Abstract
Purpose
We investigated the in vitro and in vivo anti-MM activity of mAb 1339, a high-affinity fully humanized anti-IL-6 mAb (IgG1), alone and in combination with conventional and novel anti-MM agents, as well as its effect on bone turnover.
Experimental Design
We examined the growth inhibitory effect of 1339 against MM cell lines in the absence and in the presence of bone marrow stromal cells (BMSC) alone or in combination with dexamethasone, bortezomib, perifosine and revlimid. Using the SCID-hu murine model of MM, we also examined the effect of 1339 on MM cell growth and MM bone disease.
Results
mAb 1339 significantly inhibited growth of MM cell in the presence of BMSC in vitro, associated with inhibition of phosphorylation of STAT3, ERK1/2 and Akt. In addition, mAb 1339 enhanced cytotoxicity induced by dexamethasone as well as bortezomib, lenalidomide, and perifosine in a synergistic fashion. Importantly mAb 1339 significantly enhanced growth inhibitory effects of dexamethasone in vivo in SCID-hu mouse model of MM. mAb 1339 treatment also resulted in inhibition of osteoclastogenesis in vitro and bone remodeling in SCID-hu model.
Conclusions
Our data confirm both in vitro and in vivo anti-MM activity, as well as inhibition of bone turnover by fully humanized mAb 1339 as a single agent and in combination with conventional and novel agents providing a rationale for its clinical evaluation in MM.
Introduction
IL-6 is a multifunctional cytokine regulating the immune response, the acute-phase response and the bone metabolism(1). IL-6 belongs to a family of cytokines, which all act via receptor complexes containing the cytokine receptor subunit gp-130(2). Increased production of IL-6 has been implicated in the pathogenesis of several diseases, including autoimmune disorders, B-cell malignancies and postmenopausal osteoporosis(3-5). In particular, the biologic sequelae of IL-6 in the pathogenesis of multiple myeloma (MM) are well documented(6). Autocrine and paracrine IL-6 has been recognized to play a crucial role in growth and survival of MM cells within the BM milieu(7). In the bone marrow (BM) microenvironment, IL-6 is predominantly produced by BM stromal cells (BMSCs), mediating MM cell growth and preventing apoptotic cell death. IL-6 triggers at least three major signalling pathways; Ras/MEK/ERK cascade, JAK2/signal transducer and activator of transcription (STAT-3) cascade, and PI3K/Akt cascade. Importantly, IL-6 protects against apoptotic cell death induced by a variety of agents, including dexamethasone. In addition IL-6 also controls expression of various other key growth and survival mechanisms in myeloma. For example, IL-6 plays an important role in the transcriptional regulation of myeloid cell leukemia (Mcl)-1, an anti-apoptotic B-cell lymphoma-2 family member, a critical mediator of MM cell survival, and tightly regulated by the proteasome(8). Therefore, down-regulation of IL-6 signalling would also sensitise MM cells to proteasome inhibitor-mediated apoptosis by interfering with the induction of the HSP response and Mcl-1(9). IL-6 is also a potent osteoclast activating factor (OAF) for human osteoclast precursors(10, 11). Frequent bone lesions observed in MM are due to increased osteoclast activity and decreased osteoblast activity(12). These findings suggest that inhibition of IL-6 signalling may target not only MM cells, but also BMSCs and other cellular components including osteoclasts. In addition, IL-6 promotes the antitumor activity of macrophages, helps produce lymphokine-activated killer cells, and protects neutrophils from apoptosis, which may increase their cytotoxic effect on tumor cells.
Clinical strategies using monoclonal antibodies (mAb) directed at IL-6 and IL-6R have been reported(13, 14). Although the clinical activity of single agent anti-IL-6/IL-6R in MM patients has been limited, two ongoing clinical studies of the anti-IL-6 monoclonal antibody CNTO 328 have shown evidence of encouraging activity, including one with dexamethasone and another with bortezomib.
Elsilimomab is a murine monoclonal antibody, also known as B-E8, which has been the subject of several proof-of-concept clinical studies in haematological malignancies(13, 15-17). Using the ActivMAb® technology and the murine B-E8 as template, a high-affinity, antagonist, fully human anti-IL-6 mAb 1339 (known also as the mAb OP-R003-1, IgG1), has been generated. Unpublished in vitro studies demonstrated that mAb 1339 shares similar biological properties, including affinity and epitope specificity with its “parent” murine antibody Elsilimomab (B-E8). Here, we report that inhibition of the IL-6 signalling using a fully human anti-IL-6 antibody, alone or in combination with other agents including dexamethasone, exerts anti-MM effects and prevents MM-induced bone disease in vitro and in vivo using the SCID-hu model of human MM.
Materials and Methods
Cells
The IL-6 dependent MM cell lines INA6, provided by Dr. Edward Thompson (University of Texas Medical Branch), and XG1, provided by Dr. Bernard Klein, were cultured in RPMI 1640 (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) and 1 ng/mL rhIL-6 (R&D Systems, Minneapolis, MN). MM1S and MM1R MM cell lines were provided by Dr. Steven Rosen (Northwestern University). U266 cells were obtained from American Type Culture Collection. MM1S, MM1R and U266 cell lines were cultured in RPMI 1640 supplemented with 10% FBS. Bone marrow mononuclear cells (BMMNCs) and primary MM cells were collected after obtaining informed consent using an IRB (Dana-Farber Cancer Institute) approved protocol. Cells were isolated and processed as previously described(18).
