrest and rapid turnover of thymic epithelial cells expressin Aire. J Exp Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. •• By studying the proliferative activity and turnover of mTEC subpopulations, these authors conclude that Aire-expressing mTECs represent a terminally differentiated population that goes through programmed cell death under the influence of AIRE.
- 40.Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG. Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J Immunol. 2007;178:3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- 41. Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, Maemura K, Yanagawa Y, Obata K, Takahashi S, et al. Aire controls the differentiation program of thymic epithelial cells in the the medulla for the establishment of self-tolerance. J Exp Med. 2008;205:2827–2838. doi: 10.1084/jem.20080046. •• By creating an Aire-reporter line of mice expressing GFP under the control of the Aire locus, these authors suggest that AIRE influences the proper development of the thymic medulla.
- 42.Hubert F-X, Kinkel SA, Webster KE, Cannon P, Crewther PE, Heath WR, Scott HS. A specific anti-Aire antibody reveals Aire expression is restricted to medullary thymic epithelial cells and not expressed in periphery. J Immunol. 2008;180:3824–3832. doi: 10.4049/jimmunol.180.6.3824. [DOI] [PubMed] [Google Scholar]
- 43. Akiyama T, Shimo Y, Yanai H, Qin H, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, et al. The Tumor Necrosis Factor Family Receptors RANK and CD40 Cooperatively Establish the Thymic Medullary Microenvironment and Self-Tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. • (see below, reference 45)
- 44. Hikosaka T, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, et al. The Cytokine RANKL Produced by Positively Selected Thymocytes Fosters Medullary Thymic Epithelial Cells that Express Autoimmune Regulator. Immunity. 2009;29:438–450. doi: 10.1016/j.immuni.2008.06.018. • (see below, reference 45)
- 45. Irla M, Hugues S, Gill J, Nitta T, Hikosaka T, Williams IR, Hubert F-X, Scott HS, Takahama Y, Hollander GAc, et al. Autoantigen-Specific Interactions with CD4+ Thymocytes Control Mature Medullary Thymic Epithelial Cell Cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. • These three reports offer a detailed study of the developmental pathways that control the proper development of Aire-expressing mTECs including roles for RANK, CD40, and negative selection in this process.
- 46.Schaller CE, Wang CL, Beck-Engeser G, Goss L, Scott HS, Anderson MS, Wabl M. Expression of Aire and the early wave of apoptosis in spermatogenesis. J Immunol. 2008;180:1338–1343. doi: 10.4049/jimmunol.180.3.1338. [DOI] [PubMed] [Google Scholar]
- 47.Kogawa K, Nagafuchi S, Katsuta H, Kudoh J, Tamiya S, Sakai Y, Shimizu N, Harada M. Expression of AIRE gene in peripheral monocyte/dendritic cell lineage. Immunol Lett. 2002;80:195–198. doi: 10.1016/s0165-2478(01)00314-5. [DOI] [PubMed] [Google Scholar]
- 48.Heino M, Peterson P, Sillanpaa N, Guerin S, Wu L, Anderson G, Scott HS, Antonarakis SE, Kudoh J, Shimizu N, et al. RNA and protein expression of the murine autoimmune regulator gene (Aire) in the normal, RelB-deficient and in NOD mouse. Eur J Immunol. 2000;30:1884–1893. doi: 10.1002/1521-4141(200007)30:7<1884::AID-IMMU1884>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 49.Lindh E, Lind SM, Lindmark E, Hassler S, Perheentupa J, Peltonen L, Winqvist O, Karlsson MC. AIRE regulates T-cell-independent B-cell responses through BAFF. Proc Natl Acad Sci U S A. 2008;105:18466–18471. doi: 10.1073/pnas.0808205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. •• Here, LNSCs were shown to ectopically express transgenic intestine-derived TSA to CD8+ T cells, inducing deletional tolerance. This paper was the first to show that lymph node stroma expresses mRNA for Aire and for a variety of TSAs.
- 51. Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. •• This paper identifies a tolerogenic stromal population in secondary lymphoid organs that expresses Aire and an array of AIRE-regulated TSAs distinct from the thymic AIRE-regulated repertoire.
- 52.Bjorses P, Pelto-Huikko M, Kaukonen J, Aaltonen J, Peltonen L, Ulmanen I. Localization of the APECED protein in distinct nuclear structures. Human Molecular Genetics. 1999;8:259–266. doi: 10.1093/hmg/8.2.259. [DOI] [PubMed] [Google Scholar]
- 53.Antonia SJ, Geiger T, Miller J, Flavell RA. Mechanisms of immune tolerance induction through the thymic expression of a peripheral tissue-specific protein. Int Immunol. 1995;7:715–725. doi: 10.1093/intimm/7.5.715. [DOI] [PubMed] [Google Scholar]
- 54. Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. •• This paper demonstrates that a radioresistant cell population in the peripheral lymphoid organs ectopically expresses endogenous self antigen and can mediate deletional tolerance against that antigen.
- 55.Magnusson FC, Liblau RS, von Boehmer H, Pittet MJ, Lee JW, Turley SJ, Khazaie K. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology. 2008;134:1028–1037. doi: 10.1053/j.gastro.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 56.Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci U S A. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gretz JE, Kaldijian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–499. [PubMed] [Google Scholar]
- 60.Reynoso ED, Elpek KG, Francisco L, Bronson R, Bellemare-Pelletier A, Sharpe AH, Freeman GJ, Turley SJ. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J Immunol. 2009;182:2102–2112. doi: 10.4049/jimmunol.0802769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of Autoimmunity. J Exp Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. •• These authors utilize an inducible Aire-expressing transgenic mouse to determine when the timing of Aire expression is critical for the induction of tolerance and suggest that the critical window is in the perinatal period.