Reagents
The human 1339 mAb was generated by OPi EUSA Pharma (Dardilly France) in collaboration with Vaccinex (Rochester, USA). Then 1339 Mab has been licensed to Glaxo Smith Kline. 1339 has been designed using the murine B-E8 as template. MAb 1339 contains only a few residues of the variable antigen-binding region of the murine anti IL-6 Ab and the costant region of the human IgG1k Immunoglobulin (publication submitted). Dexamethasone was purchased from Sigma (St. Louis, MO). Bortezomib, lenalidomide and perifosine were provided by Millennium Pharmaceuticals (Cambridge, MA), Celgene Corporation, and Keryx Biopharmaceuticals (New York, NY), respectively.
Cell proliferation assay
MM cell proliferation was measured by [3H]-thymidine (Perkin-Elmer, Boston, MA) incorporation assay. Multiple myeloma cells (2 × 104 cells/well) were cultured in 96-well plates (Costar) at 37°C for 24, 48 and 72 hours, in the presence or absence of 1339 mAb. Cells were pulsed with [3H]-thymidine (0.5 micro Ci/well) for 6 hours, harvested, and radioactivity was counted using the LKB Betaplate scintillation counter (Wallac, Gaithersburg, MD). In co-culture experiments, MM cells (2 × 104 cells/well) were incubated in BMSC-coated 96-well plates (Costar, Cambridge, MA) at 37°C for 24, 48 and 72 hours, in the presence or absence of 1339 mAb. In the combination treatment experiments, cells were cultured with dexamethasone, bortezomib, perifosine and revlimid, in the absence or presence of 1339 mAb for 48 hours. All experiments were carried out in triplicates.
Immunoblotting
Whole cell lysates were subjected SDS-PAGE using “Precast Gel” (Bio-Rad Laboratores, Melville, NY), transferred to a nitrocellulose membrane (Bio-Rad), and immunoblotted with anti p-STAT3, -STAT3, -pERK (Santa Cruz Biotechnology, Santa Cruz, CA), -ERK (Cell Signaling Technology, Danvers, MA), -pAkt (Cell Signaling Technology, Danvers, MA) or –Akt (Cell Signaling Technology, Danvers, MA) antibodies (Abs). After incubating with second Ab, membranes were developed by enhanced chemiluminescence (GE Healthcare, Piscataway, NJ)
Osteoclast differentiation assay
Human osteoclasts (OC) were generated from the non-adherent fraction of bone marrow mononuclear cells (BMMC) obtained from bone marrow aspirates of MM patients after 24 hours culture period. The non-adherent fraction of BMMC were cultured in 96-well plates (5× 104 cells/well) and stimulated with α-MEM osteoclastic differentiation media containing containing 10% FBS, 2% penicillin-streptomycin (Mediatech Inc., Herndon, VA), 1% of L-glutamine (Mediatech Inc., Herndon, VA), as well as 25 ng/ml of M-CSF (R&D Systems, Minneapolis, MN) and 25 ng/ml of RANKL (PeproTech, Rocky Hill, NJ) in the presence or absence of 5 ng/ml of rIL-6 (R&D systems) with or without 1 μg/ml of isotype control or mAb 1339. At the end of the culture period (7, 14 and 21 days) cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) according to manufacturers' guidelines (Takara Bio, Saint Louis, MO). Cells were observed and counted with a Nikon Eclipse TS 100 microscope, using a 100× magnification.
SCID-hu mouse model
Six- to 8-weekold male CB-17 severe combined immunodeficient (SCID) mice (Taconic, Germantown, NY) were housed and monitored in our Animal Research Facility. All experimental procedures and protocols had been approved by the Institutional Animal Care and Use Committee (VA Boston Healthcare System). Human fetal long bone grafts were s.c. implanted into SCID mice (SCID-hu), as previously described(19). Four weeks following bone implantation, 2.5 × 106 INA-6 multiple myeloma cells were injected directly into the human bone implant. The level of soluble human IL-6 receptor (shuIL-6R) in mice was used as marker of tumor burden. Mouse sera were serially monitored for shuIL-6R levels by ELISA (R&D Systems, Inc., Minneapolis, MN). After the first detection of shuIL-6R mice were injected with isotype control (100 μg per mouse/1×/week) (n=6), 1339 (100 μg per mouse, 1×/week) (n=4), dexamethasone (1mg/kg, 3×/week) (n=4), and 1339 plus dexamethasone (n=4). The pharmacokinetics of single (100 μg/mouse) intraperitoneal (i.p.) administration of 1339 in SCID mice peripheral blood was measured by ELISA.
Micro-computed tomography (micro-CT) and histological analysis
One month after treatment with mAb 1339, bone chips were retrieved, fixed in freshly prepared 4% paraformaldehyde (PFA) o/n, washed with 70% ethanol and analyzed by μ-CT and histological examination. The samples were scanned at 18 microns with 300ms integration time using μ-CT imaging system (μCT-40, Scanco Medical, Bassersdorf, Switzerland) allowing us to look at the bone samples in 3D non-invasively(20, 21). Three dimensional reconstructions were performed after segmentation for qualitative assessment of treated and corresponding untreated bone. For the bone histology, specimens fixed in 4% PFA were demineralization with 14% EDTA in phosphate buffered saline (PBS). The specimens were processed and embedded in paraffin and 5 μm sections were prepared and stained with heamatoxyline and eosin (H&E) for histological analysis. Sections were observed and photographed with a Nikon transmitted light microscope.
Statistical analysis
The statistical significance of differences between the individual and the combined treatments was analyzed using the t test; differences were considered significant when P≤ 0.05.
Results
mAb 1339 inhibits IL-6-dependant MM cell growth
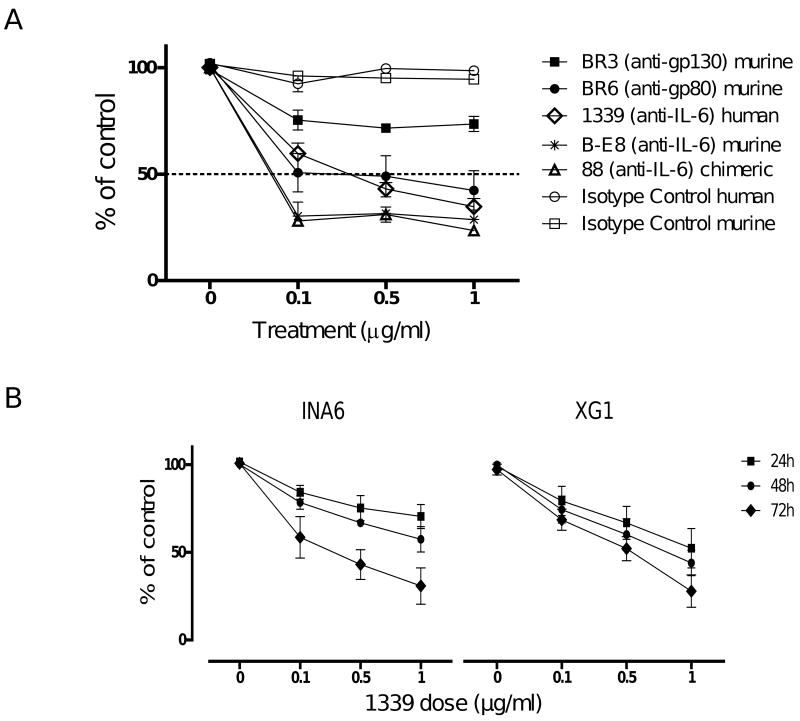
The human monoclonal antibody 1339 designed using the murine B-E8 antibody as template contains only few residues of the variable antigen-binding region of the murine anti IL-6 Ab and the constant region of the human IgG1k Immunoglobulin. We first compared the ability of mAb 1339 to inhibit MM cell growth in comparison to various murine, chimeric and humanized mAbs directed against IL-6 or IL-6R (gp80 or gp130). We cultured INA-6 MM cells in the presence of these mAbs as well as isotype controls at various concentrations. The effect on cell proliferation was assessed by [3H] Thymidine uptake after 72 hours. As seen in Figure 1A, mAbs had variable dose-dependent effect on INA-6 cell growth. For example, anti-gp130 mAb showed limited effect on MM cell growth while anti-gp80 and anti-IL-6 murine, chimeric and fully humanized mAb 1339 showed similar maximum anti-MM activity. We next evaluated both dose and time dependent activity of mAb 1339 on IL-6 dependent INA6 and XG1 MM cell lines. MM cells were cultured in the presence of different doses of mAb 1339 (0.1, 0.5 and 1 μg/ml) and cell proliferation was assessed by [3H] Thymidine uptake after 24, 48 and 72 hours. As seen in Figure 1B, mAb 1339 induced dose- and time-dependent inhibition of proliferation of both INA-6 and XG1 cell lines.
Figure 1. mAb 1339 inhibits MM cell proliferation in a time and dose-dependent manner.
(A) IL-6 dependent INA-6 cells were cultured in the presence of murine, chimeric and humanized MAbs to IL-6, to gp130 or to gp80, as well as with isotype control Abs at various concentrations. Cell proliferation was assessed by [3H]thymidine uptake at 72 hours. (B) IL-6 dependent INA6 and XG1 cells were cultured in the presence of fully humanized anti-IL-6 MAb 1339, as well as with isotype Ab control at various concentrations for variable time (24-72 h) at 37°C. Data represent mean +/- SD of 4 independent experiments performed in triplicate. mAb 1339 inhibits IL-6 dependent MM cell growth in a dose dependent manner.
mAb 1339 overcomes the growth supportive effect of BMSC on MM cells
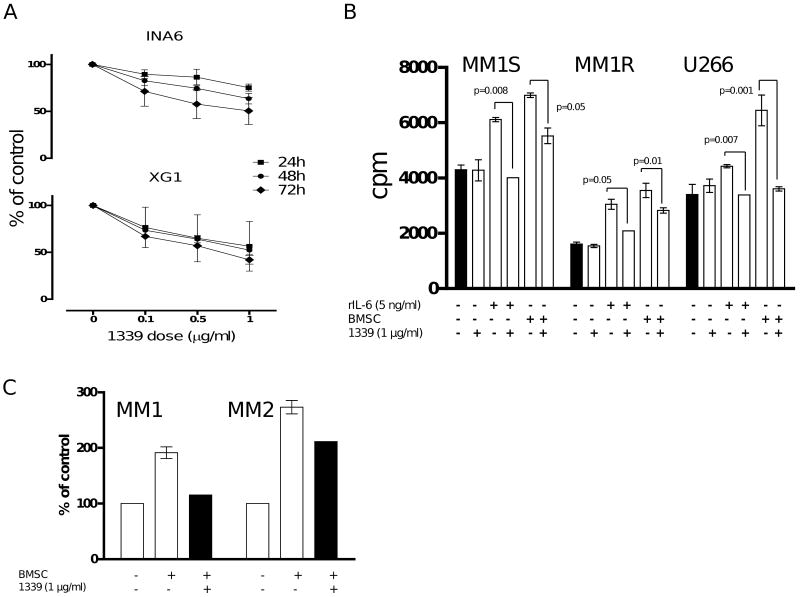
We evaluated ability of mAb 1339 to overcome growth promoting effect of BMSC on INA6 and XG1. MM cell growth was measured at 24, 48, 72 hours in presence of varying concentrations of the antibody. As seen in Figure 2A, mAb 1339 significantly inhibited growth of INA-6 and XG-1 cells in a dose-dependent fashion. We next examined whether mAb 1339 can affect BMSC-induced growth of MM cell lines which are not dependent on IL-6. MM1S, MM1R and U266 cells were cultured for 48 hours in the presence of BMSC with or without 1 μg/ml of mAb 1339. Similar to IL-6 dependent cell lines, growth promoting effects of BMSC on these MM cell lines as well as primary MM cells was significantly inhibited by mAb 1339 (Figure 2B-C).
Figure 2. mAb 1339 overcomes the effect of BMSC on both IL-6 dependent and independent MM cell growth.
(A) INA-6 and XG-1 cells were cultured with BMSC and in the presence of isotype Ab control as well as different doses of mAb 1339 for 24-72 hours. Cell proliferation was assessed by [3H]thymidine uptake assay and presented as % change from control. (B) MM1S, MM1R and U266 cells were cultured with BMSC or 5 ng/ml of rIL-6 with and without 1 μg/ml of mAb 1339, for 48 hours. DNA synthesis was assessed by [3H] thymidine uptake assay and presented as counts per minute (cpm). Data represent mean +/- SD, (N=3), (P≤ 0.05 for all samples). (C) Primary CD138+ MM cells were incubated in the absence or presence of BMSC with and without mAb 1339 for 24 hours. mAb 1339 is able to overcome the promoting effects of BMSC on MM cell growth.
mAb 1339 inhibits STAT3, AKT and ERK signalling pathways in MM1S cells
IL-6 mediates MM proliferation, survival and drug resistance via Raf/MEK/MAPK, JAK/STAT3, and PI3K/Akt pathways, respectively. To examine the effect of mAb 1339 on IL-6-induced signalling cascades, we cultured MM1S cells with mAb 1339 prior to treatment with exogenous IL-6 or BMSC culture supernatant. Both IL-6 (Figure 3A) and BMSC culture supernatant (Figure 3B) triggered STAT3, Akt and ERK1/2 phosphorylation, which is completely abrogated by mAb 1339 in a dose-dependent fashion (0.1 and 1 μg/ml), confirming that mAb 1339-induced growth inhibition of MM cells is associated with blockade of IL-6 signalling cascades.
Figure 3. 1339 inhibits STAT3, ERK1,2 and Akt signaling pathways.
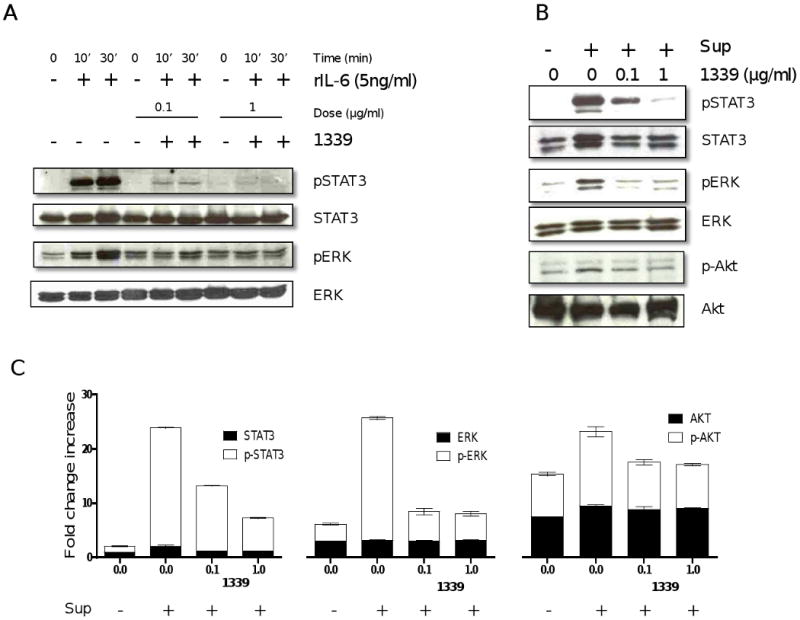
MM1S cells were incubated with (A) IL-6 (5ng/ml, 10 and 30 minutes) or (B) with BMSC supernatant (30 minutes) in the absence or presence of 0.1 and 1 ug/ml of mAb 1339. Cells were harvested and whole cell lysates were subjected to immunoblotting using indicated Abs. 1339 inhibits both IL-6- and BMSC-induced activation of ERK 1, 2 and STAT3 pathway in MM cells, while a relatively lesser effect was observed with activation of AKT, as shown by densitometric quantitation of band intensity from WB (C). Data represent mean +/- SD, (N=3), and are presented as fold change increase compared to control cells.
mAb 1339 potentiates antimyeloma activity of conventional and novel agents
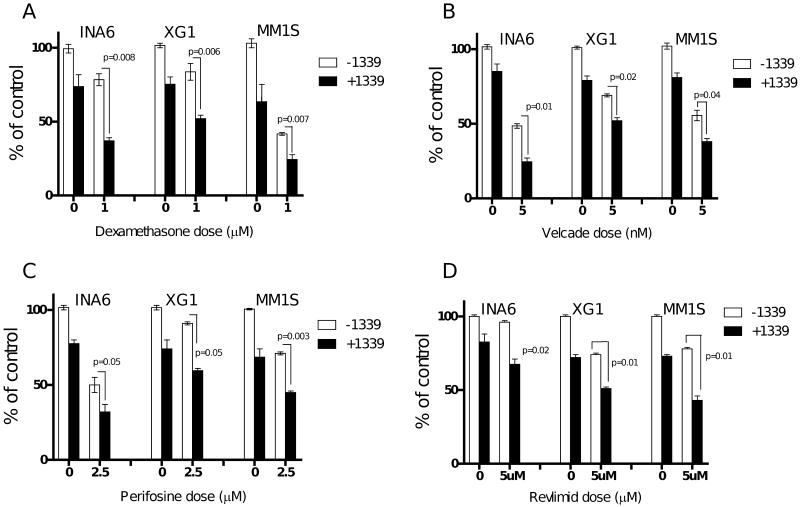
Abrogation of IL-6 signalling increases the sensitivity of MM cells to conventional and novel agents in the presence of BMSC.We next examined if 1339 mAb could enhance cytotoxicity induced by other therapeutic agents. INA-6, XG1 and MM1S cells when cultured with BMSCs in the presence of dexamethasone (1 μM), velcade (5 nM), perifosine (2.5 μM) or Revlimid (5 μM) with or without mAb 1339 (1 μg/ml) for 48 hours and cell proliferation was measured by [3H]Thymidine uptake. MM cell co-culture with BMSCs almost completely abrogated dexamethasone-induced cytotoxicity while this effect was less pronounced with other agents (data not shown). As seen in figure 4, combination of mAb 1339 with dexamethasone or other novel agents induced significantly higher inhibition of BMSC-induced MM cell proliferation compared to antibody or other agents alone. For example, dexamethasone alone triggers 21% and 58% growth inhibition in INA6 and MM1S cells, respectively, which was further enhanced to 60% and 76% inhibition in these cells, respectively (figure 4A). mAb 1339 similarly enhanced cytotoxicity induced by bortezomib (figure 4B), perifosine (figure 4C), and lenalidomide (figure 4D). These results indicate that inhibition of IL-6 signalling can augment dexamethasone-, bortezomib-, perifosine- and lenalidomide-induced cytotoxicity in MM cells in the context of BMSCs.
Figure 4. mAb 1339 enhances growth inhibitory effect of anti-MM agents in the context of BMSC.
INA-6 and MM1S cells were cultured with BMSC in the absence or presence of mAb 1339 (1 μg/ml) and dexamethasone (1 μM), velcade (5nM), perifosine (2.5 μM), revlimid (5 μM) with (1 μg/ml) or without mAb 1339 for 48 hours. DNA synthesis was assessed by [3H] thymidine uptake assay and presented as % of control cells without mAb 1339 or other agents. Data represent mean +/- SD, (N=3). The combination of mAb 1339 and other anti-MM agents resulted in significant (p < 0.05) and synergistic antiproliferative effect.
mAb 1339 promotes MM cell growth inhibition in a SCID-hu mouse model of MM
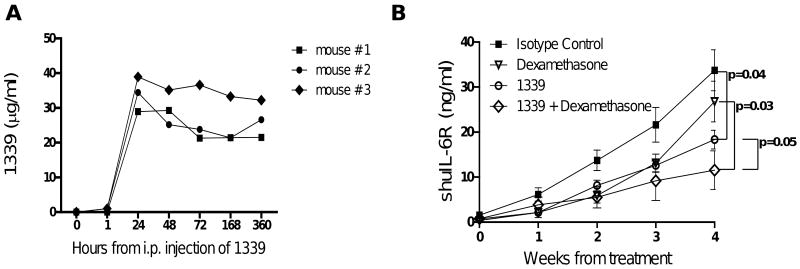
We next evaluated the in vivo effects of mAb 1339 on MM cell growth using a SCID-hu murine model of human MM(19). In this model, the human MM cell line INA6 is engrafted into a human fetal bone chip previously implanted in SCID mice (SCID-hu mice) and the level of soluble human IL-6 receptor (shuIL-6R) produced by INA-6 cells in murine serum is used as marker of tumor burden. We first determined the pharmacokinetics of single intraperitoneal (i.p.) administration (100ug/mouse) of 1339 in SCID mice. As shown in Figure 5A, peak serum concentration (Cmax) of the mAb was observed after 24 hours which was sustained upto 1 week. After the first detection of shuIL-6R, mice were injected with isotype control (100 μg per mouse, 1×/week) (n=6), mAb 1339 (100 μg per mouse, 1×/week) (n=4), dexamethasone (1mg/kg, 3×/week) (n=4), or 1339 plus dexamethasone (n=4). As seen in figure 5B, mAb 1339 alone significantly inhibited MM cells growth (p=0.04); while in the dose and scheduled used, dexamethasone alone had limited effect on MM cell growth in SCID-hu mice (p= 0.07). However, the combination of mAb 1339 and dexamethasone had the greatest effect on MM cell growth which was significantly superior to dexamethasone or mAb 1339 alone.
Figure 5. Additive in vivo effect of MAb 1339 in combination with dexamethasone in a murine model of human MM (SCID-hu).
(A) mAb 1339 (100 μg per mouse) was injected intraperitoneal (i.p.) in SCID mice and its pharmacokinetics was evaluated by quantification of MAbs concentration in serum. After a single injection of mAb 1339, peak serum levels were observed after 24 hours and the MAbs remained in circulation for more than 15 days. (B) After injection of INA-6 MM cells directly into the human bone implant, SCID-hu mice were monitored for productiron of shuIL-6R in murine sera as a marker for MM cell growth. After the first detection of shuIL-6R, mice were injected with isotype control (100 μg per mouse, 1×/week) (n=6), mAb 1339 (100 μg per mouse, 1×/week) (n=4), dexamethasone (1mg/kg, 3×/week) (n=4), or mAb 1339 plus dexamethasone (n=4). Baseline values before treatment were not significantly different among groups.
mAb 1339 preserves bone integrity in a SCID-hu mouse model of MM
IL-6 has been shown to play an important role in osteoclast differentiation and MM bone disease by affecting bone turnover. In order to evaluate the effect of inhibition of IL-6 by 1339 on osteoclast differentiation, human pre-OCs were stimulated with OC differentiation media in presence and absence of IL-6 with or without 1 μg/ml of isotype control or mAb 1339. Our results confirmed the promoting effect of IL-6 on osteoclast differentiation in a time-dependent manner, reaching the maximum effect after 14 days of stimulation (data not shown). As shown in figure 6A, 1339 was able to inhibit OC differentiation and to neutralize the promoting effect of IL-6 on osteoclastogenesis (figure 6A). Next to evaluate effect of 1339 on bone compartment in vivo, we performed micro-CT and bone histological analysis on bone chips retrieved from SCID-hu mice after 1 month of treatment. micro-CT examination demonstrated preserved human bone integrity (figure 6B), and histological analysis by H&E staining confirmed increased trabecular bone and decreased MM cell number in the mAb-treated bones compared to isotype control-treated bone (figure 6C).
Figure 6. Effect of 1339 on osteoclastogenesis in vitro and bone remodeling in vivo.
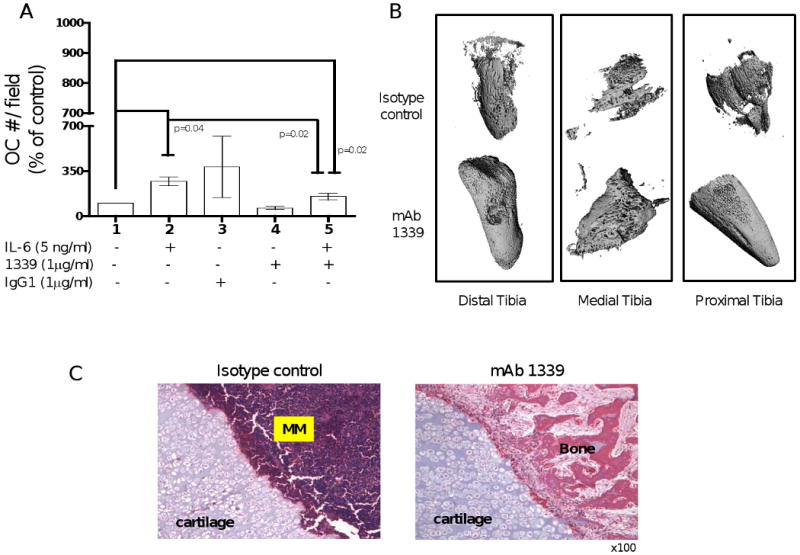
(A) In order to evaluate the effect of inhibition of IL-6 by 1339 on osteoclast differentiation, human pre-OCs were stimulated with OC differentiation media in presence and absence of IL-6 with or without 1 μg/ml of isotype control or mAb 1339 for 7 days. At the end of the treatment period, cells were fixed, stained for TRAP and counted. Data are expressed as mean +/- SD and are presented as percentage change from control. (B-C) To evaluate the effects of 1339 on the bone compartment, SCID-hu mice were injected with INA-6 cells into the implanted bone and were treated with isotype control or mAb 1339 after first detection of tumor. One month after treatment, bone chips were retrieved and analyzed by micro-CT and histological examination. (B) A qualitative assessment of images obtained from micro-CT suggests resistance to osteolytic bone destruction by mAb 1339. (C) H&E staining showed increased bone tissue and decreased MM cell number in the treated samples compared to controls. Original magnification 100×.
Discussion
IL-6 produced in the BM microenvironment by stromal cells contributes significantly to MM cell growth and survival. Patient derived MM cells and cell lines express IL-6 receptors and proliferate in vitro in response to IL-6 by activating major signaling cascades. Binding of IL-6 to its receptor induces phosphorylation of gp130, which subsequently activates Ras/MEK/MAPK, JAK2/STAT3, and PI3K/Akt signaling cascades(22). These signaling cascades mediate MM cell proliferation, survival, drug resistance and migration making IL-6 a promising therapeutic target in MM. Importantly, IL-6 protects against apoptotic cell death induced by a variety of agents, including dexamethasone(22-25).
In the past, anti-IL-6 neutralizing mAbs have been reported to exert remarkable in vitro anti-MM activity, however, their in vivo and clinical effectiveness remains unclear(13). For example, in a phase I study, using a mouse-human chimeric monoclonal anti-IL6 antibody, none of the MM patients achieved a response(26). However, two recent clinical studies of the anti-IL-6 monoclonal antibody CNTO 328, one in combination with dexamethasone and another with bortezomib, have shown evidence of encouraging activity. The study in combination with bortezomib showed a trend for a better response rate and durability than would have been expected with bortezomib alone. These finding provides the rationale to now utilize the fully humanized mAb in combination studies in MM. CNTO 328 has also been evaluated in phase I clinical trial in non-Hodgkin's lymphoma, and Castleman's disease besides MM.
One of the major problems with mAb therapy is its immunogenicity, specifically defined as human anti-mouse antibody (HAMA). To overcome this limitation, murine molecules are engineered to remove immunogenic murine content which has been initially achieved by generation of mouse-human antibodies. Chimeric antibodies are composed of murine variable regions fused with human constant regions. Although with reduced immunogenicity, and prolonged half-life, chimeric mAbs still contain a significant proportion (approximately 35%) of antigenic mouse determinants, suggesting a possibility to generate HAMA. We therefore hypothesized that fully humanized antibody might be more efficient than chimeric antibodies in vivo.
mAb 1339 is a high-affinity fully human anti-IL-6 mAb (IgG1) which was produced using murine Elsilimomab mAb (also known as B-E8) as a template. BE-8 has been used clinically in haematological malignancies(13, 15-17). Unpublished in vitro studies have demonstrated that 1339 shares biological properties with Elsilimomab (B-E8), including affinity and epitope specificity. The conversion of the murine B-E8 MAb into a fully humanized mAb may result in improved therapeutic application due to reduced immunogenicity and prolonged half-life (15-20 days versus 3-4 days(27)) by avoiding the need for daily injection of the mAb and increasing the concentration of circulating mAb in vivo.
Anti-tumor efficacy of mAb therapy may be due to number of possible mechanisms, including anti-proliferation via blockage of growth pathways, intracellular signaling leading to apoptosis, enhanced down regulation and/or turnover of receptors, and promotion of immune responses like ADCC. mAb 1339 is a neutralizing Ab that directly binds to soluble IL-6 abrogating the growth promoting effects of the ligand, however as it does not bind to tumor cells it fails to induces ADCC in MM (data not shown).
In this study, we demonstrate that mAb 1339 is able to inhibit MM growth in the presence of exogenous IL-6 or when used in co-culture experiments with BMSC including non-IL-6-dependent MM cell lines as well as primary myeloma cells. The inhibition of MM cell growth was associated with potent inhibition of STAT3 and ERK1/2 phosphorylation, confirming that mAb 1339-induced growth inhibition of MM cells is associated with blockade of IL-6 signaling cascades. Since other cytokines/chemokines also can activate these signaling cascades in the BM microenvironment, inhibition of individual cytokine may not totally inhibit myeloma cell growth and survival. For example, mAb 1339 is not able to completely block Akt phosphorylatoin in the context of BMSCs since other cytokines (ie, IGF-1, SDF-1α, and VEGF) also trigger PI3K/Akt pathway. We therefore examined and confirmed ability of mAb 1339 mAb to enhance cytotoxicity induced by conventional (dexamethasone) and novel (bortezomib, lenalidomide and perifosine) agents.
Recently, Voorhees et al. described the synergistic effect of CNTO 328 in combination with dexamethasone in a preclinical model of MM in the presence of stroma. While single agent CNTO 328 had minimal apoptotic activity(28), CNTO 328 enhanced the bortezomib-mediated cytotoxicity against MM cells, especially with added dexamethsone. We here demonstrate that mAb 1339 alone had inhibitory effect on MM cell growth in vitro in the presence of BMSC and in vivo in a SCID-hu mouse model. Furthermore, mAb 1339 significantly increased the cytotoxicity of dexamethasone, when used in combination, in the murine model of human myeloma.
IL-6 has been shown as an osteoclast activating factor (OAF) required for human osteoclast precursors proliferation(10, 11, 29). The primary effect of IL-6 on osteoclast formation is to increase the pool of the early osteoclast precursors that in turn differentiate into mature osteoclasts, inducing bone resorption(12). Bone marrow levels of IL-6 have been correlated with bone turnover markers in myeloma. This increased osteoclast activity along with decreased osteoblast activity is responsible for bone lesions in MM which is observed in more than 80% of the patients. However, the precise role that IL-6 plays in osteoclast formation and myeloma bone disease remains to be defined(30). In our in vitro study we have confirmed the osteoclast promoting effect of IL-6. Our in vitro as well as in vivo observation confirms the biologic significance of IL-6 on osteclastogenesis and its role in myeloma bone disease. It also identified IL-6 as an important target to prevent or treat bone related problems in myeloma.
In conclusion, our data demonstrate the anti-MM activity of fully humanized mAb 1339 as a single agent and in combination with conventional and novel agents. More importantly, our study also indicates utility of 1339 to treat MM-associated bone disease and provides evidences that inhibition of the IL-6 signaling pathway could help to control tumor burden and bone disease.
Acknowledgments
This work was supported in part by grants from the Dept. of Veterans Affairs Merit Review Awards and from the National Institutes of Health Grants RO1-124929 (NCM.), P50-100007 and PO1-78378 to NCM and KCA.
Footnotes
Statement of Translational relevance: Interleukin-6 (IL-6) plays important role in multiple myeloma (MM) cell growth and survival within BM milieu, making it an important therapeutic target in MM. The preclinical studies presented here are designed to identify both in vitro and in vivo efficacy of a fully human anti-IL-6-neutralizing antibody (1339) and have important implications for the therapy of multiple myeloma. mAb 1339 is generated from the murine monoclonal antibody and the conversion to a fully humanized mAb could reduce immunogenicity and prolong half-life. In the present report we describe the synergistic effect between mAb 1339 and conventional and novel agents, suggesting a potential clinical utility of these combinations. In addition, we report both in vitro and in vivo inhibition of bone turnover by mAb 1339 providing additional application in bone disease in myeloma. The data generated will help to extend this promising area of research and provide a basis for development of a clinical protocol in relapsed myeloma.
Author contributions: MF made the experimental plan, carried out in vitro and in vivo experiments, analyzed the data, and prepared the manuscript. TH assisted in data interpretation, analysis, and preparation of manuscript. CV-D provided the reagents. SP performed the osteoclast differentiation in vitro assay. PN participated in cell culture studies. ZS carried out immunohistochemistry analyses. NP performed μCT analyses. YT, RP, PT and KCA helped in the analysis and interpretation of data. NCM envisioned the study, and participated in its design and coordination, and also helped to draft the manuscript. All authors have read and approved the final manuscript.
References
- 1.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 2.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 3.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 4.Hilbert DM, Kopf M, Mock BA, Kohler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J Exp Med. 1995;182:243–8. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. Embo J. 1994;13:1189–96. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–72. [PubMed] [Google Scholar]
- 7.Klein B, Zhang XG, Jourdan M, et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517–26. [PubMed] [Google Scholar]
- 8.Podar K, Gouill SL, Zhang J, et al. A pivotal role for Mcl-1 in Bortezomib-induced apoptosis. Oncogene. 2008;27:721–31. doi: 10.1038/sj.onc.1210679. [DOI] [PubMed] [Google Scholar]
- 9.Voorhees PM, Chen Q, Kuhn DJ, et al. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin Cancer Res. 2007;13:6469–78. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- 10.Mundy GR, Raisz LG, Cooper RA, Schechter GP, Salmon SE. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med. 1974;291:1041–6. doi: 10.1056/NEJM197411142912001. [DOI] [PubMed] [Google Scholar]
- 11.Kurihara N, Bertolini D, Suda T, Akiyama Y, Roodman GD. IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release. J Immunol. 1990;144:4226–30. [PubMed] [Google Scholar]
- 12.Reddy SV, Takahashi S, Dallas M, Williams RE, Neckers L, Roodman GD. Interleukin-6 antisense deoxyoligonucleotides inhibit bone resorption by giant cells from human giant cell tumors of bone. J Bone Miner Res. 1994;9:753–7. doi: 10.1002/jbmr.5650090522. [DOI] [PubMed] [Google Scholar]
- 13.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–65. [PMC free article] [PubMed] [Google Scholar]
- 14.Gu ZJ, De Vos J, Rebouissou C, et al. Agonist anti-gp130 transducer monoclonal antibodies are human myeloma cell survival and growth factors. Leukemia. 2000;14:188–97. doi: 10.1038/sj.leu.2401632. [DOI] [PubMed] [Google Scholar]
- 15.Bataille R, Barlogie B, Lu ZY, et al. Biologic effects of anti-interleukin-6 murine monoclonal antibody in advanced multiple myeloma. Blood. 1995;86:685–91. [PubMed] [Google Scholar]
- 16.Emilie D, Wijdenes J, Gisselbrecht C, et al. Administration of an anti-interleukin-6 monoclonal antibody to patients with acquired immunodeficiency syndrome and lymphoma: effect on lymphoma growth and on B clinical symptoms. Blood. 1994;84:2472–9. [PubMed] [Google Scholar]
- 17.Rossi JF, Fegueux N, Lu ZY, et al. Optimizing the use of anti-interleukin-6 monoclonal antibody with dexamethasone and 140 mg/m2 of melphalan in multiple myeloma: results of a pilot study including biological aspects. Bone Marrow Transplant. 2005;36:771–9. doi: 10.1038/sj.bmt.1705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–9. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tassone P, Neri P, Carrasco DR, et al. A clinically relevant SCID-hu in vivo model of human multiple myeloma. Blood. 2005;106:713–6. doi: 10.1182/blood-2005-01-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller R, Hildebrand T, Ruegsegger P. Non-invasive bone biopsy: a new method to analyse and display the three-dimensional structure of trabecular bone. Phys Med Biol. 1994;39:145–64. doi: 10.1088/0031-9155/39/1/009. [DOI] [PubMed] [Google Scholar]
- 21.Ruegsegger P, Koller B, Muller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int. 1996;58:24–9. doi: 10.1007/BF02509542. [DOI] [PubMed] [Google Scholar]
- 22.Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–37. doi: 10.1038/nrc952. [DOI] [PubMed] [Google Scholar]
- 23.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan D, Kharbanda S, Ogata A, et al. Interleukin-6 inhibits Fas-induced apoptosis and stress-activated protein kinase activation in multiple myeloma cells. Blood. 1997;89:227–34. [PubMed] [Google Scholar]
- 25.Chauhan D, Pandey P, Hideshima T, et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J Biol Chem. 2000;275:27845–50. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 26.van Zaanen HC, Lokhorst HM, Aarden LA, et al. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. Br J Haematol. 1998;102:783–90. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 27.Brochier J, Legouffe E, Liautard J, et al. Immunomodulating IL-6 activity by murine monoclonal antibodies. Int J Immunopharmacol. 1995;17:41–8. doi: 10.1016/0192-0561(94)00076-z. [DOI] [PubMed] [Google Scholar]
- 28.Voorhees PM, Chen Q, Small GW, et al. Targeted inhibition of interleukin-6 with CNTO 328 sensitizes pre-clinical models of multiple myeloma to dexamethasone-mediated cell death. Br J Haematol. 2009;145:481–90. doi: 10.1111/j.1365-2141.2009.07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merico F, Bergui L, Gregoretti MG, et al. Cytokines involved in the progression of multiple myeloma. Clin Exp Immunol. 1993;92:27–31. doi: 10.1111/j.1365-2249.1993.tb05943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283:11535–40. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]